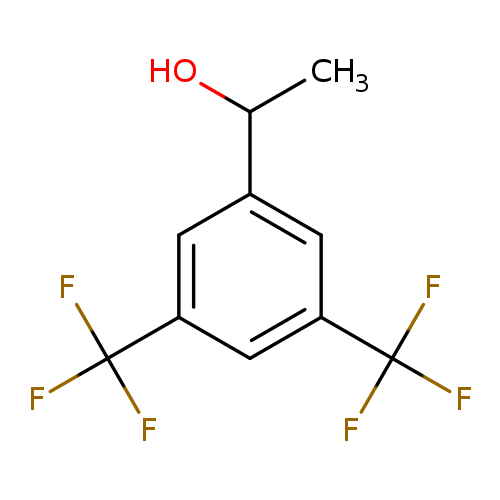
[1]JournalofOrganicChemistry,2003,vol.68,#11,p.4600-4603
[1]OrganicLetters,2003,vol.5,#23,p.4249-4251
[2]JournaloftheAmericanChemicalSociety,1990,vol.112,#15,p.5741-5747
[3]JournalofCatalysis,2004,vol.222,#1,p.117-128
[4]TetrahedronAsymmetry,2003,vol.14,#22,p.3581-3587
[5]AngewandteChemie(Internationaled.inEnglish),2004,vol.43,#21,p.2816-2819
[6]TetrahedronAsymmetry,2004,vol.15,#21,p.3413-3418
[7]AdvancedSynthesisandCatalysis,2005,vol.347,#9,p.1193-1197
[8]AdvancedSynthesisandCatalysis,2006,vol.348,#1-2,p.47-50
[9]OrganicLetters,2006,vol.8,#14,p.2969-2972
[10]JournalofOrganometallicChemistry,2006,vol.691,#10,p.2332-2334
[11]TetrahedronAsymmetry,2006,vol.17,#13,p.2000-2005
[12]Tetrahedron,2004,vol.60,#3,p.789-797
[13]TetrahedronAsymmetry,2007,vol.18,#10,p.1224-1232
[14]Patent:WO2006/67395,2006,A1,.Locationinpatent:Page/Pagecolumn20-21
[15]Patent:WO2006/67395,2006,A1,.Locationinpatent:Page/Pagecolumn25-26
[16]Patent:WO2006/67395,2006,A1,.Locationinpatent:Page/Pagecolumn25
[17]Patent:WO2006/67395,2006,A1,.Locationinpatent:Page/Pagecolumn24-25
[18]Patent:WO2006/67395,2006,A1,.Locationinpatent:Page/Pagecolumn24-25
[19]Patent:WO2006/54115,2006,A1,.Locationinpatent:Page/Pagecolumn9-10
[20]Patent:WO2006/54115,2006,A1,.Locationinpatent:Page/Pagecolumn9-10
[21]Patent:WO2006/54115,2006,A1,.Locationinpatent:Page/Pagecolumn9-10
[22]Patent:EP1927596,2008,A1,.Locationinpatent:Page/Pagecolumn32
[23]AdvancedSynthesisandCatalysis,2008,vol.350,#1,p.197-204
[24]JournalofOrganometallicChemistry,2008,vol.693,#23,p.3527-3532
[25]TetrahedronAsymmetry,2009,vol.20,#23,p.2759-2763
[26]Patent:US2010/261924,2010,A1,.Locationinpatent:Page/Pagecolumn89;91
[27]TetrahedronLetters,2009,vol.50,#6,p.688-692
[28]OrganicandBiomolecularChemistry,2011,vol.9,#16,p.5652-5654
[29]OrganicandBiomolecularChemistry,2012,vol.10,#2,p.355-360
[30]Patent:WO2012/31358,2012,A1,.Locationinpatent:Page/Pagecolumn56;70
[31]OrganicProcessResearchandDevelopment,2012,vol.16,#4,p.710-713
[32]MonatsheftefurChemie,2012,vol.143,#7,p.1045-1054
[33]Chemistry-AnAsianJournal,2012,vol.7,#11,p.2527-2530,4
[34]Chemistry-AnAsianJournal,2012,vol.7,#11,p.2527-2530
[35]AdvancedSynthesisandCatalysis,2012,vol.354,#13,p.2545-2555,11
[36]AdvancedSynthesisandCatalysis,2012,vol.354,#13,p.2545-2555
[37]AngewandteChemie-InternationalEdition,2012,vol.51,#46,p.11624-11628
[38]Angew.Chem.,2012,vol.124,#46,p.11792-11796,5
[39]ChemCatChem,2013,vol.5,#8,p.2253-2257
[40]OrganicLetters,2013,vol.15,#19,p.5110-5113
[41]OrganicProcessResearchandDevelopment,2007,vol.11,#3,p.519-523
[42]JournalofMolecularCatalysisB:Enzymatic,2014,vol.102,p.1-8
[43]JournalofMolecularCatalysisB:Enzymatic,2014,vol.102,p.1-8
[44]Patent:WO2014/68331,2014,A1,.Locationinpatent:Page/Pagecolumn82
[45]OrganicProcessResearchandDevelopment,2014,vol.18,#6,p.793-800
[46]CatalysisCommunications,2014,vol.57,p.111-114
[47]CatalysisCommunications,2014,vol.57,p.111-114
[48]JournalofOrganometallicChemistry,2014,vol.771,p.2-8
[49]JournalofOrganometallicChemistry,2014,vol.771,p.2-8
[50]Organometallics,2014,vol.33,#19,p.5517-5524
[51]Organometallics,2014,vol.33,#19,p.5517-5524
[52]Organometallics,2014,vol.33,#20,p.5791-5801
[53]OrganicProcessResearchandDevelopment,2014,vol.18,#9,p.1137-1141
[54]JournalofMolecularCatalysisA:Chemical,2016,vol.411,p.196-202
[55]RSCAdvances,2016,vol.6,#91,p.88580-88587
[56]ACSCatalysis,2016,vol.6,#10,p.6455-6464
[57]Patent:CN103232324,2016,B,.Locationinpatent:Paragraph0086-0093
[58]Chemistry-AEuropeanJournal,2017,vol.23,#30,p.7212-7216
[59]ChemicalScience,2017,vol.8,#9,p.6531-6541
[60]ChemCatChem,2017,vol.9,#16,p.3125-3130
[61]ACSCatalysis,2017,vol.7,#10,p.6827-6842
[62]ProcessBiochemistry,2017,vol.57,p.72-79
[63]Organometallics,2018,vol.37,#3,p.491-504
[64]EuropeanJournalofOrganicChemistry,2018,vol.2018,#23,p.3031-3035
[65]OrganicLetters,2018,vol.20,#19,p.6135-6139
[66]Organometallics,2018,vol.37,#24,p.4608-4618
[1]JournalofOrganicChemistry,2003,vol.68,#11,p.4600-4603
[1]RSCAdvances,2015,vol.5,#57,p.45943-45955
[2]ChemistryLetters,2005,vol.34,#8,p.1102-1103
[1]JournaloftheAmericanChemicalSociety,2019
[2]OrganicLetters,2003,vol.5,p.4249-4251
[3]JournaloftheAmericanChemicalSociety,1990,vol.112,p.5741-5747
[4]JournalofCatalysis,2004,vol.222,p.117-128
[5]TetrahedronAsymmetry,2003,vol.14,p.3581-3587
[6]AngewandteChemie-InternationalEditioninEnglish,2004,vol.43,p.2816-2819
[7]TetrahedronAsymmetry,2004,vol.15,p.3413-3418
[8]AdvancedSynthesisandCatalysis,2005,vol.347,p.1193-1197
[9]AdvancedSynthesisandCatalysis,2006,vol.348,p.47-50
[10]OrganicLetters,2006,vol.8,p.2969-2972
[11]JournalofOrganometallicChemistry,2006,vol.691,p.2332-2334
[12]TetrahedronAsymmetry,2006,vol.17,p.2000-2005
[13]Tetrahedron,2004,vol.60,p.789-797
[14]TetrahedronAsymmetry,2007,vol.18,p.1224-1232
[15]Patent:WO2006/67395,2006,A1.Locationinpatent:Page/Pagecolumn20-21
[16]Patent:WO2006/67395,2006,A1.Locationinpatent:Page/Pagecolumn25-26
[17]Patent:WO2006/67395,2006,A1.Locationinpatent:Page/Pagecolumn25
[18]Patent:WO2006/67395,2006,A1.Locationinpatent:Page/Pagecolumn24-25
[19]Patent:WO2006/67395,2006,A1.Locationinpatent:Page/Pagecolumn24-25
[20]Patent:WO2006/54115,2006,A1.Locationinpatent:Page/Pagecolumn9-10
[21]Patent:WO2006/54115,2006,A1.Locationinpatent:Page/Pagecolumn9-10
[22]Patent:WO2006/54115,2006,A1.Locationinpatent:Page/Pagecolumn9-10
[23]Patent:EP1927596,2008,A1.Locationinpatent:Page/Pagecolumn32
[24]AdvancedSynthesisandCatalysis,2008,vol.350,p.197-204
[25]JournalofOrganometallicChemistry,2008,vol.693,p.3527-3532
[26]TetrahedronAsymmetry,2009,vol.20,p.2759-2763
[27]Patent:US2010/261924,2010,A1.Locationinpatent:Page/Pagecolumn89;91
[28]TetrahedronLetters,2009,vol.50,p.688-692
[29]OrganicandBiomolecularChemistry,2011,vol.9,p.5652-5654
[30]OrganicandBiomolecularChemistry,2012,vol.10,p.355-360
[31]Patent:WO2012/31358,2012,A1.Locationinpatent:Page/Pagecolumn56;70
[32]OrganicProcessResearchandDevelopment,2012,vol.16,p.710-713
[33]MonatsheftefurChemie,2012,vol.143,p.1045-1054
[34]Chemistry-AnAsianJournal,2012,vol.7,p.2527-2530,4
[35]Advancedsynthesisandcatalysis,2012,vol.354,p.2545-2555,11
[36]AngewandteChemie-InternationalEdition,2012,vol.51,p.11624-11628 Angew.Chem.,2012,vol.124,p.11792-11796,5
[37]ChemCatChem,2013,vol.5,p.2253-2257
[38]OrganicLetters,2013,vol.15,p.5110-5113
[39]OrganicProcessResearchandDevelopment,2007,vol.11,p.519-523
[40]JournalofMolecularCatalysisB:Enzymatic,2014,vol.102,p.1-8
[41]JournalofMolecularCatalysisB:Enzymatic,2014,vol.102,p.1-8
[42]Patent:WO2014/68331,2014,A1.Locationinpatent:Page/Pagecolumn82
[43]OrganicProcessResearchandDevelopment,2014,vol.18,p.793-800
[44]CatalysisCommunications,2014,vol.57,p.111-114
[45]CatalysisCommunications,2014,vol.57,p.111-114
[46]JournalofOrganometallicChemistry,2014,vol.771,p.2-8
[47]JournalofOrganometallicChemistry,2014,vol.771,p.2-8
[48]Organometallics,2014,vol.33,p.5517-5524
[49]Organometallics,2014,vol.33,p.5517-5524
[50]Organometallics,2014,vol.33,p.5791-5801
[51]OrganicProcessResearchandDevelopment,2014,vol.18,p.1137-1141
[52]JournalofMolecularCatalysisA:Chemical,2016,vol.411,p.196-202
[53]RSCAdvances,2016,vol.6,p.88580-88587
[54]ACSCatalysis,2016,vol.6,p.6455-6464
[55]Patent:CN103232324,2016,B.Locationinpatent:Paragraph0086-0093
[56]Chemistry-AEuropeanJournal,2017,vol.23,p.7212-7216
[57]ChemicalScience,2017,vol.8,p.6531-6541
[58]ChemCatChem,2017,vol.9,p.3125-3130
[59]ACSCatalysis,2017,vol.7,p.6827-6842
[60]ProcessBiochemistry,2017,vol.57,p.72-79
[61]Organometallics,2018,vol.37,p.491-504
[62]EuropeanJournalofOrganicChemistry,2018,vol.2018,p.3031-3035
[63]OrganicLetters,2018,vol.20,p.6135-6139
[64]Organometallics,2018,vol.37,p.4608-4618
[65]ChemCatChem,2019,vol.11,p.820-830
[66]AngewandteChemie-InternationalEdition,2019,vol.58,p.4973-4977 Angew.Chem.,2019,vol.131,p.5027-5031,5
[67]AdvancedSynthesisandCatalysis,2019,vol.361,p.4691-4706
[68]Patent:CN110590859,2019,A.Locationinpatent:Paragraph0063-0066;0069-0083
[69]AngewandteChemie-InternationalEdition,2020,vol.59,p.4498-4504 Angew.Chem.,2020,vol.132,p.4528-4534,7
[1]JournalofOrganicChemistry,2003,vol.68,p.4600-4603
[2]ChemicalCommunications,2019,vol.55,p.15033-15036
[1]RSCAdvances,2015,vol.5,p.45943-45955
[2]ChemistryLetters,2005,vol.34,p.1102-1103
[1]JournaloftheAmericanChemicalSociety,2003,vol.125,p.2129-2135
[2]JournaloftheAmericanChemicalSociety,2003,vol.125,p.2129-2135
[3]JournaloftheAmericanChemicalSociety,2003,vol.125,p.2129-2135
[4]JournaloftheAmericanChemicalSociety,2003,vol.125,p.2129-2135
[5]JournaloftheAmericanChemicalSociety,2003,vol.125,p.2129-2135
[6]JournaloftheAmericanChemicalSociety,2003,vol.125,p.2129-2135
[7]JournalofOrganicChemistry,2002,vol.67,p.6743-6747
[1]Patent:US2004/23960,2004,A1