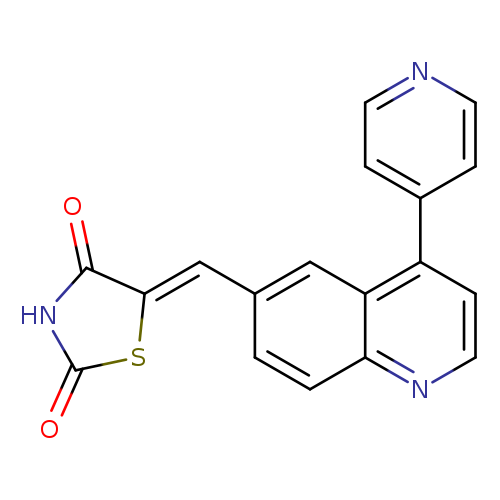
[1]CurrentPatentAssignee:GLAXOSMITHKLINEPLC-WO2007/136940,2007,A2Locationinpatent:Page/Pagecolumn41
[1]CurrentPatentAssignee:GLAXOSMITHKLINEPLC-WO2007/136940,2007,A2
[1]CurrentPatentAssignee:GLAXOSMITHKLINEPLC-WO2007/136940,2007,A2
[1]CurrentPatentAssignee:GLAXOSMITHKLINEPLC-WO2007/136940,2007,A2
Title: GSK1059615 kills head and neck squamous cell carcinoma cells possibly via activating mitochondrial programmed necrosis pathway.
Journal: Oncotarget 20170801
Title: Discovery of GSK2126458, a Highly Potent Inhibitor of PI3K and the Mammalian Target of Rapamycin.
Journal: ACS medicinal chemistry letters 20100408
Title: Carnero A. Novel inhibitors of the PI3K family. Expert Opin Investig Drugs. 2009 Sep;18(9):1265-77.
Title: Maira SM, et al. From the bench to the bed side: PI3K pathway inhibitors in clinical development. Curr Top Microbiol Immunol, 2010, 347, 209-239.
Title: Takashi Kei Kishimoto, et al. Methods and compositions for attenuating gene therapy anti-viral transfer vector immune responses. US 20160074531 A1.