Title: Group VIA phospholipase A2 is a target for vasopressin signaling in the thick ascending limb.
Journal: American journal of physiology. Renal physiology 20120401
Title: Combination therapy of an inhibitor of group VIA phospholipase A2 with paclitaxel is highly effective in blocking ovarian cancer development.
Journal: The American journal of pathology 20110701
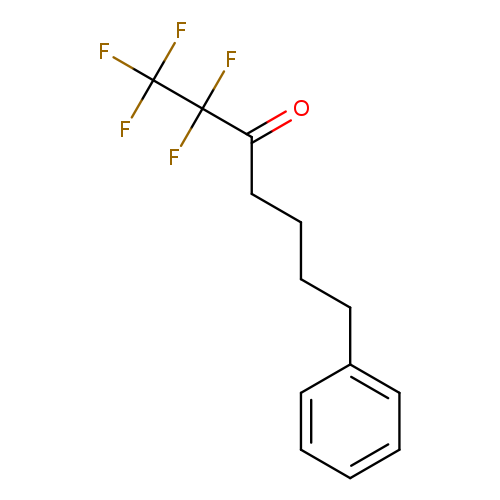
Title: Potent and selective fluoroketone inhibitors of group VIA calcium-independent phospholipase A2.
Journal: Journal of medicinal chemistry 20100513
Title: Synthesis of polyfluoro ketones for selective inhibition of human phospholipase A2 enzymes.
Journal: Journal of medicinal chemistry 20081225
Title: Intracellular phospholipase A(2) group IVA and group VIA play important roles in Wallerian degeneration and axon regeneration after peripheral nerve injury.
Journal: Brain : a journal of neurology 20081001
Title: Kokotos G, et al. Potent and selective fluoroketone inhibitors of group VIA calcium-independent phospholipase A2. J Med Chem. 2010 May 13;53(9):3602-10.
Title: Yan Xu, et al. Abstract 3525: Targeting Group VIA phospholipase A2 using small molecules for ovarian cancer treatment. Cancer Res (2011) 71 (8_Supplement): 3525.
Title: López-Vales R, et al. Intracellular phospholipase A(2) group IVA and group VIA play important roles in Wallerian degeneration and axon regeneration after peripheral nerve injury. Brain. 2008 Oct;131(Pt 10):2620-31.