[1]Patent:WO2005/9389,2005,A2,.Locationinpatent:Page/Pagecolumn139
[2]Patent:WO2005/9389,2005,A2,.Locationinpatent:Page/Pagecolumn139
[3]JournalofOrganicChemistry,1985,vol.50,#2,p.217-231
[4]BioorganicandMedicinalChemistryLetters,2002,vol.12,#20,p.2893-2897
[5]OrganicandBiomolecularChemistry,2016,vol.14,#11,p.3061-3068
[6]Patent:WO2006/117653,2006,A1,.Locationinpatent:Page/Pagecolumn46
[7]ChemicalCommunications,2003,#10,p.1170-1171
[8]BioorganicandMedicinalChemistryLetters,2010,vol.20,#2,p.718-721
[9]IndianJournalofChemistry-SectionBOrganicandMedicinalChemistry,2014,vol.53B,#10,p.1269-1274
[10]JournalofOrganicChemistry,2017,vol.82,#7,p.3990-3995
[11]BioorganicandMedicinalChemistryLetters,2011,vol.21,#12,p.3637-3640
[12]Patent:WO2008/125867,2008,A2,.Locationinpatent:Page/Pagecolumn25-26
[13]Patent:CN108752248,2018,A,.Locationinpatent:Paragraph0036-0038;0045-0047;0054-0056
[14]JournalofHeterocyclicChemistry,1990,vol.27,#7,p.1957-1961
[15]Tetrahedron,2001,vol.57,#2,p.319-329
[16]JournaloftheChemicalSociety,1939,p.1159
[17]JournaloftheChemicalSociety,1948,p.265
[18]JournaloftheChemicalSociety,1930,p.817,818
[19]ChemischeBerichte,1941,vol.74,p.79,94
[20]JournalofAgriculturalandFoodChemistry,2003,vol.51,#24,p.6953-6956
[21]JournalofMedicinalChemistry,2005,vol.48,#9,p.3290-3312
[22]Patent:WO2007/58338,2007,A2,.Locationinpatent:Page/Pagecolumn45
[23]Patent:EP2119702,2009,A1,.Locationinpatent:Page/Pagecolumn107
[24]Patent:EP1217000,2002,A1,.Locationinpatent:Page38
[25]ACSMedicinalChemistryLetters,2010,vol.1,#4,p.145-149
[26]Patent:WO2014/34958,2014,A1,.Locationinpatent:Page/Pagecolumn59
[27]ChemistryofNaturalCompounds,2015,vol.51,#4,p.620-625
[28]Khim.Prir.Soedin.,2015,vol.51,#4,p.536-540,5
[29]AngewandteChemie-InternationalEdition,2016,vol.55,#27,p.7821-7825
[30]Angew.Chem.,2016,vol.128,p.7952-7956,5
[31]Patent:WO2017/199227,2017,A1,.Locationinpatent:Page/Pagecolumn9-10
[32]JournalofMedicinalChemistry,2017,vol.60,#24,p.10188-10204
[33]JournalofOrganicChemistry,2018,vol.83,#17,p.9667-9681
[34]Patent:EP973746,2003,B1,.Locationinpatent:Page/Pagecolumn52
[1]Patent:CN105153065,2017,B,.Locationinpatent:Paragraph0157;0158;0159
[2]OrganicandBiomolecularChemistry,2005,vol.3,#12,p.2271-2281
[3]JournalofMolecularLiquids,2017,vol.242,p.1085-1095
[1]Patent:US2002/173656,2002,A1,
[2]Patent:US2002/198195,2002,A1,
[3]Patent:US5208247,1993,A,
[4]Patent:US5603868,1997,A,
[5]Patent:US6268387,2001,B1,
[6]Patent:US6384080,2002,B1,
[1]Patent:WO2005/9389,2005,A2.Locationinpatent:Page/Pagecolumn139
[2]JournalofOrganicChemistry,1985,vol.50,p.217-231
[3]BioorganicandMedicinalChemistryLetters,2002,vol.12,p.2893-2897
[4]OrganicandBiomolecularChemistry,2016,vol.14,p.3061-3068
[5]Patent:WO2006/117653,2006,A1.Locationinpatent:Page/Pagecolumn46
[6]ChemicalCommunications,2003,p.1170-1171
[7]BioorganicandMedicinalChemistryLetters,2010,vol.20,p.718-721
[8]IndianJournalofChemistry-SectionBOrganicandMedicinalChemistry,2014,vol.53B,p.1269-1274
[9]JournalofOrganicChemistry,2017,vol.82,p.3990-3995
[10]BioorganicandMedicinalChemistryLetters,2011,vol.21,p.3637-3640
[11]Patent:WO2008/125867,2008,A2.Locationinpatent:Page/Pagecolumn25-26
[12]Patent:CN108752248,2018,A.Locationinpatent:Paragraph0036-0038;0045-0047;0054-0056
[13]JournalofHeterocyclicChemistry,1990,vol.27,p.1957-1961
[14]Tetrahedron,2001,vol.57,p.319-329
[15]JournaloftheChemicalSociety,1939,p.1159 JournaloftheChemicalSociety,1948,p.265
[16]ChemischeBerichte,1941,vol.74,p.79,94
[17]JournalofAgriculturalandFoodChemistry,2003,vol.51,p.6953-6956
[18]JournalofMedicinalChemistry,2005,vol.48,p.3290-3312
[19]Patent:WO2007/58338,2007,A2.Locationinpatent:Page/Pagecolumn45
[20]Patent:EP2119702,2009,A1.Locationinpatent:Page/Pagecolumn107
[21]Patent:EP1217000,2002,A1.Locationinpatent:Page38
[22]ACSMedicinalChemistryLetters,2010,vol.1,p.145-149
[23]Patent:WO2014/34958,2014,A1.Locationinpatent:Page/Pagecolumn59
[24]ChemistryofNaturalCompounds,2015,vol.51,p.620-625 Khim.Prir.Soedin.,2015,vol.51,p.536-540,5
[25]AngewandteChemie-InternationalEdition,2016,vol.55,p.7821-7825 Angew.Chem.,2016,vol.128,p.7952-7956,5
[26]Patent:WO2017/199227,2017,A1.Locationinpatent:Page/Pagecolumn9-10
[27]JournalofMedicinalChemistry,2017,vol.60,p.10188-10204
[28]JournalofOrganicChemistry,2018,vol.83,p.9667-9681
[29]Patent:EP973746,2003,B1.Locationinpatent:Page/Pagecolumn52
[1]IndianJournalofChemistry-SectionBOrganicandMedicinalChemistry,2014,vol.53B,p.1269-1274
[2]JournaloftheChemicalSociety,1930,p.817,818
[1]Patent:WO2015/161830,2015,A1.Locationinpatent:Paragraph00326
[2]JournalofHeterocyclicChemistry,1990,vol.27,p.1957-1961
[3]EuropeanJournalofMedicinalChemistry,2018,vol.151,p.389-400
[1]Tetrahedron,2013,vol.69,p.6959-6968
[2]BioorganicandMedicinalChemistryLetters,2009,vol.19,p.738-741
[3]OrganicLetters,2017,vol.19,p.2889-2892
[4]JournalofChemicalResearch,Miniprint,1991,p.1240-1254
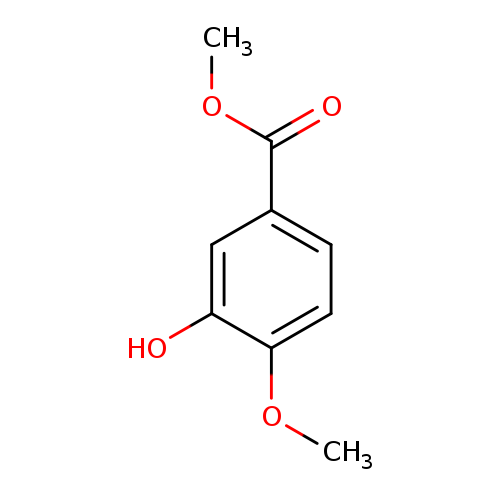
Title: Synthesis of gefitinib from methyl 3-hydroxy-4-methoxy-benzoate.
Journal: Molecules (Basel, Switzerland) 20070328
Title: Withdrawn paper: Li, M.D.; Zheng, Y.G.; Ji, M. Synthesis of gefitinib from methyl 3-hydroxy-4-methoxybenzoate. Molecules 2007,12,673-678.
Journal: Molecules (Basel, Switzerland) 20070101
Title: Protective constituents against sepsis in mice from the root cortex of Paeonia suffruticosa.
Journal: Archives of pharmacal research 20041101
Title: Tyrosinase inhibitor from black rice bran.
Journal: Journal of agricultural and food chemistry 20031119