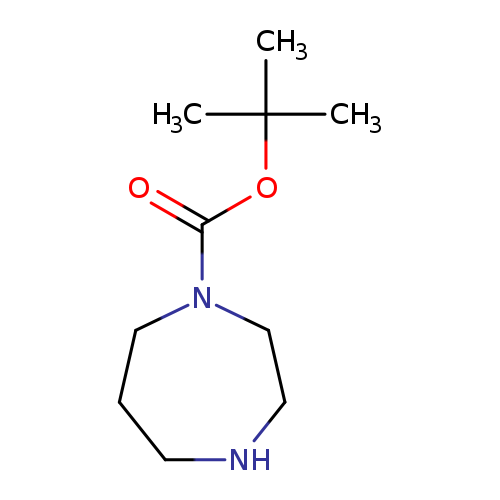
[1]Patent:EP2578588,2013,A1,.Locationinpatent:Paragraph0110;0111
[2]BioorganicandMedicinalChemistry,2010,vol.18,#16,p.6143-6148
[3]Patent:US2016/168156,2016,A1,.Locationinpatent:Paragraph0592;0593
[4]Patent:US2003/216375,2003,A1,
[5]BioorganicandMedicinalChemistryLetters,2012,vol.22,#17,p.5493-5497
[6]BioorganicandMedicinalChemistry,2008,vol.16,#15,p.7291-7301
[7]Patent:EP914319,2001,B1,
[8]Patent:US6686353,2004,B1,.Locationinpatent:Page/Pagecolumn120-121
[9]TetrahedronLetters,2008,vol.49,#34,p.5047-5049
[10]JournaloftheAmericanChemicalSociety,2009,vol.131,#40,p.14413-14418
[11]Patent:US6337326,2002,B1,.Locationinpatent:Example1
[12]Patent:WO2017/219083,2017,A1,.Locationinpatent:Page/Pagecolumn71;72
[13]Patent:US2003/153556,2003,A1,
[14]Patent:US6335324,2002,B1,.Locationinpatent:Pagecolumn92
[15]JournalofMedicinalChemistry,2011,vol.54,#4,p.1033-1058
[16]BioorganicandMedicinalChemistry,2013,vol.21,#11,p.2988-2998
[1]Patent:CN106810467,2017,A,.Locationinpatent:Paragraph0020;0044;0046
[1]Patent:US6262046,2001,B1,
[1]Patent:US5380724,1995,A,
[1]Patent:WO2003/101994,2003,A1,.Locationinpatent:Page84
[1]Locationinpatent:experimentalpartSchön,Uwe;Messinger,Josef;Buckendahl;Prabhu;Konda[Tetrahedron,2009,vol.65,#39,p.8125-8131]
[2]Nielsen,SimonFeldbœk;Nielsen,ElsebetØstergaard;Olsen,GunnarM.;Liljefors,Tommy;Peters,Dan[JournalofMedicinalChemistry,2000,vol.43,#11,p.2217-2226]
[3]CurrentPatentAssignee:DANPET-US2002/45618,2002,A1
[4]CurrentPatentAssignee:DANPET-US2004/72823,2004,A1Locationinpatent:Page/Pagecolumn17
[5]CurrentPatentAssignee:NEUROPORETHERAPIES-WO2022/67114,2022,A1Locationinpatent:Paragraph0259
[6]CurrentPatentAssignee:NEUROPORETHERAPIES-WO2022/67114,2022,A1Locationinpatent:Paragraph0259
[1]CurrentPatentAssignee:DAIICHISANKYOCOMPANY,LIMITED-EP1764367,2007,A1Locationinpatent:Page/Pagecolumn96
[2]CurrentPatentAssignee:IPSENSA-US6340700,2002,B1Locationinpatent:Pagecolumn40
[3]CurrentPatentAssignee:UNIVERSITYOFLONDON-WO2020/99886,2020,A1Locationinpatent:Paragraph00342
[4]Koshio,Hiroyuki;Hirayama,Fukushi;Ishihara,Tsukasa;Taniuchi,Yuta;Sato,Kazuo;Sakai-Moritani,Yumiko;Kaku,Seiji;Kawasaki,Tomihisa;Matsumoto,Yuzo;Sakamoto,Shuichi;Tsukamoto,Shin-Ichi[BioorganicandMedicinalChemistry,2004,vol.12,#9,p.2179-2191]
[5]CurrentPatentAssignee:NUVATIONBIOINC-US2019/106436,2019,A1Locationinpatent:Paragraph0648-0650
[6]CurrentPatentAssignee:SYNTAPHARMACEUTICALSCORP.-US2010/35864,2010,A1Locationinpatent:Page/Pagecolumn7
[7]VanderWel,ScottN.;Harvey,PatriciaJ.;McNamara,DennisJ.;Repine,JosephT.;Keller,PaulR.;QuinIII,John;Booth,R.John;Elliott,WilliamL.;Dobrusin,EllenM.;Fry,DavidW.;Toogood,PeterL.[JournalofMedicinalChemistry,2005,vol.48,#7,p.2371-2387]
[8]Locationinpatent:experimentalpartPerez-Silanes,Silvia;Berrade,Luis;Garcia-Sanchez,RoryN.;Mendoza,Adela;Galiano,Silvia;Perez-Solorzano,BertaMartin;Nogal-Ruiz,JuanJ.;Martinez-Fernandez,AntonioR.;Aldana,Ignacio;Monge,Antonio[Molecules,2009,vol.14,#10,p.4120-4135]
[9]CurrentPatentAssignee:SHIJIAZHUANGSAGACITYNEWDRUGDEVELOPMENTCOLTD-US2020/325145,2020,A1Locationinpatent:Paragraph0167-0170;0302-0304
[10]Kang,Dongwei;Sun,Yanying;Feng,Da;Gao,Shenghua;Wang,Zhao;Jing,Lanlan;Zhang,Tao;Jiang,Xiangyi;Lin,Hao;DeClercq,Erik;Pannecouque,Christophe;Zhan,Peng;Liu,Xinyong[JournalofMedicinalChemistry,2022,vol.65,#3,p.2458-2470]
[1]JournalofMedicinalChemistry,2008,vol.51,p.6581-6591
[2]BioorganicandMedicinalChemistryLetters,2006,vol.16,p.1040-1044
[1]JournalofMedicinalChemistry,2008,vol.51,p.6581-6591
[2]BioorganicandMedicinalChemistryLetters,2006,vol.16,p.1040-1044
[3]Patent:WO2012/71684,2012,A1.Locationinpatent:Page/Pagecolumn26
[4]Patent:US2013/224107,2013,A1.Locationinpatent:Paragraph0160;0161;0162;0163
[1]Patent:WO2007/126935,2007,A2.Locationinpatent:Page/Pagecolumn29
Title: Synthesis of a histamine H(3) receptor antagonist-manipulation of hydroxyproline stereochemistry, desymmetrization of homopiperazine, and nonextractive sodium triacetoxyborohydride reaction workup.
Journal: The Journal of organic chemistry 20100702