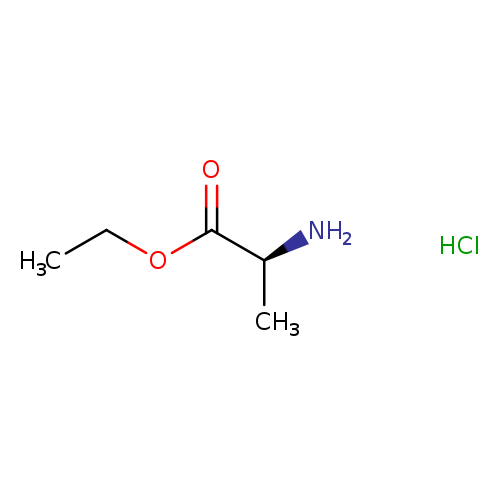
[1]Patent:US5162307,1992,A,
[1]ChemicalCommunications,2011,vol.47,#26,p.7347-7349
[2]DaltonTransactions,2015,vol.44,#3,p.1170-1177
[3]Tetrahedron,1999,vol.55,#11,p.3337-3354
[4]IndianJournalofChemistry-SectionBOrganicandMedicinalChemistry,2006,vol.45,#8,p.1942-1944
[5]JournaloftheChemicalSociety,PerkinTransactions2:PhysicalOrganicChemistry(1972-1999),1994,#7,p.1455-1462
[6]ACSMedicinalChemistryLetters,2016,vol.7,#12,p.1197-1201
[7]EuropeanJournalofInorganicChemistry,2003,#11,p.2113-2122
[8]JournaloftheChemicalSociety,FaradayTransactions1:PhysicalChemistryinCondensedPhases,1980,vol.76,p.915-922
[9]JournaloftheIndianChemicalSociety,2001,vol.78,#3,p.137-141
[10]EuropeanJournalofMedicinalChemistry,2011,vol.46,#1,p.11-20
[11]Tetrahedron,2008,vol.64,#7,p.1301-1308
[12]ChemistryofNaturalCompounds,1994,vol.30,#2,p.238-244
[13]KhimiyaPrirodnykhSoedinenii,1994,#2,p.261-268
[14]Tetrahedron,1990,vol.46,#15,p.5325-5332
[15]JournalofOrganometallicChemistry,1981,vol.221,#2,p.203-222
[16]HelveticaChimicaActa,1995,vol.78,p.109-121
[17]Tetrahedron,2007,vol.63,#14,p.3031-3041
[18]AdvancedSynthesisandCatalysis,2012,vol.354,#2-3,p.295-300
[19]GreenChemistry,2012,vol.14,#5,p.1350-1356
[20]ChemistryofNaturalCompounds,2012,vol.48,#2,p.194-197
[21]Tetrahedron,2012,vol.68,#49,p.10218-10229,12
[22]Tetrahedron,2012,vol.68,#49,p.10218-10229
[23]Nucleosides,NucleotidesandNucleicAcids,2013,vol.32,#4,p.161-173
[24]Patent:US2016/264611,2016,A1,.Locationinpatent:Paragraph0222;0223;0224
[25]InternationalJournalofPharmaceutics,2018,vol.546,#1-2,p.31-38
[1]EuropeanJournalofMedicinalChemistry,2009,vol.44,#9,p.3765-3770
[1]TetrahedronAsymmetry,1999,vol.10,#3,p.411-427
[1]CanadianJournalofChemistry,1976,vol.54,p.733-737
[1]JournaloftheChemicalSociety.PerkintransactionsII,1994,p.1455-1462
[2]CollectionofCzechoslovakChemicalCommunications,1968,vol.33,p.1911-1916
 1115-59-9
1115-59-9


[1]AngewandteChemie,1993,vol.105,p.785-786
[1]TetrahedronLetters,2017,vol.58,p.860-863
[2]Tetrahedron,1983,vol.39,p.3419
[1]ChemicalCommunications,2011,vol.47,p.7347-7349
[2]DaltonTransactions,2015,vol.44,p.1170-1177
[3]EuropeanJournalofMedicinalChemistry,2020,vol.190
[4]Tetrahedron,1999,vol.55,p.3337-3354
[5]ChemicalCommunications,2020,vol.56,p.3373-3376
[6]IndianJournalofChemistry-SectionBOrganicandMedicinalChemistry,2006,vol.45,p.1942-1944
[7]JournaloftheChemicalSociety.PerkintransactionsII,1994,p.1455-1462
[8]ACSMedicinalChemistryLetters,2016,vol.7,p.1197-1201
[9]EuropeanJournalofInorganicChemistry,2003,p.2113-2122
[10]JournaloftheChemicalSociety,FaradayTransactions1:PhysicalChemistryinCondensedPhases,1980,vol.76,p.915-922
[11]JournaloftheIndianChemicalSociety,2001,vol.78,p.137-141
[12]EuropeanJournalofMedicinalChemistry,2011,vol.46,p.11-20
[13]Tetrahedron,2008,vol.64,p.1301-1308
[14]ChemistryofNaturalCompounds,1994,vol.30,p.238-244 KhimiyaPrirodnykhSoedinenii,1994,p.261-268
[15]Tetrahedron,1990,vol.46,p.5325-5332
[16]JournalofOrganometallicChemistry,1981,vol.221,p.203-222
[17]HelveticaChimicaActa,1995,vol.78,p.109-121
[18]Tetrahedron,2007,vol.63,p.3031-3041
[19]AdvancedSynthesisandCatalysis,2012,vol.354,p.295-300
[20]GreenChemistry,2012,vol.14,p.1350-1356
[21]ChemistryofNaturalCompounds,2012,vol.48,p.194-197
[22]Tetrahedron,2012,vol.68,p.10218-10229,12
[23]Nucleosides,nucleotidesandnucleicacids,2013,vol.32,p.161-173
[24]Patent:US2016/264611,2016,A1.Locationinpatent:Paragraph0222;0223;0224
[25]InternationalJournalofPharmaceutics,2018,vol.546,p.31-38
[26]RSCAdvances,2019,vol.9,p.26302-26314
[27]BioorganicChemistry,2020,vol.99