Title: Isolation of methyl syringate as a specific aflatoxin production inhibitor from the essential oil of Betula alba and aflatoxin production inhibitory activities of its related compounds.
Journal: International journal of food microbiology 20120215
Title: Methyl-3-O-methyl gallate and gallic acid from the leaves of Peltiphyllum peltatum: isolation and comparative antioxidant, prooxidant, and cytotoxic effects in neuronal cells.
Journal: Journal of medicinal food 20111101
Title: Complete genome sequence of 'Enterobacter lignolyticus' SCF1.
Journal: Standards in genomic sciences 20111015
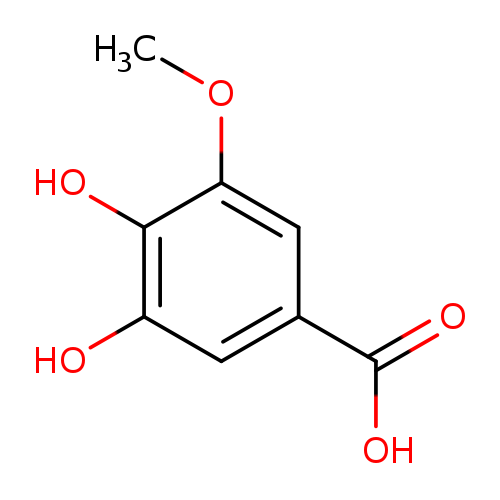
Title: Gut metabolites of anthocyanins, gallic acid, 3-O-methylgallic acid, and 2,4,6-trihydroxybenzaldehyde, inhibit cell proliferation of Caco-2 cells.
Journal: Journal of agricultural and food chemistry 20100512
Title: Comparative genome analysis provides insights into the evolution and adaptation of Pseudomonas syringae pv. aesculi on Aesculus hippocastanum.
Journal: PloS one 20100101
Title: Anti-ischemic activity and endothelium-dependent vasorelaxant effect of hydrolysable tannins from the leaves of Rhus coriaria (Sumac) in isolated rabbit heart and thoracic aorta.
Journal: Planta medica 20091101
Title: Synthesis of (-)-epicatechin 3-(3-O-methylgallate) and (+)-catechin 3-(3-O-methylgallate), and their anti-inflammatory activity.
Journal: Chemistry & biodiversity 20090401
Title: Identification of Cabernet Sauvignon anthocyanin gut microflora metabolites.
Journal: Journal of agricultural and food chemistry 20081008
Title: Degradation of 3-O-methylgallate in Sphingomonas paucimobilis SYK-6 by pathways involving protocatechuate 4,5-dioxygenase.
Journal: FEMS microbiology letters 20070901
Title: Biotransformation of gallic acid by Beauveria sulfurescens ATCC 7159.
Journal: Applied microbiology and biotechnology 20070301
Title: Genetic and biochemical investigations on bacterial catabolic pathways for lignin-derived aromatic compounds.
Journal: Bioscience, biotechnology, and biochemistry 20070101
Title: Characterization of the 3-O-methylgallate dioxygenase gene and evidence of multiple 3-O-methylgallate catabolic pathways in Sphingomonas paucimobilis SYK-6.
Journal: Journal of bacteriology 20040801
Title: Antioxidant activity of phenolic and related compounds: a density functional theory study on the O-H bond dissociation enthalpy.
Journal: Redox report : communications in free radical research 20040101
Title: [Studies on chemical constituents in fruits of Tibetan medicine Phyllanthus emblica].
Journal: Zhongguo Zhong yao za zhi = Zhongguo zhongyao zazhi = China journal of Chinese materia medica 20031001
Title: Molecular mechanisms controlling the rate and specificity of catechol O-methylation by human soluble catechol O-methyltransferase.
Journal: Molecular pharmacology 20010201
Title: Forester SC, et al. Gut metabolites of anthocyanins, gallic acid, 3-O-methylgallic acid, and 2,4,6-trihydroxybenzaldehyde, inhibit cell proliferation of Caco-2 cells. J Agric Food Chem. 2010 May 12;58(9):5320-7.
Title: Forester SC, The anthocyanin metabolites gallic acid, 3-O-methylgallic acid, and 2,4,6-trihydroxybenzaldehyde decrease human colon cancer cell viability by regulating pro-oncogenic signals. Mol Carcinog. 2014 Jun;53(6):432-9.