Title: Comparison of pharmacokinetic variability of fesoterodine vs. tolterodine extended release in cytochrome P450 2D6 extensive and poor metabolizers.
Journal: British journal of clinical pharmacology 20110801
Title: Pharmacokinetics of tolterodine in Japanese and Koreans: physiological and stochastic assessment of ethnic differences.
Journal: Drug metabolism and pharmacokinetics 20110601
Title: Effects of hepatic dysfunction on the single-dose pharmacokinetics of fesoterodine.
Journal: Journal of clinical pharmacology 20110301
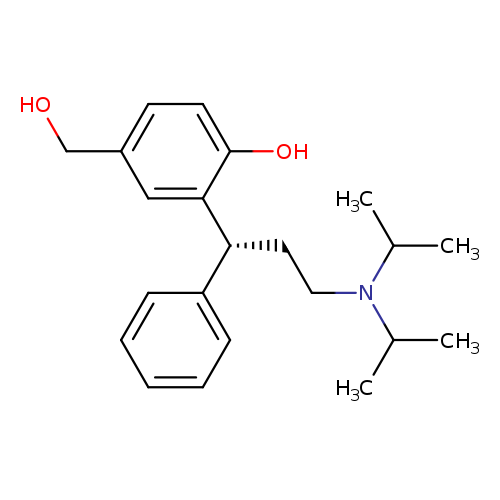
Title: Spinal effects of the fesoterodine metabolite 5-hydroxymethyl tolterodine and/or doxazosin in rats with or without partial urethral obstruction.
Journal: The Journal of urology 20100801
Title: The pharmacokinetic profile of fesoterodine 8 mg with daytime or nighttime dosing.
Journal: European journal of clinical pharmacology 20100201
Title: The design and development of fesoterodine as a prodrug of 5-hydroxymethyl tolterodine (5-HMT), the active metabolite of tolterodine.
Journal: Current medicinal chemistry 20090101
Title: Pharmacokinetic profile of fesoterodine.
Journal: International journal of clinical pharmacology and therapeutics 20081101
Title: Effect of the proton pump inhibitor omeprazole on the pharmacokinetics of extended-release formulations of oxybutynin and tolterodine.
Journal: Journal of clinical pharmacology 20050801
Title: Tolterodine and its active 5-hydroxymethyl metabolite: pure muscarinic receptor antagonists.
Journal: Pharmacology & toxicology 20020501
Title: The effect of tolterodine on the pharmacokinetics and pharmacodynamics of a combination oral contraceptive containing ethinyl estradiol and levonorgestrel.
Journal: Clinical therapeutics 20011101
Title: Food increases the bioavailability of tolterodine but not effective exposure.
Journal: Journal of clinical pharmacology 20010301
Title: Nilvebrant L, Gillberg PG, Sparf B. Antimuscarinic potency and bladder selectivity of PNU-200577, a major metabolite of tolterodine. Pharmacol Toxicol. 1997 Oct;81(4):169-72.
Title: Fullhase, Claudius; Soler, Roberto; Gratzke, Christian et al. Spinal effects of the fesoterodine metabolite 5-hydroxymethyl tolterodine and/or doxazosin in rats with or without partial urethral obstruction. Journal of Urology (New York, NY, United States) (2010), 184(2), 783-789.
Title: B Malhotra, et al. The Design and Development of Fesoterodine as a Prodrug of 5-hydroxymethyl Tolterodine (5-HMT), the Active Metabolite of Tolterodine. Curr Med Chem. 2009;16(33):4481-9.
Title: Naoki Aizawa, et al. Selective Inhibitory Effect of Imidafenacin and 5-hydroxymethyl Tolterodine on Capsaicin Sensitive C Fibers of the Primary Bladder Mechanosensitive Afferent Nerves in the Rat. J Urol. 2015 Apr;193(4):1423-32.