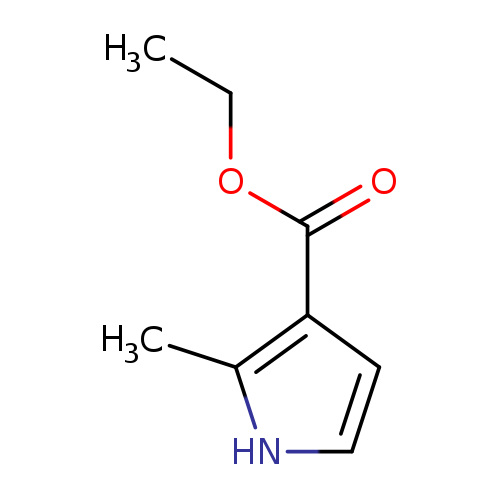
[1]Patent:US2014/275079,2014,A1,.Locationinpatent:Paragraph0604
[2]OrganicandBiomolecularChemistry,2015,vol.13,#29,p.7911-7914
[3]BioorganicandMedicinalChemistryLetters,2017,vol.27,#10,p.2225-2233
[4]ChemischeBerichte,1911,vol.44,p.490
[5]ChemischeBerichte,1918,vol.51,p.569
[6]Patent:WO2017/59191,2017,A1,.Locationinpatent:Paragraph00916-00918
[1]Patent:WO2015/11164,2015,A1,
[1]GazzettaChimicaItaliana,1959,vol.89,p.1457,1458
[1]JournalofOrganicChemistry,1984,vol.49,p.3327-3336
[1]ChemischeBerichte,1980,vol.113,p.2036-2037
[1]LiebigsAnnalenderChemie,1981,p.396-401
Title: Synthesis and SAR studies of 1,4-benzoxazine MenB inhibitors: novel antibacterial agents against Mycobacterium tuberculosis.
Journal: Bioorganic & medicinal chemistry letters 20101101
Title: Microsphere-based protease assays and screening application for lethal factor and factor Xa.
Journal: Cytometry. Part A : the journal of the International Society for Analytical Cytology 20060501