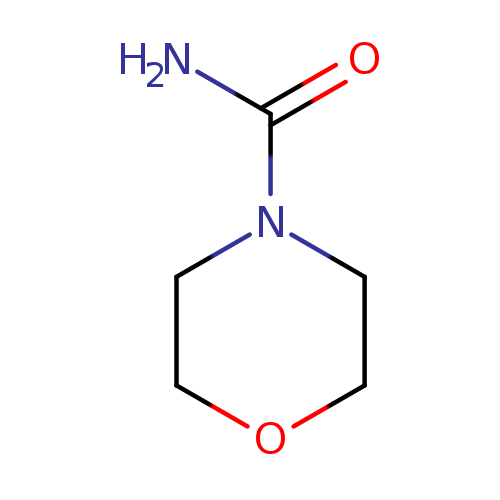
[1]Schroth,W.;Kluge,H.;Frach,R.;Hodek,W.;Schaedler,H.D.[JournalfurpraktischeChemie(Leipzig1954),1983,vol.325,#5,p.787-802]
[2]Schroth,Werner;Kluge,Helmut;Matthias,Michael;Krieg,Reimar;Schaedler,Hans-Dieter[ZeitschriftfurChemie,1981,vol.21,#1,p.25-27]
[1]BioorganicandMedicinalChemistry,2005,vol.13,p.3705-3720
[1]Patent:US2012/172320,2012,A1
[1]Aksinenko,AlexeyYu.;Goreva,TatyanaV.;Epishina,TatyanaA.;Trepalin,SergeyV.;Sokolov,VladimirB.[JournalofFluorineChemistry,2017,vol.201,p.19-23]
Title: Synthesis, antitumor, antitrypanosomal and antileishmanial activities of benzo[4,5]canthin-6-ones bearing the N'-(Substituted benzylidene)-carbohydrazide and N-Alkylcarboxamide groups at C-2.
Journal: Chemical & pharmaceutical bulletin 20120101
Title: Asymmetric synthesis of (+)-galbelgin, (-)-kadangustin J, (-)-cyclogalgravin and (-)-pycnanthulignenes A and B, three structurally distinct lignan classes, using a common chiral precursor.
Journal: The Journal of organic chemistry 20110819
Title: New classes of alanine racemase inhibitors identified by high-throughput screening show antimicrobial activity against Mycobacterium tuberculosis.
Journal: PloS one 20110101
Title: Modeling of human prokineticin receptors: interactions with novel small-molecule binders and potential off-target drugs.
Journal: PloS one 20110101
Title: The oxytocin-oxytocin receptor system and its antagonists as tocolytic agents.
Journal: International journal of endocrinology 20110101
Title: Structural insights into substrate specificity in variants of N-acetylneuraminic Acid lyase produced by directed evolution.
Journal: Journal of molecular biology 20101119
Title: In vitro metabolism of indomethacin morpholinylamide (BML-190), an inverse agonist for the peripheral cannabinoid receptor (CB(2)) in rat liver microsomes.
Journal: European journal of pharmaceutical sciences : official journal of the European Federation for Pharmaceutical Sciences 20100911
Title: BML-111, a lipoxin receptor agonist, modulates the immune response and reduces the severity of collagen-induced arthritis.
Journal: Inflammation research : official journal of the European Histamine Research Society ... [et al.] 20080401
Title: Lewis base activation of Lewis acids: catalytic, enantioselective vinylogous aldol addition reactions.
Journal: The Journal of organic chemistry 20070720
Title: Cannabinoids stimulate fibroblastic colony formation by bone marrow cells indirectly via CB2 receptors.
Journal: Calcified tissue international 20070101
Title: Lewis base activation of Lewis acids. Vinylogous aldol addition reactions of conjugated N,O-silyl ketene acetals to aldehydes.
Journal: Journal of the American Chemical Society 20060201
Title: Syntheses and neuraminidase inhibitory activity of multisubstituted cyclopentane amide derivatives.
Journal: Journal of medicinal chemistry 20040408
Title: Reduction of human monocytic cell neurotoxicity and cytokine secretion by ligands of the cannabinoid-type CB2 receptor.
Journal: British journal of pharmacology 20030601
Title: BML-190 and AM251 act as inverse agonists at the human cannabinoid CB2 receptor: signalling via cAMP and inositol phosphates.
Journal: FEBS letters 20030211
Title: Design, synthesis, and structure-activity relationships of macrocyclic hydroxamic acids that inhibit tumor necrosis factor alpha release in vitro and in vivo.
Journal: Journal of medicinal chemistry 20010802
Title: Effects of cannabinoids on LPS-stimulated inflammatory mediator release from macrophages: involvement of eicosanoids.
Journal: Journal of cellular biochemistry 20010101