Title: Detoxification of toxins by bacillithiol in Staphylococcus aureus.
Journal: Microbiology (Reading, England) 20120401
Title: Synthesis and structure-activity relationships of novel substituted 8-amino, 8-thio, and 1,8-pyrazole congeners of antitubercular rifamycin S and rifampin.
Journal: Bioorganic & medicinal chemistry letters 20111015
Title: Direct generation of acyclic polypropionate stereopolyads via double diastereo- and enantioselective iridium-catalyzed crotylation of 1,3-diols: beyond stepwise carbonyl addition in polyketide construction.
Journal: Journal of the American Chemical Society 20110817
Title: Rifamycin S and its geometric isomer produced by a newly found actinomycete, Micromonospora rifamycinica.
Journal: Antonie van Leeuwenhoek 20090201
Title: Preparation and in vitro anti-staphylococcal activity of novel 11-deoxy-11-hydroxyiminorifamycins.
Journal: Bioorganic & medicinal chemistry letters 20071015
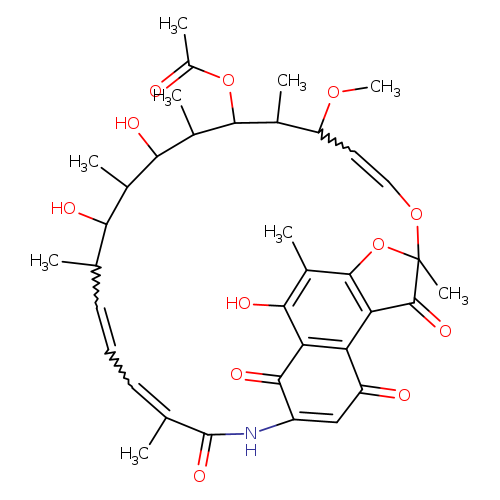
Title: Hygrocins a and B, naphthoquinone macrolides from Streptomyces hygroscopicus.
Journal: Journal of natural products 20051201
Title: NMR spectroscopic analysis of new spiro-piperidylrifamycins.
Journal: Magnetic resonance in chemistry : MRC 20050401
Title: Expeditious asymmetric synthesis of a stereoheptad corresponding to the C(19)-C(27)-ansa chain of rifamycins: formal total synthesis of Rifamycin S.
Journal: Chemistry (Weinheim an der Bergstrasse, Germany) 20050107
Title: Characterization of Mycobacterium tuberculosis mycothiol S-conjugate amidase.
Journal: Biochemistry 20031021
Title: Solid state cultivation of Curvularia lunata for transformation of rifamycin B to S.
Journal: Indian journal of experimental biology 20020801
Title: Efficient strategy for the synthesis of stereopentad subunits of scytophycin, rifamycin S, and discodermolide.
Journal: Organic letters 20011213
Title: Oxabicyclo[3.2.1]octenes in organic synthesis--direct ring opening of oxabicyclo[3.2.1] systems employing silyl ketene acetals in concentrated solutions of lithium perchlorate-diethyl ether: application to the synthesis of the C(19)-C(27) fragment of rifamycin S.
Journal: Organic letters 20010208
Title: Structure-activity relationships in open ansa-chain rifamycin S derivatives as inhibitors of HIV-1 reverse transcriptase.
Journal: Farmaco (Societa chimica italiana : 1989) 19950901
Title: Rao DN, et al. A comparative study of the redox-cycling of a quinone (rifamycin S) and a quinonimine (rifabutin) antibiotic by rat liver microsomes. Free Radic Biol Med. 1997;22(3):439-46.
Title: Kono Y. Oxygen Enhancement of bactericidal activity of rifamycin SV on Escherichia coli and aerobic oxidation of rifamycin SV to rifamycin S catalyzed by manganous ions: the role of superoxide. J Biochem. 1982 Jan;91(1):381-95.
Title: Huang H, et al. Rifamycin S and its geometric isomer produced by a newly found actinomycete, Micromonospora rifamycinica. Antonie Van Leeuwenhoek. 2009 Feb;95(2):143-8.