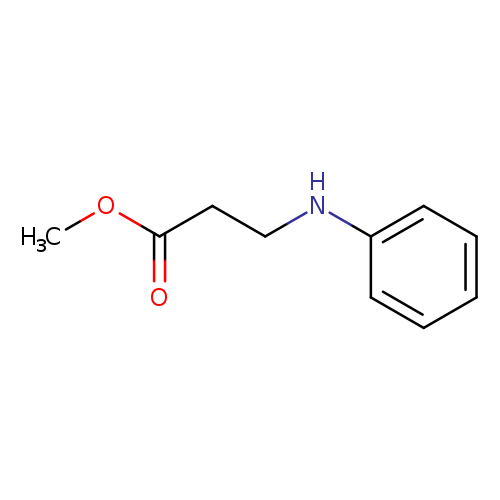
[1]TetrahedronLetters,2006,vol.47,#48,p.8583-8586
[2]OrganicLetters,2011,vol.13,#15,p.4008-4011
[1]SyntheticCommunications,2009,vol.39,#6,p.1109-1119
[2]RSCAdvances,2016,vol.6,#98,p.95951-95956
[3]MonatsheftefurChemie,2008,vol.139,#9,p.1041-1047
[4]InorganicChemistryCommunications,2015,vol.53,p.92-96
[5]Tetrahedron,2013,vol.69,#6,p.1712-1716
[6]Tetrahedron,2010,vol.66,#5,p.1091-1097
[7]CatalysisLetters,2018,vol.148,#9,p.2918-2928
[8]AsianJournalofChemistry,2011,vol.23,#9,p.3792-3794
[9]ComptesRendusChimie,2011,vol.14,#12,p.1059-1064
[10]MonatsheftefurChemie,2012,vol.143,#1,p.109-112
[11]TetrahedronLetters,2006,vol.47,#13,p.2125-2127
[12]JournaloftheChineseChemicalSociety,2010,vol.57,#6,p.1221-1226
[13]JournaloftheIranianChemicalSociety,2011,vol.8,#3,p.775-781
[14]IndianJournalofHeterocyclicChemistry,2010,vol.20,#2,p.169-170
[15]IndianJournalofChemistry-SectionBOrganicandMedicinalChemistry,2005,vol.44,#4,p.838-841
[16]TetrahedronLetters,2016,vol.57,#46,p.5026-5032
[17]RSCAdvances,2015,vol.5,#51,p.40950-40952
[18]TetrahedronLetters,2006,vol.47,#48,p.8583-8586
[19]Synthesis,2008,#24,p.3931-3936
[20]OrganicProcessResearchandDevelopment,2008,vol.12,#6,p.1078-1088
[21]SyntheticCommunications,2013,vol.43,#2,p.191-197,7
[22]OrganicProcessResearchandDevelopment,2008,vol.12,#1,p.41-57
[23]OrganicPreparationsandProceduresInternational,2013,vol.45,#1,p.28-43
[24]SyntheticCommunications,2010,vol.40,#19,p.2830-2836
[25]JournalofOrganicChemistry,2016,vol.81,#10,p.4048-4057
[26]OrganicLetters,2016,vol.18,#23,p.6140-6143
[27]JournalofOrganometallicChemistry,2003,vol.665,#1-2,p.250-257
[28]Tetrahedron,2010,vol.66,#29,p.5373-5377
[29]Synlett,1998,#9,p.975-976
[30]ChemicalCommunications,2010,vol.46,#43,p.8234-8236
[31]JournalofOrganicChemistry,2009,vol.74,#16,p.6260-6265
[32]Tetrahedron,2004,vol.60,#2,p.383-387
[33]Tetrahedron,2006,vol.62,#4,p.672-677
[34]Tetrahedron,2007,vol.63,#4,p.904-909
[35]Patent:US2008/9482,2008,A1,.Locationinpatent:Page/Pagecolumn121
[36]ResearchonChemicalIntermediates,2011,vol.37,#8,p.883-890
[37]JournaloftheAmericanChemicalSociety,1949,vol.71,p.2532,2537
[38]JournaloftheAmericanChemicalSociety,1949,vol.71,p.1901,1905
[39],1967,vol.3,p.520-526
[40]ZhurnalOrganicheskoiKhimii,1967,vol.3,p.542-550
[41]Synthesis,1981,#5,p.375-376
[42]EuropeanJournalofMedicinalChemistry,1993,vol.28,#11,p.837-852
[43]JournaloftheAmericanChemicalSociety,2006,vol.128,#5,p.1446-1447
[44]Tetrahedron,2007,vol.63,#29,p.6764-6773
[45]TetrahedronLetters,2007,vol.48,#49,p.8735-8738
[46]OrganicandBiomolecularChemistry,2009,vol.7,#11,p.2452-2457
[47]Synlett,2010,#3,p.379-382
[48]TetrahedronLetters,2010,vol.51,#33,p.4455-4458
[49]ComptesRendusChimie,2011,vol.14,#11,p.973-977
[50]ACSCatalysis,2013,vol.3,#12,p.2891-2904
[51]Organometallics,2013,vol.32,#23,p.6986-6995
[52]ProcessBiochemistry,2016,vol.51,#10,p.1420-1433
[1]Tetrahedron,2011,vol.67,#1,p.167-172
[1]Tetrahedron,2014,vol.70,#14,p.2449-2454
[2]JournalofOrganicChemistry,2009,vol.74,#16,p.6260-6265
[1]OrganicandBiomolecularChemistry,2015,vol.13,#16,p.4637-4641
[1]Patent:US2470094,1945,
[1]JournaloftheAmericanChemicalSociety,1959,vol.81,p.5957,5959
[1]Arzneimittel-Forschung/DrugResearch,1973,vol.23,p.290-295
[1]Arzneimittel-Forschung/DrugResearch,1973,vol.23,p.290-295
[1]Arzneimittel-Forschung/DrugResearch,1973,vol.23,p.290-295