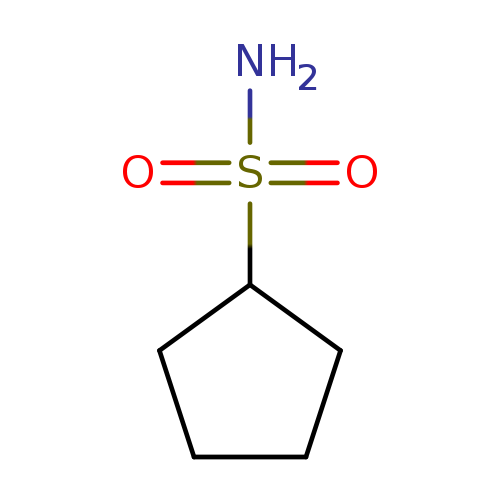
[1]CurrentPatentAssignee:ROCHEHOLDINGAG-WO2021/116050,2021,A1Locationinpatent:Page/Pagecolumn42
[2]CurrentPatentAssignee:UNIVERSITYOFDUBLIN;THEUNIVERSITYOFQUEENSLAND-WO2016/131098,2016,A1Locationinpatent:Page/Pagecolumn90;91
[3]CurrentPatentAssignee:BRISTOL-MYERSSQUIBBCO-US2006/183694,2006,A1Locationinpatent:Page/Pagecolumn31
[4]CurrentPatentAssignee:BRISTOL-MYERSSQUIBBCO-US2007/10455,2007,A1Locationinpatent:Page/Pagecolumn33
[5]CurrentPatentAssignee:BRISTOL-MYERSSQUIBBCO-US2008/107625,2008,A1Locationinpatent:Page/Pagecolumn31
[6]CurrentPatentAssignee:BRISTOL-MYERSSQUIBBCO-WO2003/99274,2003,A1Locationinpatent:Page66
[7]CurrentPatentAssignee:BRISTOL-MYERSSQUIBBCO-WO2008/64061,2008,A1Locationinpatent:Page/Pagecolumn61
[8]CurrentPatentAssignee:BRISTOL-MYERSSQUIBBCO-US2007/93414,2007,A1Locationinpatent:Page/Pagecolumn44-45
[9]CurrentPatentAssignee:BRISTOL-MYERSSQUIBBCO-US2007/99825,2007,A1Locationinpatent:Page/Pagecolumn34
[10]Bravo,Yalda;Baccei,ChristopherS.;Broadhead,Alex;Bundey,Richard;Chen,Austin;Clark,Ryan;Correa,Lucia;Jacintho,JasonD.;Lorrain,DanielS.;Messmer,Davorka;Stebbins,Karin;Prasit,Peppi;Stock,Nicholas[BioorganicandMedicinalChemistryLetters,2014,vol.24,#10,p.2267-2272]
[11]CurrentPatentAssignee:BRISTOL-MYERSSQUIBBCO-US2008/107623,2008,A1Locationinpatent:Page/Pagecolumn30
[12]CurrentPatentAssignee:BRISTOL-MYERSSQUIBBCO-US2008/107624,2008,A1Locationinpatent:Page/Pagecolumn30-31
[13]CurrentPatentAssignee:BRISTOL-MYERSSQUIBBCO-US2008/119461,2008,A1Locationinpatent:Page/Pagecolumn29
[14]CurrentPatentAssignee:BRISTOL-MYERSSQUIBBCO-WO2008/64057,2008,A1Locationinpatent:Page/Pagecolumn58-59
[15]CurrentPatentAssignee:ROCHEHOLDINGAG-WO2022/129259,2022,A1Locationinpatent:Page/Pagecolumn54-55
[16]CurrentPatentAssignee:ROCHEHOLDINGAG-WO2022/129259,2022,A1Locationinpatent:Page/Pagecolumn54-55
[17]CurrentPatentAssignee:ASTRAZENECAPLC-WO2022/155294,2022,A1
[1]CurrentPatentAssignee:BRISTOL-MYERSSQUIBBCO-US2008/14173,2008,A1Locationinpatent:Page/Pagecolumn32
[2]CurrentPatentAssignee:BRISTOL-MYERSSQUIBBCO-WO2005/51410,2005,A1Locationinpatent:Page/Pagecolumn79
[3]CurrentPatentAssignee:BRISTOL-MYERSSQUIBBCO-US2004/48802,2004,A1
[4]CurrentPatentAssignee:BRISTOL-MYERSSQUIBBCO-US2009/274648,2009,A1
[5]CurrentPatentAssignee:BRISTOL-MYERSSQUIBBCO-US2009/304626,2009,A1
[6]CurrentPatentAssignee:BRISTOL-MYERSSQUIBBCO-US2008/107623,2008,A1CurrentPatentAssignee:BRISTOL-MYERSSQUIBBCO-US2008/107624,2008,A1
[7]CurrentPatentAssignee:BRISTOL-MYERSSQUIBBCO-US2008/119461,2008,A1CurrentPatentAssignee:BRISTOL-MYERSSQUIBBCO-WO2008/64057,2008,A1
[1]CurrentPatentAssignee:BRISTOL-MYERSSQUIBBCO-US2002/111313,2002,A1
[1]CurrentPatentAssignee:ELILILLY&CO-US5216026,1993,A
[1]CurrentPatentAssignee:ELILILLY&CO-US5216026,1993,A