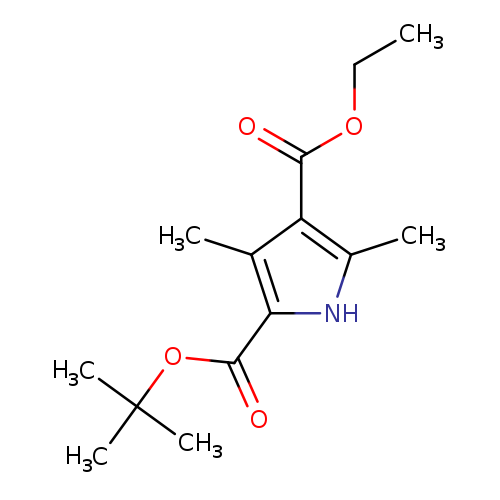
[1]Synlett,2010,#17,p.2561-2564
[2]BioorganicandMedicinalChemistryLetters,2011,vol.21,#10,p.3062-3065
[3]JournaloftheAmericanChemicalSociety,
[4]JournaloftheAmericanChemicalSociety,2009,vol.131,p.8578-8586
[5]JournalofMedicinalChemistry,2010,vol.53,#1,p.139-146
[6]OrganicandBiomolecularChemistry,2016,vol.14,#21,p.4829-4841
[7]MedicinalChemistryResearch,2013,vol.22,#4,p.1723-1729
[8]JournaloftheChemicalSociety,PerkinTransactions1:OrganicandBio-OrganicChemistry(1972-1999),1983,p.633-636
[9]Patent:WO2009/157011,2009,A1,.Locationinpatent:Page/Pagecolumn28
[10]Patent:US2011/92717,2011,A1,.Locationinpatent:Page/Pagecolumn14
[11]Patent:CN108191835,2018,A,.Locationinpatent:Paragraph0026-0027
[12]Patent:WO2008/33743,2008,A1,.Locationinpatent:Page/Pagecolumn53;56
[13]Patent:WO2008/33747,2008,A2,.Locationinpatent:Page/Pagecolumn200-201
[14]ChemischeBerichte,1954,vol.87,p.1163,1166
[1]JournalofMedicinalChemistry,2003,vol.46,#7,p.1116-1119
[2]Bioorganicandmedicinalchemistryletters,2002,vol.12,#16,p.2153-2157
[3]Patent:US2011/92717,2011,A1,
[4]BioorganicandMedicinalChemistryLetters,2011,vol.21,#10,p.3062-3065
[5]Patent:WO2011/119777,2011,A2,
[6]MedicinalChemistryResearch,2013,vol.22,#4,p.1723-1729
[7]OrganicandBiomolecularChemistry,2016,vol.14,#21,p.4829-4841
[8]JournalofChromatographyB:AnalyticalTechnologiesintheBiomedicalandLifeSciences,2018,vol.1092,p.515-523
[1]Bioorganicandmedicinalchemistryletters,2002,vol.12,#16,p.2153-2157
[2]Patent:US2004/266843,2004,A1,.Locationinpatent:Page10;11
[3]ChemicalandPharmaceuticalBulletin,2007,vol.55,#10,p.1439-1441
[4]Patent:US6878733,2005,B1,.Locationinpatent:Page/Pagecolumn181-182
[5]Patent:US2004/204407,2004,A1,.Locationinpatent:Page/Pagecolumn14-15
[6]Patent:US2003/69297,2003,A1,
[7]Patent:US2003/125370,2003,A1,
[1]Patent:US2011/92717,2011,A1,
[2]BioorganicandMedicinalChemistryLetters,2011,vol.21,#10,p.3062-3065
[3]Patent:WO2011/119777,2011,A2,.Locationinpatent:Page/Pagecolumn32
[4]MedicinalChemistryResearch,2013,vol.22,#4,p.1723-1729
[5]OrganicandBiomolecularChemistry,2016,vol.14,#21,p.4829-4841
[6]Patent:CN108191835,2018,A,
[1]JournalofChromatographyB:AnalyticalTechnologiesintheBiomedicalandLifeSciences,2018,vol.1092,p.515-523
[2]JournalofMedicinalChemistry,2003,vol.46,#7,p.1116-1119
[1]ChemischeBerichte,1954,vol.87,p.1163,1166
[2]JournalofMedicinalChemistry,2003,vol.46,p.1116-1119
[3]BioorganicandMedicinalChemistryLetters,2011,vol.21,p.3062-3065
[4]MedicinalChemistryResearch,2013,vol.22,p.1723-1729
[5]OrganicandBiomolecularChemistry,2016,vol.14,p.4829-4841
[6]JournalofChromatographyB:AnalyticalTechnologiesintheBiomedicalandLifeSciences,2018,vol.1092,p.515-523
[7]Patent:US6878733,2005,B1
[1]Patent:WO2008/33743,2008,A1.Locationinpatent:Page/Pagecolumn53;55-56
[1]ChemicalandPharmaceuticalBulletin,2007,vol.55,p.1439-1441
[1]ChemicalandPharmaceuticalBulletin,2007,vol.55,p.1439-1441
[1]JournalofOrganicChemistry,2005,vol.70,p.688-691