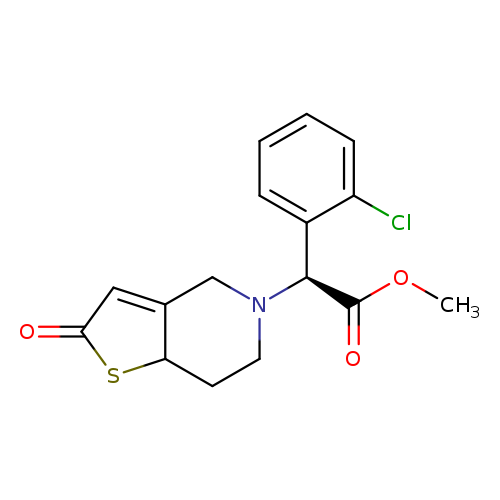
[1]Patent:WO2012/25942,2012,A1
[2]Patent:WO2012/25942,2012,A1
[3]JournalofMedicinalChemistry,2012,vol.55,p.3342-3352
[4]Patent:EP2532668,2012,A1
[5]Patent:US2013/165476,2013,A1
[1]JournalofMedicinalChemistry,2012,vol.55,p.3342-3352
[2]Patent:EP2532668,2012,A1.Locationinpatent:Page/Pagecolumn7-8
[3]Patent:WO2012/25942,2012,A1.Locationinpatent:Page/Pagecolumn20-21
[4]Patent:US2013/165476,2013,A1.Locationinpatent:Paragraph0092;0093
[1]Locationinpatent:experimentalpartShan,Jiaqi;Zhang,Boyu;Zhu,Yaoqiu;Jiao,Bo;Zheng,Weiyi;Qi,Xiaowei;Gong,Yanchun;Yuan,Fang;Lv,Fusheng;Sun,Hongbin[JournalofMedicinalChemistry,2012,vol.55,#7,p.3342-3352]
[2]CurrentPatentAssignee:JILINYATAI(GROUP)CO.,LTD.-EP2532668,2012,A1Locationinpatent:Page/Pagecolumn10
[3]CurrentPatentAssignee:JILINYATAI(GROUP)CO.,LTD.-US2013/165476,2013,A1Locationinpatent:Paragraph0103;0104
[1]CurrentPatentAssignee:UNIVERSITYOFMICHIGAN-WO2014/109987,2014,A1Locationinpatent:Page/Pagecolumn38
[1]Patent:WO2014/109987,2014,A1.Locationinpatent:Page/Pagecolumn45;46
Title: Glutaredoxin is involved in the formation of the pharmacologically active metabolite of clopidogrel from its GSH conjugate.
Journal: Drug metabolism and disposition: the biological fate of chemicals 20120901
Title: Formation of the thiol conjugates and active metabolite of clopidogrel by human liver microsomes.
Journal: Molecular pharmacology 20120801
Title: Overcoming clopidogrel resistance: discovery of vicagrel as a highly potent and orally bioavailable antiplatelet agent.
Journal: Journal of medicinal chemistry 20120412
Title: Cytochromes P450 catalyze both steps of the major pathway of clopidogrel bioactivation, whereas paraoxonase catalyzes the formation of a minor thiol metabolite isomer.
Journal: Chemical research in toxicology 20120220
Title: Dissecting the activation of thienopyridines by cytochromes P450 using a pharmacodynamic assay in vitro.
Journal: The Journal of pharmacology and experimental therapeutics 20111101
Title: Identification of the human cytochrome P450 enzymes involved in the two oxidative steps in the bioactivation of clopidogrel to its pharmacologically active metabolite.
Journal: Drug metabolism and disposition: the biological fate of chemicals 20100101
Title: Cytochrome P-450 polymorphisms and response to clopidogrel.
Journal: The New England journal of medicine 20090521
Title: Shan J, et, al. Overcoming clopidogrel resistance: discovery of vicagrel as a highly potent and orally bioavailable antiplatelet agent. J Med Chem. 2012 Apr 12;55(7):3342-52.
Title: Hagihara K, et, al. Comparison of formation of thiolactones and active metabolites of prasugrel and clopidogrel in rats and dogs. Xenobiotica. 2009 Mar;39(3):218-26.