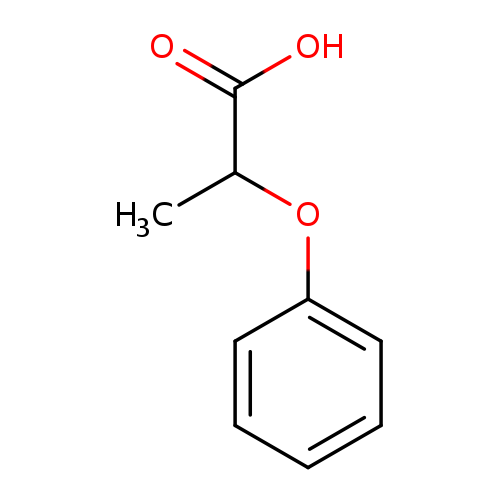
Title: Enantioselective enzymatic hydrolysis of racemic drugs by encapsulation in sol-gel magnetic sporopollenin.
Journal: Bioprocess and biosystems engineering 20120501
Title: Poly[(μ(2)-4,4'-bipyridine-κN:N')bis-(μ(2)-2-phen-oxy-propionato-κO:O')cobalt(II)].
Journal: Acta crystallographica. Section E, Structure reports online 20111101
Title: Tetra-kis(μ(2)-2-phen-oxy-propionato)-κO,O':O';κO:O,O';κO:O'-bis-[(1,10-phenanthroline-κN,N')(2-phen-oxy-propionato-κO,O')lanthanum(III)].
Journal: Acta crystallographica. Section E, Structure reports online 20111001
Title: Bis(μ(2)-2-phen-oxy-propionato-κO:O')bis-[(1,10-phenanthroline-κN,N')bis-(2-phen-oxy-propionato-κO,O')ytterbium(III)].
Journal: Acta crystallographica. Section E, Structure reports online 20111001
Title: Tetra-kis(μ-2-phen-oxy-propionato)-κO,O':O';κO:O,O';κO:O'-bis-[(1,10-phenanthroline-κN,N')(2-phen-oxy-propionato-κO,O')holmium(III)].
Journal: Acta crystallographica. Section E, Structure reports online 20111001
Title: Tetra-kis(μ(2)-2-phen-oxy-propionato)-κO,O':O';κO:O,O';κO:O'-bis-[(1,10-phenanthroline-κN,N')(2-phen-oxy-propionato-κO,O')cerium(III)].
Journal: Acta crystallographica. Section E, Structure reports online 20111001
Title: Tetra-kis(μ(2)-2-phen-oxy-propionato)-κO,O':O';κO:O,O';κO:O'-bis-[(1,10-phenanthroline-κN,N')(2-phen-oxy-propionato-κO,O')gadolinium(III)].
Journal: Acta crystallographica. Section E, Structure reports online 20111001
Title: Tetra-kis(μ-2-phen-oxy-propionato)-κO,O':O';κO:O,O';κO:O'-bis-[(1,10-phenanthroline-κN,N')(2-phen-oxy-propionato-κO,O')terbium(III)].
Journal: Acta crystallographica. Section E, Structure reports online 20110901
Title: Tetra-kis(μ-2-phen-oxy-propionato)-κO,O':O';κO:O,O',κO:O'-bis-[(1,10-phenanthroline-κN,N')(2-phen-oxy-propionato-κO,O')dysprosium(III)].
Journal: Acta crystallographica. Section E, Structure reports online 20110901
Title: Tetra-kis(μ-2-phen-oxy-propionato)-κO,O':O';κO:O,O',κO:O'-bis-[(1,10-phenanthroline-κN,N')(2-phen-oxy-propionato-κO,O')praseodymium(III)].
Journal: Acta crystallographica. Section E, Structure reports online 20110901
Title: Bis(μ-2-phen-oxy-propionato-κO:O')bis-[(1,10-phenanthroline-κN,N')bis-(2-phen-oxy-propionato-κO,O')samarium(III)].
Journal: Acta crystallographica. Section E, Structure reports online 20110901
Title: Synthesis and SAR studies of 1,4-benzoxazine MenB inhibitors: novel antibacterial agents against Mycobacterium tuberculosis.
Journal: Bioorganic & medicinal chemistry letters 20101101
Title: CYP2C8- and CYP3A-mediated C-demethylation of (3-{[(4-tert-butylbenzyl)-(pyridine-3-sulfonyl)-amino]-methyl}-phenoxy)-acetic acid (CP-533,536), an EP2 receptor-selective prostaglandin E2 agonist: characterization of metabolites by high-resolution liquid chromatography-tandem mass spectrometry and liquid chromatography/mass spectrometry-nuclear magnetic resonance.
Journal: Drug metabolism and disposition: the biological fate of chemicals 20081001
Title: A rapid and easy multiresidue method for the determination of pesticide residues in vegetables, fruits, and cereals using liquid Chromatography/tandem mass spectrometry.
Journal: Journal of AOAC International 20080101
Title: Chlorination of 2-phenoxypropanoic acid with NCP in aqueous acetic acid: using a novel ortho-para relationship and the para/meta ratio of substituent effects for mechanism elucidation.
Journal: The Journal of organic chemistry 20070706
Title: Enantioseparation of 2-aryloxypropionic acids on chiral porous monolithic columns by capillary electrochromatography. Evaluation of column performance and enantioselectivity.
Journal: Journal of chromatography. A 20060707
Title: Solubilization of two structurally related anticancer drugs: XK-469 and PPA.
Journal: Journal of pharmaceutical sciences 20060101
Title: Metabolism, excretion, and pharmacokinetics of (3-{[4-tert-butyl-benzyl)-(pyridine-3-sulfonyl)-amino]-methyl}-phenoxy)-acetic acid, an EP2 receptor-selective prostaglandin E2 agonist, in male and female Sprague-Dawley rats.
Journal: Drug metabolism and disposition: the biological fate of chemicals 20050801
Title: In vitro reactivity of carboxylic acid-CoA thioesters with glutathione.
Journal: Chemical research in toxicology 20040101
Title: Theoretical studies on the gas-phase pyrolysis of 2-phenoxycarboxylic acids: an ONIOM approach.
Journal: Journal of computational chemistry 20030601
Title: Hydrogen bonding in enantiomeric versus racemic mono-carboxylic acids; a case study of 2-phenoxypropionic acid.
Journal: Acta crystallographica. Section B, Structural science 20030201
Title: Origin of enantiomeric selectivity in the aryloxyphenoxypropionic acid class of herbicidal acetyl coenzyme A carboxylase (ACCase) inhibitors.
Journal: Journal of agricultural and food chemistry 20020731
Title: Microbial deracemization of alpha-substituted carboxylic acids.
Journal: Organic letters 20020207
Title: Enantiomeric separation of chiral phenoxy acid herbicides by electrokinetic chromatography. Application to the determination of analyte-selector apparent binding constants for enantiomers.
Journal: Electrophoresis 20010901
Title: Solvent-free optical resolution of N-methylamphetamine by distillation after partial diastereoisomeric salt formation.
Journal: Chirality 20010801