Title: Kinetic and mechanistic aspects of atom transfer radical addition (ATRA) catalyzed by copper complexes with tris(2-pyridylmethyl)amine.
Journal: Inorganic chemistry 20121105
Title: Core shell hybrids based on noble metal nanoparticles and conjugated polymers: synthesis and characterization.
Journal: Nanoscale research letters 20110101
Title: Validation of Different Methods of Preparation of Adhatoda vasica Leaf Juice by Quantification of Total Alkaloids and Vasicine.
Journal: Indian journal of pharmaceutical sciences 20080101
Title: Palladium-catalyzed, copper(I)-mediated coupling of boronic acids and benzylthiocyanate. A cyanide-free cyanation of boronic acids.
Journal: Organic letters 20060914
Title: Phenethyl isothiocyanate triggers apoptosis in Jurkat cells made resistant by the overexpression of Bcl-2.
Journal: Cancer research 20060701
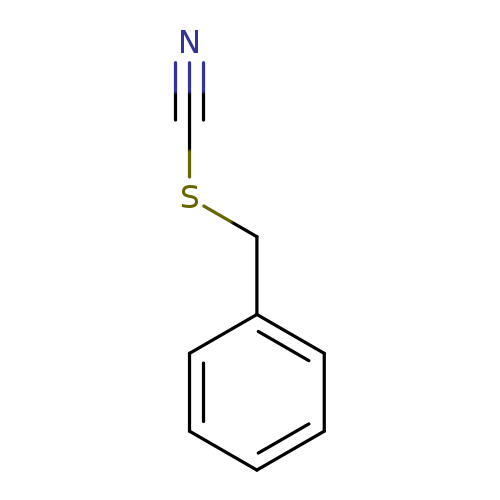
Title: Regioselective bond cleavage in the dissociative electron transfer to benzyl thiocyanates: the role of radical/ion pair formation.
Journal: Journal of the American Chemical Society 20060524
Title: Structure-activity relationship of S-benzylisothiourea derivatives to induce spherical cells in Escherichia coli.
Journal: Bioscience, biotechnology, and biochemistry 20041101
Title: Regioselective bond cleavage in the dissociative electron transfer to benzyl thiocyanates.
Journal: Journal of the American Chemical Society 20031022
Title: Involvement of toxicity as an early event in urinary bladder carcinogenesis induced by phenethyl isothiocyanate, benzyl isothiocyanate, and analogues in F344 rats.
Journal: Toxicologic pathology 20030101
Title: Composition of the essential oil of Lepidium meyenii (Walp).
Journal: Phytochemistry 20020901
Title: Preparation of 2,6-dimethyl-4-arylpyridine- 3,5-dicarbonitrile: a paired electrosynthesis.
Journal: The Journal of organic chemistry 20020405
Title: Major proteins related to chlortetracycline biosynthesis in a Streptomyces aureofaciens production strain studied by quantitative proteomics.
Journal: Applied microbiology and biotechnology 20011201
Title: Benzyl isothiocyanate is the chief or sole anthelmintic in papaya seed extracts.
Journal: Phytochemistry 20010601
Title: Polarographic investigation of reduction process of some azodyes and their complexes with rare earths.
Journal: Talanta 20010412