2020-01-20 09:56:04
Aimee K. Clarke+, Hon E. Ho+, James A. Rossi-Ashton+, Richard J. K. Taylor, and William P. Unsworth
1.Introduction
The indole core is a key structural component in many natural products and pharmaceuticals and serves as a fundamental building block in organic synthesis.[1] The synthesis of indole scaffolds has therefore been the focus of much research and a myriad of methods to construct indole rings have been devel- oped over the years.[2] Classical methods include the Fischer indole[3] and Bartoli syntheses, which are widely and routinely used by the synthetic community.[4] Nonetheless, limitations as- sociated with these classical procedures mean that establishing novel strategies to prepare indoles is still important and con- tinues to be actively pursued.
Many indole syntheses make use of alkyne activation ap- proaches, typically involving coordination of a metal catalyst to the alkyne to activate it towards cyclization.[5] Silver, a member of the “coinage metal” family, can be readily obtained in the form of silver(I) salts with a variety of different counter- ions. These salts, which have a d10 electronic configuration at silver, are well-established as being good s-/p-Lewis acids and are recognized as being powerful catalysts in alkyne activa- tion.[6] In addition to their ability to interact with p-systems to promote useful reactivity, the use of silver in organic transfor- mations has important economic benefits relative to other more expensive transition metals such as gold, palladium and platinum.[5,6]
Indole synthesis has been extensively reviewed previously,[2] however, a comprehensive review focusing specifically on silver-catalyzed approaches has not been reported before. As the use of silver catalysis in heterocycle synthesis is becoming more prevalent,[5] a review of this topic in the context of indole synthesis is timely. To the best of our knowledge, this Minireview summarizes all silver-catalyzed indole syntheses to date, with a cut-off period of papers published before January 2019. Note that whilst we believe that all publications that fea- ture examples within the remit of this review are discussed, we have not reproduced all of the individual examples from these studies.
Many indole syntheses utilize silver in mixed catalytic sys- tems (e.g. mixed gold/silver systems),[5] but this Minieview is limited to examples in which the silver species have been shown to be competent at catalyzing the reaction without the influence of another metal species. The review is organized in chronological order and is divided based on the type of reac- tion used to construct the indole core, starting with the most commonly used hydroamination pathway, before moving on to other methods. Mechanisms are included and described in more detail whenever they deviate from the generally accept- ed hydroamination mechanism.
2.Hydroamination Strategies
Alkyne hydroamination[7] is by far the most common synthetic strategy used for silver-catalyzed indole syntheses. In such re- actions, anilines 1 substituted with an alkyne at their 2-position are treated with a silver(I) species which acts as a p-acid to ac- tivate the alkyne towards attack from a pendant aniline nitro- gen via a 5-endo-dig cyclization mode (3!4). Protodemetalla- tion then liberates the silver(I) species (meaning that the reac- tions can be catalytic in the silver species) and deprotonation completes the synthesis of the indole product 2 (Scheme 1).
To the best of our knowledge, the earliest example of the silver-catalyzed hydroamination strategy being used to prepare indole derivatives was reported by Rutjes and co-workers in 2004.[8] This group described the transition metal-catalyzed cyclization of o-alkynylanilines to access indole 2-propargylgly- cine (isotryptophan) derivatives. o-Alkynylpropargylglycine ani- lines 5 and 6 were prepared using Sonogashira cross-coupling between o-iodoanilines and enantiopure propargylic glycine precursors. The use of 10 mol% AgOTf in MeCN at reflux for 20 h furnished the isotryptophan products 7 and 8 in good yields. By comparison, the use of a PdII catalytic system result- ed in formation of the undesired cyclization product 9, which was not observed when AgOTf was employed (Scheme 2).
In 2007, Li et al. reported a gold and silver co-catalyzed double hydroamination of o-alkynylanilines with terminal al- kynes to access N-vinyl indole derivatives.[9] During the catalyst screening process, the separate use of both 5 mol% AgOTf and 5 mol % AgBF4 at 60 8C for 2 h under neat conditions gave the N-vinyl indole product 12 in 62 % and 59 % yields, respec- tively (Scheme 3). Although a silver(I) species can facilitate the cascade hydroamination process alone, it was later revealed that the combination of 5 mol % of AuCl3/AgOTf at RT was more efficient and hence was the main focus of the study.
In 2009, Liu et al. reported a gold and silver co-catalyzed mi- crowave-assisted intramolecular hydroamination of o-alkynyla- mides to construct N1-carbamide indole derivatives.[10] Although the combination of AuI/AgI in aqueous media using microwave irradiation at 150 8C was chosen as the optimal re- action conditions, using 10 mol % AgOTf or Ag2CO3 alone dis- played catalytic activity to afford the cyclized indole product 14 in 23 % and 75 % yields, respectively (Scheme 4 A). It was also found that the reaction conditions were exclusive to o-ter- minal alkynes as no reaction was observed when 2-substituted o-alkynylcarbamides 15 and 16 were used as substrates (Scheme 4 B).
In 2009, Ding et al. reported a silver-catalyzed hydroamina- tion process using (o-alkynylphenyl)guanidines 17 to access N- carboximidamide or N-carboximidoate indole derivatives 18 (Scheme 5).[11] By using 5 mol% AgNO3 at RT and MeCN as the solvent, guanidines 17 were found to selectively undergo 5- endo-dig cyclization to afford a range of indole derivatives 18 in good yields. The authors also conducted a comparison study between AgI and other commonly used p-acids such as PdII and CuI salts. It was reported that the reaction using a AgNO3 catalyst was the most effective, proceeding efficiently and in high yield; meanwhile, the analogous reactions using both PdII and CuI catalytic systems were incomplete, even after extended reaction times. Overall, this silver(I)-catalyzed cycliza- tion provides access to N-carboximidamide or N-carboximi- doate indole-2-phenyl derivatives under simple and mild reac- tion conditions.
In 2010, Oh et al. reported a silver(I)-catalyzed cascade pro- cess based on the reaction of o-alkynylformidates 19 and acti- vated methylene compounds 20 to synthesize 3-vinyl indole derivatives 21 (Scheme 6).[12] Typically, these reactions were performed using 5 mol% AgOTf in toluene at 808C for 12 h, enabling a range of 3-vinyl indoles 21 to be prepared in mod- erate to good yields.
The authors suggested a plausible mechanism for this trans- formation, involving an interesting 3-alkenyl migration process (Scheme 7). First, coordination of silver(I) to the alkyne facilitates enolate addition into imine 22 to form 23. This is fol- lowed by p-acid activation of the alkyne by silver(I) to induce a 5-endo-dig cyclization to form the indole core. 1,3-Alkenyl mi- gration is then proposed to occur via a silver-carbene inter- mediate 26, which is followed by rapid protodemetallation under acidic conditions to furnish the 3-vinyl indole product 21. Note that similar migration patterns have also been report- ed by using other transition metals such as PdII, PtII, and AuIII.[5, 13]
The 1,3-alkenyl migration mechanism shown in Scheme 7 was supported by a series of control experiments. For example, when o-alkynylenamine 28 was subjected to the standard reac- tion conditions, only the hydroamination product 29 was iso- lated in 55 % yield (Scheme 8). This suggested that fast proto- demetallation was competing with the 1,3-alkenyl migration pathway in some instances.
In 2010 Chan et al. described a system for the synthesis of indoles via gold-catalyzed cycloisomerization reactions.[14] During this investigation, as a control experiment, 1,3-diphen- yl-1-(2-(tosylamino)phenyl)prop-2-yn-1-ol 30 was treated with 5 mol% AgOTf, which yielded the corresponding indenyl-fused indole 31 in 16 % yield, alongside the alcohol-tethered indole
32 in 48 % yield (Scheme 9). Although it was proven that AgOTf could facilitate indole formation, a gold-catalyzed method was shown to be more efficient and was the main focus of this investigation.
Two years later, Chan et al. developed a silver-catalyzed tandem heterocyclization/alkynylation process using propargyl- ic 1,4-diols 33 to generate o-alkynyl indoles 34, liberating two molecules of water as the sole by-products (Scheme 10).
This was the first reported indole synthesis that introduced alkyne moieties at the 2-position of the indole ring without re- lying on traditional cross-coupling methods. A variety of tosyl- protected o-alkynyl indoles 34, bearing additional substituents in the 3-, 5- and 6-positions, were generated in good to excel- lent yields employing AgOTf as the catalyst. Interestingly, the reaction proceeds well in the absence of a group in the R1 po- sition, which leads to the formation of 3H-indole products; this is particularly noteworthy as these products cannot be formed using traditional cross-coupling approaches. The authors sug- gested that the silver catalyst activates the C@OH bonds in the diol substrates, rather than the alkyne moiety directly, and that this subsequently triggers cyclization/hydroamination.
In 2012, Van der Eycken et al. reported the microwave-assist- ed syntheses of pyrazino-quinazolines and indolyl-pyrazinones from alkyne-tethered pyrazinones using either silver or gold catalysis.[16] Treatment of alkyne-tethered pyrazinone 35 with AgOTf, using conventional heating, resulted in the synthesis of indole 36 in 18 % yield, alongside quinazoline product 37 in 75 % yield (Scheme 11). Ag(I) was found to be the superior cat- alyst for the formation of the quinazoline products, but AuCl was in fact identified as the optimum catalyst for formation of the indole products.
In 2012, Tang et al. reported a silver-catalyzed process for the synthesis of bis(indolyl)methanes 40 from o-alkynylanilines 38 and aryl aldehydes 39 (Scheme 12).[17] Their simple one-pot procedure was performed in the presence of 5 mol % AgNO3 in DMSO at 808C for 12 h. A wide range of o-alkynylanilines 38 and aryl aldehydes 39 were tolerated in this process, providing access to the corresponding bis(indolyl)methanes 40 in moder- ate to excellent yields. Based on previously reported mecha- nisms, the authors suggested that these reactions proceed via a hydroamination pathway in which the silver catalyst activates both alkyne and aldehyde starting materials.
In 2013, Liu et al. reported the synthesis of (3-indolyl)stan- nanes 42 via a silver-catalyzed cyclization/stannylation cascade process.[18] Starting from a series of o-alkynylanilines 41 and re- acting with 5 mol% AgSbF6 and two equivalents of 2-tributyl- stannylfuran, a wide range of N1-protected-(3-indolyl)stan- nanes 42 were synthesized (Scheme 13 A). The procedure was shown to tolerate both electron-donating and electron-with- drawing groups on the alkyne phenyl ring and substituents at the 4-position of the parent aniline ring. It was found that the presence of an electron-withdrawing protecting group is es- sential to the success of the reaction, as the non-stannylated 3H-indole product was isolated when a N-methyl aniline start- ing material was tested. It was also found that indoles bearing electron-withdrawing protecting groups other than sulfonyl were unstable during purification via column chromatography. The authors showcased the utility of the 3-stannylated indole products 42 by performing a series of elaboration reactions. To probe the reaction mechanism, 3H-indole was subjected to the optimized reaction conditions and no stannylated product was observed, which indicated that the stannylation did not occur via C@H functionalization of the indole product but instead through a silver-tin transmetallation process as shown in Scheme 13 B. In this mechanism, the silver is proposed to have a dual role; activating the alkyne towards attack from the amino group via the silver-coordinated alkyne 43 whilst also catalyzing the destannylation of 2-tributylstannylfuran through a transmetallation protodemetallation pathway, thus liberating Bu3Sn+ which goes on to react with the 3-indolyl silver(I) intermediate 44.
In 2014, McNulty et al. reported the synthesis and applica- tion of a series of homogenous silver(I) acetate complexes as catalysts in intramolecular hydroamination reactions using N- protected o-alkynylanilines 46.[19] A range of N-protected in- doles 48 were formed in high yields with the process per- formed at room temperature in the absence of base or other additives (Scheme 14). The authors conducted a catalyst screen of heterogeneous silver(I) salts at 100 8C, which furnished indole products from their corresponding o-alkynylanilines. It was found that those salts with weakly coordinating anions were ineffective in the intramolecular hydroamination. Next, they screened a series of homogeneous ligated silver species and found catalyst 47 to be the most efficient at catalyzing indole formation. Further investigations revealed that the reac- tions could be performed at room temperature and that polar solvents were required, with DMF being optimal. A wide range of N-tosylindoles 48 were then accessed in high yields and the procedure was shown to tolerate sensitive functionalities such as a TMS-functionalized alkyne.
In 2014, Song and You et al. reported a one-pot silver-cata- lyzed cyclization/fluorination cascade to deliver structurally di- verse fluorinated indole derivatives 50 from o-alkynylanilines 49 (Scheme 15).[20] Overall, four different indolenine, indoline and indole derivatives were accessed in good yields by altera- tion of the reaction conditions. A limited range of 3-fluorinated indoles 50 were synthesized as it was found that an electron- withdrawing group on the alkynyl phenyl ring was required for a successful reaction. The reaction was shown to tolerate both unprotected and N-methyl protected aniline starting materials. A tentative general mechanism was proposed, whereby silver(I) activates the alkynyl aniline triple bond towards attack by the nitrogen, forming the corresponding 3H-indole upon protode- metallation, at which point the mechanism for each derivative varies according to the reaction conditions. In the case of the 3-fluorinated indole products 50, the 3H-indole is simply mon- ofluorinated by the electrophilic SelectfluorS reagent, forming the corresponding 3-fluorinated indole.
In 2014, Rang and Fan et al. reported the synthesis of N-pro- tected 4-acetonylindoles 53 from o-alkynylanilines 51 and silyl enol ethers 52 (Scheme 16).[21] Their two-step, one-pot proce- dure involves a hypervalent iodide mediated oxidative dearomatisation and silver-catalyzed cyclization and includes an in- teresting conjugate addition step of a silyl enol ether (mecha- nism discussed later, see Scheme 17). Iodosylbenzene was iden- tified as the optimum oxidant due to the absence of acidic contaminants that were produced when using other hyperva- lent iodine sources. Although the acidic species did not impede the oxidation step, they were found to be harmful to the subsequent silyl enol ether reaction. To avoid decomposi- tion of the silyl enol ether, methanol from the iodosylbenzene step was removed under reduced pressure prior to addition of the silyl enol ethers. The catalytic activity of a variety of metal salts was investigated, and AgOTf was found to be the opti- mum salt for catalyzing the process. Gold(I) salts gave reduced yields of the 4-acetonylindole products 53 relative to that of AgOTf, whereas other metal salts, such as BiIII, InIII, ZnII, CuII, AuIII, PdII, or PtII gave rise to the 4-methoxyindole product.
A range of substituted o-alkynylanilines were shown to un- dergo the one-pot procedure in good yields with groups at the para-position required for a successful reaction. The N-pro- tecting group was also shown to play a crucial role. The reac- tion proceeded smoothly in the presence of an extensive series of silyl enol ethers furnishing the corresponding 4-aceto- nylindole products 53. The authors proposed an iodosylben- zene-mediated oxidative dearomatization of para-substituted o-alkynylanilines 51 (Scheme 17), forming o-alkynylcyclohexa- dienimines 55. AgOTf is then proposed to activate the triple bond to induce heterocyclization, to form intermediate 57, which then undergoes a Mukaiyama-Michael-type addition with the silyl enol ether to generate intermediate 58. Me3SiOTf generated in situ then promotes rearomatization to give indole 60. Subsequent protodemetallation by TfOH produces 4-acetonylindole 53 and regenerates the AgOTf catalyst.
In an extension to their previous work (shown in Scheme 16), Fan and co-workers went on to report another ox- idative dearomatization/silver-catalyzed cyclization domino process, this time involving an interesting [3+3]-dipolar cyclo- addition reaction.[22] This procedure afforded 3,4-fused indoles 63 directly in a one-pot process from o-alkynylanilines 61 and o-alkynylbenzaldoximes 62 (Scheme 18). The reaction was
shown to tolerate an extensive range of N-protecting groups, aniline ring substituents, alkynyl phenyl ring substituents and a variety of o-alkynylbenzaldoximes 62.
The mechanism of this process (shown in Scheme 19) is thought to proceed via an oxidative dearomatization of a para-substituted o-alkynylaniline 61, which furnishes inter- mediate 64 following a silver-catalyzed cyclization (see Scheme 17 for details of these steps). This species is then pro- posed to undergo a [3+3]-dipolar cycloaddition (this step is best thought of as operating on resonance form 65) to form an unstable cycloadduct product 66. The authors then suggest two possible radical pathways (not shown) for the thermal re- arrangement of cycloadduct 66 resulting in the formation of 3,4-fused indole 63.
In 2014, Bi et al. reported silver-catalyzed heteroaromatiza- tion of propargylic alcohols to form a range of 3-tosyl benzo- furans, benzothiophenes and indoles.[23] Key to this work is the dual role of p-toluenesulfonylmethyl isocyanide, which serves as both the sulfonyl source and the ligand for the silver catalyst. An extensive mechanistic study was conducted on the silver-catalyzed benzofuran forming part of this work, resulting in the proposal of a deoxysulfonylation/hydration/condensa- tion reaction pathway, which was extended to the analogous indole formation. While the main focus of this investigation was on benzofuran synthesis, a smaller series of indoles 68 were synthesized from propargylic alcohols 67 with a variety of aryl and heteroaryl functionality on the C2 indole tether (Scheme 20). The scope of the investigation was expanded to isomeric propargylic alcohols 69, which resulted in the forma- tion of 3H-indoles 70 with tosyl functionality on the C2 indole tether. A similar reaction pathway was proposed to operate when isomeric propargylic alcohols 69 were reacted under broadly the same reactions conditions.
In 2016, Michelet and co-workers described the synthesis of benzoxazinone and benzisoxazole derivatives using silver or gold catalysts in the presence of oxone.[24] During optimization studies, the formation of an indole side product 71 was ob- served when reacting o-alkynylaniline 10 with catalytic quanti- ties of AgNO3 under an oxygen atmosphere (A) or in the pres- ence of 2,6-lutidine N-oxide (B). There were just two examples of the 2-phenylindole side product 71 being formed in low yield (15 % and 20 %, Scheme 21). These were the only report- ed examples of silver-catalyzed indole formation in the publi- cation but note that this was not the main focus of the study. The oxidants themselves are not involved in the formation of the indole product 71, but they were added in an attempt to promote subsequent oxidation reactions.
The authors also observed the same silver-catalyzed intra- molecular hydroamination reaction in a follow-up paper; this time indole 71 was isolated in a 37% yield when aniline 10 was heated in the presence of AgNO3 and a large excess of H2O2 in a methanol/water solvent system (Scheme 22).
Again, the oxidant appears not to be involved in this reaction. A silver-catalyzed indolization of o-alkynylanilines 72 fol- lowed by ring-opening of donor-acceptor cyclopropanes (DACs) 73 in one-pot was reported by Singh et al. in 2016 (Scheme 23).[26] This cascade process features an initial intramolecular hydroamination reaction catalyzed by AgSbF6 and is fol- lowed by an unprecedented AgI-mediated ring-opening/trap- ping of DAC 73, which functionalizes the resulting indole at the C3 position. When unprotected anilines were used, most DACs tested underwent ring-opening prior to cyclization lead- ing to a diminished indole product yield, therefore protection of the free amine is necessary for these reactions to proceed successfully. This methodology tolerates a wide range of DACs incorporating aryl, heteroaryl and vinyl functionalities as well as a variety of substituted o-alkynylaniline partners, furnishing 2,3-disubstituted indole derivatives 74 in good to excellent yields. The authors also described the elaboration of their cas- cade products into useful intermediates, further showcasing their synthetic utility.
Following on from the work reported by Tang et al. in 2012 on the preparation of bis(indolyl)methanes 40 (see Scheme 12), a similar procedure was reported by Chattopad- hyay et al. in 2016.[27] An efficient one-pot domino process for the formation of symmetrical bis(indolyl)methanes 40 from o- alkynylanilines 38 and aldehydes 39 using silver catalysis was described (Scheme 24). Although conceptually similar to Tang’s work, this paper did significantly extend the scope of this reac- tion; in particular, this study focused on exploring substituents on the pyrrole ring to include aryl, heteroaryl and alkyl groups, which were limited to aryl moieties in Tang’s earlier work. Inter- estingly, substrates featuring a n-butyl or an ester group on their alkyne unit proceeded particularly well in this transformation, which is surprising given that a hexyl substituted alkyne in Tang’s work did not generate any of the desired bis(indolyl)- methane.
In view of the importance of 2-substituted indoles in medici- nal applications, a facile silver-catalyzed route towards N- cyano-2-substituted indole derivatives was developed by Trive- di et al. and reported in 2017.[28] Hydroamination reactions commonly use o-alkynylanilines as starting substrates for indole formation but, in contrast, Trivedi and co-workers uti- lized a unique intramolecular cyclization of alkynyl tetrazoles 75 instead, which was catalyzed by AgOAc under ambient con- ditions (Scheme 25). Linear alkyl, cycloalkyl and aryl substituted alkynes are tolerated in the starting tetrazoles 75, with this method used to prepare a range of 2/7-substituted N-cyanoin- doles 76 in high yields. The authors propose that the mecha- nism proceeds via a typical AgI-alkyne activation pathway fol- lowed by nucleophilic intramolecular cyclization, whereby the nucleophilic nitrogen is generated from deprotonation of the acidic tetrazole proton and subsequent loss of N2 produces an N-phenylcyanamide species.
A “greener” AgNO3-catalyzed approach to indole and 7-aza- indole derivatives 78 using water as the reaction solvent has been reported by Shao et al. (Scheme 26).[29] The majority of the indoles and aza-indoles 78 obtained using this method were isolated in excellent yields following a simple filtration and drying process. The authors also explored recycling the aqueous AgNO3 medium, whereby the product from each cycle was obtained by filtration and the resulting aqueous filtrate (containing AgNO3) could be used in the next reaction immediately; three reaction cycles were successfully achieved in yields of 93 % and above, although a prolonged reaction time of 36 h compared to 10 h was required for the third cycle. Interestingly, none of the desired indole products were formed when the reactions were carried out in organic solvents such as DMF, toluene or ethanol. The authors suggested that this was because their process belonged to the “on-water” reaction class, which requires a water-oil phase boundary for the chemi- cal transformation to take place. They proposed that a cooper- ative hydrogen bond network forms at the water-oil interface between the substrate and solvent which helps to lower the energy of the cyclization transition state and hence promote hydroamination.
In 2018, Samanta and co-workers reported a one-pot silver- catalyzed intramolecular hydroamination of o-alkynylanilines 49 to construct complex 2,3-substituted indole derivatives 81 and 82 (Scheme 27).[30] Various additives including AuIII, CuI, ZnII, FeIII reagents as well as TfOH and HBF4 were screened in the optimization studies; 2 mol% AgSbF6 in DCE at 45 8C was found to be optimal for the synthesis of indoles 81 and 82. A wide range of substituents were tolerated on o-alkynylanilines 49, as well as the o-alkynyl cyclic enynones 79 and 80, to afford the 2,3-disubstituted indoles 81 and 82 in moderate to excellent yields.
A mechanism involving a silver(I)-catalyzed intramolecular hydroamination and Friedel–Crafts alkylation/oxacyclization cascade was proposed, as depicted in Scheme 28. First electro- philic activation of the alkynylaniline by AgI occurs to form species 83 which then undergoes hydroamination (83!84) followed by fast protodemetallation to furnish indole inter- mediate 85 whilst regenerating the silver catalyst. Then, indole 85, generated in situ, undergoes a C3 Friedel–Crafts type alky- lation at the b-position of enynone-Ag complex 86, followed by a 5-endo-dig oxacyclization in a concerted fashion to form the vinyl-Ag species 87. Finally, fast protodemetallation of 87 produces the 2,3-disubstituted indole 81.
In 2018, a silver-catalyzed “anti-Michael” hydroamination procedure to access 2-acylindoles 89 was developed by Ma- rinelli and co-workers (Scheme 29).[31] This method comple- ments existing procedures for the synthesis of 2-acylindoles and the fact that it is fully atom-economical and does not require any additional protection/deprotection strategies is par- ticularly advantageous. Other coinage metals were explored in this procedure; CuOTf also promoted the desired transforma- tion, albeit with lower efficiency and interestingly, AuI salts were completely ineffective. Silver(I) salts, in particular AgOTf, were the best catalysts for this transformation. A variety of 2- acylindoles 89 bearing aryl, heteroaryl, vinyl and alkyl groups were prepared in good to excellent yields. Thienyl and cyclo- hexyl substituted products unfortunately led to lower isolated yields and propargyl alcohols and alkynoate esters were not suitable substrates for this cyclization.
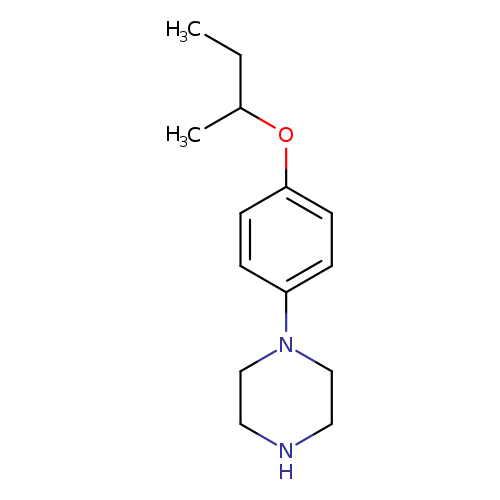
1-[4-(Butan-2-yloxy)phenyl]piperazineCatalog No.:AA01B1UE CAS No.:1082381-12-1 MDL No.:MFCD11586573 MF:C14H22N2O MW:234.3373 |
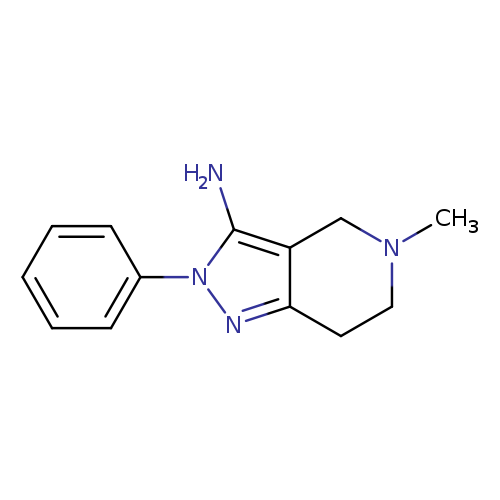
5-Methyl-2-phenyl-4,5,6,7-tetrahydro-2H-pyrazolo[4,3-c]pyridin-3-amineCatalog No.:AA01APHU CAS No.:1082387-17-4 MDL No.:MFCD11574116 MF:C13H16N4 MW:228.2929 |
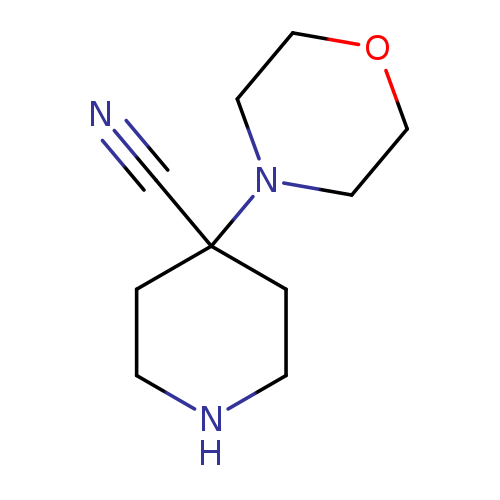
4-(morpholin-4-yl)piperidine-4-carbonitrileCatalog No.:AA01BCAS CAS No.:1082390-96-2 MDL No.:MFCD11585212 MF:C10H17N3O MW:195.2615 |
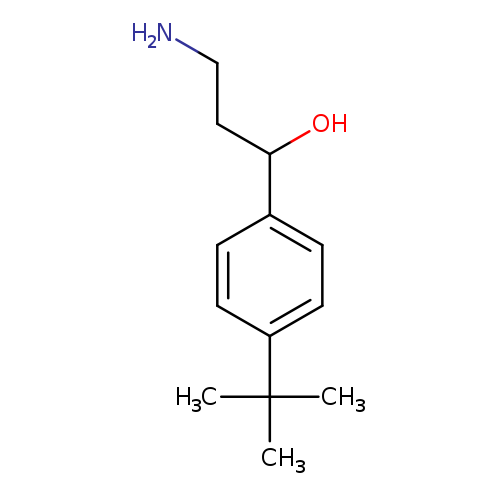
3-AMINO-1-(4-TERT-BUTYLPHENYL)PROPAN-1-OLCatalog No.:AA01APEM CAS No.:1082392-21-9 MDL No.:MFCD11581643 MF:C13H21NO MW:207.3119 |
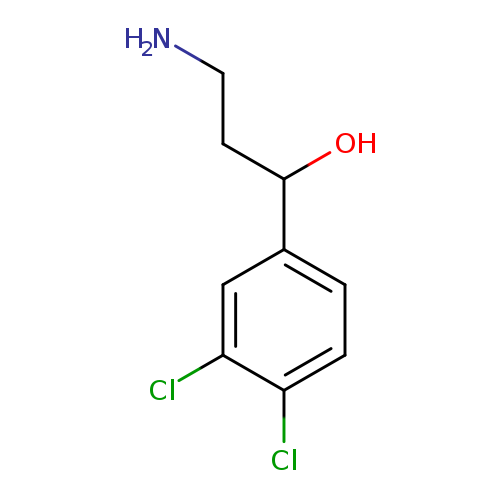
3-amino-1-(3,4-dichlorophenyl)propan-1-olCatalog No.:AA01BDKL CAS No.:1082392-23-1 MDL No.:MFCD11581654 MF:C9H11Cl2NO MW:220.0957 |
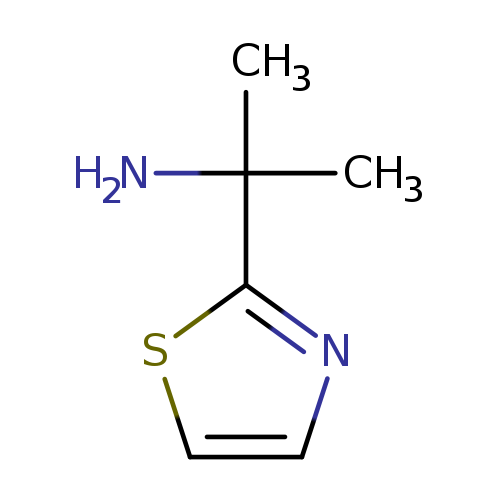
2-(1,3-Thiazol-2-yl)propan-2-amineCatalog No.:AA019UMD CAS No.:1082393-38-1 MDL No.:MFCD11587497 MF:C6H10N2S MW:142.2220 |
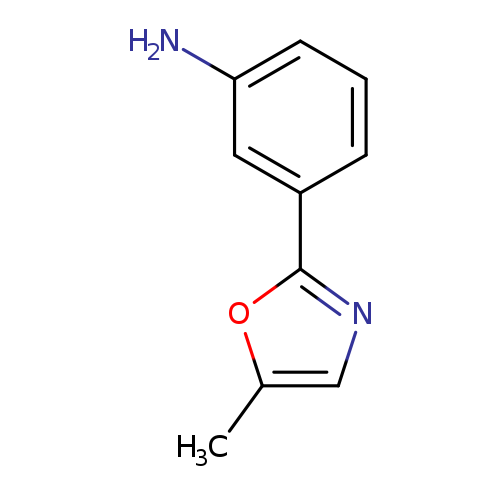
3-(5-Methyl-1,3-oxazol-2-yl)anilineCatalog No.:AA01AGXB CAS No.:1082398-99-9 MDL No.:MFCD14529697 MF:C10H10N2O MW:174.1992 |
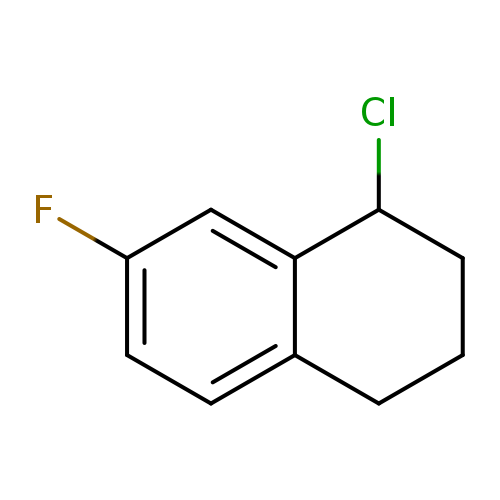
1-Chloro-7-fluoro-1,2,3,4-tetrahydronaphthaleneCatalog No.:AA01A24D CAS No.:1082399-51-6 MDL No.:MFCD11590904 MF:C10H10ClF MW:184.6378 |
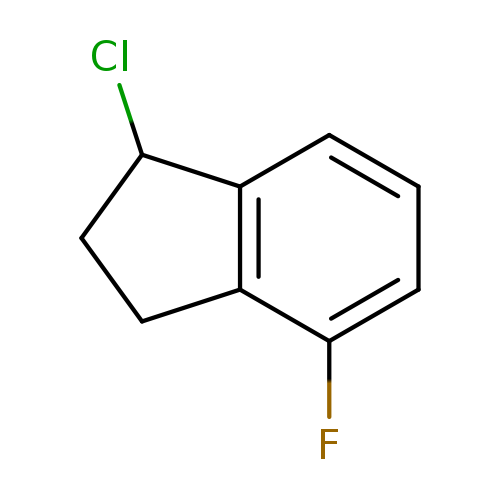
1-chloro-4-fluoro-2,3-dihydro-1H-indeneCatalog No.:AA01A1YA CAS No.:1082399-60-7 MDL No.:MFCD11219559 MF:C9H8ClF MW:170.6112 |
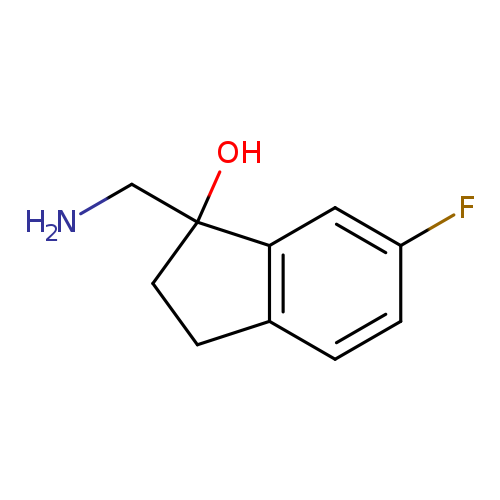
"1-(aminomethyl)-6-fluoro-2,3-dihydroinden-1-ol"Catalog No.:AA01FMA2 CAS No.:1082399-74-3 MDL No.:MFCD11591013 MF:C10H12FNO MW:181.2068 |
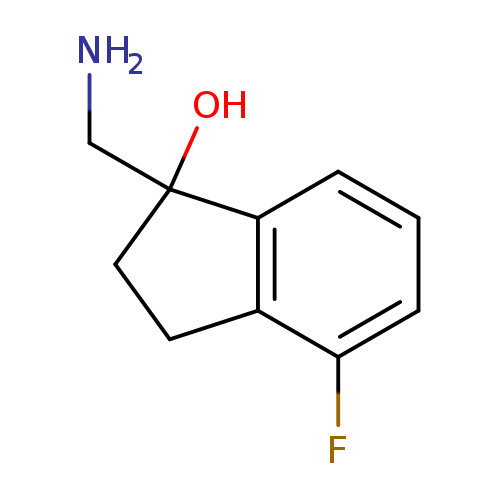
"1-(aminomethyl)-4-fluoro-2,3-dihydroinden-1-ol"Catalog No.:AA01FMA7 CAS No.:1082399-76-5 MDL No.:MFCD11591022 MF:C10H12FNO MW:181.2068 |
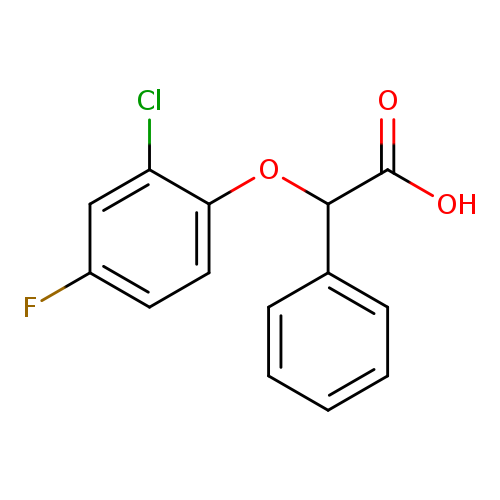
2-(2-chloro-4-fluorophenoxy)-2-phenylacetic acidCatalog No.:AA01ABFH CAS No.:1082401-01-1 MDL No.:MFCD11580100 MF:C14H10ClFO3 MW:280.6788 |
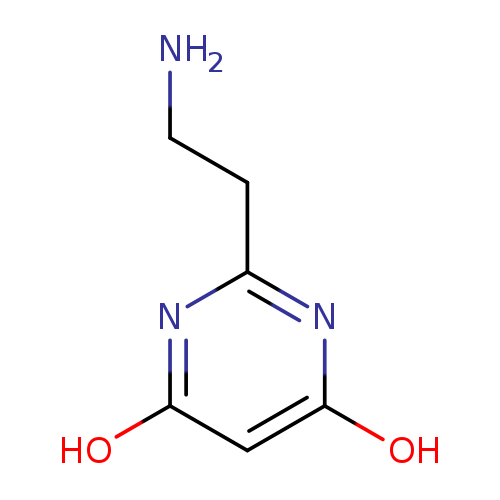
2-(2-Aminoethyl)-4,6-pyrimidinediol hydrochloride hydrateCatalog No.:AA00J16O CAS No.:1082402-61-6 MDL No.:MFCD09055285 MF:C6H9N3O2 MW:155.1546 |
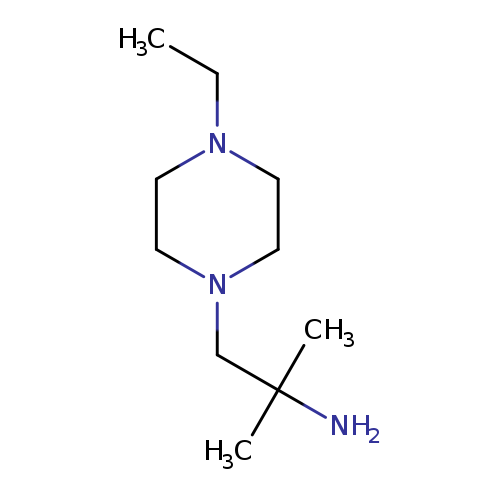
1-(4-ethylpiperazin-1-yl)-2-methylpropan-2-amineCatalog No.:AA01C1KA CAS No.:1082411-69-5 MDL No.:MFCD11585405 MF:C10H23N3 MW:185.3097 |
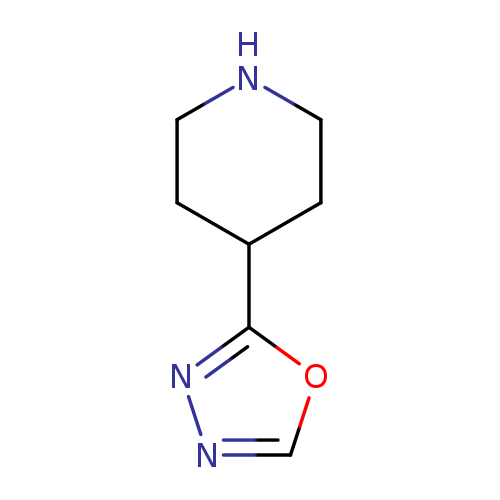
2-(Piperidin-4-yl)-1,3,4-oxadiazoleCatalog No.:AA008RT0 CAS No.:1082413-19-1 MDL No.:MFCD11042734 MF:C7H11N3O MW:153.1817 |
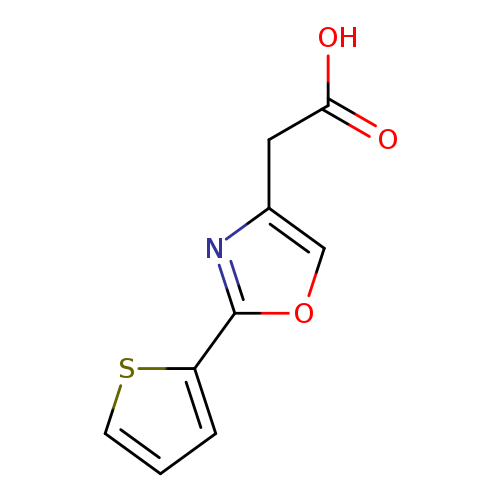
2-[2-(thiophen-2-yl)-1,3-oxazol-4-yl]acetic acidCatalog No.:AA01AB26 CAS No.:1082414-36-5 MDL No.:MFCD11587880 MF:C9H7NO3S MW:209.2218 |
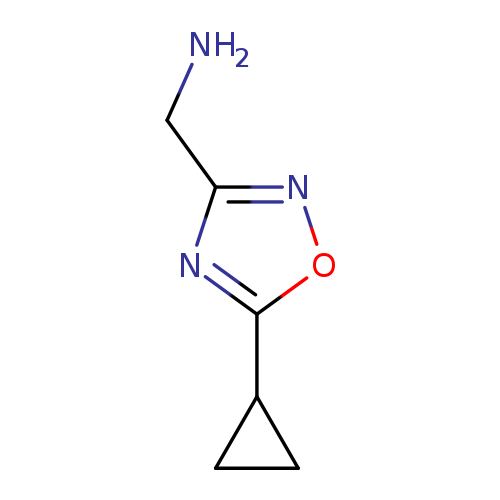
[(5-Cyclopropyl-1,2,4-oxadiazol-3-yl)methyl]amine hydrochlorideCatalog No.:AA00996N CAS No.:1082420-52-7 MDL No.:MFCD09971289 MF:C6H9N3O MW:139.1552 |
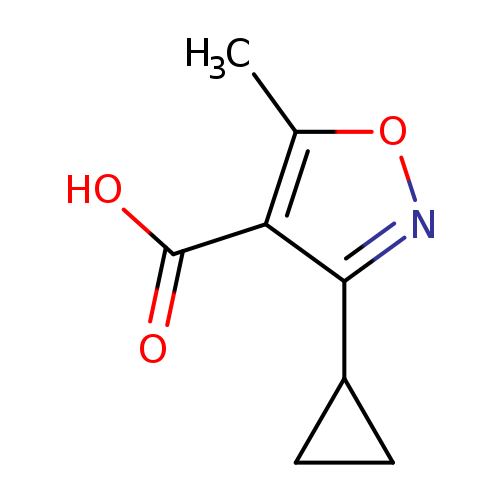
3-cyclopropyl-5-methyl-1,2-oxazole-4-carboxylic acidCatalog No.:AA01AFIZ CAS No.:1082420-70-9 MDL No.:MFCD11584319 MF:C8H9NO3 MW:167.1620 |
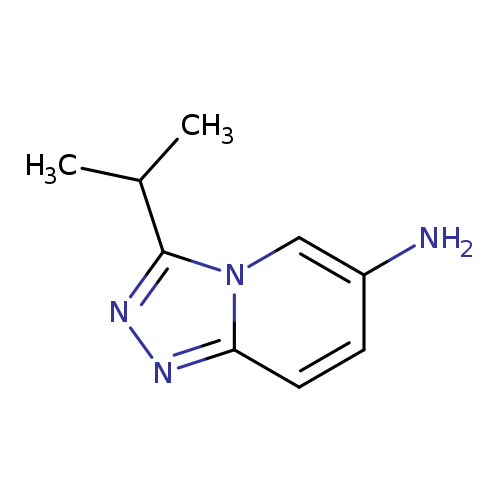
3-Isopropyl-[1,2,4]triazolo[4,3-a]pyridin-6-amineCatalog No.:AA01AG7M CAS No.:1082428-28-1 MDL No.:MFCD11597185 MF:C9H12N4 MW:176.2184 |
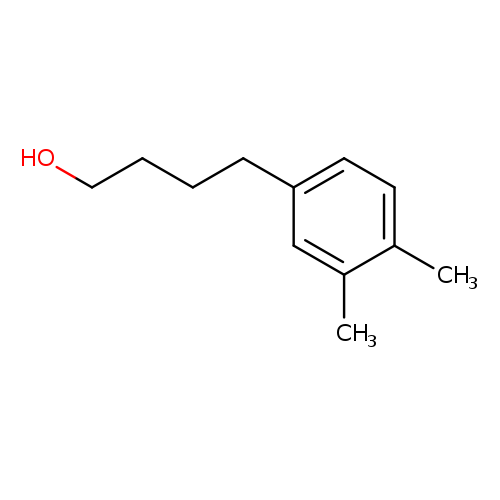
4-(3,4-dimethylphenyl)butan-1-olCatalog No.:AA01A6AA CAS No.:1082435-12-8 MDL No.:MFCD11592105 MF:C12H18O MW:178.2707 |
4-(3,4-dimethylphenyl)butanalCatalog No.:AA01A6DB CAS No.:1082435-13-9 MDL No.:MFCD11592122 MF:C12H16O MW:176.2548 |
1-(2-isocyanatoethyl)-4-(propan-2-yl)benzeneCatalog No.:AA01B2LJ CAS No.:1082435-41-3 MDL No.:MFCD11592302 MF:C12H15NO MW:189.2536 |
3-(3-METHOXYPHENYL)-3H-[1,2,3]TRIAZOLO[4,5-D]PYRIMIDINE-7-THIOLCatalog No.:AA01AQFK CAS No.:1082437-20-4 MDL No.:MFCD16653068 MF:C11H9N5OS MW:259.2871 |
2-chloro-6-ethyl-5-methylpyridine-3-carbonitrileCatalog No.:AA01EL2O CAS No.:108244-43-5 MDL No.:MFCD10478932 MF:C9H9ClN2 MW:180.6342 |
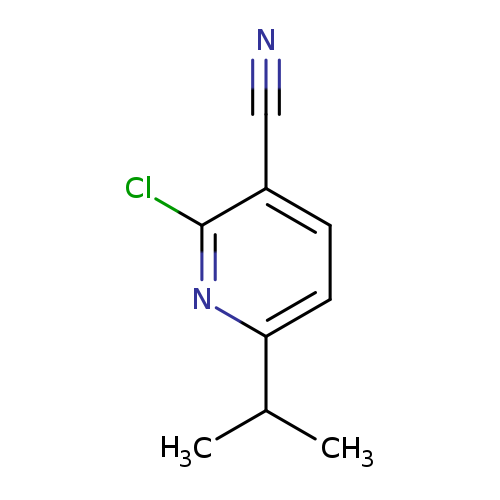
2-Chloro-6-isopropylnicotinonitrileCatalog No.:AA009TG2 CAS No.:108244-44-6 MDL No.:MFCD14581659 MF:C9H9ClN2 MW:180.6342 |
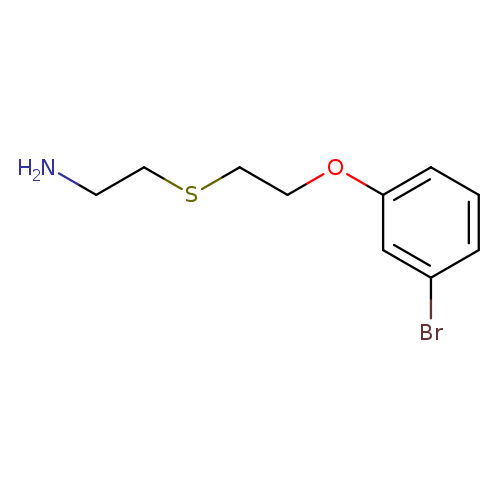
1-{2-[(2-aminoethyl)sulfanyl]ethoxy}-3-bromobenzeneCatalog No.:AA01AHDR CAS No.:1082443-87-5 MDL No.:MFCD11577801 MF:C10H14BrNOS MW:276.1933 |
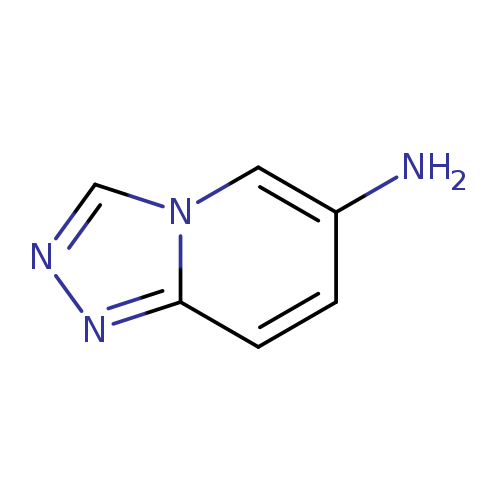
[1,2,4]Triazolo[4,3-a]pyridin-7-amineCatalog No.:AA008Z07 CAS No.:1082448-58-5 MDL No.:MFCD19689668 MF:C6H6N4 MW:134.1386 |
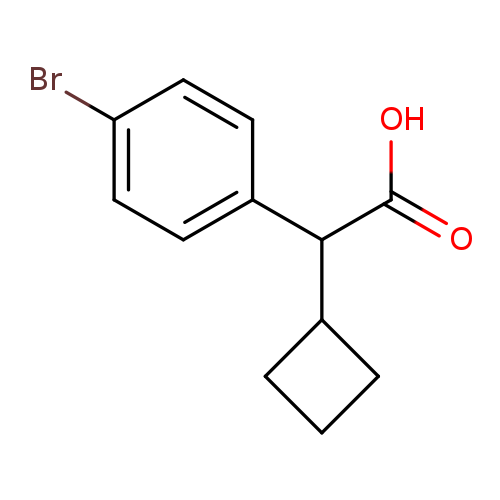
(4-Bromo-phenyl)-cyclobutyl-acetic acidCatalog No.:AA0093IO CAS No.:1082453-52-8 MDL No.:MFCD11590487 MF:C12H13BrO2 MW:269.1344 |
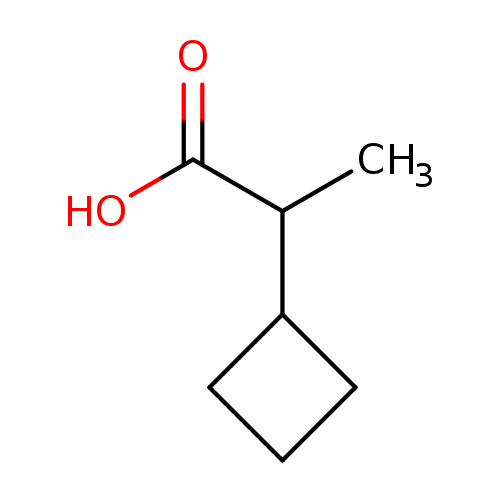
2-Cyclobutylpropanoic acidCatalog No.:AA01AUI5 CAS No.:1082453-55-1 MDL No.:MFCD09908184 MF:C7H12O2 MW:128.1690 |
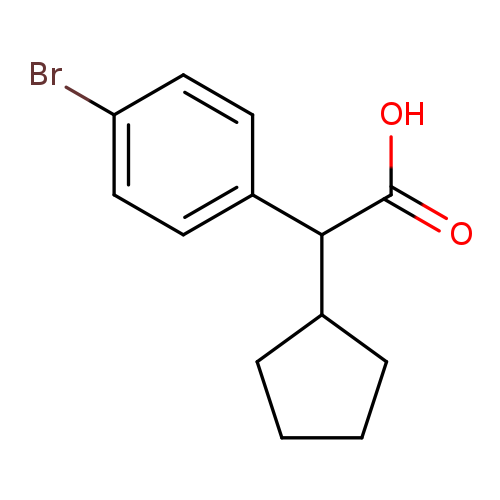
(4-Bromo-phenyl)-cyclopentyl-acetic acidCatalog No.:AA0093J2 CAS No.:1082453-57-3 MDL No.:MFCD11590509 MF:C13H15BrO2 MW:283.1610 |
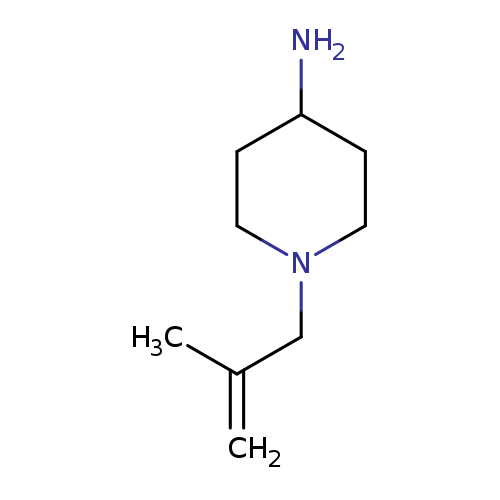
1-(2-methylprop-2-en-1-yl)piperidin-4-amineCatalog No.:AA01AB4A CAS No.:1082454-34-9 MDL No.:MFCD11586523 MF:C9H18N2 MW:154.2526 |
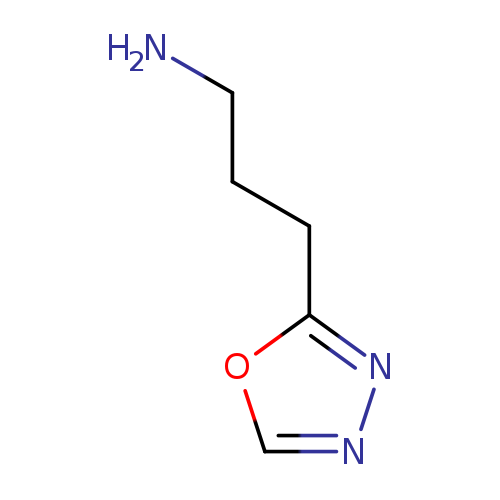
3-(1,3,4-oxadiazol-2-yl)propan-1-amineCatalog No.:AA01DX7D CAS No.:1082469-12-2 MDL No.:MFCD11582060 MF:C5H9N3O MW:127.1445 |
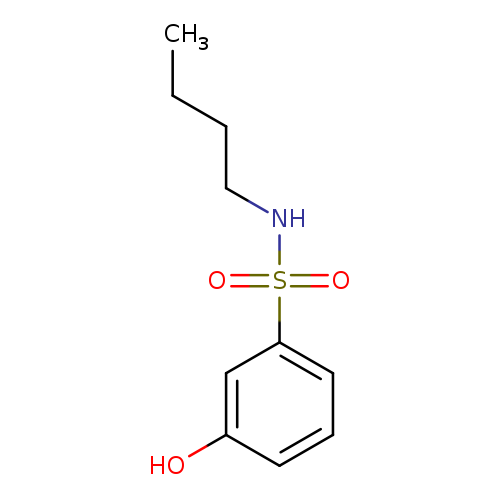
N-Butyl-3-hydroxybenzene-1-sulfonamideCatalog No.:AA00HB1N CAS No.:1082469-96-2 MDL No.:MFCD11594629 MF:C10H15NO3S MW:229.2960 |
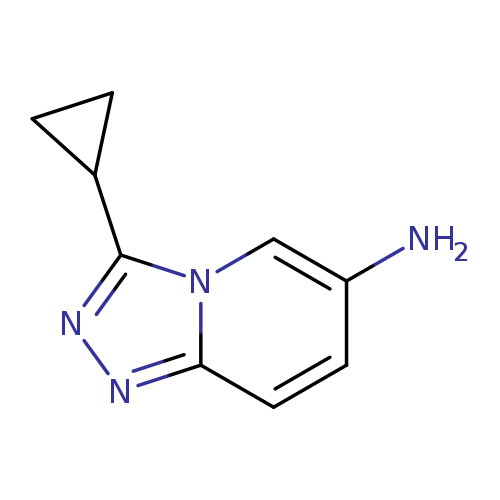
3-cyclopropyl-[1,2,4]triazolo[4,3-a]pyridin-6-amineCatalog No.:AA01AG4F CAS No.:1082471-97-3 MDL No.:MFCD11597188 MF:C9H10N4 MW:174.2025 |
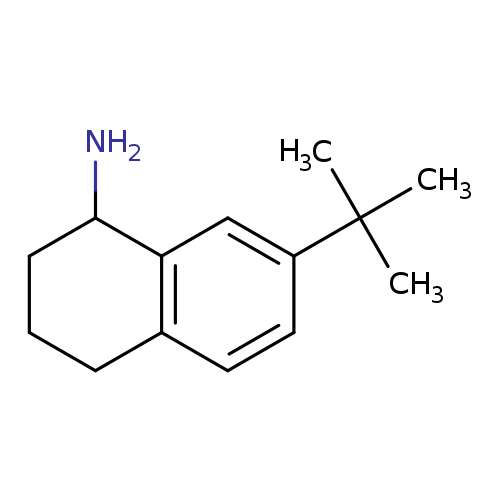
7-tert-butyl-1,2,3,4-tetrahydronaphthalen-1-amineCatalog No.:AA01AFY6 CAS No.:1082475-32-8 MDL No.:MFCD11591035 MF:C14H21N MW:203.3232 |
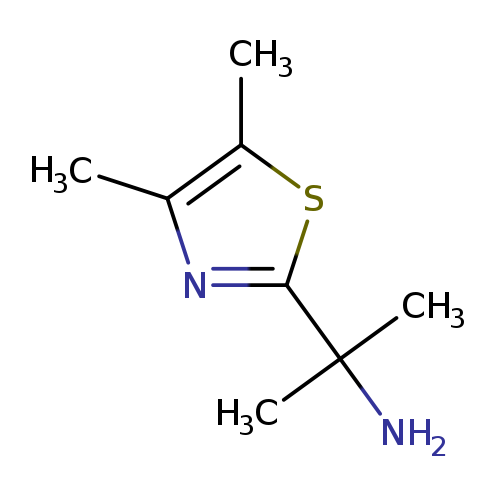
2-(dimethyl-1,3-thiazol-2-yl)propan-2-amineCatalog No.:AA01AITF CAS No.:1082478-78-1 MDL No.:MFCD11587499 MF:C8H14N2S MW:170.2752 |
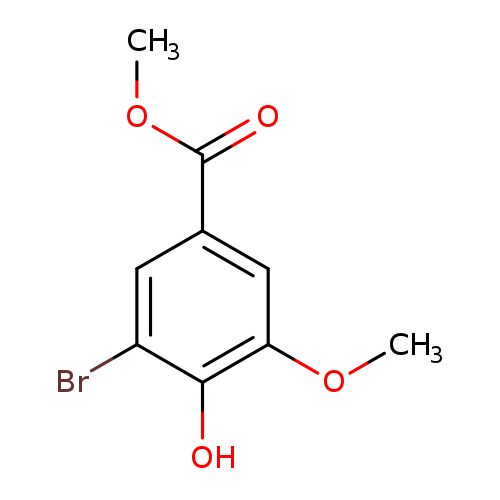
Methyl 3-bromo-4-hydroxy-5-methoxybenzoateCatalog No.:AA007V2H CAS No.:108249-43-0 MDL No.:MFCD06203663 MF:C9H9BrO4 MW:261.0694 |
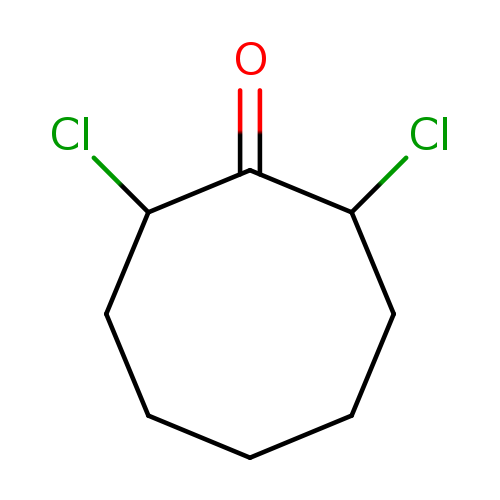
2,8-DichlorocyclooctanoneCatalog No.:AA003G4G CAS No.:108249-93-0 MDL No.:MFCD06797089 MF:C8H12Cl2O MW:195.0863 |
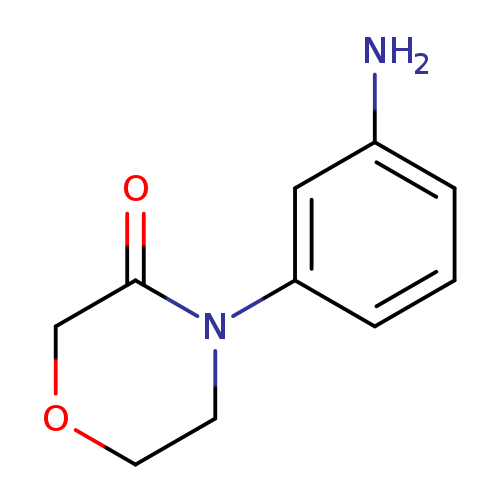
4-(3-AMino-phenyl)-Morpholin-3-oneCatalog No.:AA0093X3 CAS No.:1082495-22-4 MDL No.:MFCD11577324 MF:C10H12N2O2 MW:192.2145 |
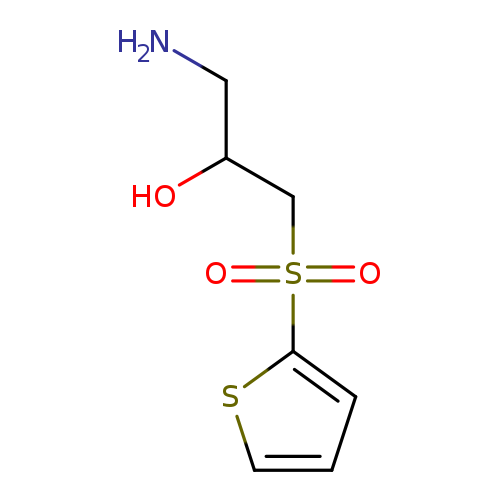
1-amino-3-(thiophene-2-sulfonyl)propan-2-olCatalog No.:AA01A1WP CAS No.:1082500-83-1 MDL No.:MFCD11585543 MF:C7H11NO3S2 MW:221.2971 |
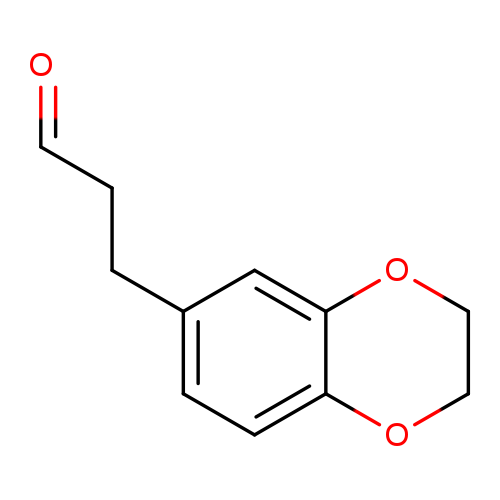
3-(2,3-dihydro-1,4-benzodioxin-6-yl)propanalCatalog No.:AA01A4H9 CAS No.:1082503-42-1 MDL No.:MFCD11584418 MF:C11H12O3 MW:192.2112 |
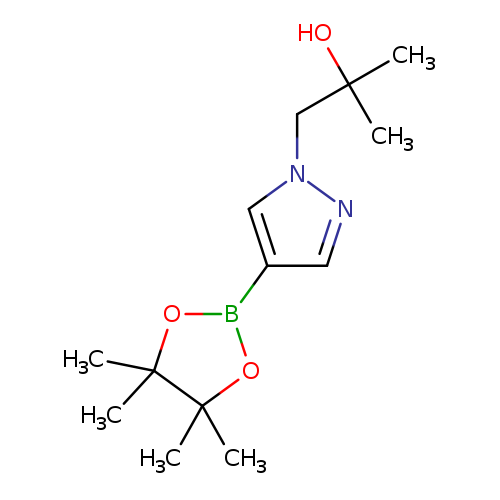
[1-(2-Hydroxy-2-methyl-propyl)pyrazol-4-yl]boronic acid pinacol esterCatalog No.:AA0094QJ CAS No.:1082503-77-2 MDL No.:MFCD22378011 MF:C13H23BN2O3 MW:266.1443 |
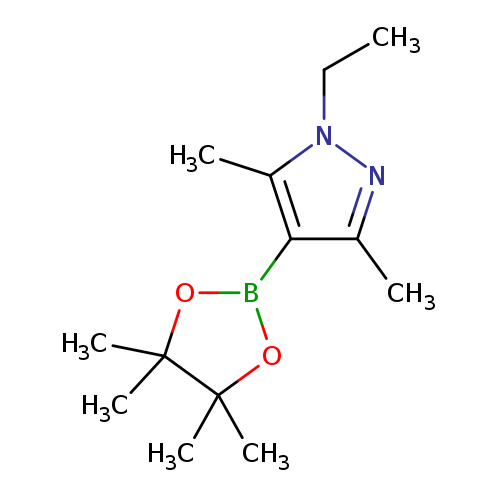
1-Ethyl-3,5-dimethyl-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1h-pyrazoleCatalog No.:AA0083SQ CAS No.:1082503-79-4 MDL No.:MFCD16659792 MF:C13H23BN2O2 MW:250.1449 |
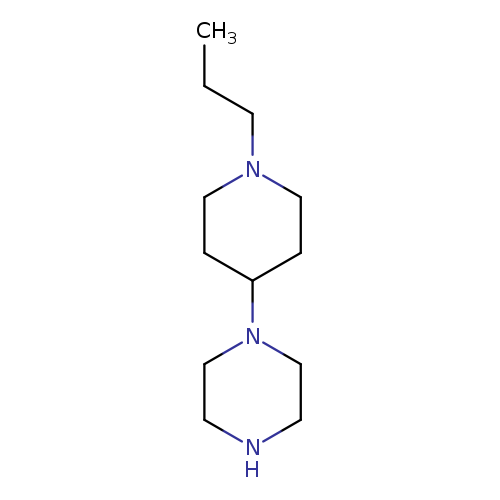
1-(1-Propylpiperidin-4-yl)piperazineCatalog No.:AA01AHK5 CAS No.:1082511-85-0 MDL No.:MFCD11586644 MF:C12H25N3 MW:211.3470 |
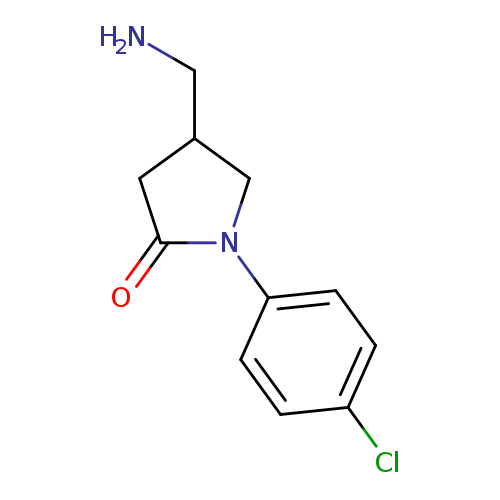
4-(aminomethyl)-1-(4-chlorophenyl)pyrrolidin-2-oneCatalog No.:AA01AFLB CAS No.:1082524-78-4 MDL No.:MFCD11579982 MF:C11H13ClN2O MW:224.6867 |
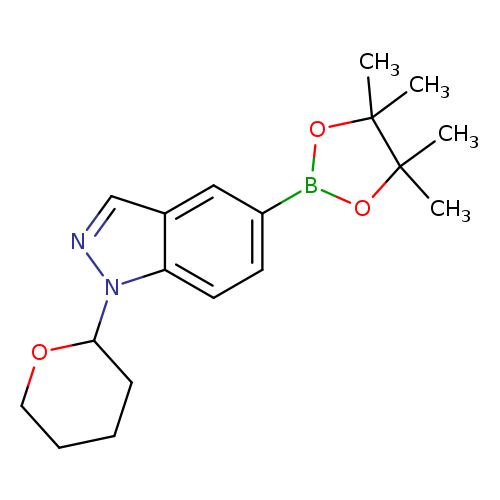
1-(Tetrahydro-2H-pyran-2-yl)-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-indazoleCatalog No.:AA008T87 CAS No.:1082525-64-1 MDL No.:MFCD12922967 MF:C18H25BN2O3 MW:328.2137 |
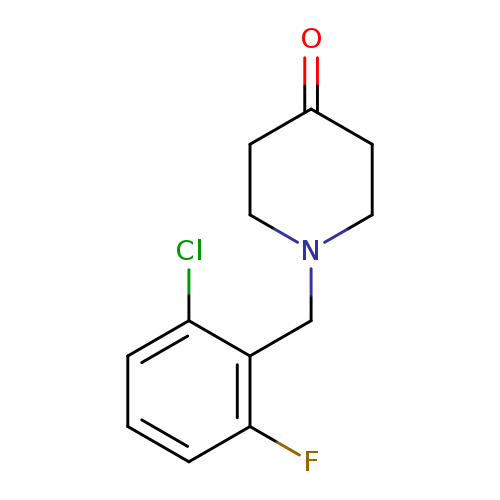
1-[(2-chloro-6-fluorophenyl)methyl]piperidin-4-oneCatalog No.:AA019WL2 CAS No.:1082545-19-4 MDL No.:MFCD11586480 MF:C12H13ClFNO MW:241.6891 |
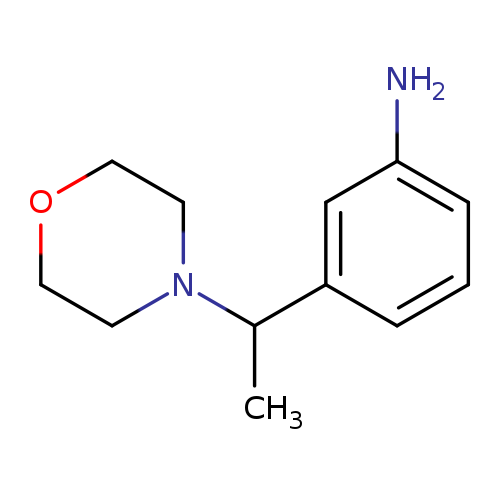
3-[1-(morpholin-4-yl)ethyl]anilineCatalog No.:AA01AAUG CAS No.:1082549-24-3 MDL No.:MFCD11593206 MF:C12H18N2O MW:206.2841 |
4-(3-chlorophenyl)-1,2,5-oxadiazol-3-amineCatalog No.:AA00LAP9 CAS No.:1082550-10-4 MDL No.:MFCD11574180 MF:C8H6ClN3O MW:195.6057 |
5-amino-6-(1-pyrrolidinyl)-1,3-dihydro-2H-benzimidazol-2-oneCatalog No.:AA008VBH CAS No.:1082550-33-1 MDL No.:MFCD11574286 MF:C11H14N4O MW:218.2551 |
1-(2,3-dihydro-1,4-benzodioxine-6-sulfonyl)-1,4-diazepaneCatalog No.:AA01A85C CAS No.:1082556-61-3 MDL No.:MFCD11583412 MF:C13H18N2O4S MW:298.3580 |
2-cyclopropyl-1,1,3-trioxo-2,3-dihydro-1,2-benzothiazole-6-carboxylic acidCatalog No.:AA019VSU CAS No.:1082558-21-1 MDL No.:MFCD10690738 MF:C11H9NO5S MW:267.2579 |
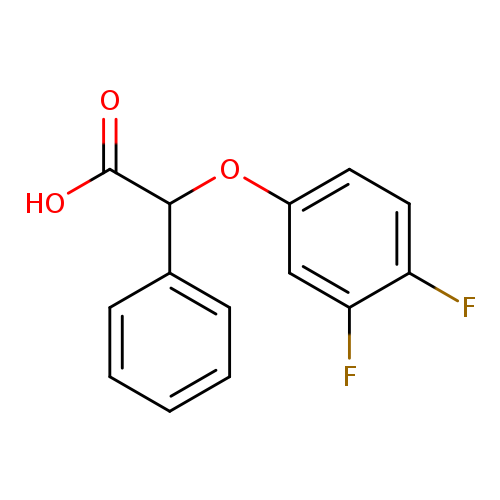
2-(3,4-difluorophenoxy)-2-phenylacetic acidCatalog No.:AA01AGET CAS No.:1082558-35-7 MDL No.:MFCD11580097 MF:C14H10F2O3 MW:264.2242 |
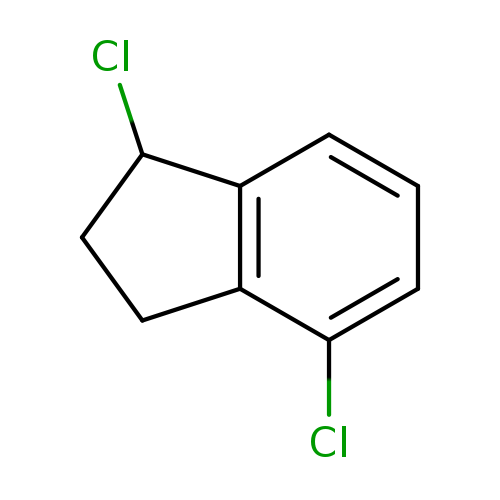
1,4-dichloro-2,3-dihydro-1H-indeneCatalog No.:AA01A21A CAS No.:1082562-97-7 MDL No.:MFCD11219558 MF:C9H8Cl2 MW:187.0658 |
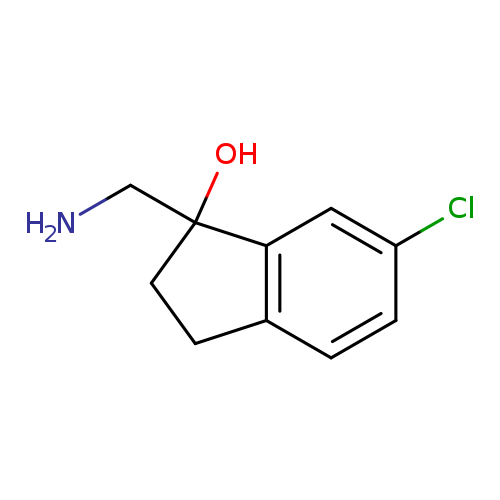
"1-(aminomethyl)-6-chloro-2,3-dihydroinden-1-ol"Catalog No.:AA01FM8W CAS No.:1082563-11-8 MDL No.:MFCD11591014 MF:C10H12ClNO MW:197.6614 |
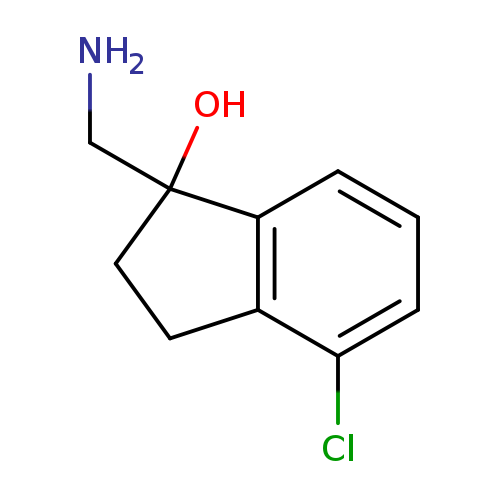
"1-(aminomethyl)-4-chloro-2,3-dihydroinden-1-ol"Catalog No.:AA01FM96 CAS No.:1082563-13-0 MDL No.:MFCD11591023 MF:C10H12ClNO MW:197.6614 |
1-[(diethylamino)methyl]cyclopentan-1-amineCatalog No.:AA01B6WF CAS No.:1082563-25-4 MDL No.:MFCD11591092 MF:C10H22N2 MW:170.2951 |
5-Methyl-1h-imidazole-4-carbonitrileCatalog No.:AA008STK CAS No.:108257-41-6 MDL No.:MFCD19219933 MF:C5H5N3 MW:107.1133 |
2-(Phenoxymethyl)thiazole-4-carbaldehydeCatalog No.:AA008VMS CAS No.:1082576-04-2 MDL No.:MFCD11587920 MF:C11H9NO2S MW:219.2597 |
2-(4-methoxyphenoxymethyl)-1,3-thiazole-4-carbaldehydeCatalog No.:AA019WRZ CAS No.:1082576-05-3 MDL No.:MFCD11587924 MF:C12H11NO3S MW:249.2856 |
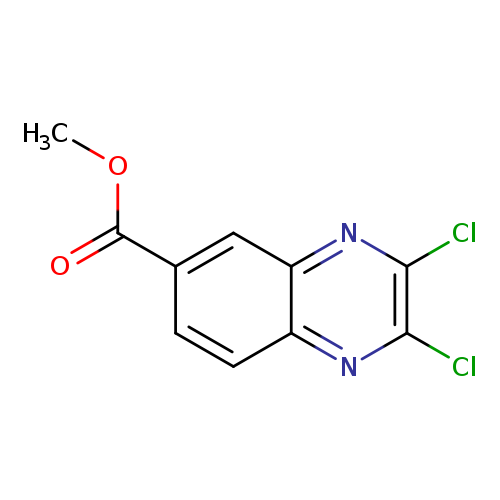
Methyl 2,3-dichloroquinoxaline-6-carboxylateCatalog No.:AA0093XN CAS No.:108258-54-4 MDL No.:MFCD12827814 MF:C10H6Cl2N2O2 MW:257.0728 |

Ethyl-d5 2-MethylbutyrateCatalog No.:AA01CB4D CAS No.:1082581-95-0 MDL No.: MF:C7H9D5O2 MW:135.2157 |

Isovaleric Acid Ethyl-d5 EsterCatalog No.:AA01CBX4 CAS No.:1082581-99-4 MDL No.: MF:C7H9D5O2 MW:135.2157 |
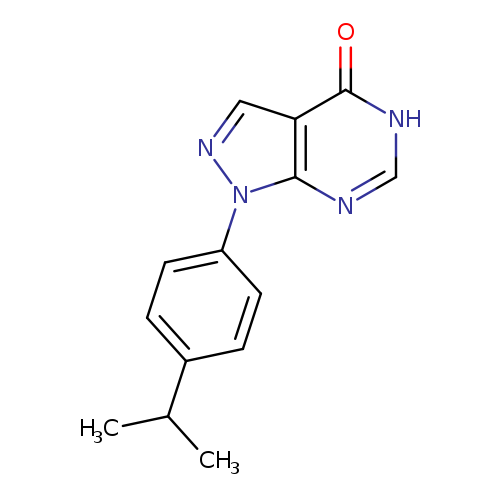
1-(4-Isopropylphenyl)-1H-pyrazolo[3,4-d]pyrimidin-4(5H)-oneCatalog No.:AA01FFO3 CAS No.:1082584-10-8 MDL No.:MFCD11588444 MF:C14H14N4O MW:254.2872 |
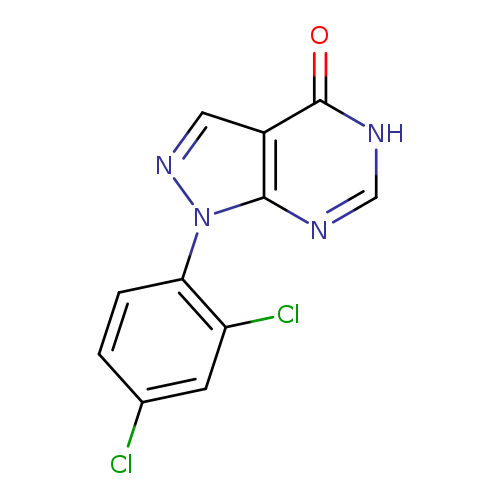
1-(2,4-dichlorophenyl)-1H-pyrazolo[3,4-d]pyrimidin-4(5H)-oneCatalog No.:AA01FFPM CAS No.:1082584-19-7 MDL No.:MFCD11544215 MF:C11H6Cl2N4O MW:281.0975 |
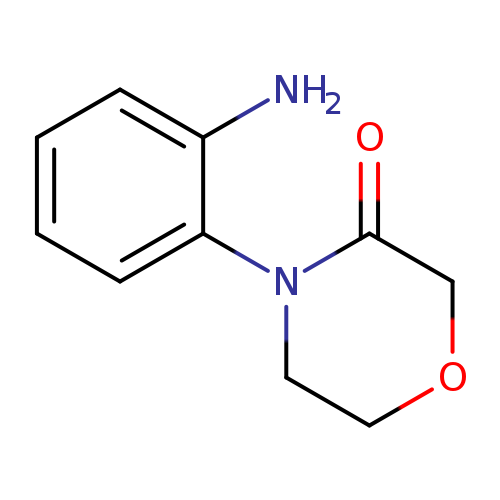
3-Morpholinone, 4-(2-aMinophenyl)-Catalog No.:AA0091VG CAS No.:1082588-73-5 MDL No.:MFCD11577323 MF:C10H12N2O2 MW:192.2145 |
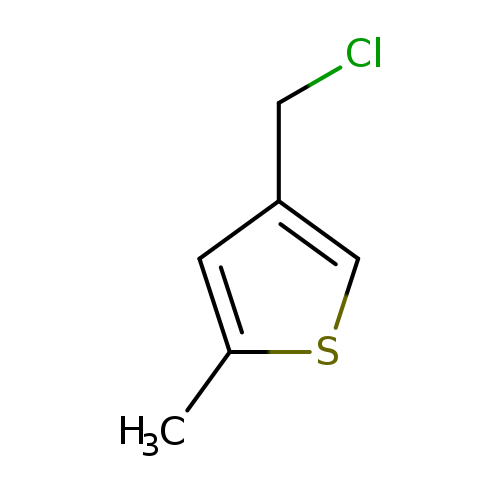
4-(chloromethyl)-2-methylthiopheneCatalog No.:AA01B96O CAS No.:1082602-29-6 MDL No.:MFCD11597597 MF:C6H7ClS MW:146.6378 |
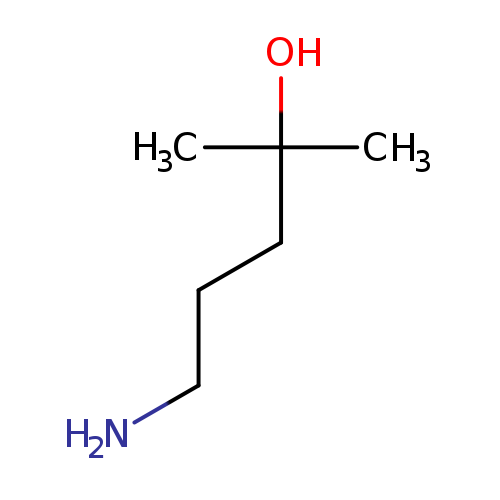
5-Amino-2-methyl-2-pentanolCatalog No.:AA003MAN CAS No.:108262-66-4 MDL No.:MFCD19210993 MF:C6H15NO MW:117.1894 |
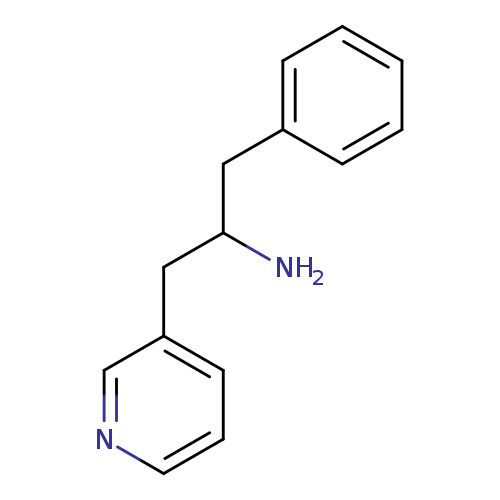
1-PHENYL-3-(PYRIDIN-3-YL)PROPAN-2-AMINECatalog No.:AA01EJCV CAS No.:1082648-77-8 MDL No.:MFCD16786262 MF:C14H16N2 MW:212.2902 |
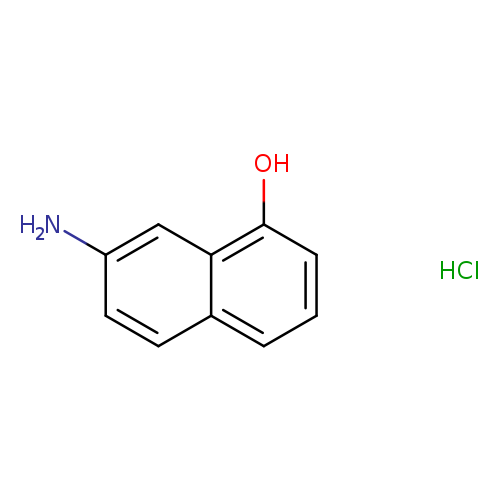
7-Aminonaphthalen-1-ol hydrochlorideCatalog No.:AA01D907 CAS No.:1082649-42-0 MDL No.:MFCD31536272 MF:C10H10ClNO MW:195.6455 |
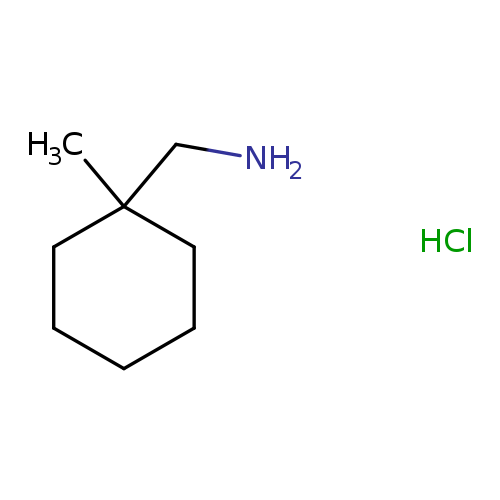
(1-methylcyclohexyl)methanamine hydrochlorideCatalog No.:AA019ZIS CAS No.:1082650-61-0 MDL No.:MFCD22369988 MF:C8H18ClN MW:163.6882 |
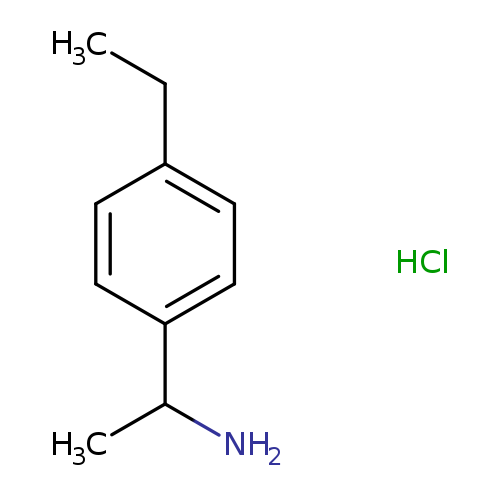
[1-(4-ethylphenyl)ethyl]amine hydrochlorideCatalog No.:AA00J0YV CAS No.:1082653-64-2 MDL No.:MFCD18071215 MF:C10H16ClN MW:185.6937 |
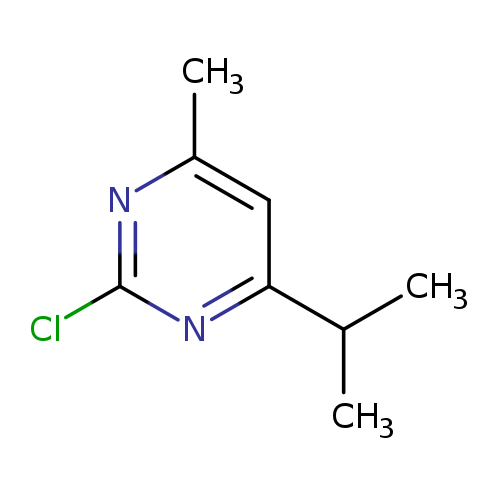
2-chloro-4-methyl-6-(propan-2-yl)pyrimidineCatalog No.:AA01A6XE CAS No.:1082658-26-1 MDL No.:MFCD21790514 MF:C8H11ClN2 MW:170.6393 |
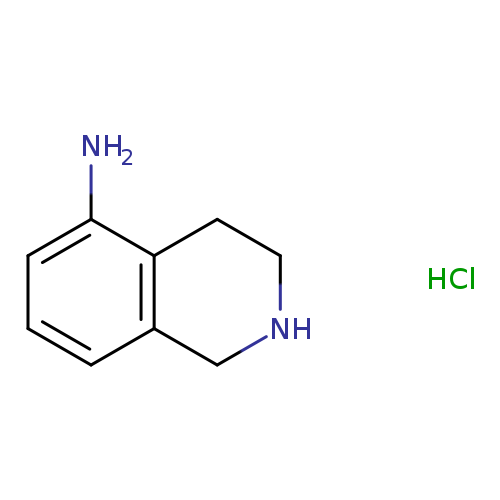
1,2,3,4-Tetrahydroisoquinolin-5-amine hydrochlorideCatalog No.:AA0090Z2 CAS No.:1082658-92-1 MDL No.:MFCD08461265 MF:C9H13ClN2 MW:184.6659 |
1-Phenylpiperidin-4-amine dihydrochlorideCatalog No.:AA007D0J CAS No.:1082662-38-1 MDL No.:MFCD23135281 MF:C11H18Cl2N2 MW:249.1800 |
6-Bromoisoquinoline-1-carbonitrileCatalog No.:AA008Y3M CAS No.:1082674-24-5 MDL No.:MFCD18633063 MF:C10H5BrN2 MW:233.0641 |
7-Bromoheptan-1-amine hydrobromideCatalog No.:AA008W7L CAS No.:1082678-45-2 MDL No.:MFCD28118492 MF:C7H17Br2N MW:275.0246 |
Ethoxy(methyl)amine hydrochlorideCatalog No.:AA01AG3L CAS No.:1082680-18-9 MDL No.:MFCD27920356 MF:C3H10ClNO MW:111.5706 |
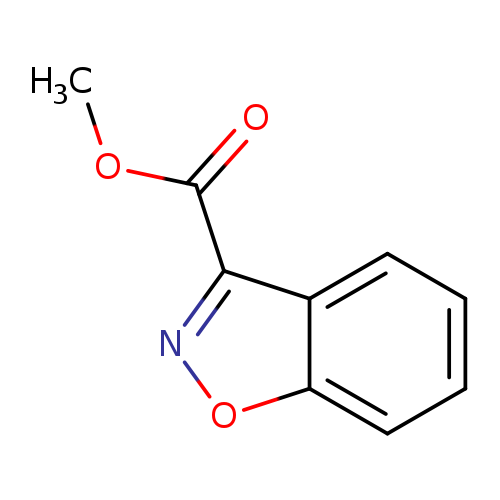
Methyl benzo[d]isoxazole-3-carboxylateCatalog No.:AA0095TK CAS No.:1082682-56-1 MDL No.:MFCD21362332 MF:C9H7NO3 MW:177.1568 |
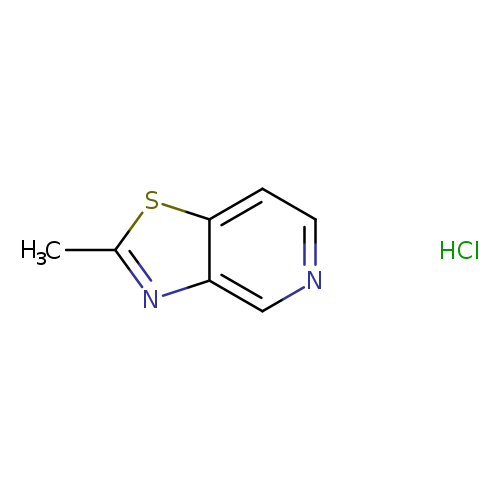
2-methyl-[1,3]thiazolo[4,5-c]pyridine hydrochlorideCatalog No.:AA01BVBA CAS No.:1082684-56-7 MDL No.:MFCD30476352 MF:C7H7ClN2S MW:186.6619 |
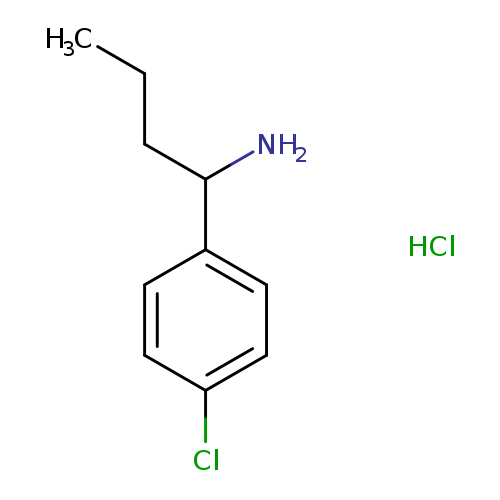
1-(4-chlorophenyl)butan-1-amine hydrochlorideCatalog No.:AA019ZVN CAS No.:1082688-38-7 MDL No.:MFCD22375376 MF:C10H15Cl2N MW:220.1388 |
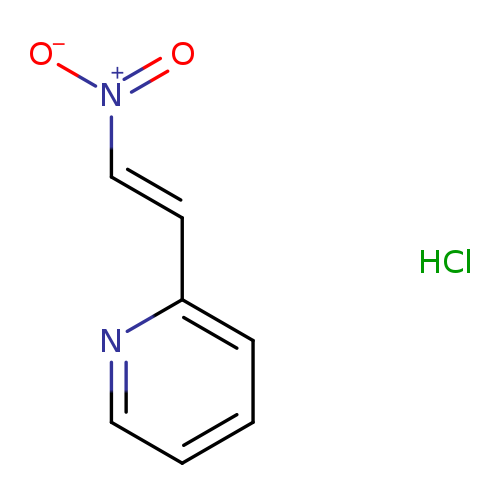
2-[(E)-2-nitroethenyl]pyridine hydrochloride, ECatalog No.:AA01BUTW CAS No.:1082693-24-0 MDL No.:MFCD18917276 MF:C7H7ClN2O2 MW:186.5957 |
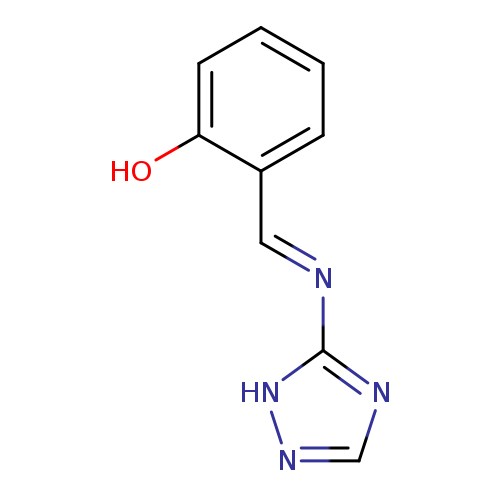
2-[(1E)-[(1H-1,2,4-triazol-5-yl)imino]methyl]phenolCatalog No.:AA00J0OA CAS No.:1082704-14-0 MDL No.:MFCD00773600 MF:C9H8N4O MW:188.1860 |
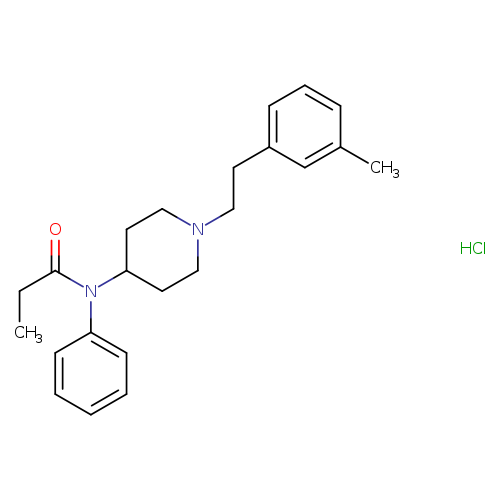
N-[1-[2-(3-methylphenyl)ethyl]-4-piperidinyl]-N-phenyl-propanamide,monohydrochlorideCatalog No.:AA01EQ5Q CAS No.:1082721-49-0 MDL No.: MF:C23H31ClN2O MW:386.9580 |
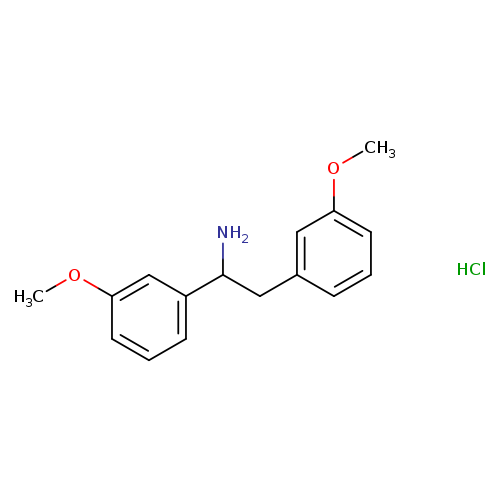
1,2-bis(3-methoxyphenyl)ethan-1-amine hydrochlorideCatalog No.:AA01BIDV CAS No.:1082722-50-6 MDL No.:MFCD30734276 MF:C16H20ClNO2 MW:293.7885 |
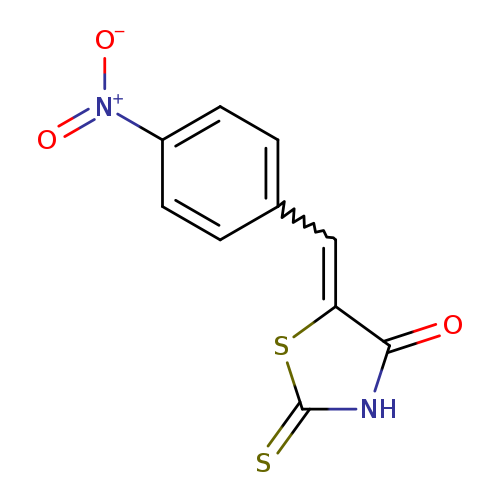
(5E)-2-mercapto-5-(4-nitrobenzylidene)-1,3-thiazol-4(5H)-oneCatalog No.:AA00J0TJ CAS No.:1082724-70-6 MDL No.:MFCD04969014 MF:C10H6N2O3S2 MW:266.2962 |
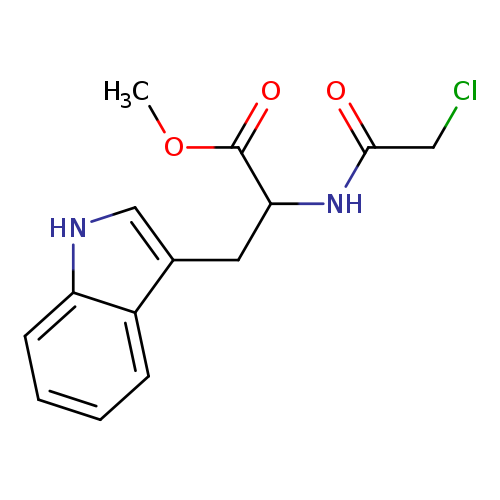
Methyl 2-[(chloroacetyl)amino]-3-(1h-indol-3-yl)propanoateCatalog No.:AA0083SI CAS No.:108273-71-8 MDL No.:MFCD01566900 MF:C14H15ClN2O3 MW:294.7335 |
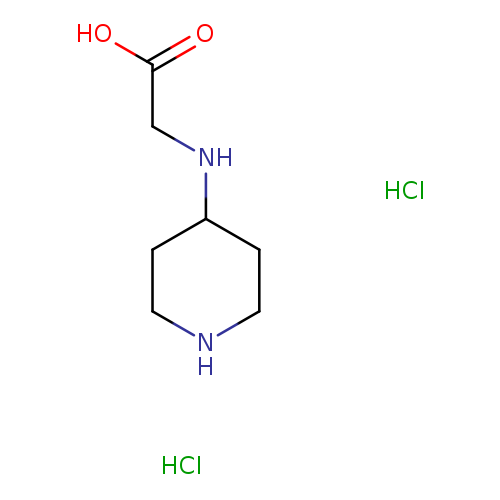
2-[(piperidin-4-yl)amino]acetic acid dihydrochlorideCatalog No.:AA01ABTU CAS No.:1082735-89-4 MDL No.:MFCD26407765 MF:C7H16Cl2N2O2 MW:231.1201 |
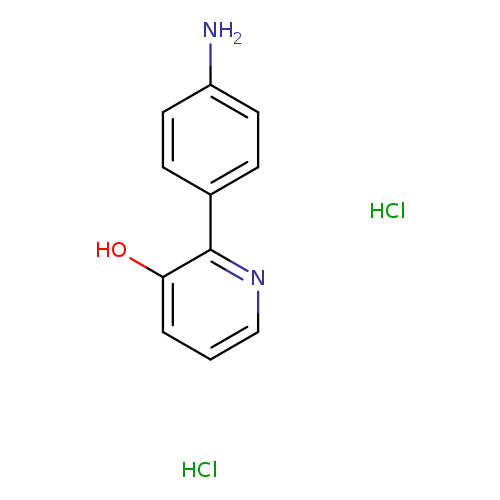
2-(4-Aminophenyl)-3-pyridinol dihydrochlorideCatalog No.:AA00J38D CAS No.:1082739-91-0 MDL No.:MFCD18483563 MF:C11H12Cl2N2O MW:259.1318 |
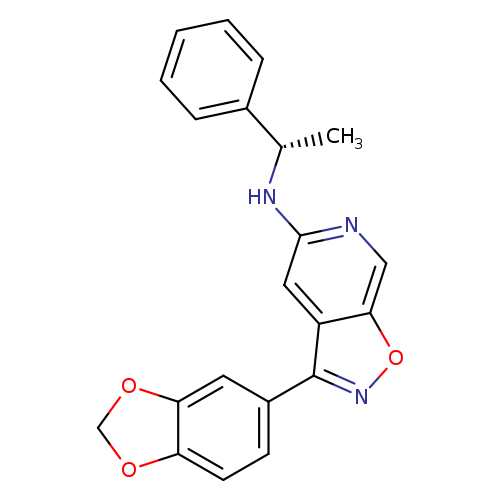
3-(1,3-Benzodioxol-5-yl)-N-[(1S)-1-phenylethyl]-isoxazolo[5,4-c]pyridin-5-amineCatalog No.:AA01ENLK CAS No.:1082739-92-1 MDL No.:MFCD27991269 MF:C21H17N3O3 MW:359.3780 |
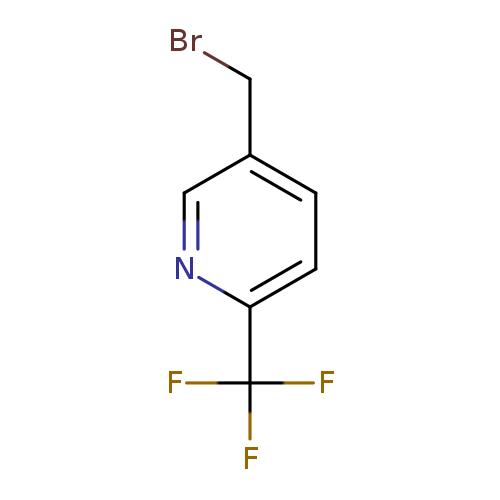
5-(Bromomethyl)-2-(trifluoromethyl)pyridineCatalog No.:AA003897 CAS No.:108274-33-5 MDL No.:MFCD10697684 MF:C7H5BrF3N MW:240.0205 |
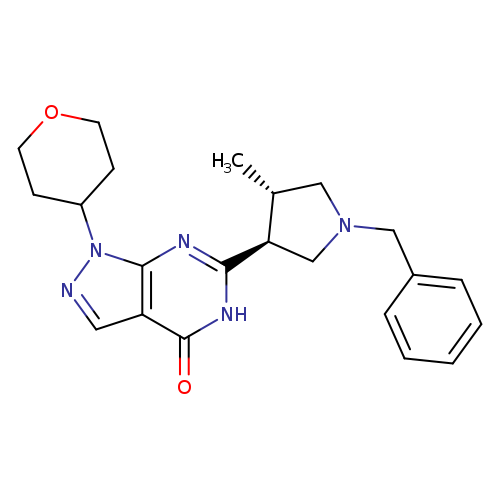
PDE-9 inhibitorCatalog No.:AA008TG1 CAS No.:1082743-70-1 MDL No.:MFCD13184806 MF:C22H27N5O2 MW:393.4821 |
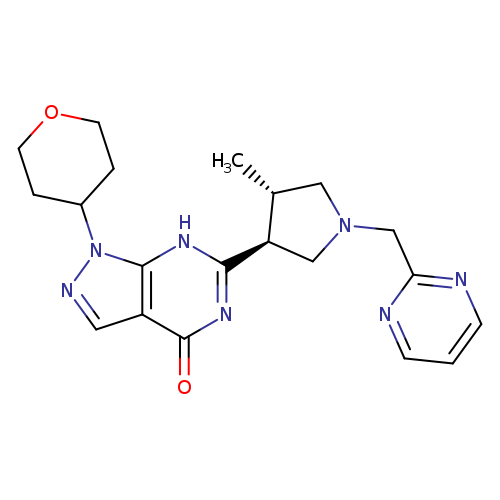
PF 04447943Catalog No.:AA008TG4 CAS No.:1082744-20-4 MDL No.:MFCD22665724 MF:C20H25N7O2 MW:395.4582 |
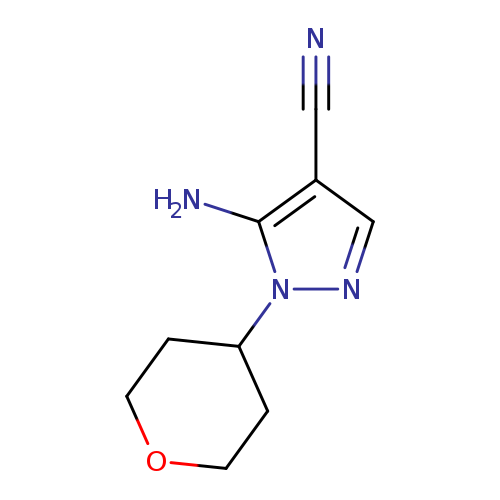
5-Amino-1-(tetrahydro-2h-pyran-4-yl)-1h-pyrazole-4-carbonitrileCatalog No.:AA003896 CAS No.:1082745-49-0 MDL No.:MFCD12405838 MF:C9H12N4O MW:192.2178 |
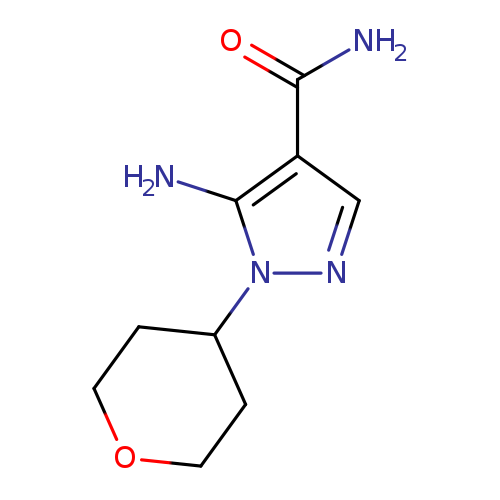
5-Amino-1-(tetrahydro-2h-pyran-4-yl)-1h-pyrazole-4-carboxamideCatalog No.:AA0083SH CAS No.:1082745-50-3 MDL No.:MFCD12405839 MF:C9H14N4O2 MW:210.2331 |
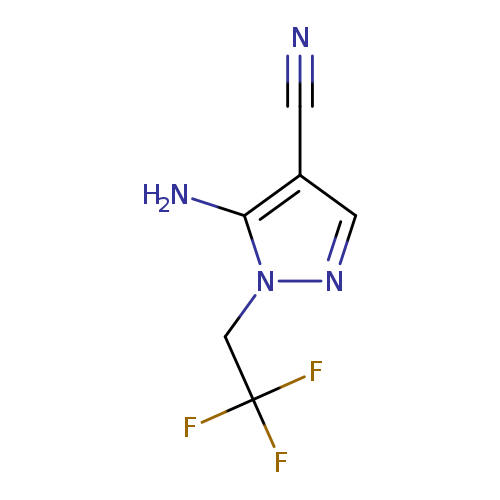
5-amino-1-(2,2,2-trifluoroethyl)-1H-pyrazole-4-carbonitrileCatalog No.:AA00HB21 CAS No.:1082745-56-9 MDL No.:MFCD00128420 MF:C6H5F3N4 MW:190.1259 |
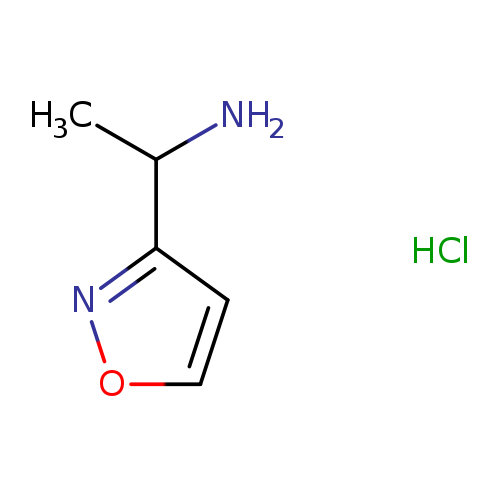
1-(Isoxazol-3-yl)ethanamine hydrochlorideCatalog No.:AA01A3F2 CAS No.:1082746-81-3 MDL No.:MFCD08276220 MF:C5H9ClN2O MW:148.5908 |
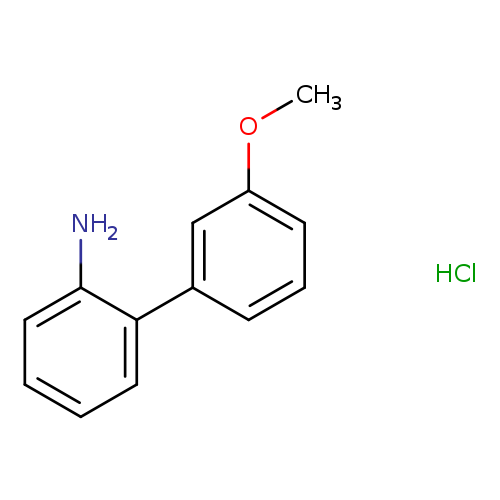
3'-methoxy-[1,1'-biphenyl]-2-amine hydrochlorideCatalog No.:AA00ITPB CAS No.:1082749-15-2 MDL No.:MFCD06739419 MF:C13H14ClNO MW:235.7094 |
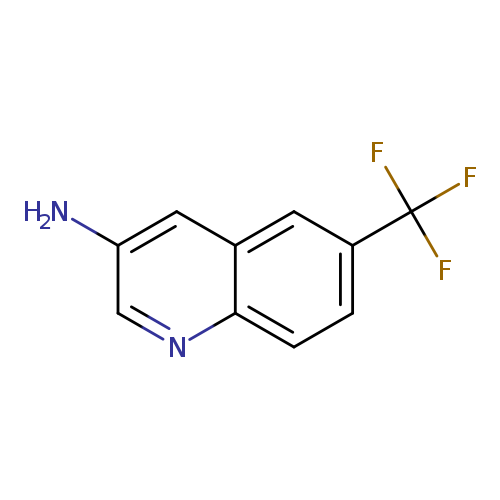
6-(Trifluoromethyl)quinolin-3-amineCatalog No.:AA0095RE CAS No.:1082750-49-9 MDL No.:MFCD26406712 MF:C10H7F3N2 MW:212.1712 |
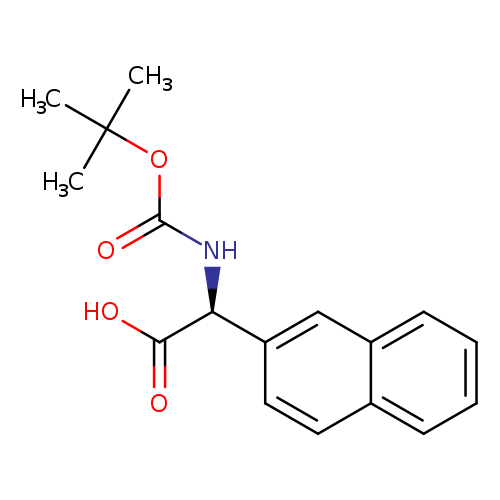
(S)-2-((tert-Butoxycarbonyl)amino)-2-(naphthalen-2-yl)acetic acidCatalog No.:AA00965L CAS No.:1082750-59-1 MDL No.:MFCD07371920 MF:C17H19NO4 MW:301.3371 |
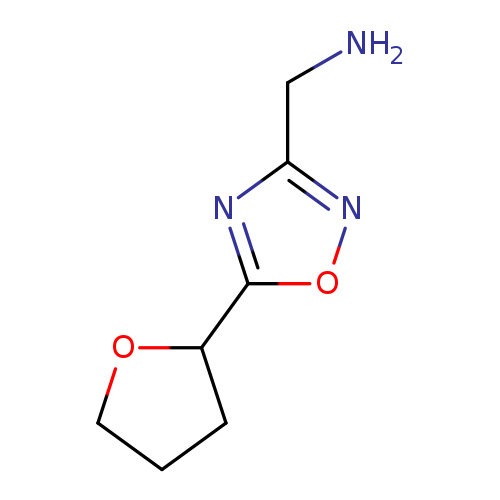
(5-(Tetrahydrofuran-2-yl)-1,2,4-oxadiazol-3-yl)methanamineCatalog No.:AA008VEB CAS No.:1082766-07-1 MDL No.:MFCD11505280 MF:C7H11N3O2 MW:169.1811 |
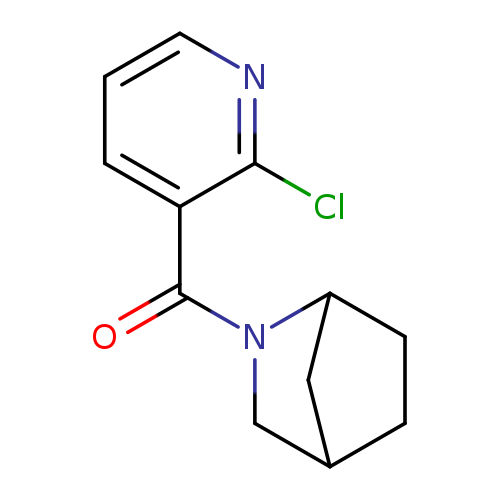
2-[(2-CHLORO-3-PYRIDINYL)CARBONYL]-2-AZABICYCLO[2.2.1]HEPTANECatalog No.:AA0083SF CAS No.:1082766-10-6 MDL No.:MFCD11505286 MF:C12H13ClN2O MW:236.6974 |
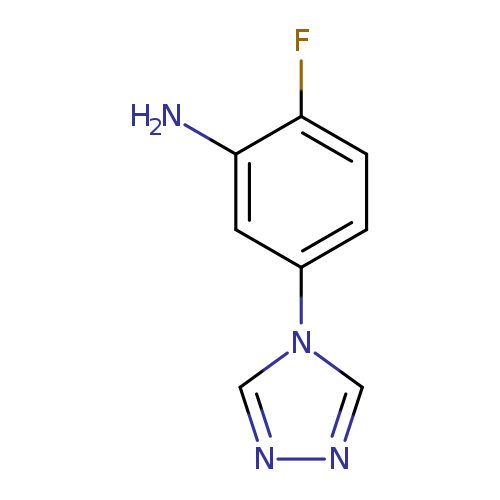
2-Fluoro-5-(4h-1,2,4-triazol-4-yl)anilineCatalog No.:AA0083SE CAS No.:1082766-13-9 MDL No.:MFCD11505288 MF:C8H7FN4 MW:178.1664 |
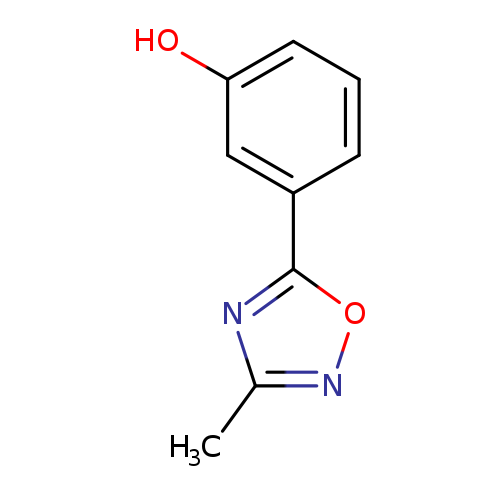
3-(3-Methyl-1,2,4-oxadiazol-5-yl)phenolCatalog No.:AA007V2A CAS No.:1082766-16-2 MDL No.:MFCD11505289 MF:C9H8N2O2 MW:176.1720 |
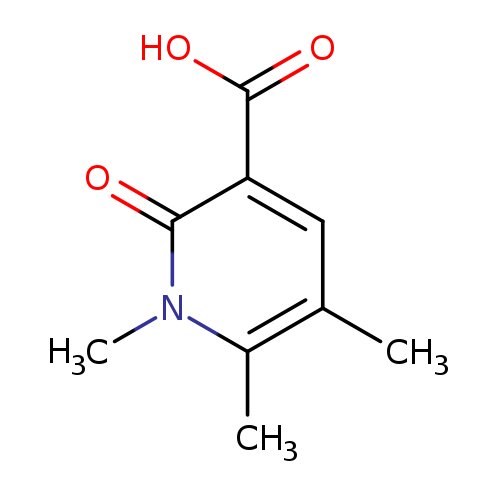
1,5,6-Trimethyl-2-oxo-1,2-dihydro-3-pyridinecarboxylic acidCatalog No.:AA0083SD CAS No.:1082766-19-5 MDL No.:MFCD11505293 MF:C9H11NO3 MW:181.1885 |
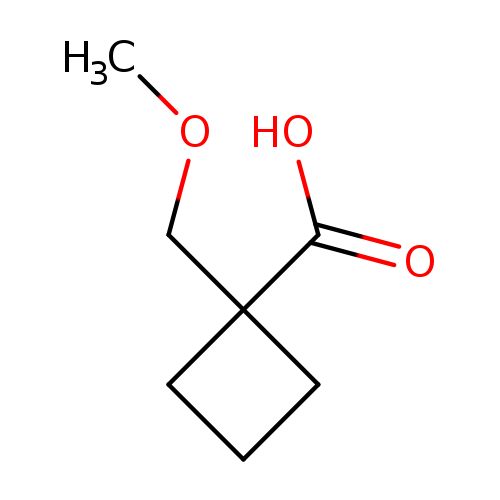
1-(Methoxymethyl)cyclobutanecarboxylic acidCatalog No.:AA00996S CAS No.:1082766-22-0 MDL No.:MFCD11505299 MF:C7H12O3 MW:144.1684 |
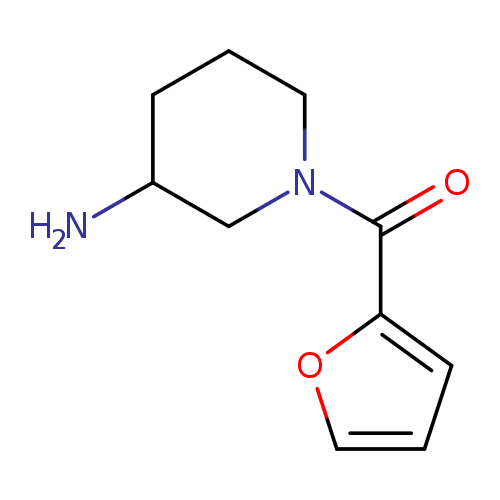
1-(2-Furoyl)piperidin-3-amineCatalog No.:AA007V29 CAS No.:1082766-25-3 MDL No.:MFCD11505301 MF:C10H14N2O2 MW:194.2304 |
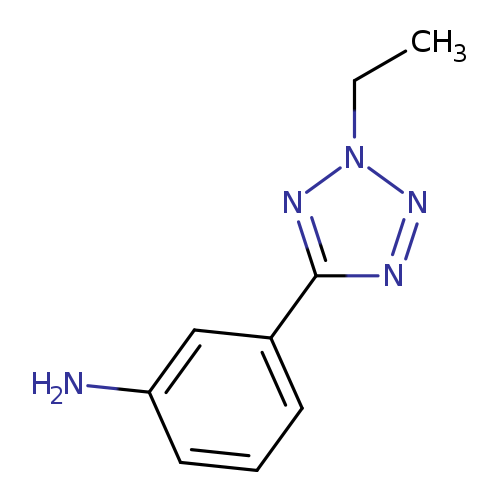
3-(2-Ethyl-2h-tetrazol-5-yl)anilineCatalog No.:AA007D0F CAS No.:1082766-28-6 MDL No.:MFCD11505303 MF:C9H11N5 MW:189.2171 |
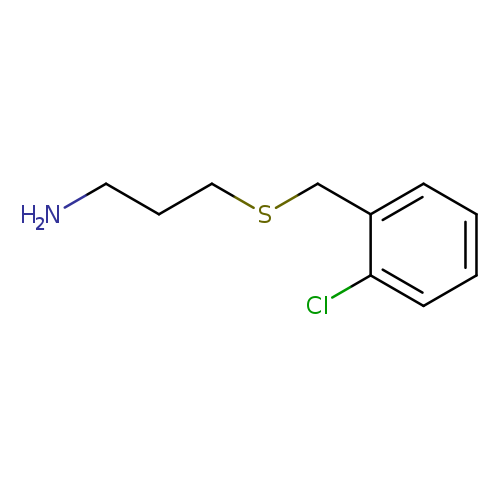
3-[(2-Chlorobenzyl)thio]-1-propanamineCatalog No.:AA007D0E CAS No.:1082766-31-1 MDL No.:MFCD11505309 MF:C10H14ClNS MW:215.7429 |
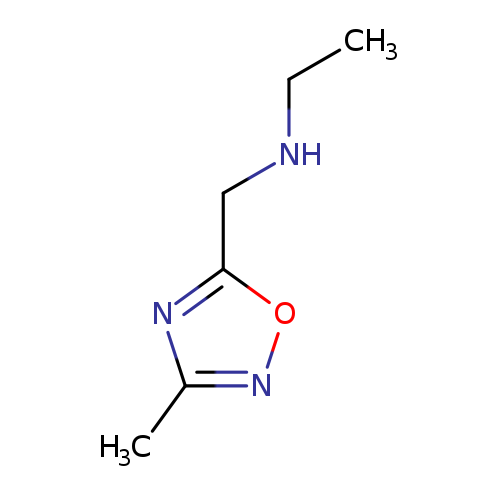
N-[(3-Methyl-1,2,4-oxadiazol-5-yl)methyl]ethanamineCatalog No.:AA007V28 CAS No.:1082766-34-4 MDL No.:MFCD11505312 MF:C6H11N3O MW:141.1710 |
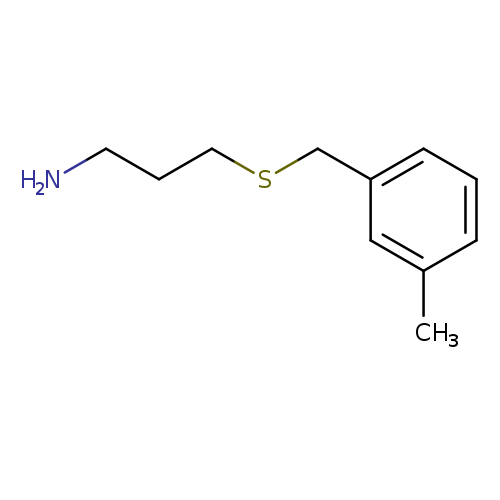
3-[(3-Methylbenzyl)thio]-1-propanamineCatalog No.:AA007D0D CAS No.:1082766-37-7 MDL No.:MFCD11505318 MF:C11H17NS MW:195.3244 |
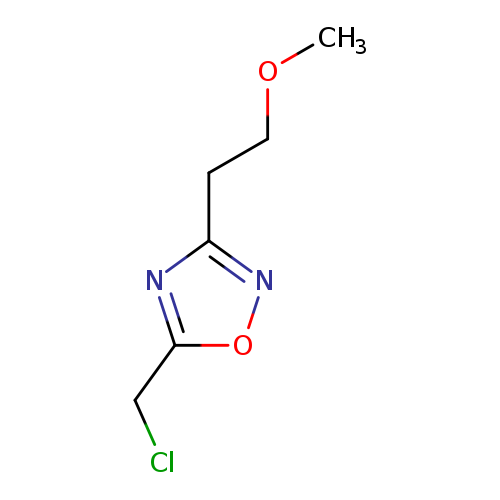
5-(Chloromethyl)-3-(2-methoxyethyl)-1,2,4-oxadiazoleCatalog No.:AA008VIA CAS No.:1082766-40-2 MDL No.:MFCD11505322 MF:C6H9ClN2O2 MW:176.6009 |
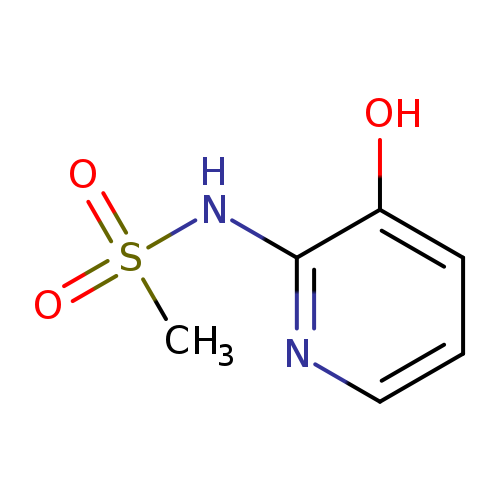
N-(3-Hydroxypyridin-2-yl)methanesulfonamideCatalog No.:AA007D0C CAS No.:1082766-43-5 MDL No.:MFCD11505329 MF:C6H8N2O3S MW:188.2043 |
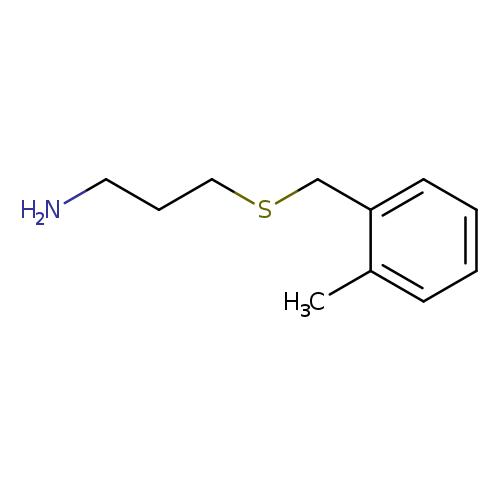
3-[(2-METHYLBENZYL)THIO]-1-PROPANAMINECatalog No.:AA0083SC CAS No.:1082766-46-8 MDL No.:MFCD11505334 MF:C11H17NS MW:195.3244 |
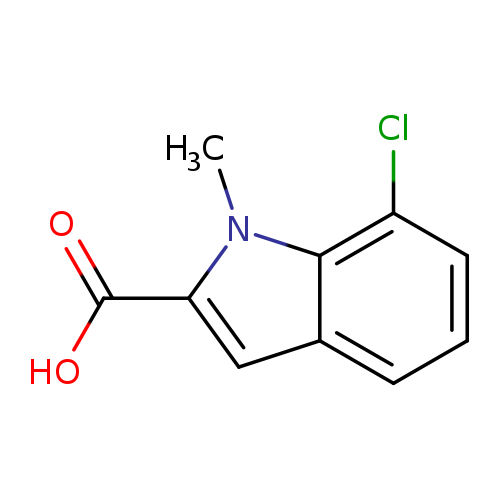
7-Chloro-1-methyl-1H-indole-2-carboxylic acidCatalog No.:AA0083SB CAS No.:1082766-49-1 MDL No.:MFCD11505335 MF:C10H8ClNO2 MW:209.6290 |
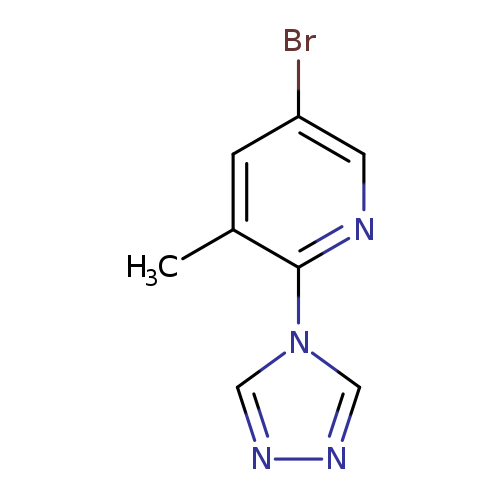
5-Bromo-3-methyl-2-(4H-1,2,4-triazol-4-yl)pyridineCatalog No.:AA0083SA CAS No.:1082766-52-6 MDL No.:MFCD11505342 MF:C8H7BrN4 MW:239.0720 |
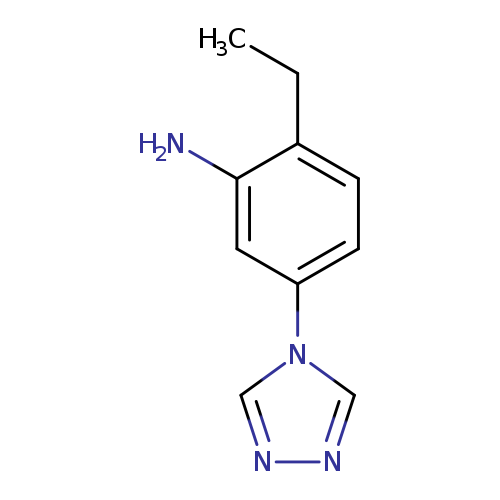
2-Ethyl-5-(4h-1,2,4-triazol-4-yl)anilineCatalog No.:AA008V3F CAS No.:1082766-55-9 MDL No.:MFCD11505344 MF:C10H12N4 MW:188.2291 |
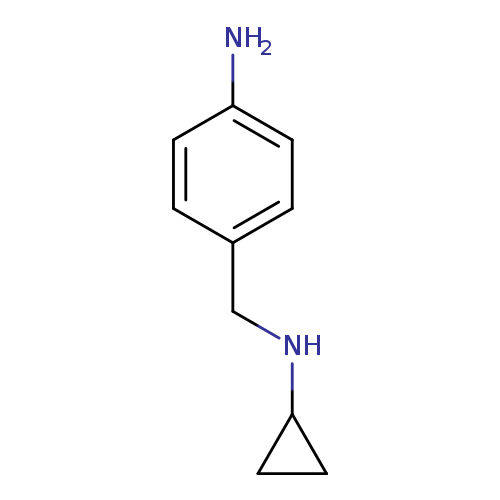
N-Cyclopropyl-4-aminobenzylamineCatalog No.:AA003SUX CAS No.:1082768-71-5 MDL No.:MFCD11223484 MF:C10H14N2 MW:162.2316 |
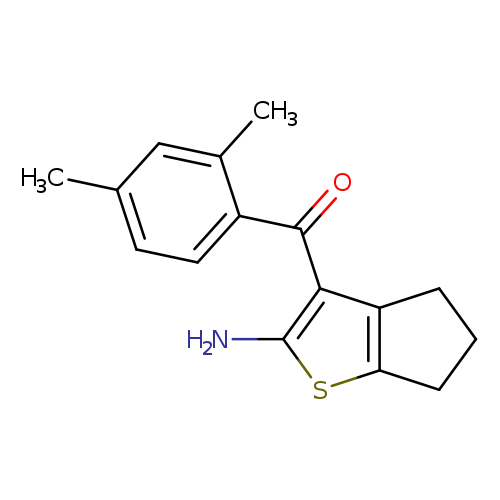
3-(2,4-dimethylbenzoyl)-4H,5H,6H-cyclopenta[b]thiophen-2-amineCatalog No.:AA01A5RJ CAS No.:1082778-95-7 MDL No.:MFCD11607096 MF:C16H17NOS MW:271.3773 |
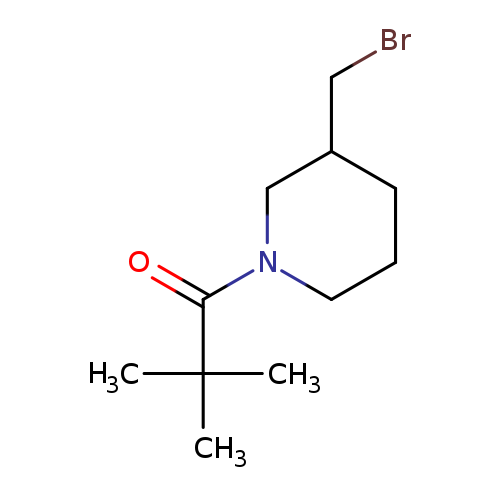
1-[3-(bromomethyl)piperidin-1-yl]-2,2-dimethylpropan-1-oneCatalog No.:AA01AM9F CAS No.:1082786-50-2 MDL No.:MFCD11612805 MF:C11H20BrNO MW:262.1866 |