2020-03-09 15:13:55
Lucas Schirmera, Karolina Chwaleka, Mikhail V. Tsurkana, Uwe Freudenberga, Carsten Wernera, Leibniz-Institut für Polymerforschung Dresden e.V., Max Bergmann Center of Biomaterials Dresden, Hohe Str. 6, 01069, Dresden, Germany
Technische Universität Dresden, Center for Regenerative Therapies Dresden, Fetscherstr, 105, 01307, Dresden, Germany
1.Introduction
Tris(2-carboxyethyl)phosphine (TCEP) and N-(2- aminoethyl)maleimide trifluoroacetate salt were purchased from Sigma-Aldrich. Polymer hydrogels are widely used in tissue engineering technologies as transient artificial extracellular matrix (ECM) substitutes to provide mechanical support, cell adhesion sites, cell-responsive remodeling, and transport of nutrients and metabolites [3–5]. Glycosaminoglycan (GAG)-based polymer networks have emerged as a particularly powerful hydrogel variant as they allow for the biomimetic administering of cytokines to direct cell fate decisions [6]. To exploit the resulting options, we have previously developed a theory-driven design concept of GAG-based hydrogels enabling the independent modulation of multiple cell-instructive matrix cues [7]: biohybrid hydrogels formed from multi-armed (star-shaped) poly(ethylene glycol) (star PEG) and the GAG heparin were crosslinked by incorporating matrix metalloproteinase-sensitive (MMP)-peptides to enable cell responsive remodeling and integrin-binding RGD peptides for control of cell adhesion [8–12]. Varying the molar ratio of star PEG to heparin and the solid content of the reaction mixture created unprecedented options for adjusting the crosslinking degree – and thus the mechanical material properties – at fixed concentrations of the bioactive components of the hydrogels [7,12–14]. The highly negatively charged GAG heparin af- fords the non-covalent complexation of a plethora of different chemo- kines and growth factors, including vascular endothelial growth factor (VEGFa), fibroblast growth factor 2 (FGF2), stromal cell-derived factor (SDF1α) [10,15–23]. Rationally designed star PEG-GAG hydrogels can be formed using different crosslinking reactions: isopeptide bonds in between carboxylic acid groups of GAGs and amine-terminated star PEG-peptide units can be formed by active ester (carbodiimide) chemistry [13]. Alternatively, maleimide-functionalized GAGs can be converted with thiol-terminated star PEG-peptide conjugates using a Michael-type addition reaction scheme [12]. The latter approach allows for the cell- and tissue-compatible in situ gelation and is thus suitable for minimally invasive implantation techniques.
While previous in vivo studies have been exploring biomedical applications of star PEG-GAG hydrogels include the accumulation of progenitor cells in ischemic tissue [10], the anti-inflammatory treatment and healing of chronic cutaneous wounds [24,25] and new cell transplantation strategies for Parkinson's disease [20], they mostly focused on the cell-instructive characteristics of the materials and did not investigate the host reaction to the implants. Upon implantation, all biomaterials induce a foreign body response to a different degree, de- pending on their interactions with the host tissue [26]. Degradation and the rate of cellular infiltration - as the first stage of tissue reconstruction - as well as the dynamically changing physical properties of the materials and their capability of presenting soluble mediator molecules (in particular growth factors) have been reported to determine the fate of the implants [28]. When implanted subcutaneously, pure PEG-based hydrogels – without a GAG-component – have been shown to induce a significant inflammatory reaction, which can be partially attenuated by the incorporation of RGD cell adhesion ligands [29,30]. Furthermore, PEG hydrogels have been loaded with various types of growth factors to promote desired pro-regenerative processes such as vascularization (VEG Fa) [31] or bone repair (bone morphogenetic protein-2 (BMP-2)) [27]. However, no study has so far evaluated the foreign body reaction of in situ-assembling star PEG-GAG-hydrogels that sustainably deliver growth factors due to the incorporated GAG units serving as high-affinity binding sites and allow for the independent tuning of physical network properties, adhesiveness, and cell-responsive remodeling. As an integral part of the extracellular matrix, GAGs fulfil a number of diverse functions in living tissues. Creating reservoirs of signaling mediators and modulating their functionalities, they fulfil multifaceted roles in embryogenesis and development [32,33], homeostasis [34], inflammation [35], tumor formation and progression [36]. Referring to the resulting importance of GAG-based, cell-instructive materials, this study aimed to systematically evaluating host response patterns to modular in situ-forming starPEG-GAG-hydrogels of distinct physical and adhesive properties, biodegradation characteristics, and pro-angiogenic growth factor functionalization. Subcutaneous implantation in wild- type, immunocompetent mice (C57BL/6 J) was chosen as a representative model [37–40]. The immune and angiogenic responses to the subcutaneously implanted materials were determined by histology, including H&E, CD31, and CD68 stains. The integration of the hydrogels within target tissues was assessed by evaluating the cellular in- filtration of the materials and the vascularization of adjacent tissues. The reported data prove the excellent tissue compatibility, suggesting tunable in situ-assembling star PEG-GAG hydrogels for safe and effective in vivo tissue engineering applications.
2.Materials and methods
2.1.Synthesis and purification of cysteine-terminated PEG-(MMP)4
PEG-peptide conjugate (PEG-(MMP)4) was synthesized, as previously described [12]. Briefly, MMP-cleavable Ac-GGPQGIWGQG- GCG-NH2 (MMP) peptide was synthesized by standard Fmoc-based solid-phase method on a peptide synthesizer (Activo P14, Activotec) and its purity was assessed by analytical HPLC chromatography (1100 Series; Agilent Technologies) and MALDI mass spectroscopy (Bruker Daltonik GmbH). Next, 500 mg of four-arm maleimide-terminated PEG- (Maleimide)4 (MW 10000) was dissolved in 5 ml of water and mixed with 350 mg of MMP peptide (5% excess) which was dissolved in 5 ml of water. Next, 5 ml of phosphate buffer pH 7.4 was added. The pH of the reaction mixture was adjusted to pH 7.5–8 by addition of 1 M NaOH. The reaction was run for 5 h under the N2 atmosphere, and the completion of the reaction was followed by HPLC. In order to remove the StBu protection groups, a five times molar excess (to the peptide) TCEP solution (pH 7–8) was added to the reaction mixture and was stirred at room temperature for several hours. Next, the reaction mixture was evaporated, dissolved in water, and purified by preparative HPLC. The product was collected from the HPLC, mixed with 0.1 ml of 1 M HCl and freeze-dried for at least 24 h. The resulting PEG-peptide conjugate (PEG-(MMP)4) in the form of white powder was stored at −20 °C.
2.2.Synthesis and purification of heparin-maleimide conjugates
All the heparin maleimide conjugates were synthesized as previously described [12,41]. Briefly, threefold molar excess of EDC and 1.5-fold molar excess of sNHS (referring to the maleimide amine) were added to a solution of 0.5 g of heparin dissolved in 3 ml of ultrapure water. The reaction mixtures were kept at 4 °C for 20 min. Next, N-(2- aminoethyl)maleimide trifluoroacetate salt dissolved in ultrapure water (at 4 °C) was added to the reaction mixtures and stirred overnight at room temperature. The conjugates were purified by dialysis (membrane with 8 kDa molecular weight cut-off) against 1 l of 1 M sodium chloride three times, followed by dialysis against 1 l of water five times in order to remove any unreacted maleimide amine. The products were then freeze-dried for at least 24 h and stored at −20 °C. The purity and reactively of the formed conjugates were controlled by HPSEC (1100 Series; Agilent Technologies).
All solvents and reagents for peptide synthesis were purchased from IRIS Biotech GmbH. Tris(2-carboxyethyl)phosphine (TCEP) and N-(2- aminoethyl)maleimide trifluoroacetate salt were purchased from Sigma-Aldrich. Four-armed maleimide terminated polyethylene glycol (Mn = 10.0 × 103; PDI = 1.06) was purchased from JenKem Technology USA Inc. All the reagents were used as is without preliminary purification.
2.3.Hydrogel preparation
Hydrogels were prepared as described previously [9]. The gel preparation took place on the same day as in vivo implantation. Briefly, 10 mg of heparin-maleimide conjugate (MW 15000) was dissolved in 107 μl of phosphate-buffered saline (PBS) (Biochrom). The heparin solution was cooled down on ice and added to 0.79 mg cRGD (Peptides International) at a final concentration of 3 mM. Heparin-RGD solution was mixed with mouse recombinant growth factors (VEG Fa, FGF2, or SDF1α; PeproTech). The final amount of growth factor per implant was varied, as stated in Table 1. 10 mg of PEG-MMP conjugate (MW 15920) or PEG (MW 15000), which was dissolved in 372 μl of PBS. Heparin- RGD solution was mixed gently with the PEG or PEG-MMP conjugate solution in ratio 1:1, and 30 μl of solution was quickly sandwiched between two 5 mm glass coverslips coated with Sigmacote (Sigma-Aldrich). The gel formation occurred at room temperature within a minute. Upon crosslinking, the coverslips were removed, leaving a discshaped hydrogel. Each of the gel discs was immediately immersed in 400 μl of PBS to prevent dehydration and stored at 4 °C till implantation.
2.4.Rheology
Storage moduli of the hydrogels were determined with oscillating measurements on swollen gel disks carried out on a rotational rheometer (Ares LN2, TA Instruments) fitted with a 25 mm parallel plate geometry. Frequency sweeps were carried out at 25 °C in a shear frequency range of 100–102 rad/s with the strain amplitude of 2%. Both storage and loss modulus were measured as a function of the shear frequency. Due to the elastic nature of the hydrogel, the loss modulus was several magnitudes lower compared to the storage moduli, and under the measuring limit of the rheometer. Mean values of the storage modulus were calculated from at least three independent experiments including three technical replicates each. To assess the effects of collagenase degradation on the storage modulus, the hydrogel was formed at 4 °C with the addition of 1 U/ml collagenase type IV (Biochrome) at 1 Hz sweeps until no further increase stiffness was de- tectable. Then the temperature was increased to 37 °C to activate the collagenase, and 1 Hz sweeps were continued until degradation of the implant.
2.5.Animals and surgical procedure
All animal care and experimental procedures were approved by the Regional Office Sachsen (Landesdirektion Sachsen) Animal Care and Use Committee of as well as by Technical University Dresden Institutional Animal Care and Use Committee (24-9168.11-6/2012-1). 12-week old male mice of the strain C57BL/6 J OlaHsd (Harlan Laboratories GmbH) were housed in single ventilated cages (3–4 mice per cage).
Before the surgical procedure, the mice were weighed and anesthetized by intraperitoneal injection of ketamine 100 mg/kg and xylazine 10 mg/kg. The back of the animals was shaved and disinfected with ethanol. The hydrogel discs were implanted subcutaneously by inserting them in small pockets under the skin and closing the skin with sutures. Silicon discs (Silastic MDX4-4210 Biomedical Grade Elastomer, Dow Corning) were implanted as a control. Each mouse obtained two implants on the right and left side of the back. One of the two implants contained growth factors. The experimental setup included eight mice per condition, although not all hydrogel scaffolds could be retrieved at the termination of the experiment. All mice were monitored until they recovered from anesthesia. Upon either 7 or 28 days, the mice were euthanized by CO2 asphyxiation and cervical dislocation. The implant and the adjacent area of full-thickness dermal tissue were excised together and immediately fixed in 10% buffered formalin (Fisher Scientific) for subsequent analysis.
2.6.Histology
The fixed tissue samples were subjected to automated tissue processing, including gradual dehydration in ethanol and xylene solutions. Afterward, the samples were embedded in paraffin blocks (Paraplast Plus), and sagittal sections, including the adjacent repair tissue, were cut using a rotary microtome and stained with antibodies against CD31 and CD68 using the avidin-biotin-peroxidase complex (ABC) technique with diaminobenzidine (DAB) substrate reagent. Additionally, all the sections were stained with hematoxylin-eosin (H&E) stain (cell nuclei- blue; cytoplasm, elastin, and collagen-pink). The stained sections were observed and photographed using automated slide scanner Axio Scan.Z1 with a Plan-Apochromat 20×/0.8 M27 (Zeiss).
2.7.Image analysis
Images were analyzed with ImageJ. Degradation of the hydrogel was scored from blinded images from 1 to 5, according to Table 2. Foreign body reaction was assessed by repeatedly measuring the width of the formed capsule at the adjacent to the implant. Effects on angiogenesis were quantified as visible CD31-positive blood vessels in the tissue below the panniculus carnosus within the remodeled hydrogel or in the directly adjacent tissue.
2.8.Statistical analysis
In all in vitro experiments, at least three specimens from each hydrogel were tested. Data are presented as mean ± standard deviation (SD). Statistical analyses were performed by one-way analysis of variance (ANOVA). Data are presented as mean ± standard deviation (SD). Statistical analyses were performed by one-way analysis of variance (ANOVA) if not described otherwise. Any P value less than 0.05 was statistically significant.
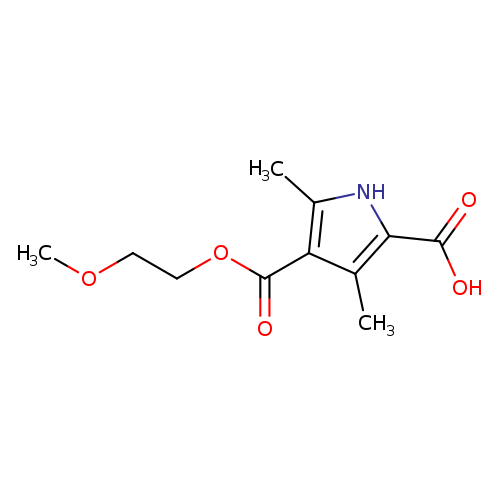
4-[(2-methoxyethoxy)carbonyl]-3,5-dimethyl-1H-pyrrole-2-carboxylic acidCatalog No.:AA019NFN CAS No.:1114822-74-0 MDL No.:MFCD11814079 MF:C11H15NO5 MW:241.2405 |
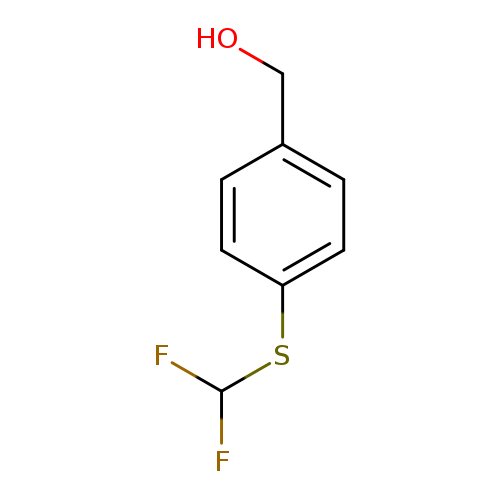
{4-[(difluoromethyl)sulfanyl]phenyl}methanolCatalog No.:AA019U65 CAS No.:1114822-77-3 MDL No.:MFCD11857780 MF:C8H8F2OS MW:190.2103 |
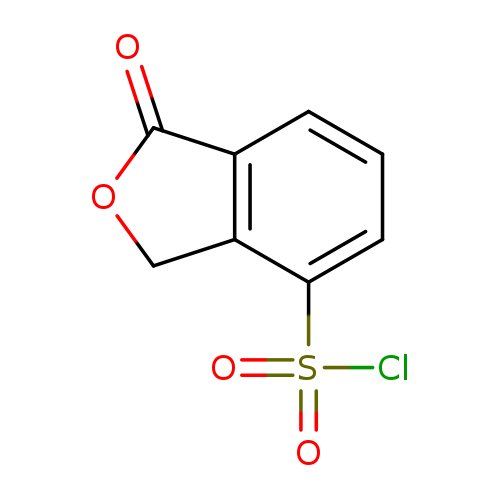
1-Oxo-1,3-dihydroisobenzofuran-4-sulfonyl chlorideCatalog No.:AA019U6V CAS No.:1114822-78-4 MDL No.:MFCD11857785 MF:C8H5ClO4S MW:232.6409 |
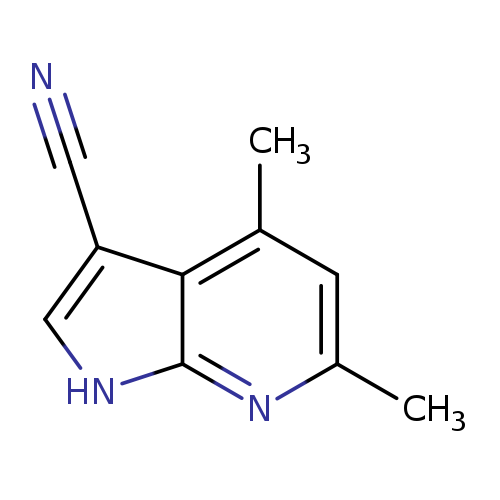
4,6-Dimethyl-1h-pyrrolo[2,3-b]pyridine-3-carbonitrileCatalog No.:AA019U8M CAS No.:1114822-80-8 MDL No.:MFCD11837415 MF:C10H9N3 MW:171.1986 |
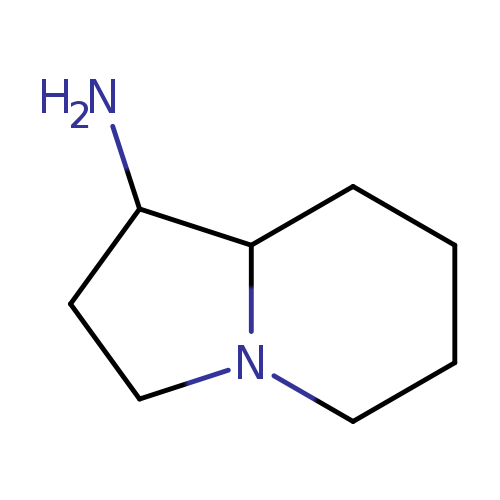
Octahydroindolizin-1-amineCatalog No.:AA019U8V CAS No.:1114822-81-9 MDL No.:MFCD11857793 MF:C8H16N2 MW:140.2260 |
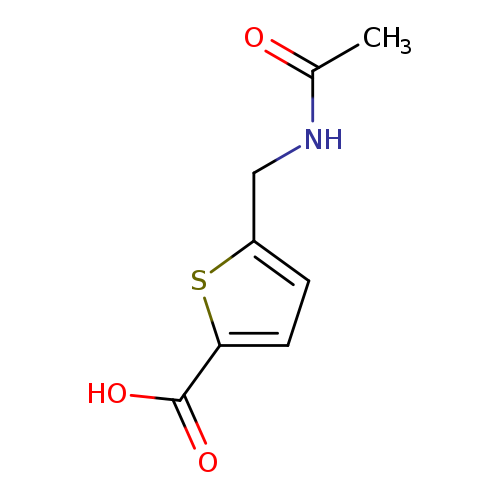
5-(acetamidomethyl)thiophene-2-carboxylic acidCatalog No.:AA019U9J CAS No.:1114822-83-1 MDL No.:MFCD11857799 MF:C8H9NO3S MW:199.2270 |
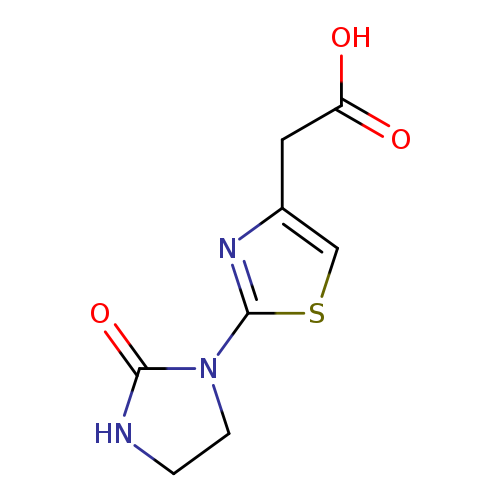
2-[2-(2-Oxoimidazolidin-1-yl)-1,3-thiazol-4-yl]acetic acidCatalog No.:AA019UAO CAS No.:1114822-84-2 MDL No.:MFCD09041463 MF:C8H9N3O3S MW:227.2404 |
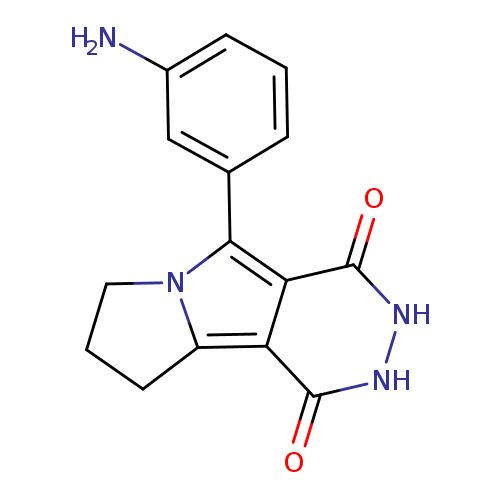
5-(3-aminophenyl)-1H,2H,3H,4H,7H,8H,9H-pyridazino[4,5-a]pyrrolizine-1,4-dioneCatalog No.:AA019UCH CAS No.:1114822-85-3 MDL No.:MFCD11838894 MF:C15H14N4O2 MW:282.2973 |
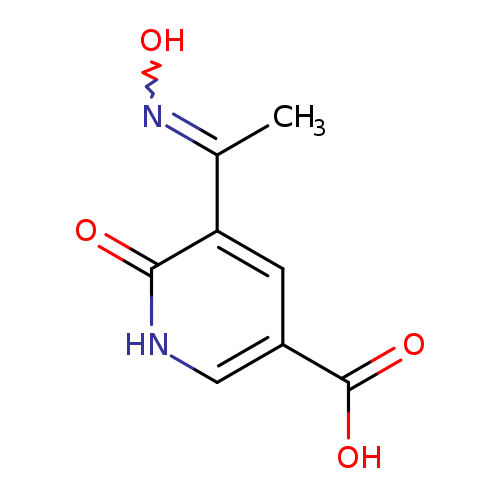
5-[(1E)-1-(Hydroxyimino)ethyl]-6-oxo-1,6-dihydropyridine-3-carboxylic acidCatalog No.:AA019WW9 CAS No.:1114822-87-5 MDL No.:MFCD11505643 MF:C8H8N2O4 MW:196.1601 |
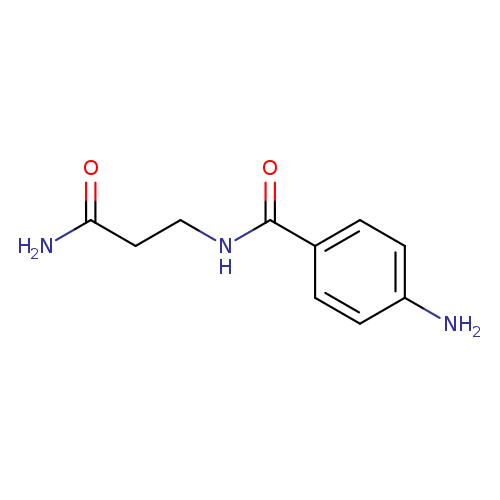
4-Amino-n-(3-amino-3-oxopropyl)benzamideCatalog No.:AA019QVL CAS No.:1114822-89-7 MDL No.:MFCD11505649 MF:C10H13N3O2 MW:207.2291 |
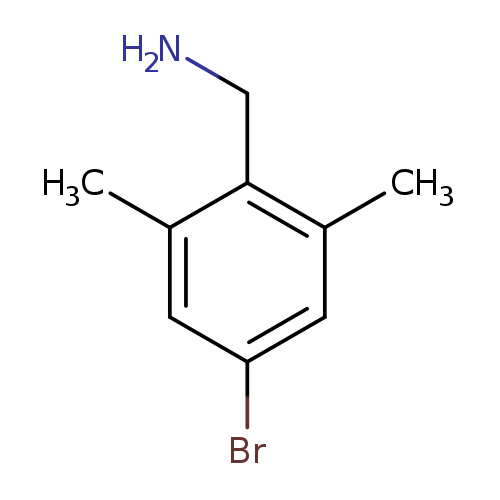
(4-bromo-2,6-dimethylphenyl)methanamineCatalog No.:AA00952F CAS No.:1114822-90-0 MDL No.:MFCD11227153 MF:C9H12BrN MW:214.1023 |
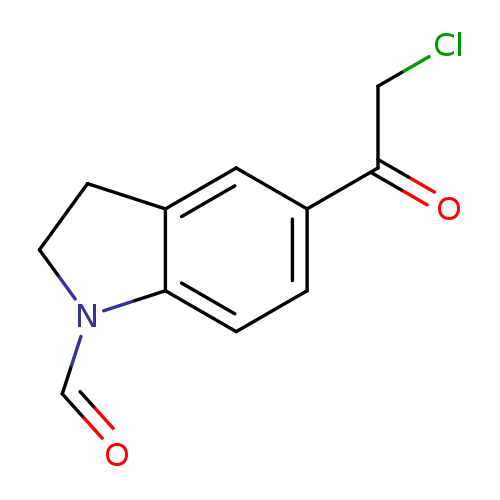
5-(Chloroacetyl)indoline-1-carbaldehydeCatalog No.:AA019MSP CAS No.:1114823-88-9 MDL No.:MFCD11505378 MF:C11H10ClNO2 MW:223.6556 |
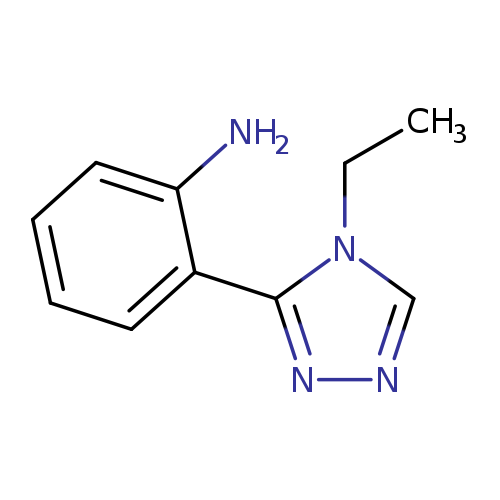
2-(4-ethyl-4H-1,2,4-triazol-3-yl)anilineCatalog No.:AA019MYL CAS No.:1114823-89-0 MDL No.:MFCD11857767 MF:C10H12N4 MW:188.2291 |
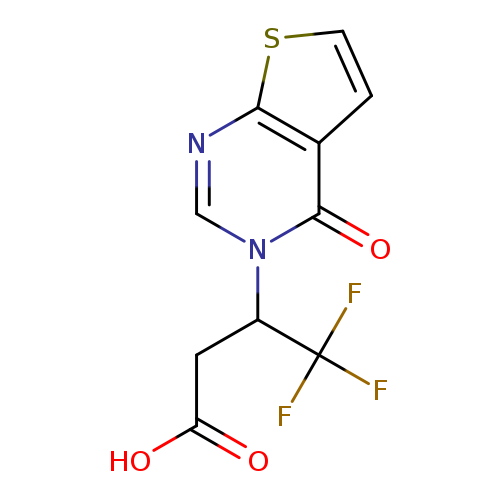
4,4,4-Trifluoro-3-(4-oxothieno[2,3-d]pyrimidin-3(4h)-yl)butanoic acidCatalog No.:AA019N97 CAS No.:1114823-92-5 MDL No.:MFCD11505539 MF:C10H7F3N2O3S MW:292.2344 |
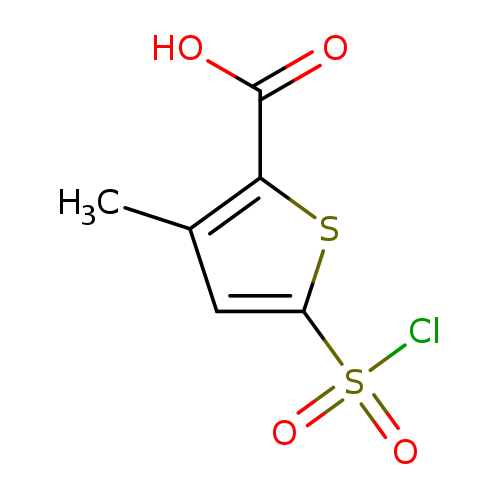
5-(Chlorosulfonyl)-3-methylthiophene-2-carboxylic acidCatalog No.:AA019NAC CAS No.:1114823-93-6 MDL No.:MFCD11505555 MF:C6H5ClO4S2 MW:240.6845 |
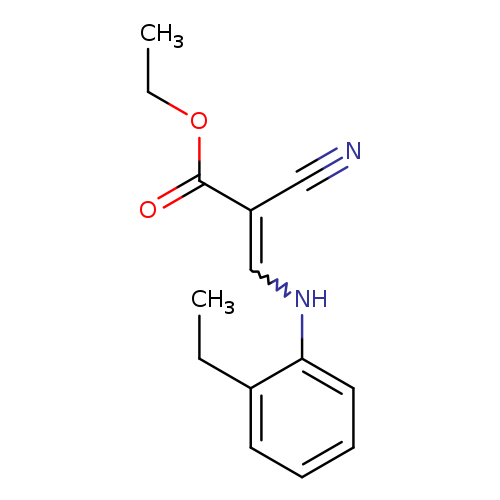
ethyl 2-cyano-3-[(2-ethylphenyl)amino]prop-2-enoateCatalog No.:AA019NFU CAS No.:1114823-95-8 MDL No.:MFCD11761953 MF:C14H16N2O2 MW:244.2890 |
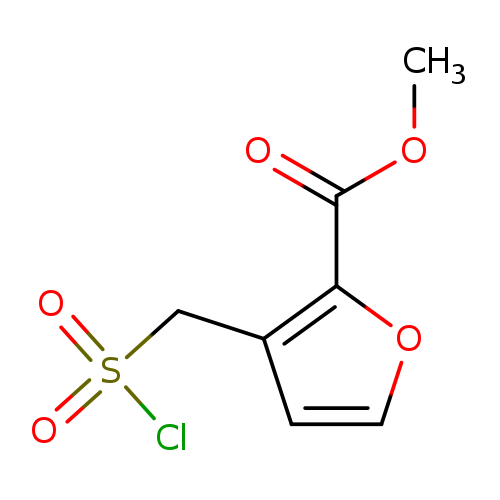
methyl 3-[(chlorosulfonyl)methyl]furan-2-carboxylateCatalog No.:AA019U5V CAS No.:1114823-97-0 MDL No.:MFCD11505634 MF:C7H7ClO5S MW:238.6455 |
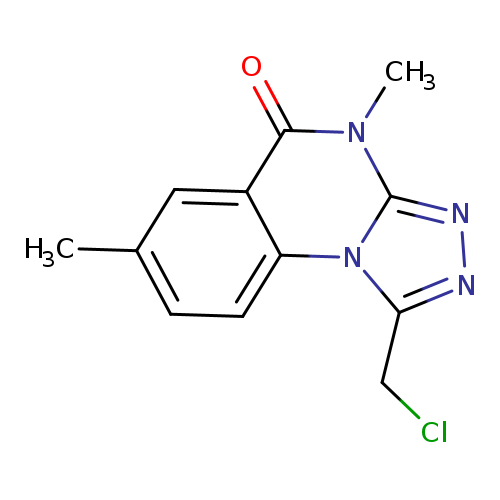
1-(chloromethyl)-4,7-dimethyl-4H,5H-[1,2,4]triazolo[4,3-a]quinazolin-5-oneCatalog No.:AA019U8E CAS No.:1114824-01-9 MDL No.:MFCD11839771 MF:C12H11ClN4O MW:262.6949 |
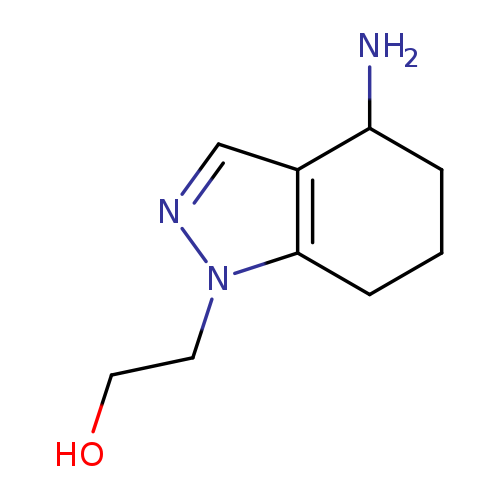
2-(4-amino-4,5,6,7-tetrahydro-1H-indazol-1-yl)ethan-1-olCatalog No.:AA019U8Y CAS No.:1114824-03-1 MDL No.:MFCD11857795 MF:C9H15N3O MW:181.2349 |
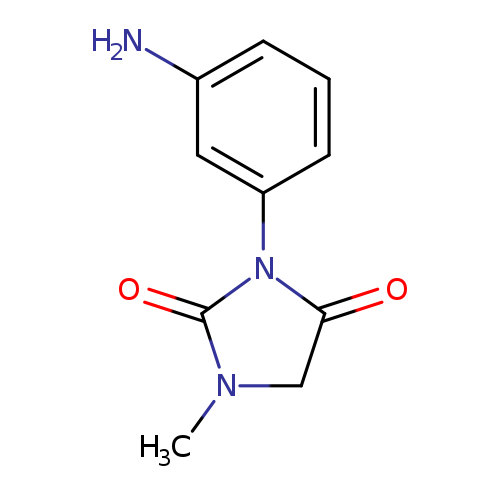
3-(3-Aminophenyl)-1-methylimidazolidine-2,4-dioneCatalog No.:AA008VEI CAS No.:1114824-11-1 MDL No.:MFCD11857888 MF:C10H11N3O2 MW:205.2132 |

(2S)-2-Methylbutanoic Acid (1S,3S,7S,8S,8aR)-8-[2-[(2R)-3,6-Dihydro-6-oxo-2H-pyran-2-yl]ethyl]-1,2,3,7,8,8a-hexahydro-3-hydroxy-7-Methyl-1-naphthalenyl EsterCatalog No.:AA008WC5 CAS No.:1114834-63-7 MDL No.:MFCD29045548 MF:C23H32O5 MW:388.4972 |
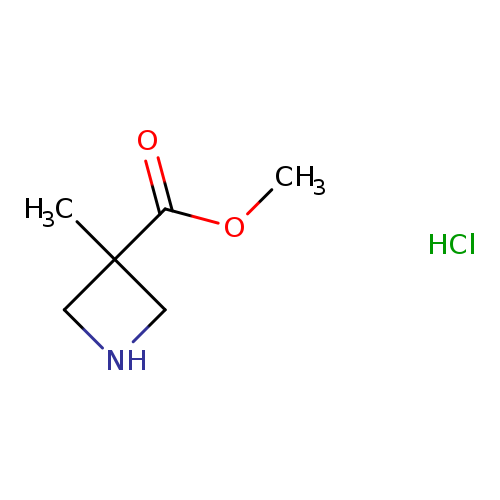
Methyl 3-methylazetidine-3-carboxylate hydrochlorideCatalog No.:AA00HC35 CAS No.:1114876-08-2 MDL No.:MFCD12408554 MF:C6H12ClNO2 MW:165.6180 |
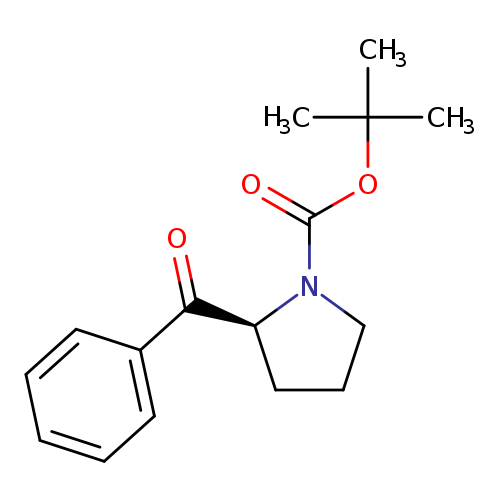
tert-butyl (2s)-2-benzoylpyrrolidine-1-carboxylateCatalog No.:AA007ABG CAS No.:111492-61-6 MDL No.:MFCD24368824 MF:C16H21NO3 MW:275.3428 |
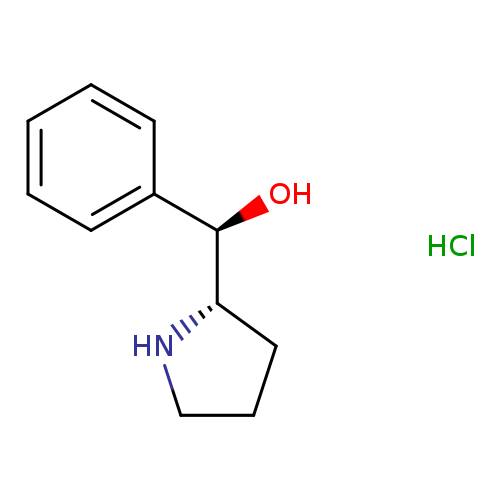
(R)-Phenyl[(2s)-pyrrolidin-2-yl]methanol hydrochlorideCatalog No.:AA01B6UJ CAS No.:111492-62-7 MDL No.:MFCD30344752 MF:C11H16ClNO MW:213.7038 |
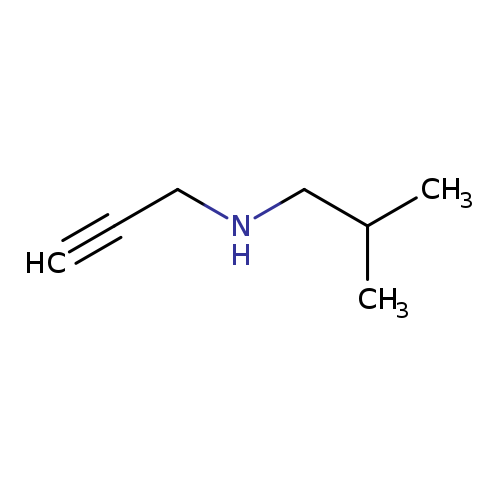
(2-METHYLPROPYL)(PROP-2-YN-1-YL)AMINECatalog No.:AA01E5ZI CAS No.:111493-01-7 MDL No.:MFCD04035012 MF:C7H13N MW:111.1848 |
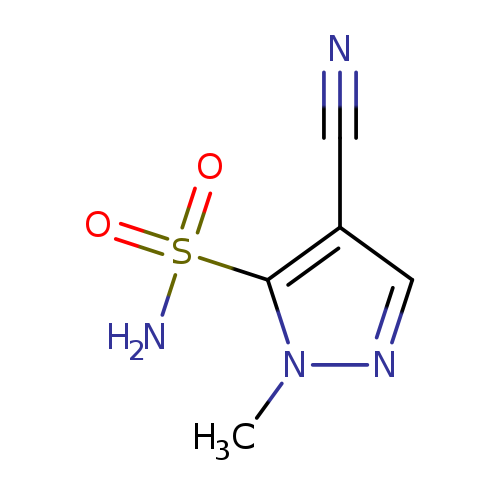
4-Cyano-1-methyl-1H-pyrazole-5-sulfonamideCatalog No.:AA009PTR CAS No.:111493-51-7 MDL No.:MFCD18809745 MF:C5H6N4O2S MW:186.1917 |
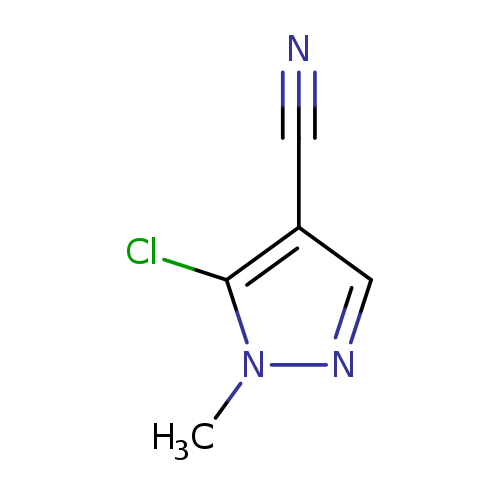
5-Chloro-1-methyl-1h-pyrazole-4-carbonitrileCatalog No.:AA0092TR CAS No.:111493-52-8 MDL No.:MFCD16619821 MF:C5H4ClN3 MW:141.5584 |
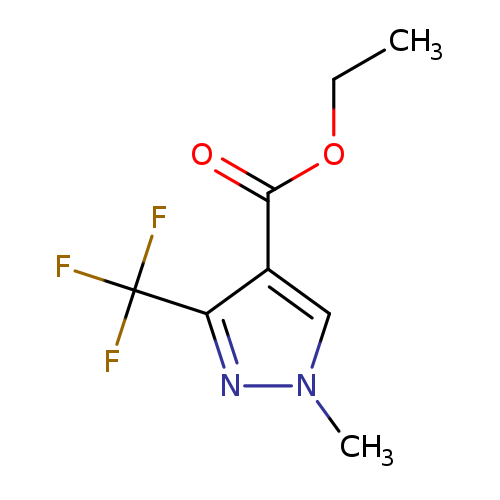
ethyl 1-methyl-3-(trifluoromethyl)pyrazole-4-carboxylateCatalog No.:AA0034R7 CAS No.:111493-74-4 MDL No.:MFCD00009136 MF:C8H9F3N2O2 MW:222.1645 |
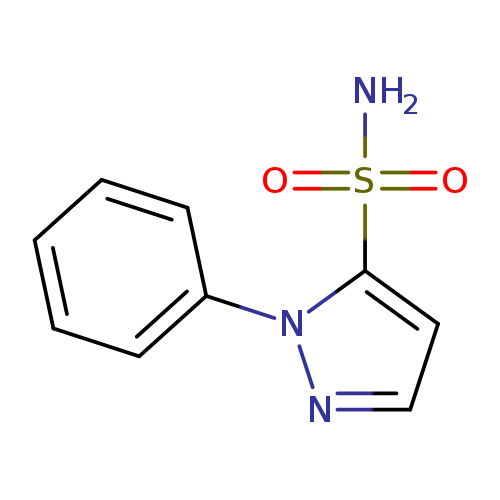
1-phenyl-1h-pyrazole-5-sulfonamideCatalog No.:AA01EE53 CAS No.:111494-00-9 MDL No.:MFCD31718376 MF:C9H9N3O2S MW:223.2517 |
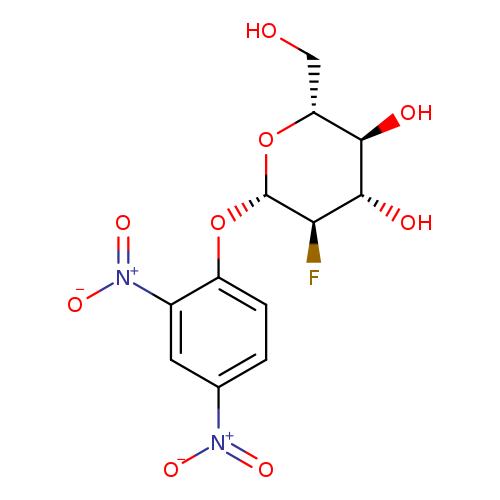
2,4-DINITROPHENYL-2-FLUORO-2-DEOXY-BETA-D-GLUCOPYRANOSIDECatalog No.:AA008V2S CAS No.:111495-86-4 MDL No.:MFCD00132931 MF:C12H13FN2O9 MW:348.2380 |
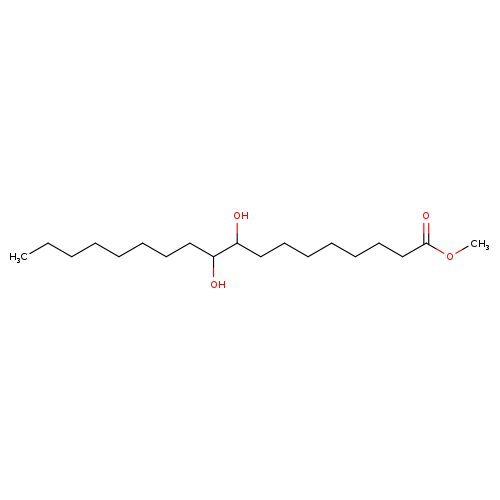
METHYL 9,10-DIHYDROXYOCTADECANOATECatalog No.:AA008ULI CAS No.:1115-01-1 MDL No.:MFCD00144775 MF:C19H38O4 MW:330.5026 |
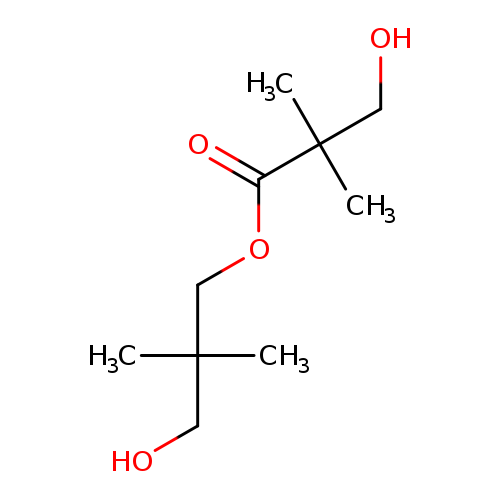
3-Hydroxy-2,2-dimethylpropyl 3-hydroxy-2,2-dimethylpropanoateCatalog No.:AA003SW3 CAS No.:1115-20-4 MDL No.:MFCD00059597 MF:C10H20O4 MW:204.2634 |
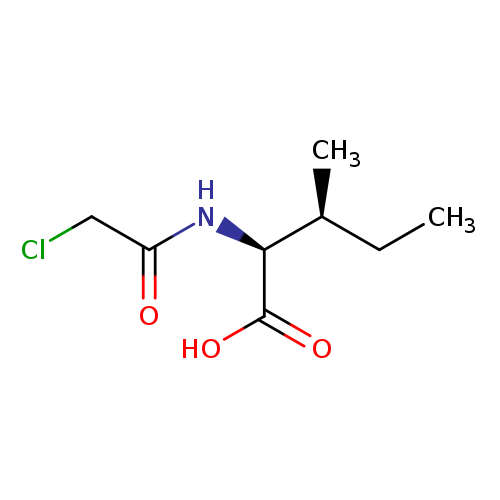
Chloroacetyl-dl-isoleucineCatalog No.:AA003OQD CAS No.:1115-24-8 MDL No.:MFCD00018911 MF:C8H14ClNO3 MW:207.6547 |
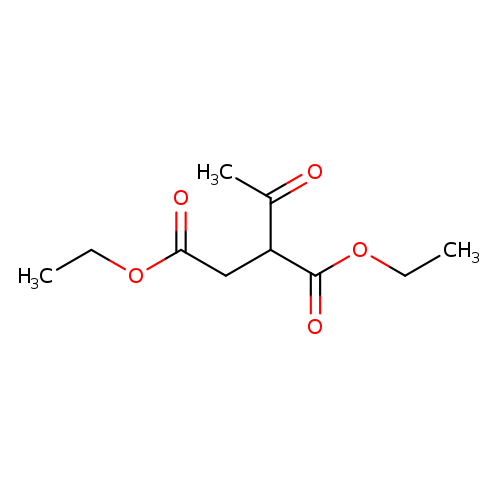
Diethyl acetylsuccinateCatalog No.:AA003PDC CAS No.:1115-30-6 MDL No.:MFCD00009157 MF:C10H16O5 MW:216.2310 |
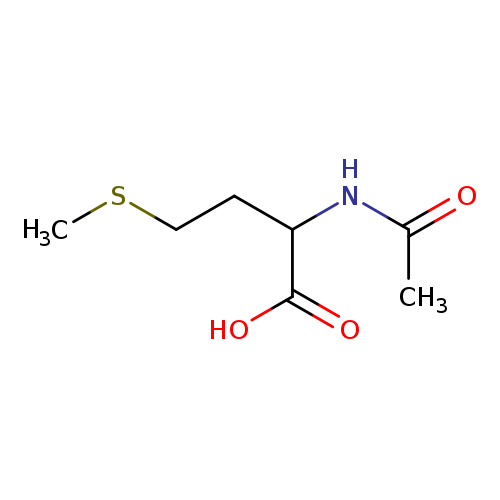
N-Acetyl-dl-methionineCatalog No.:AA003SLZ CAS No.:1115-47-5 MDL No.:MFCD00008681 MF:C7H13NO3S MW:191.2480 |
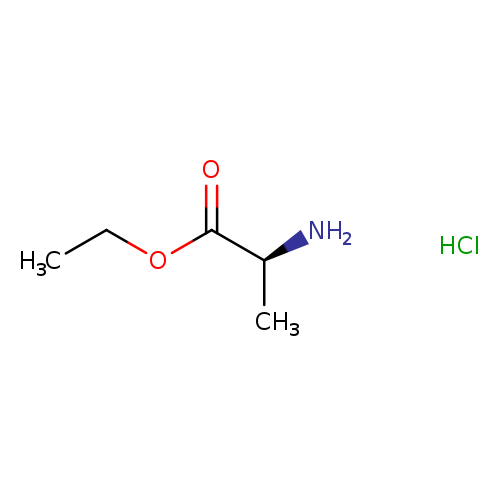
L-Alanine ethyl ester HClCatalog No.:AA003506 CAS No.:1115-59-9 MDL No.:MFCD00063662 MF:C5H12ClNO2 MW:153.6073 |
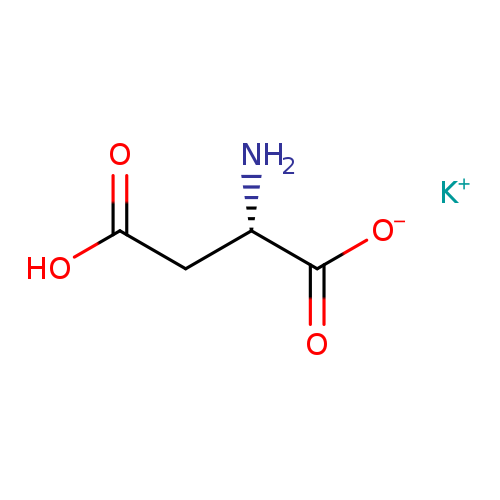
L-Aspartic acid potassium saltCatalog No.:AA0035FA CAS No.:1115-63-5 MDL No.:MFCD00021083 MF:C4H6KNO4 MW:171.1930 |
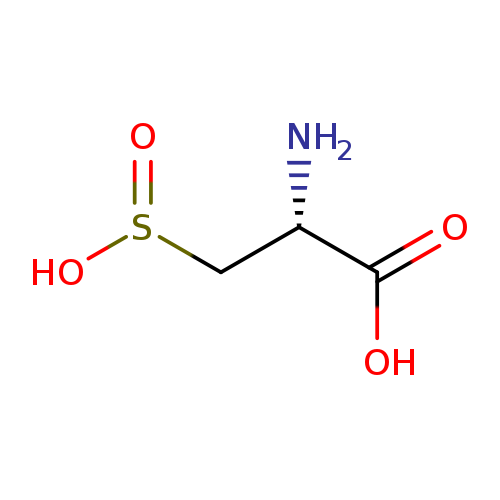
L-CYSTEINESULFINIC ACIDCatalog No.:AA008RGK CAS No.:1115-65-7 MDL No.:MFCD00150732 MF:C3H7NO4S MW:153.1570 |
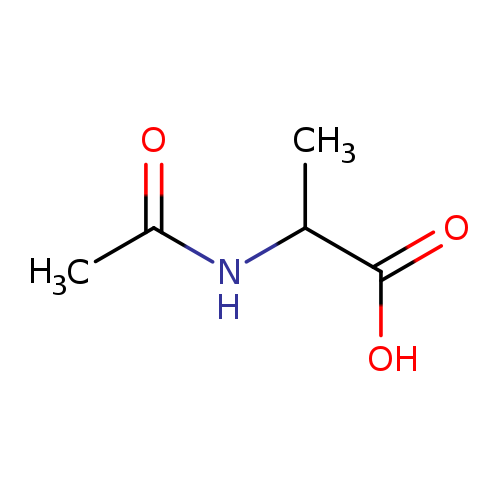
N-Acetyl-DL-alanineCatalog No.:AA00HC37 CAS No.:1115-69-1 MDL No.:MFCD00037238 MF:C5H9NO3 MW:131.1299 |
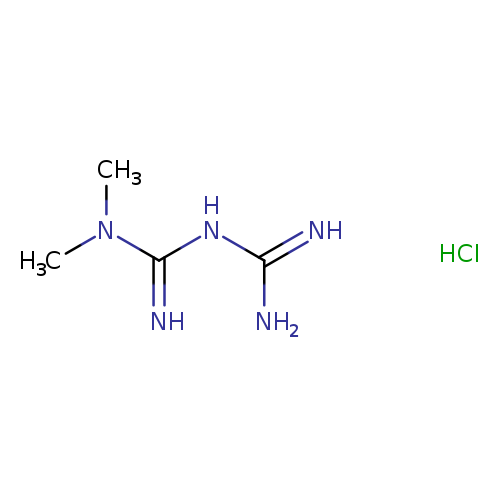
1-carbamimidamido-N,N-dimethylmethanimidamide hydrochlorideCatalog No.:AA003REQ CAS No.:1115-70-4 MDL No.:MFCD00012582 MF:C4H12ClN5 MW:165.6246 |
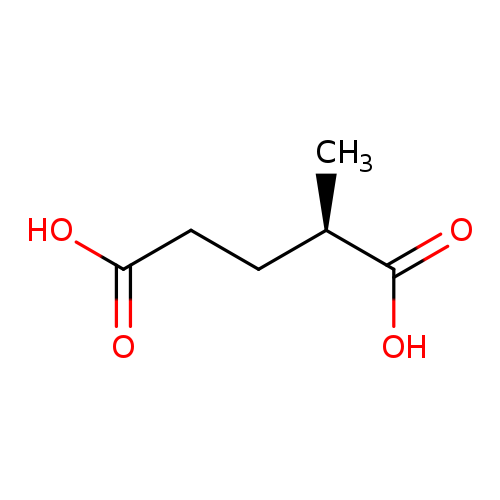
(R)-(-)-2-Methylglutaric acidCatalog No.:AA003C2L CAS No.:1115-81-7 MDL No.:MFCD00671562 MF:C6H10O4 MW:146.1412 |
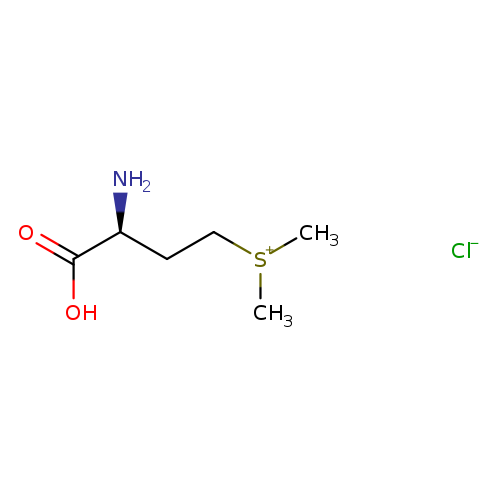
L-Methionine-S-methylSulfoniumChlorideCatalog No.:AA01E60C CAS No.:1115-84-0 MDL No.:MFCD00067196 MF:C6H14ClNO2S MW:199.6989 |
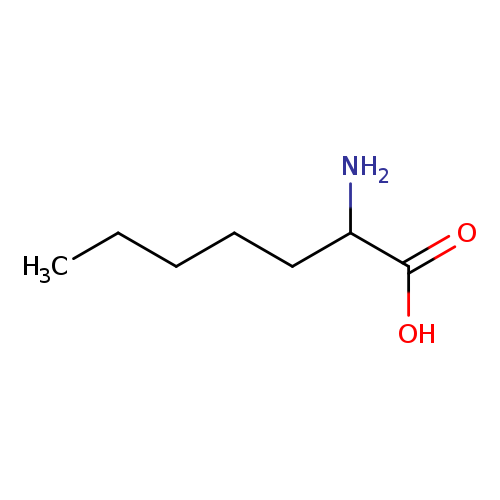
2-Aminoheptanoic acidCatalog No.:AA003GGN CAS No.:1115-90-8 MDL No.:MFCD00008103 MF:C7H15NO2 MW:145.1995 |
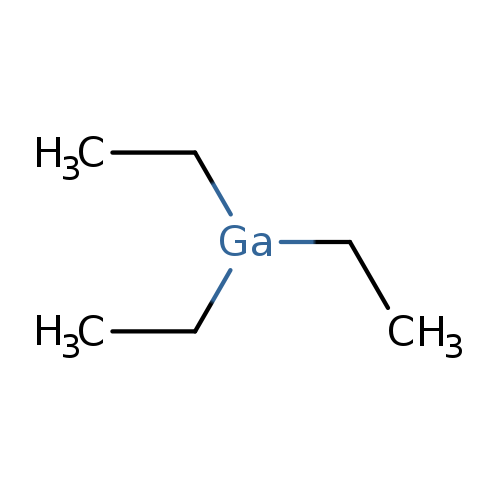
Gallium, triethyl-Catalog No.:AA01EIBV CAS No.:1115-99-7 MDL No.:MFCD00015094 MF:C6H15Ga MW:156.9063 |
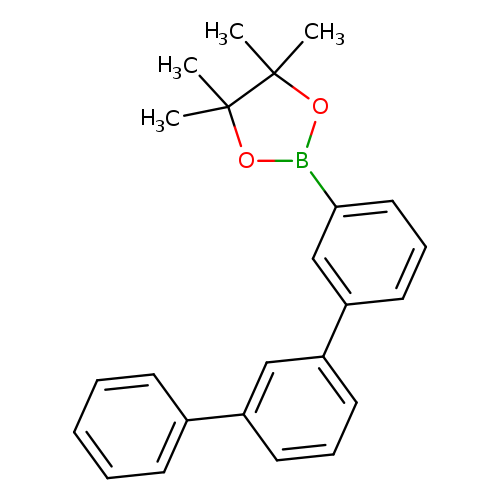
2-([1,1':3',1''-Terphenyl]-3-yl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolaneCatalog No.:AA0095ZS CAS No.:1115023-84-1 MDL No.:MFCD28134531 MF:C24H25BO2 MW:356.2651 |
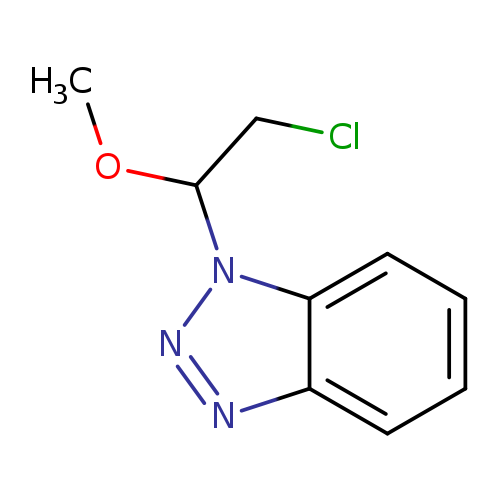
1-(2-Chloro-1-methoxyethyl)-1H-1,2,3-benzotriazoleCatalog No.:AA01EQOJ CAS No.:111507-99-4 MDL No.:MFCD00956917 MF:C9H10ClN3O MW:211.6482 |
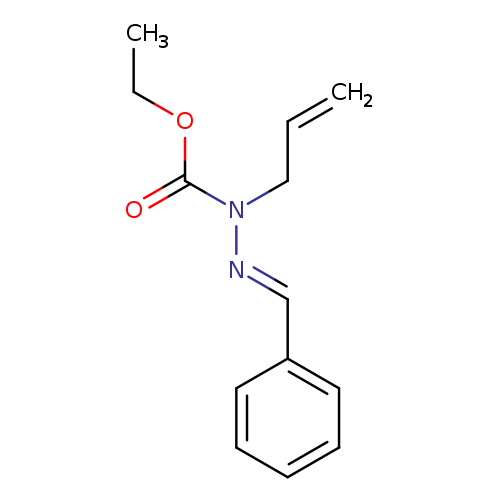
Ethyl 1-allyl-2-benzylidenehydrazinecarboxylateCatalog No.:AA019EOO CAS No.:111508-26-0 MDL No.:MFCD28991896 MF:C13H16N2O2 MW:232.2783 |
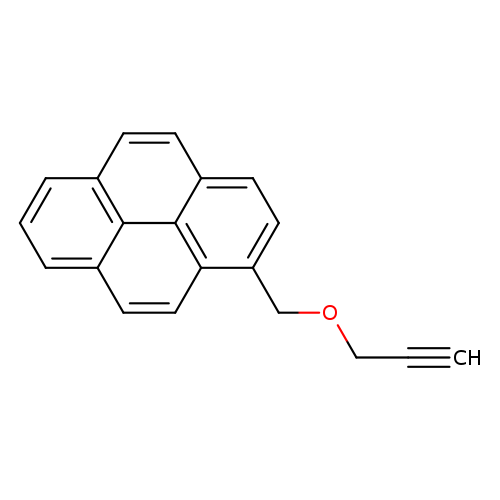
1-[(2-Propynyloxy)methyl]pyreneCatalog No.:AA00942Y CAS No.:1115084-83-7 MDL No.:MFCD28384153 MF:C20H14O MW:270.3246 |
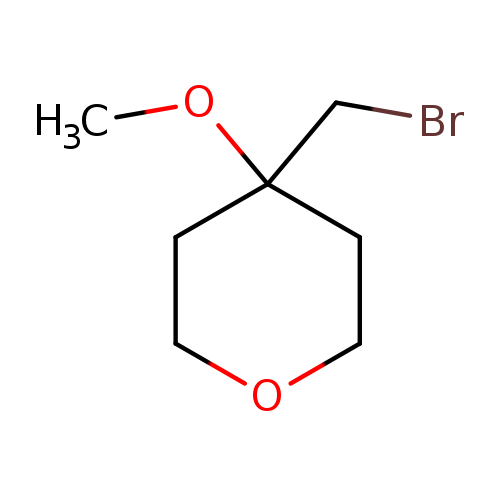
4-(bromomethyl)-4-methoxyoxaneCatalog No.:AA01AC31 CAS No.:111511-47-8 MDL No.:MFCD27500678 MF:C7H13BrO2 MW:209.0809 |
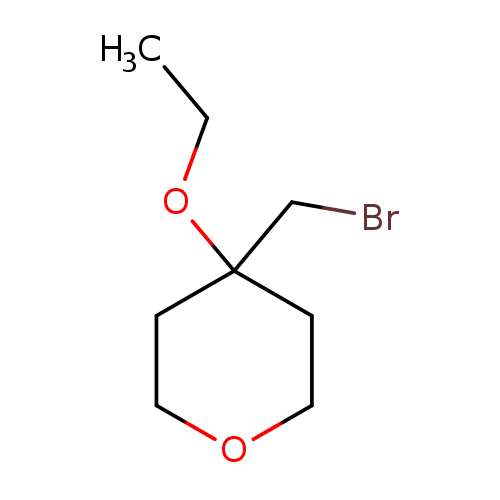
4-(bromomethyl)-4-ethoxyoxaneCatalog No.:AA01B6IX CAS No.:111511-48-9 MDL No.:MFCD30344934 MF:C8H15BrO2 MW:223.1075 |