2020-01-31 10:40:49
Santigopal Mondal,† Santhivardhana Reddy Yetra,† Subrata Mukherjee,† and Akkattu T. Biju*,‡
†Organic Chemistry Division, CSIR-National Chemical Laboratory, Dr. Homi Bhabha Road, Pune 411008, India
‡Department of Organic Chemistry, Indian Institute of Science, Bangalore 560012, India
1.INTRODUCTION
Since the seminal, independent, isolation of free carbenes by the Bertrand1 and Arduengo2 groups, N-heterocyclic carbene (NHC)-based organocatalysis has emerged as an effective and sophisticated synthetic strategies for the rapid construction of medicinally and biologically important molecules from simple starting materials. In general, NHCs are useful for the umpolung of aldehydes. The addition of carbenes to aldehydes generate the nucleophilic enaminol intermediate known as the “Breslow intermediates” (Figure 1).4 The two important transformations proceeding via the umpolung strategy are benzoin condensation5 and Stetter reaction.6 Moreover, NHCs are also employed in the conjugate umpolung of α,β-unsaturated aldehydes, and these reactions proceed via the generation of homoenolate equivalents.7 In addition, the reaction of NHC with α-functionalized aldehydes such as α-chloroaldehydes, α- epoxyaldehydes or ketenes could result in the generation of NHC-bound enolates.8 A wide variety of carbocycles, hetero- cycles and acyclic molecules can be accessed using the NHC- umpolung concept, and the use of chiral NHCs in the process results in the synthesis of enantiomerically enriched compounds. In addition to the application of NHCs in umpolung strategies, carbenes are also useful as catalysts in non-umpolung transformations.9 An important mode of reactivity in this domain proceeds through the α,β-unsaturated acylazoliums. In many cases, α,β-unsaturated acylazolium intermediate acts as a bis-electrophile allowing the conjugate addition of various bis- nucleophiles followed by 1,2-addition to form a wide variety of carbocycles and heterocycles. Important methods for the generation of α,β-unsaturated acylazoliums include (i) the reaction of α,β-unsaturated aldehydes with NHCs in the presence of stoichiometric oxidants, (ii) the reaction of ynals with NHCs in the absence of external oxidants, (iii) the reaction of 2-bromoenals and rarely 3-bromoenals with NHCs, (iv) the reaction of NHCs with α,β-unsaturated esters, thioester, or acyl fluorides and more recently, and (v) the reaction of NHCs with α,β-unsaturated acids and amides (Figure 2). The purpose of this Account is to summarize the recent developments in our laboratory on the NHC-catalyzed generation of α,β-unsaturated acylazolium intermediates and their subsequent reactions with bis-nucleophiles thereby shedding light on the power of this NHC-bound intermediate in organocatalysis. To put things in proper perspective, adequate description of related work carried out by others is also included in this Account.
Analogous to the NHC-catalyzed umpolung of aldehydes, the chemistry of α,β-unsaturated acylazoliums also has a biological origin. α,β-Unsaturated acylazoliums were found to be the key intermediates in the biosynthesis of clavulanic acid (2). In 2007, Merski and Townsend successfully demonstrated that the conjugate addition of L-arginine to the α,β-unsaturated acylazolium 1 derived from thiamine diphosphate (ThDP, vitamin B1) is the key step in the biosynthesis of the potent β- lactamase inhibitor clavulanic acid (2) (Scheme 1).
2.[3 + 3] ANNULATION OF α,β-UNSATURATED ACYLAZOLIUMS WITH 1,3-BISNUCLEOPHILES
In 2009, Lupton and co-workers demonstrated that NHCs can catalyze the intramolecular cyclization reactions of α,β- unsaturated enol esters 3 leading to the synthesis of dihydropyranones 4. The reaction proceeds via the intermediacy of the α,β-unsaturated acylazolium 5 (Scheme 2, eq 1). The reaction is initiated by the 1,2-addition of NHC generated from the imidazolium salt A to the enol ester leading to the α,β- unsaturated acylazolium 5 and the enolate 6. Michael addition of the enolate 6 to the azolium 5 generates the NHC-bound enolate 7, which undergoes a proton transfer to form the azolium 8. The intramolecular acylation then affords the dihydropyranone 4. A variety of enol esters with β,β-disubstitutions underwent smooth annulation reactions to afford the dihydropyranone products in good yields (up to 92%). Moreover, the Lupton group demonstrated that NHCs can catalyze the [3 + 3] annulation reaction of TMS enol ethers 9 with α,β-unsaturated acid fluorides 10 leading to the synthesis of dihydropyranones 11 in 37−76% yields (eq 2).
After Zeitler successfully demonstrated that α,β-unsaturated
acylazoliums can be reliably generated from ynals,12 coupling of ynals with enolic C-nucleophiles such as kojic acids 12 proceeding via the enantioselective Claisen rearrangement was disclosed by Bode and co-workers (Scheme 3).13 The expected dihydropyranone product was unstable under the reaction conditions. In the presence of methanol, the ring-opened product 13 was formed in good yields (78−98%) and excellent enantioselectivities (up to >99%). In addition to kojic acid, pyruvic esters and β-naphthol afforded the corresponding functionalized dihydropyranone under identical conditions.
The reaction afforded functionalized dihydropyranones 19 in good to excellent yields (51−92%) using NHC generated from the triazolium salt D. A wide variety of α,β-unsaturated aldehydes were well tolerated under the optimized reaction conditions and nucleophiles such as β-diketones and β- ketoesters underwent smooth annulation reaction to afford the desired products. In the same year, Xiao and co-workers disclosed an efficient and atom-economic NHC-catalyzed annulation reaction of ynals with 1,3-diketones or 1,3-keto esters for the synthesis of dihydropyranones (eq 4).15 In the presence of NHC generated from the imidazolium salt A and KOt-Bu, the desired dihydropyranones were obtained in moderate to good yields (41−74%). Subsequently, Xiao and co-workers developed the enantioselective version of this [3 + 3] annulation reaction using NHC generated from the chiral triazolium salt ent-C under base-free conditions.16 In addition, an enantioselective version of the oxidative NHC-catalyzed dihydropyranone synthesis was developed by the You group.17 Furthermore, the generation of α,β-unsaturated acylazoliums from 2-bromoenals followed by their interception with 1,3- dicarbonyl compounds was demonstrated by the Ye18 and Yao groups.19
Inspired by these studies, we envisioned a unified strategy for the enantioselective synthesis of dihydropyranones and dihydropyridinones by the NHC-catalyzed formal [3 + 3] annulation of 2-bromoenals with readily available 1,3-dicarbonyl compounds or primary vinylogous amides. Treatment of 2- bromoenal 21 with 1,3-dicarbonyl compound 22 in the presence of carbene generated from the chiral triazolium salt C using DABCO as the base and LiOAc as the additive furnished the dihydropyranones 23 in excellent yields (up to 96%) and enantioselectivities (up to 99%) (Table 1).20 For good reactivity and selectivity, the combination of two bases was found to be important. A broad range of 2-bromoenals as well as 1,3- dicarbonyl compounds were well tolerated under the optimized reaction condition. Notably, the reaction proceeds under mild conditions and relatively low catalyst loading (5 mol %). Employing primary vinylogous amides 24 as the bisnucleophile resulted in the enantioselective synthesis of dihydropyridinones 25 (Table 2).20 The protection of the vinylogous amide nitrogen was not required for this annulation and the amide-bond forming side reaction was not observed under the present conditions.
Mechanistically, the reaction proceeds via the generation of free carbene from C followed by its addition to 2-bromoenal 21 and subsequent proton transfer. The resulting Breslow intermediate 26,4 then undergoes debromination to afford the key α,β-unsaturated acylazolium intermediate 27 (Scheme 5). Nucleophilic addition of 22 or 24 to intermediate 27 generates the NHC-bound enol intermediate 28, which undergoes proton transfer and a subsequent intramolecular acylation resulting in the formation of the dihydropyranones 23 or dihydropyridi- nones 25 via the azolium 29. To get insight into the mode of enantioinduction, DFT studies were carried out. These studies indicated that the addition of acetyl acetone from below the plane containing the triazolium moiety (leading to the intermediate 28a) is energetically more favored than the approach from above the plane of the triazolium (leading to the intermediate 28b). This is because the addition of nucleophile from below the plane is stabilized by possible intramolecular hydrogen bonding between the carbonyl oxygen and enol hydrogen, which is absent when the nucleophile approaches from above the plane of the azolium moiety.
Motivated by the success of this reaction, we have envisioned the interception of the chiral α,β-unsaturated acylazolium intermediates with various cyclic and acyclic bisnucleophiles. Employing enolizable aldehydes 30 as the coupling partner for 2-bromoenals 21 resulted in a chemoselective NHC-catalyzed enantioselective cross-coupling for the synthesis of 4,5- disubstituted dihydropyranones 31 (Table 3).21 It is noteworthy that the enantioselective formation of dihydropyranones took place in favor of eight possible byproducts (two γ-butyrolac- tones, four benzoin products, and two Stetter products). The use of NHC generated from the chiral triazolium salt C using Na2CO3 as the base afforded the desired products in good yields (41−96%) and excellent enantioselectivities (87−99%) under mild reaction conditions and relatively low catalyst loading. Various electron-donating and -withdrawing substituents on β- aryl ring of 2-bromoenals as well as aliphatic 2-bromoenals are well tolerated under the optimized reaction conditions. Additionally, a series of enolizable aldehydes also underwent smooth annulation reaction to form the desired product.
Moreover, the heterocyclic C−H acids such as 4-hydroxy coumarins (32) were successfully employed as the 1,3- bisnucleophilic component for the interception of α,β- unsaturated acylazolium, generated from 2-bromoenals and NHCs, for the synthesis of biologically important coumarin- fused dihydropyranones 33.22 The NHC generated from imidazolium salt A was efficient for this transformation, resulting in various oxygen heterocycles, and the target products 33 were obtained in 37−95% yield. The use of N-methyl quinolinones as the bisnucleophile afforded quinolinone-fused dihydropyra- nones in high yields. The enantioselective version of this reaction using NHC generated from the chiral triazolium salt C furnished the coumarin-fused dihydropyranones in up to 86% ee (Table 4). Competition experiments revealed that the presence of electron-withdrawing groups on β-aryl ring of 21 enhances the reaction rate.
Subsequently, the use of pyrazolones as bisnucleophiles in oxidative NHC-catalysis was investigated. The formal [3 + 3] annulation of pyrazolones 34 with α,β-unsaturated acylazoliums generated from enals 18 under oxidative conditions (using bisquinone 20) afforded the dihydropyranone-fused pyrazoles 35 in good yields (up to 82%) and ee values (up to 96%) (Table 5).23 The reaction proceeds under mild, operationally simple and base-free conditions. Interestingly, in the absence of the NHC catalyst, simple Knoevenagel condensation of enals with 34 occurred. A wide variety of functional groups on the enal as well as on the pyrazolone moiety were well tolerated under the reaction conditions. Moreover, the β,β-disubstituted enal underwent smooth annulation reaction to afford the desired product. Considering the importance of functionalized pyrazoles in the pharmaceutical and agricultural industries, the NHC-catalyzed enantioselective routes to these compounds under metal-free conditions are attractive.
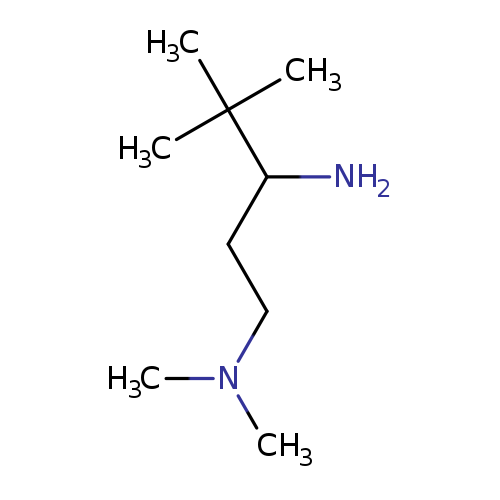
(3-amino-4,4-dimethylpentyl)dimethylamineCatalog No.:AA01AITR CAS No.:1092301-75-1 MDL No.:MFCD11558162 MF:C9H22N2 MW:158.2844 |
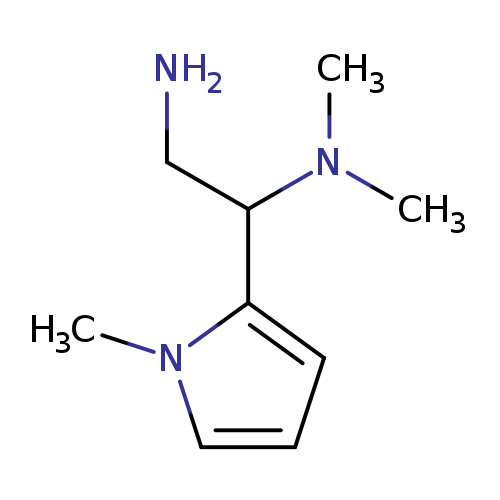
N1,N1-DIMETHYL-1-(1-METHYL-1H-PYRROL-2-YL)ETHANE-1,2-DIAMINECatalog No.:AA01APGE CAS No.:1092302-01-6 MDL No.:MFCD11558177 MF:C9H17N3 MW:167.2514 |
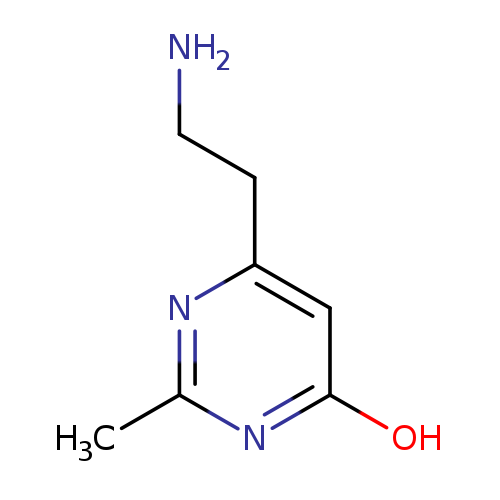
6-(2-Aminoethyl)-2-methylpyrimidin-4-olCatalog No.:AA00987Z CAS No.:1092302-30-1 MDL No.:MFCD11054028 MF:C7H11N3O MW:153.1817 |
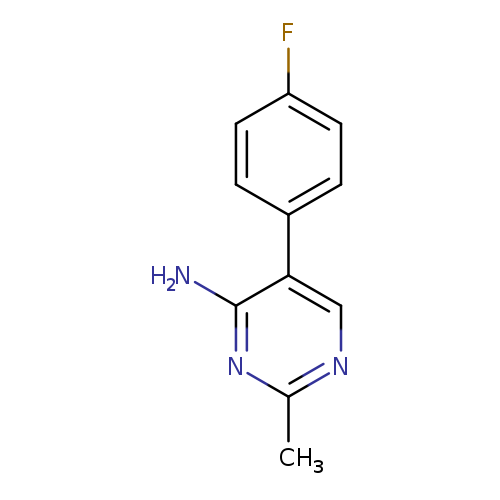
5-(4-fluorophenyl)-2-methylpyrimidin-4-amineCatalog No.:AA01B1FL CAS No.:1092303-48-4 MDL No.:MFCD11107224 MF:C11H10FN3 MW:203.2156 |
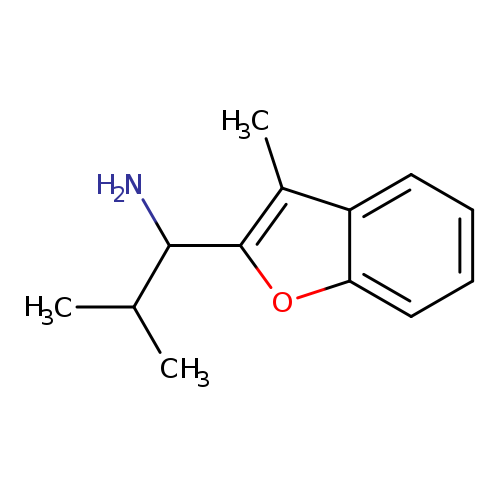
2-Methyl-1-(3-methyl-1-benzofuran-2-yl)propan-1-amineCatalog No.:AA01AHIQ CAS No.:1092304-31-8 MDL No.:MFCD10689162 MF:C13H17NO MW:203.2802 |
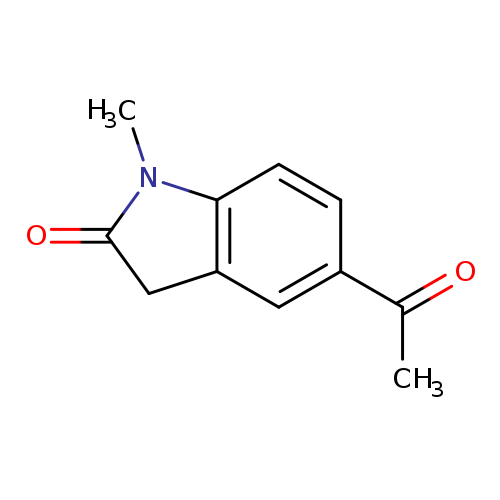
5-acetyl-1-methyl-2,3-dihydro-1H-indol-2-oneCatalog No.:AA019UI0 CAS No.:1092304-72-7 MDL No.:MFCD10689513 MF:C11H11NO2 MW:189.2105 |
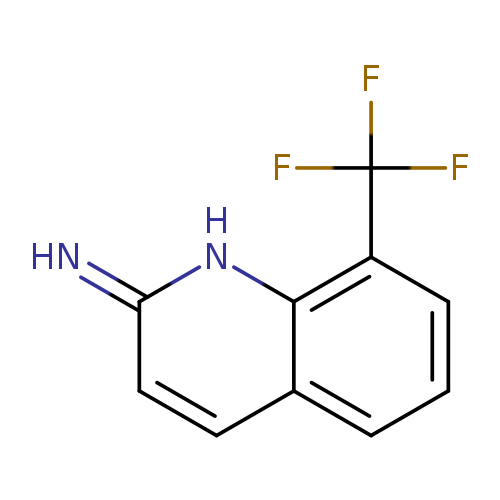
8-(Trifluoromethyl)quinolin-2-amineCatalog No.:AA0095RF CAS No.:1092304-80-7 MDL No.:MFCD11108677 MF:C10H7F3N2 MW:212.1712 |
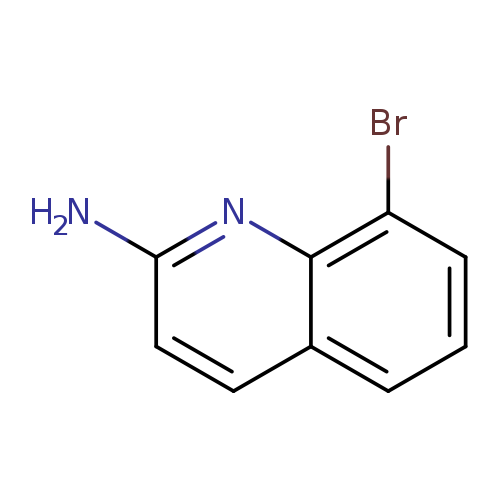
8-Bromoquinolin-2-amineCatalog No.:AA008STR CAS No.:1092304-85-2 MDL No.:MFCD08062652 MF:C9H7BrN2 MW:223.0693 |
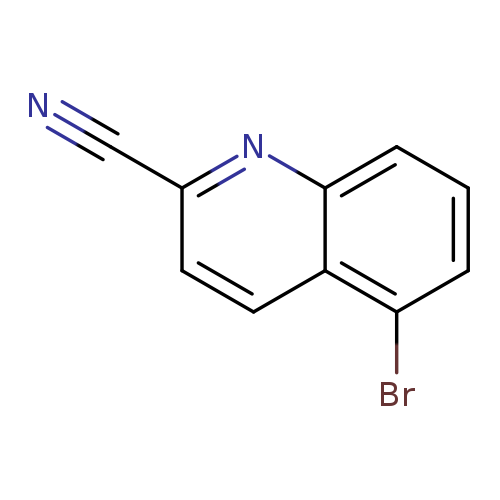
5-Bromoquinoline-2-carbonitrileCatalog No.:AA009LVM CAS No.:1092304-90-9 MDL No.:MFCD11108680 MF:C10H5BrN2 MW:233.0641 |
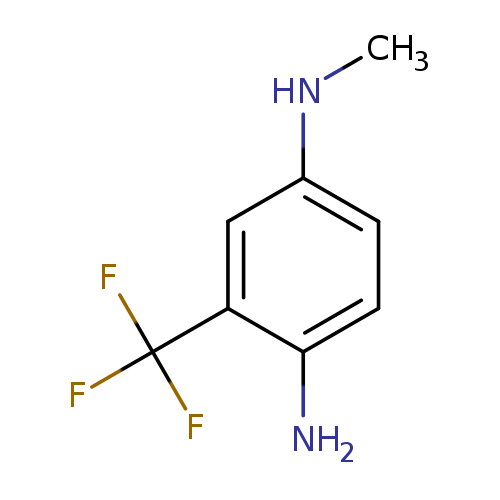
1-N-methyl-3-(trifluoromethyl)benzene-1,4-diamineCatalog No.:AA019RZP CAS No.:1092305-02-6 MDL No.:MFCD10690843 MF:C8H9F3N2 MW:190.1657 |
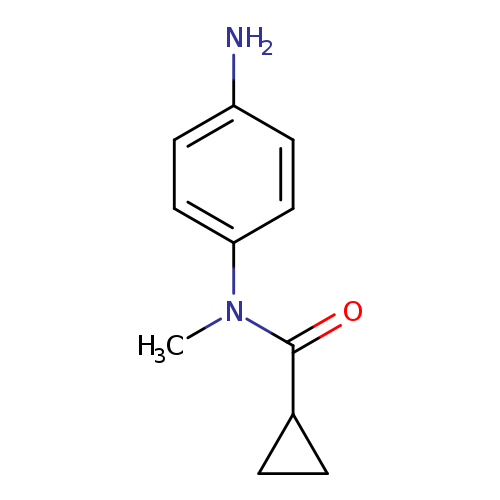
N-(4-aminophenyl)-N-methylcyclopropanecarboxamideCatalog No.:AA01BIN1 CAS No.:1092305-17-3 MDL No.:MFCD10689257 MF:C11H14N2O MW:190.2417 |
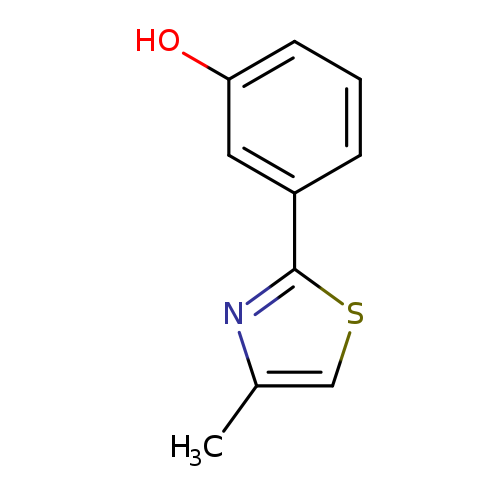
3-(4-Methyl-1,3-thiazol-2-yl)phenolCatalog No.:AA01A9BD CAS No.:1092305-67-3 MDL No.:MFCD10689922 MF:C10H9NOS MW:191.2496 |
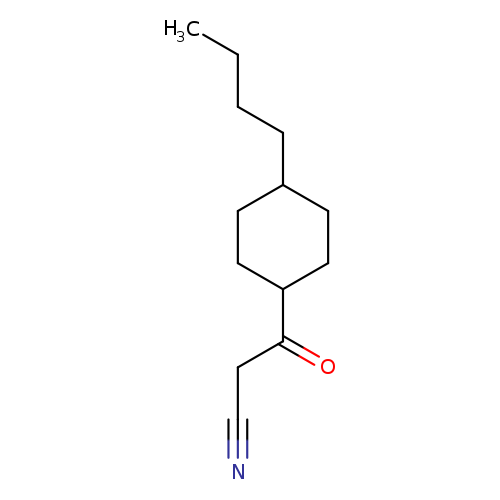
3-(4-butylcyclohexyl)-3-oxopropanenitrileCatalog No.:AA01AKU1 CAS No.:1092306-03-0 MDL No.:MFCD10690732 MF:C13H21NO MW:207.3119 |
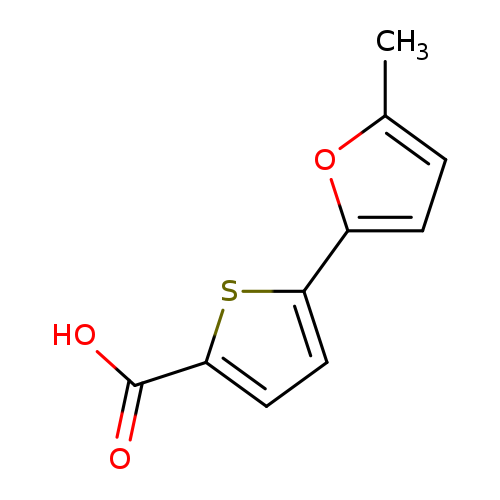
5-(5-Methylfuran-2-yl)thiophene-2-carboxylic acidCatalog No.:AA01A5OQ CAS No.:1092306-23-4 MDL No.:MFCD07376000 MF:C10H8O3S MW:208.2337 |
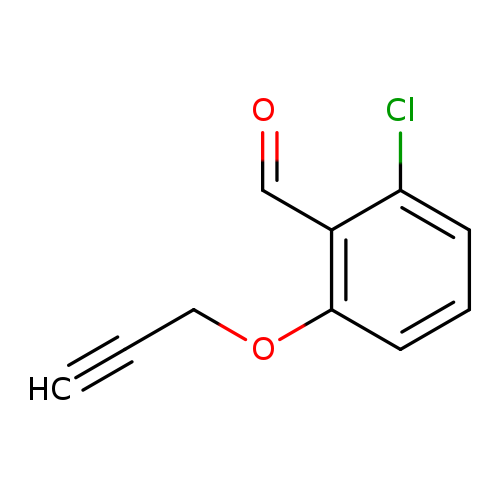
2-chloro-6-(prop-2-yn-1-yloxy)benzaldehydeCatalog No.:AA01AFJL CAS No.:1092306-71-2 MDL No.:MFCD10691258 MF:C10H7ClO2 MW:194.6144 |
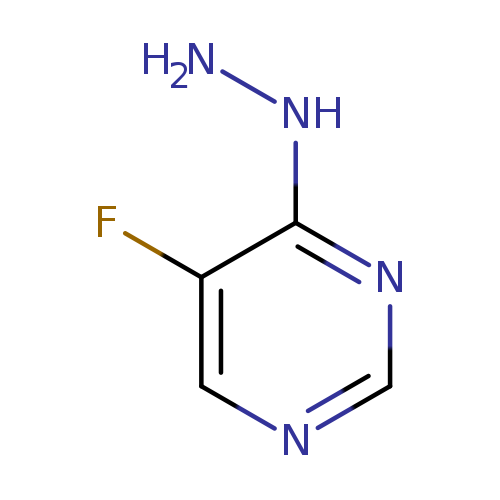
5-fluoro-4-hydrazinylpyrimidineCatalog No.:AA01ALKE CAS No.:1092307-06-6 MDL No.:MFCD11107457 MF:C4H5FN4 MW:128.1077 |
4-methyl-3-(pyridin-3-yl)-1,2-oxazol-5-amineCatalog No.:AA019YAA CAS No.:1092307-33-9 MDL No.:MFCD11209331 MF:C9H9N3O MW:175.1873 |
5-(3-Methylthiophen-2-yl)-1,3,4-oxadiazole-2-thiolCatalog No.:AA01AB5R CAS No.:1092308-05-8 MDL No.:MFCD10690986 MF:C7H6N2OS2 MW:198.2653 |
Isobutyl 5-chloro-2,2-dimethylvalerateCatalog No.:AA007B8I CAS No.:109232-37-3 MDL No.:MFCD03265308 MF:C11H21ClO2 MW:220.7362 |
2-[(1-Benzyl-4-methyl-1H-pyrazol-3-yl)oxy]-2-methylbutanoic acidCatalog No.:AA01F5P2 CAS No.:109232-39-5 MDL No.:MFCD00966926 MF:C16H20N2O3 MW:288.3416 |
2-Morpholin-4-yl-7-(2-thienyl)[1,3]thiazolo[4,5-d]pyridazin-4(5H)-oneCatalog No.:AA01ARRD CAS No.:1092329-04-8 MDL No.:MFCD11986460 MF:C13H12N4O2S2 MW:320.3900 |
2,6-Bis(trifluoromethyl)isonicotinic acidCatalog No.:AA00HB9C CAS No.:1092343-70-8 MDL No.:MFCD11501683 MF:C8H3F6NO2 MW:259.1053 |
N-(2-{[3-chloro-5-(trifluoromethyl)pyridin-2-yl]oxy}ethyl)-2-methylbenzene-1-sulfonamideCatalog No.:AA00IQMX CAS No.:1092344-09-6 MDL No.:MFCD11501693 MF:C15H14ClF3N2O3S MW:394.7965 |
N-(2-{[3-chloro-5-(trifluoromethyl)pyridin-2-yl]oxy}ethyl)-2,6-difluorobenzene-1-sulfonamideCatalog No.:AA00IQMY CAS No.:1092344-14-3 MDL No.:MFCD11501694 MF:C14H10ClF5N2O3S MW:416.7508 |
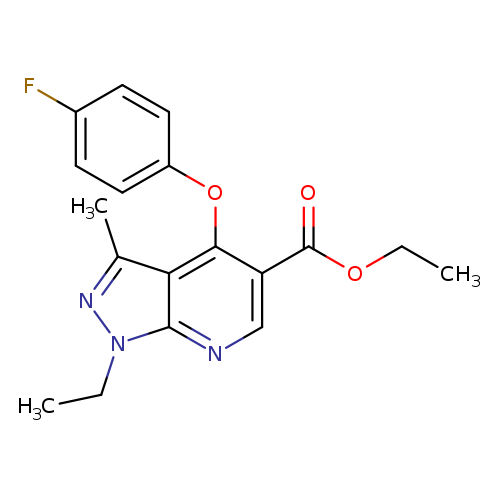
ethyl 1-ethyl-4-(4-fluorophenoxy)-3-methyl-1H-pyrazolo[3,4-b]pyridine-5-carboxylateCatalog No.:AA00IRA4 CAS No.:1092344-19-8 MDL No.:MFCD11501695 MF:C18H18FN3O3 MW:343.3522 |

N~2~,N~6~-dicyclopropyl-4-(trifluoromethyl)-2,6-pyridinedicarboxamideCatalog No.:AA01FOKZ CAS No.:1092344-44-9 MDL No.: MF: MW: |

5-methyl-1H,2H,7H-pyrido[3,2-d][1,2]diazepin-2-oneCatalog No.:AA00IRYG CAS No.:1092344-97-2 MDL No.:MFCD11501765 MF:C9H9N3O MW:175.1873 |
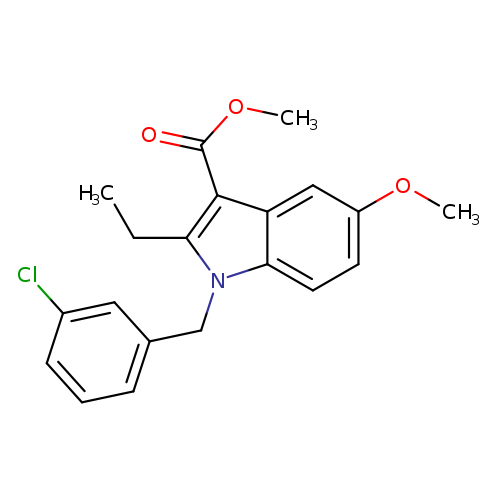
methyl 1-[(3-chlorophenyl)methyl]-2-ethyl-5-methoxy-1H-indole-3-carboxylateCatalog No.:AA00IZ8A CAS No.:1092345-30-6 MDL No.:MFCD11501778 MF:C20H20ClNO3 MW:357.8307 |
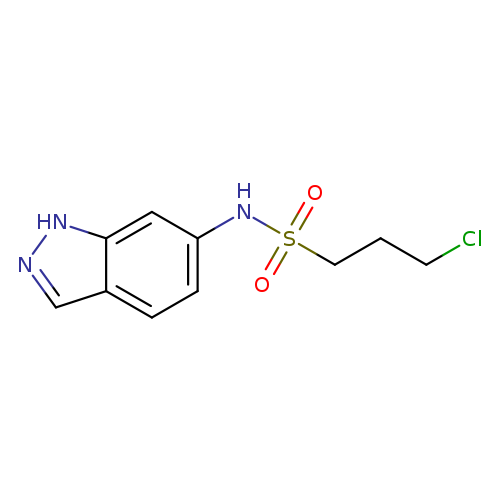
3-chloro-N-(1H-indazol-6-yl)propane-1-sulfonamideCatalog No.:AA00IRYZ CAS No.:1092345-50-0 MDL No.:MFCD11501785 MF:C10H12ClN3O2S MW:273.7392 |
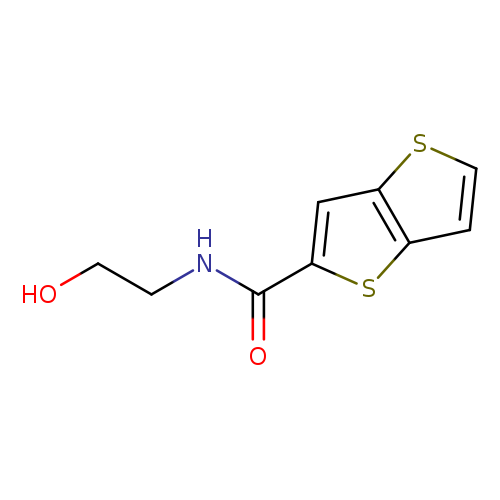
N-(2-hydroxyethyl)thieno[3,2-b]thiophene-2-carboxamideCatalog No.:AA00IRZ8 CAS No.:1092345-52-2 MDL No.:MFCD11501786 MF:C9H9NO2S2 MW:227.3033 |
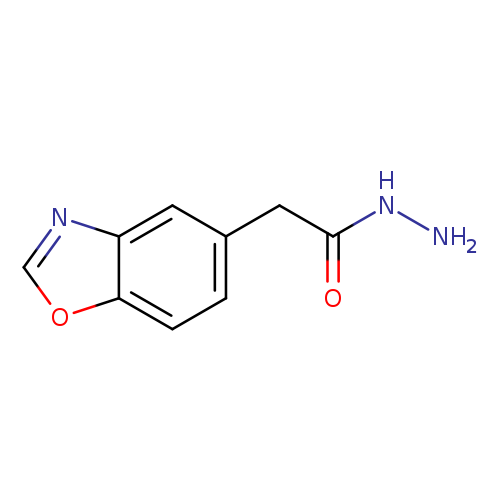
2-(1,3-benzoxazol-5-yl)acetohydrazideCatalog No.:AA00IS3G CAS No.:1092345-58-8 MDL No.:MFCD11501791 MF:C9H9N3O2 MW:191.1867 |
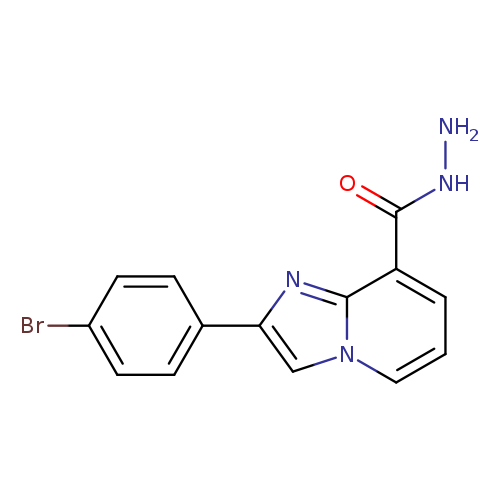
2-(4-bromophenyl)imidazo[1,2-a]pyridine-8-carbohydrazideCatalog No.:AA00IS3H CAS No.:1092345-68-0 MDL No.:MFCD11501797 MF:C14H11BrN4O MW:331.1673 |
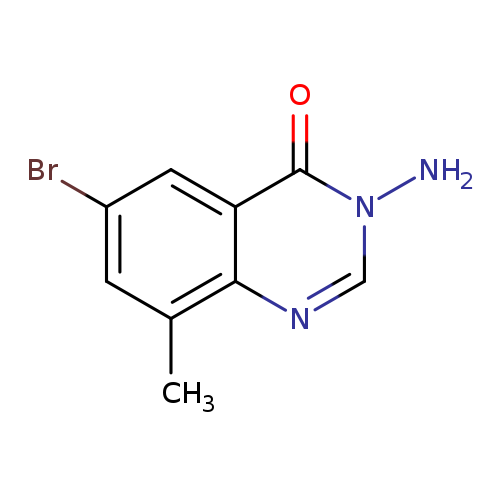
3-amino-6-bromo-8-methyl-3,4-dihydroquinazolin-4-oneCatalog No.:AA00ITZF CAS No.:1092345-74-8 MDL No.:MFCD11501800 MF:C9H8BrN3O MW:254.0833 |
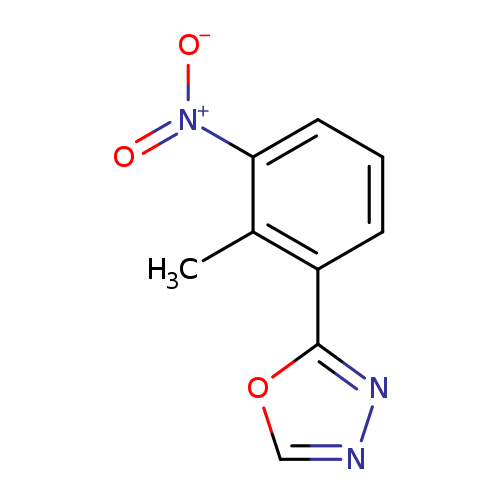
2-(2-methyl-3-nitrophenyl)-1,3,4-oxadiazoleCatalog No.:AA00IZBZ CAS No.:1092345-76-0 MDL No.:MFCD11501801 MF:C9H7N3O3 MW:205.1702 |
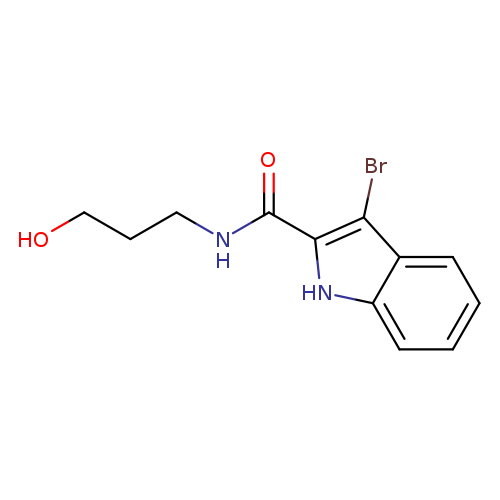
3-bromo-N-(3-hydroxypropyl)-1H-indole-2-carboxamideCatalog No.:AA00IWG6 CAS No.:1092345-78-2 MDL No.:MFCD11501802 MF:C12H13BrN2O2 MW:297.1478 |
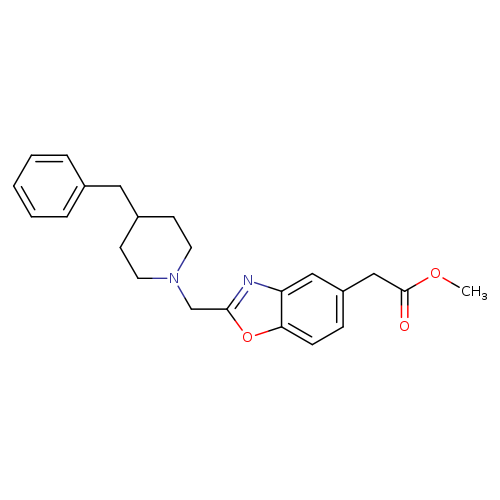
methyl 2-{2-[(4-benzylpiperidin-1-yl)methyl]-1,3-benzoxazol-5-yl}acetateCatalog No.:AA00IS3S CAS No.:1092345-82-8 MDL No.:MFCD11501805 MF:C23H26N2O3 MW:378.4641 |
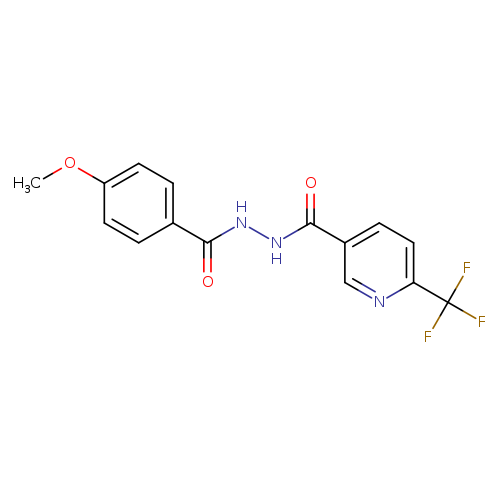
4-methoxy-N'-[6-(trifluoromethyl)pyridine-3-carbonyl]benzohydrazideCatalog No.:AA00IS89 CAS No.:1092346-04-7 MDL No.:MFCD11501828 MF:C15H12F3N3O3 MW:339.2693 |
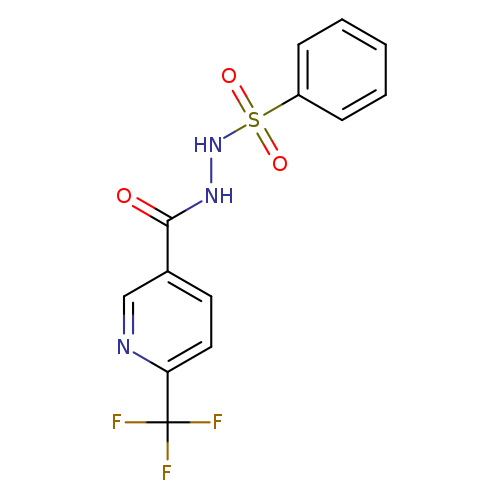
N'-(benzenesulfonyl)-6-(trifluoromethyl)pyridine-3-carbohydrazideCatalog No.:AA00IS8A CAS No.:1092346-06-9 MDL No.:MFCD11501830 MF:C13H10F3N3O3S MW:345.2970 |
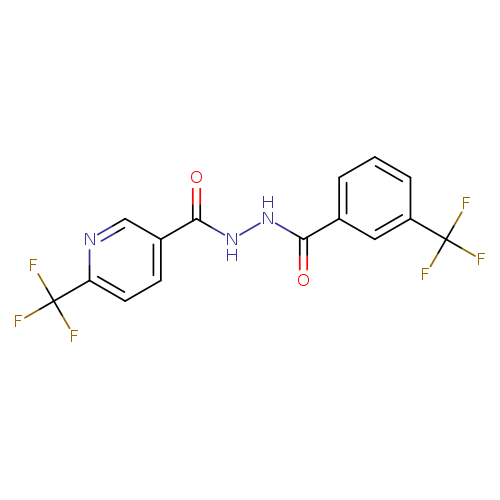
3-(trifluoromethyl)-N'-[6-(trifluoromethyl)pyridine-3-carbonyl]benzohydrazideCatalog No.:AA00IWJK CAS No.:1092346-10-5 MDL No.:MFCD11501833 MF:C15H9F6N3O2 MW:377.2413 |
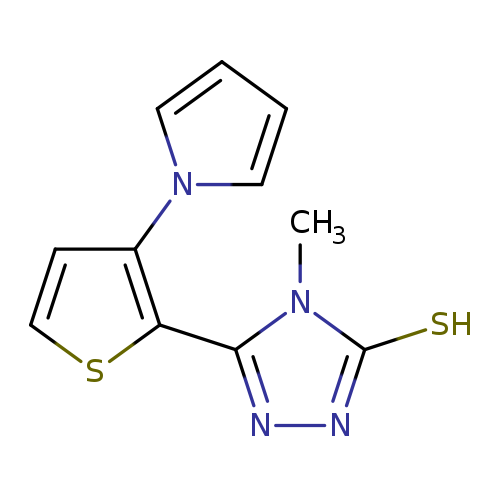
4-methyl-5-[3-(1H-pyrrol-1-yl)thiophen-2-yl]-4H-1,2,4-triazole-3-thiolCatalog No.:AA00IQQ6 CAS No.:1092346-11-6 MDL No.:MFCD11501696 MF:C11H10N4S2 MW:262.3539 |
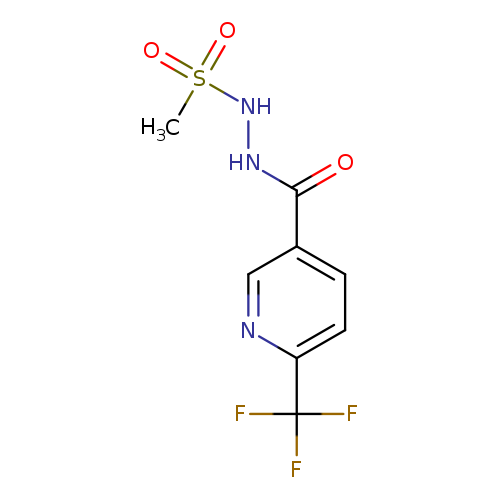
N'-methanesulfonyl-6-(trifluoromethyl)pyridine-3-carbohydrazideCatalog No.:AA00IU5V CAS No.:1092346-12-7 MDL No.:MFCD11501834 MF:C8H8F3N3O3S MW:283.2276 |
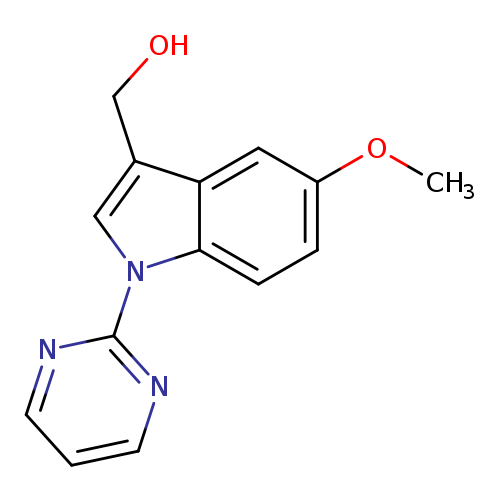
[5-methoxy-1-(pyrimidin-2-yl)-1H-indol-3-yl]methanolCatalog No.:AA00IQQ7 CAS No.:1092346-13-8 MDL No.:MFCD11501697 MF:C14H13N3O2 MW:255.2719 |
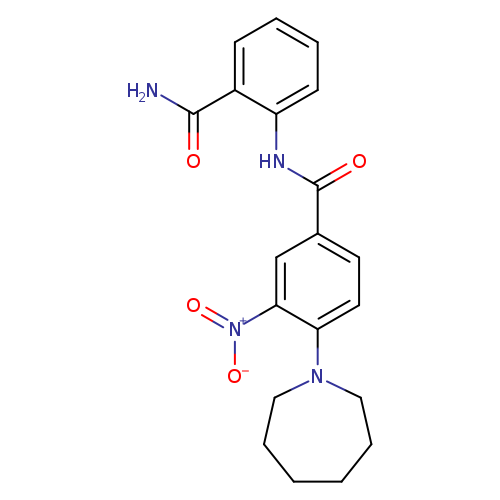
4-(azepan-1-yl)-N-(2-carbamoylphenyl)-3-nitrobenzamideCatalog No.:AA00ISGO CAS No.:1092346-15-0 MDL No.:MFCD11501698 MF:C20H22N4O4 MW:382.4131 |
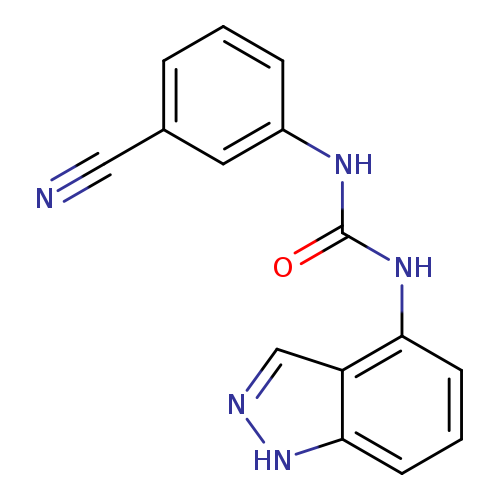
1-(3-cyanophenyl)-3-(1H-indazol-4-yl)ureaCatalog No.:AA00IZ4R CAS No.:1092346-46-7 MDL No.:MFCD11501718 MF:C15H11N5O MW:277.2807 |
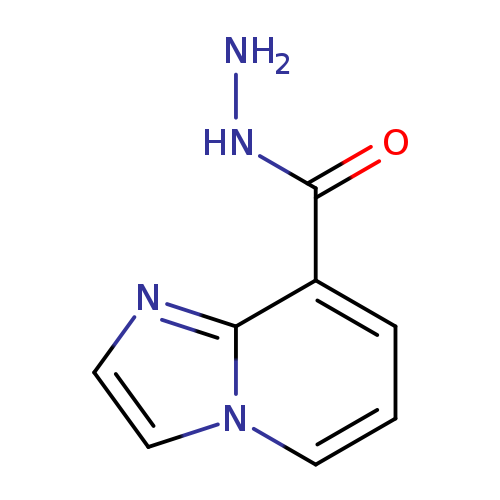
imidazo[1,2-a]pyridine-8-carbohydrazideCatalog No.:AA00IZDW CAS No.:1092346-49-0 MDL No.:MFCD01105846 MF:C8H8N4O MW:176.1753 |
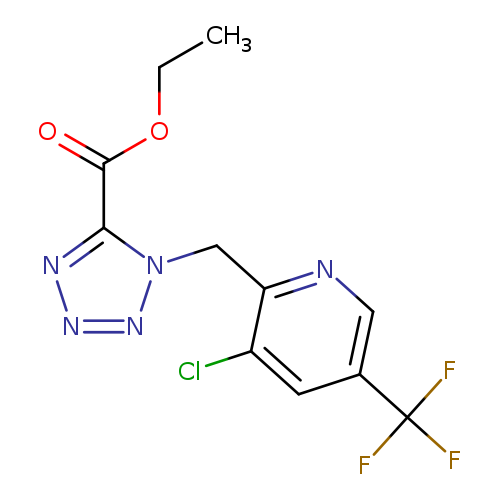
ethyl 1-{[3-chloro-5-(trifluoromethyl)pyridin-2-yl]methyl}-1H-1,2,3,4-tetrazole-5-carboxylateCatalog No.:AA00IWJU CAS No.:1092346-51-4 MDL No.:MFCD11501854 MF:C11H9ClF3N5O2 MW:335.6697 |
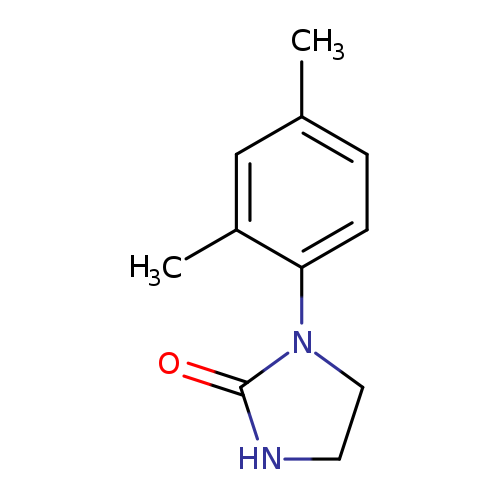
1-(2,4-dimethylphenyl)imidazolidin-2-oneCatalog No.:AA00IZDY CAS No.:1092346-55-8 MDL No.:MFCD11501857 MF:C11H14N2O MW:190.2417 |
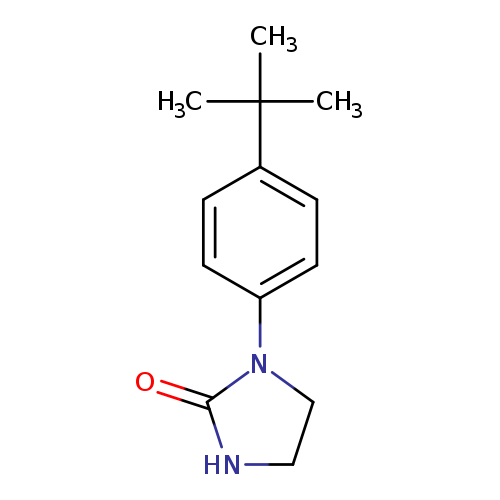
1-(4-tert-butylphenyl)imidazolidin-2-oneCatalog No.:AA00IWJV CAS No.:1092346-57-0 MDL No.:MFCD11501858 MF:C13H18N2O MW:218.2948 |
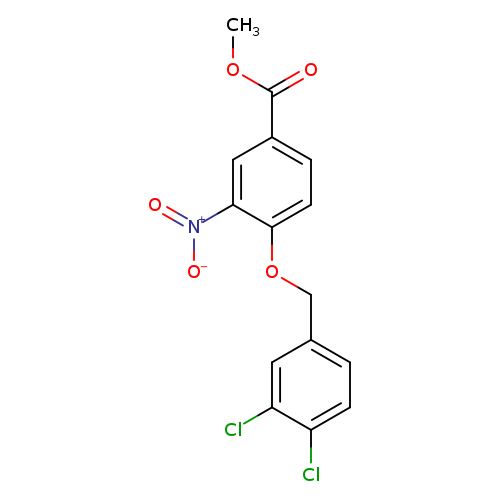
methyl 4-[(3,4-dichlorophenyl)methoxy]-3-nitrobenzoateCatalog No.:AA00IZ4Y CAS No.:1092346-58-1 MDL No.:MFCD11501725 MF:C15H11Cl2NO5 MW:356.1575 |
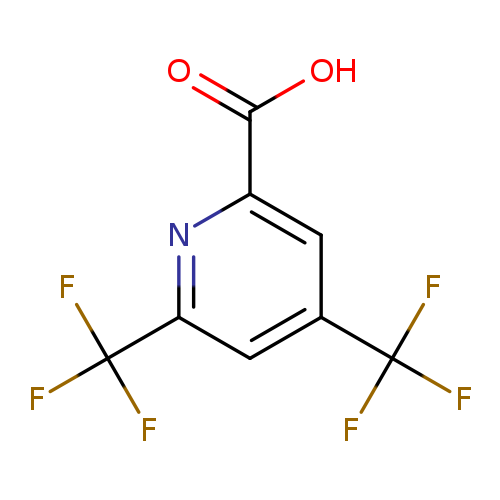
4,6-Bis-trifluoroMethyl-pyridine-2-carboxylic acidCatalog No.:AA009562 CAS No.:1092346-60-5 MDL No.:MFCD11501727 MF:C8H3F6NO2 MW:259.1053 |
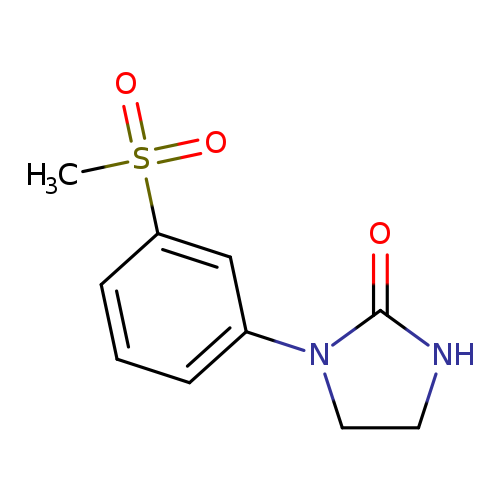
1-(3-methanesulfonylphenyl)imidazolidin-2-oneCatalog No.:AA00IWLP CAS No.:1092346-63-8 MDL No.:MFCD11501863 MF:C10H12N2O3S MW:240.2789 |
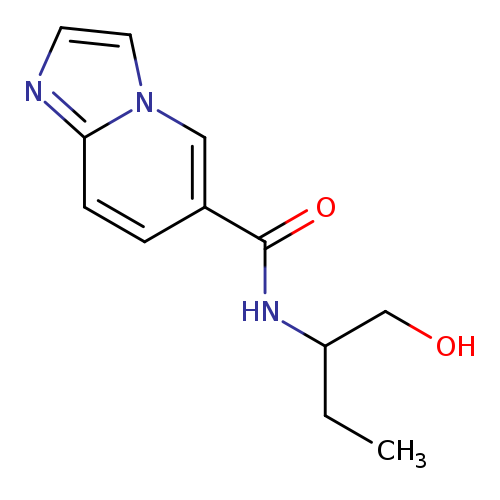
N-(1-hydroxybutan-2-yl)imidazo[1,2-a]pyridine-6-carboxamideCatalog No.:AA00IZF2 CAS No.:1092346-65-0 MDL No.:MFCD11501865 MF:C12H15N3O2 MW:233.2664 |
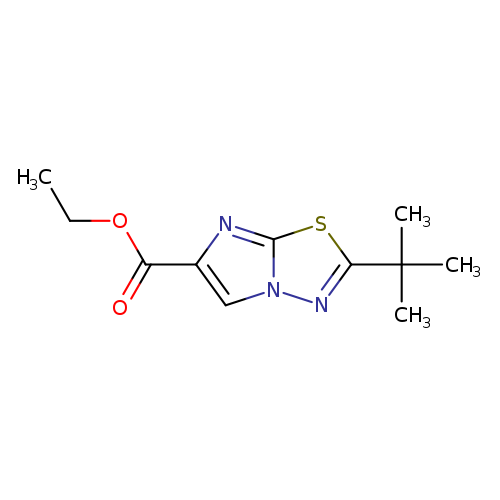
ethyl 2-tert-butylimidazo[2,1-b][1,3,4]thiadiazole-6-carboxylateCatalog No.:AA00IUJI CAS No.:1092346-67-2 MDL No.:MFCD11501866 MF:C11H15N3O2S MW:253.3207 |
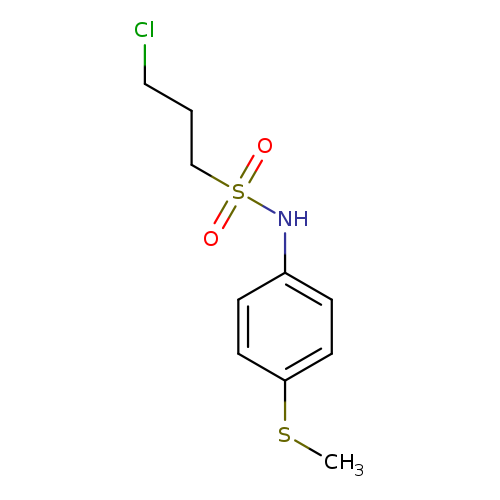
3-chloro-N-[4-(methylsulfanyl)phenyl]propane-1-sulfonamideCatalog No.:AA00ISPP CAS No.:1092346-71-8 MDL No.:MFCD11501871 MF:C10H14ClNO2S2 MW:279.8067 |
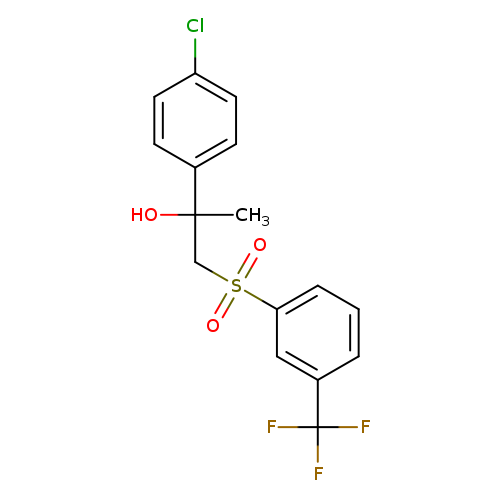
2-(4-chlorophenyl)-1-[3-(trifluoromethyl)benzenesulfonyl]propan-2-olCatalog No.:AA00IUR0 CAS No.:1092346-75-2 MDL No.:MFCD11501874 MF:C16H14ClF3O3S MW:378.7938 |
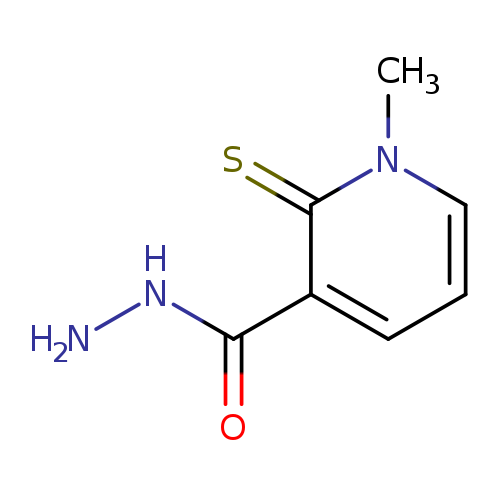
1-methyl-2-sulfanylidene-1,2-dihydropyridine-3-carbohydrazideCatalog No.:AA00IZOZ CAS No.:1092346-77-4 MDL No.:MFCD11501875 MF:C7H9N3OS MW:183.2309 |
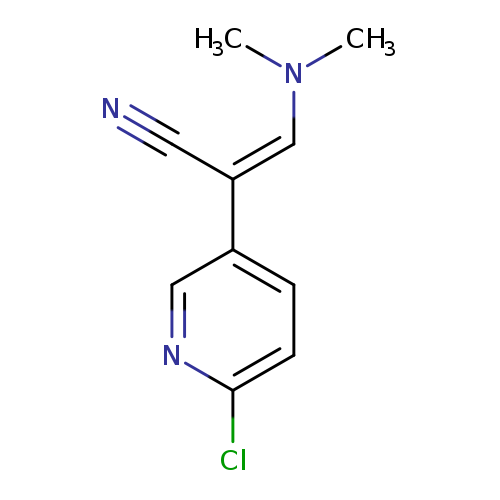
(2Z)-2-(6-chloropyridin-3-yl)-3-(dimethylamino)prop-2-enenitrileCatalog No.:AA00IUUU CAS No.:1092346-79-6 MDL No.:MFCD11501876 MF:C10H10ClN3 MW:207.6595 |
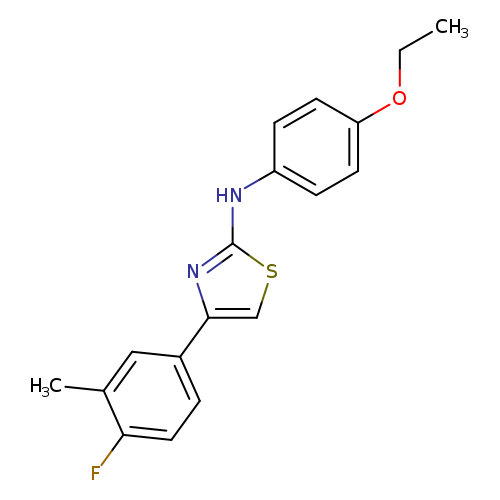
2-(4-Ethoxyphenylamino)-4-(3-methyl-4-fluorophenyl)thiazoleCatalog No.:AA00HB9D CAS No.:1092346-81-0 MDL No.:MFCD00129327 MF:C18H17FN2OS MW:328.4038 |

1-{4-[(2-chloro-1,3-thiazol-5-yl)methyl]-1,4-diazepan-1-yl}-1-ethanoneCatalog No.:AA01FOIJ CAS No.:1092346-82-1 MDL No.: MF: MW: |
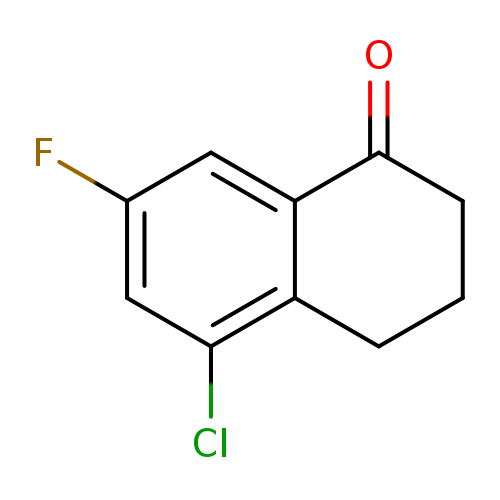
5-Chloro-7-fluoro-1,2,3,4-tetrahydronaphthalen-1-oneCatalog No.:AA00HB9F CAS No.:1092348-26-9 MDL No.:MFCD09864772 MF:C10H8ClFO MW:198.6213 |
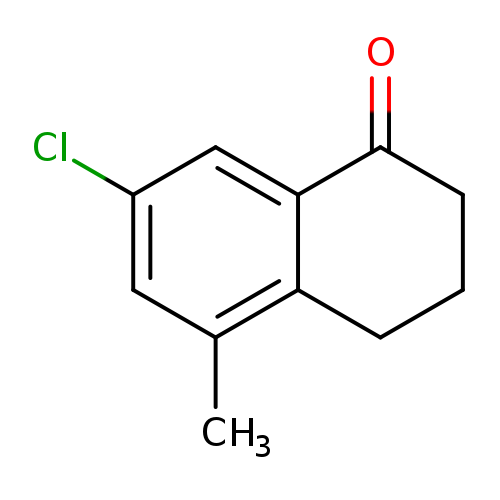
7-Chloro-5-methyl-2,3,4-trihydronaphthalen-1-oneCatalog No.:AA01DU2V CAS No.:1092348-30-5 MDL No.:MFCD11518687 MF:C11H11ClO MW:194.6574 |
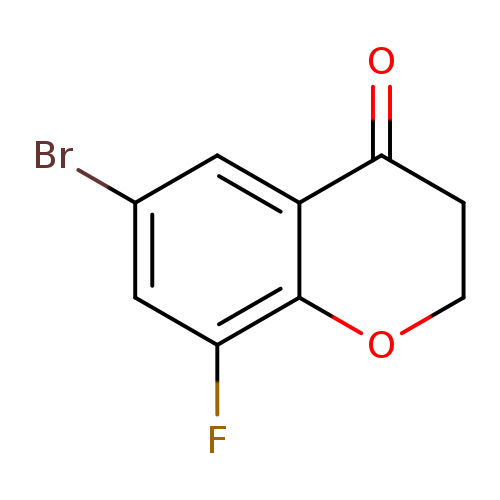
6-bromo-8-fluoro-3,4-dihydro-2H-1-benzopyran-4-oneCatalog No.:AA01AKUH CAS No.:1092348-68-9 MDL No.:MFCD11207542 MF:C9H6BrFO2 MW:245.0451 |
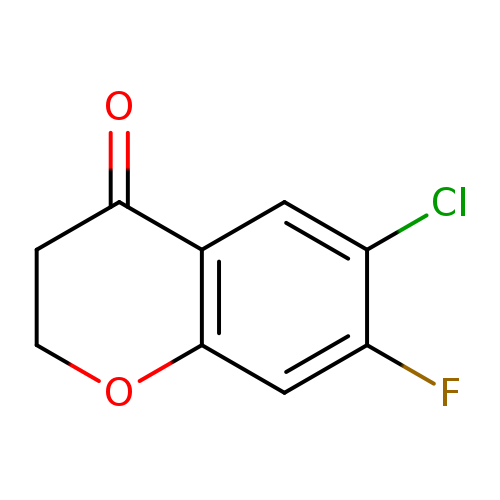
6-chloro-7-fluoro-3,4-dihydro-2H-1-benzopyran-4-oneCatalog No.:AA01B57O CAS No.:1092349-25-1 MDL No.:MFCD11518440 MF:C9H6ClFO2 MW:200.5941 |
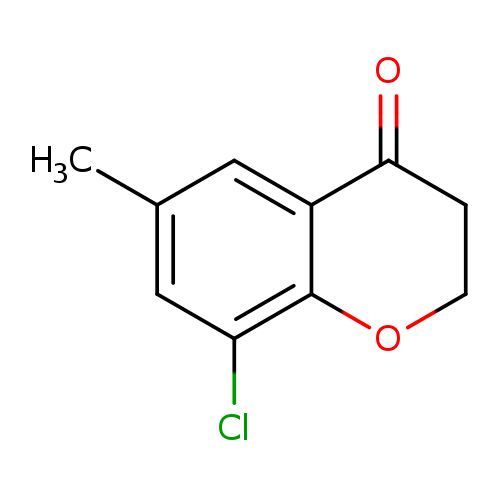
8-chloro-6-methyl-3,4-dihydro-2H-1-benzopyran-4-oneCatalog No.:AA01DT89 CAS No.:1092349-58-0 MDL No.:MFCD11518455 MF:C10H9ClO2 MW:196.6303 |
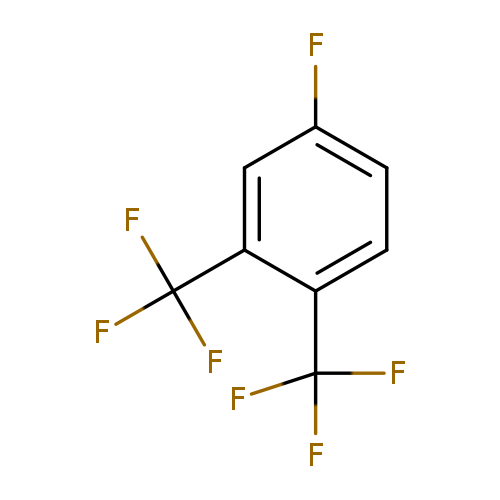
3,4-Bis(trifluoromethyl)fluorobenzeneCatalog No.:AA01F3UD CAS No.:1092349-75-1 MDL No.:MFCD11100160 MF:C8H3F7 MW:232.0982 |
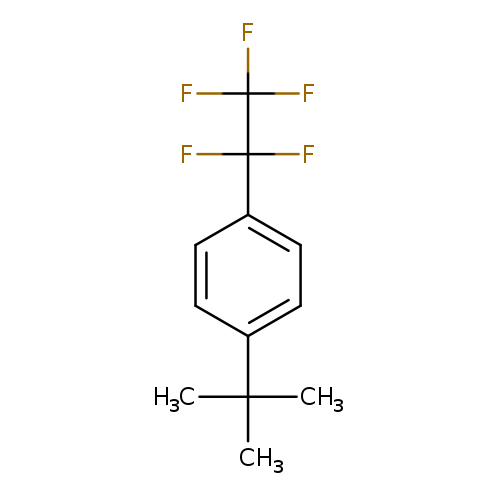
4-tert-ButylpentafluoroethylbenzeneCatalog No.:AA01FDMX CAS No.:1092349-84-2 MDL No.:MFCD11041190 MF:C12H13F5 MW:252.2236 |
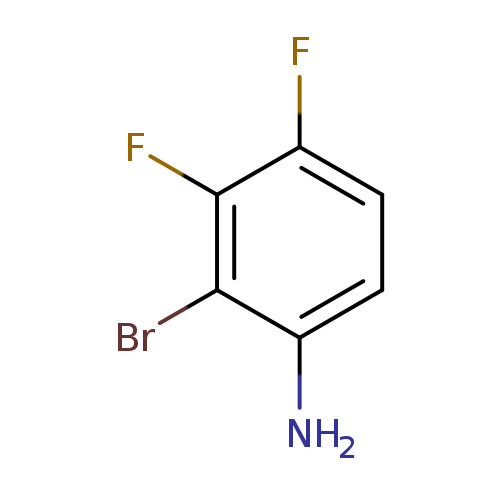
2-Bromo-3,4-difluoroanilineCatalog No.:AA007TBQ CAS No.:1092349-87-5 MDL No.:MFCD11035924 MF:C6H4BrF2N MW:208.0035 |
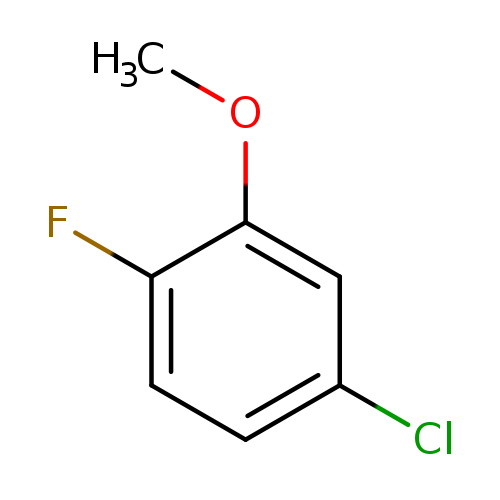
4-Chloro-1-fluoro-2-methoxybenzeneCatalog No.:AA00HB9G CAS No.:1092349-89-7 MDL No.:MFCD00042204 MF:C7H6ClFO MW:160.5733 |
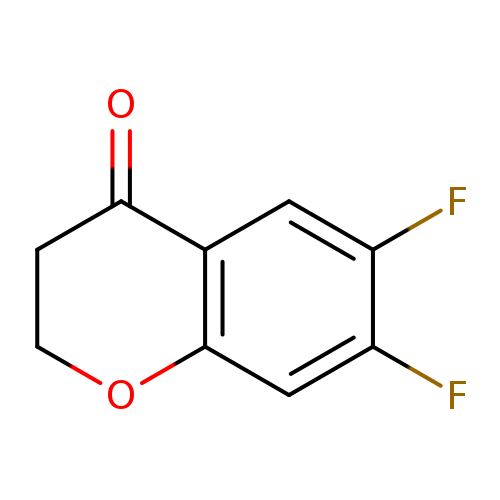
6,7-Difluorochroman-4-oneCatalog No.:AA008UE5 CAS No.:1092349-93-3 MDL No.:MFCD18822570 MF:C9H6F2O2 MW:184.1395 |
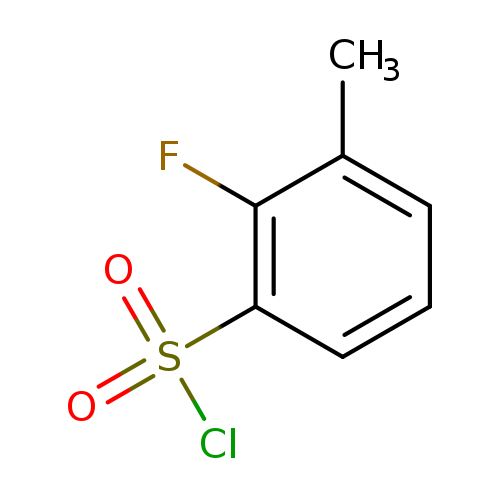
2-Fluoro-3-methylbenzenesulfonyl chlorideCatalog No.:AA008UIP CAS No.:1092349-98-8 MDL No.:MFCD11036242 MF:C7H6ClFO2S MW:208.6377 |
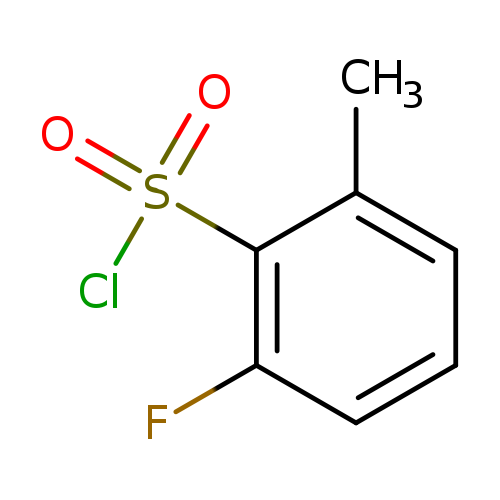
2-Fluoro-6-methylbenzene-1-sulfonyl chlorideCatalog No.:AA008UIO CAS No.:1092350-02-1 MDL No.:MFCD11036243 MF:C7H6ClFO2S MW:208.6377 |
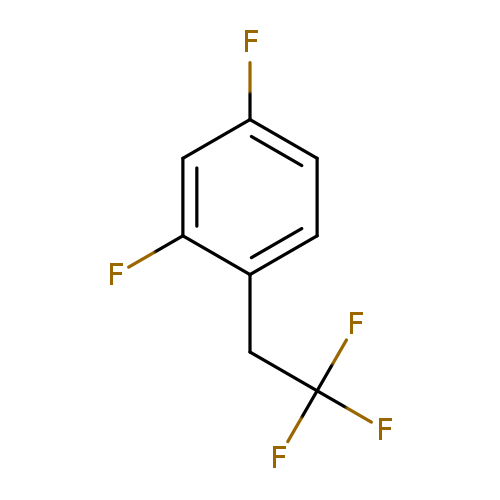
2,4-Difluoro-1-(2,2,2-trifluoroethyl)benzeneCatalog No.:AA01FAGP CAS No.:1092350-12-3 MDL No.:MFCD11100168 MF:C8H5F5 MW:196.1173 |
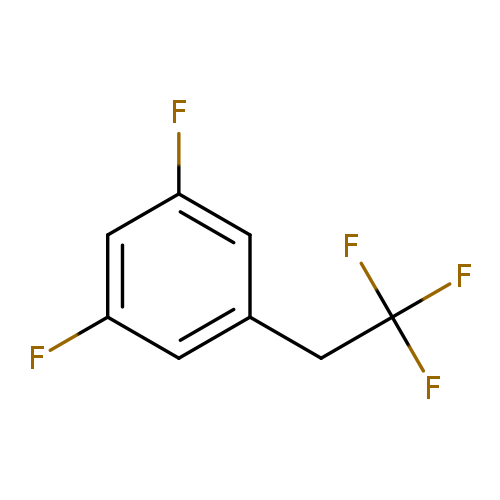
1,3-difluoro-5-(2,2,2-trifluoroethyl)benzeneCatalog No.:AA01EQNI CAS No.:1092350-20-3 MDL No.:MFCD11100170 MF:C8H5F5 MW:196.1173 |
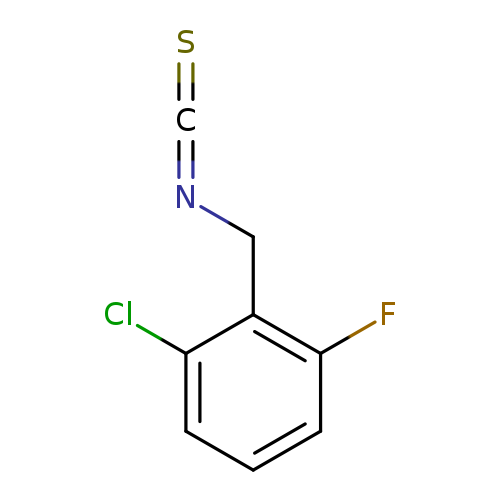
2-Chloro-6-fluorobenzyl isothiocyanateCatalog No.:AA01FCFV CAS No.:1092350-26-9 MDL No.:MFCD06740514 MF:C8H5ClFNS MW:201.6484 |
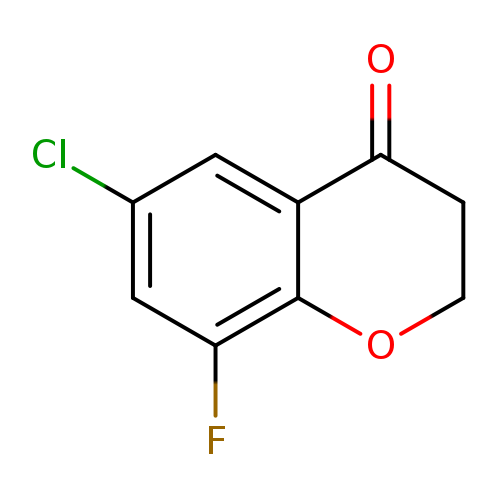
6-Chloro-8-fluorochroman-4-oneCatalog No.:AA00964Y CAS No.:1092350-27-0 MDL No.:MFCD11518488 MF:C9H6ClFO2 MW:200.5941 |
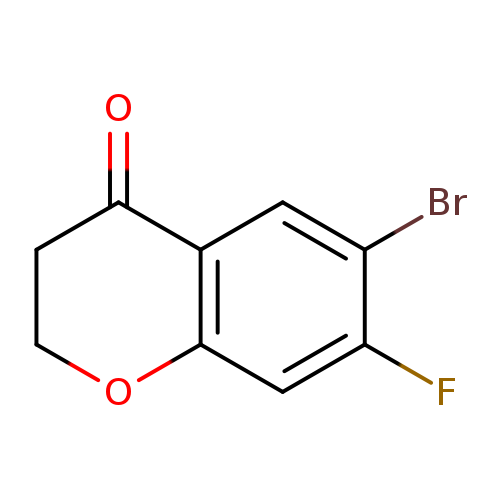
6-Bromo-7-fluorochroman-4-oneCatalog No.:AA00964W CAS No.:1092350-30-5 MDL No.:MFCD11518489 MF:C9H6BrFO2 MW:245.0451 |
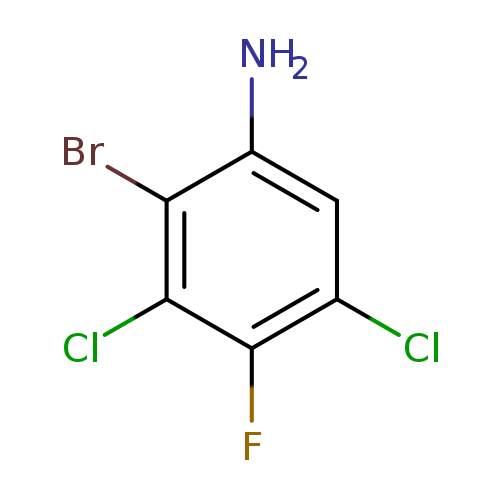
2-Bromo-3,5-dichloro-4-fluoroanilineCatalog No.:AA008V8F CAS No.:1092350-32-7 MDL No.:MFCD11505662 MF:C6H3BrCl2FN MW:258.9031 |
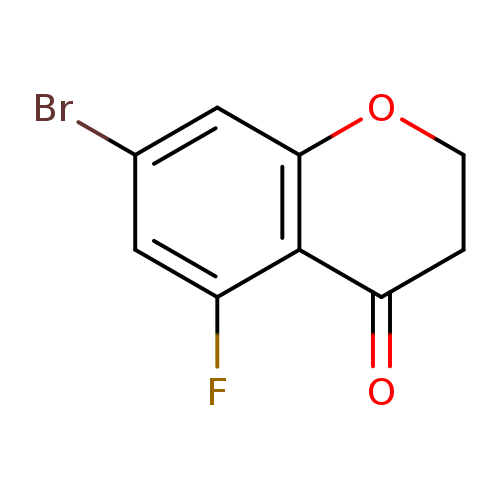
7-Bromo-5-fluorochroman-4-oneCatalog No.:AA00HB9J CAS No.:1092350-58-7 MDL No.:MFCD11518503 MF:C9H6BrFO2 MW:245.0451 |
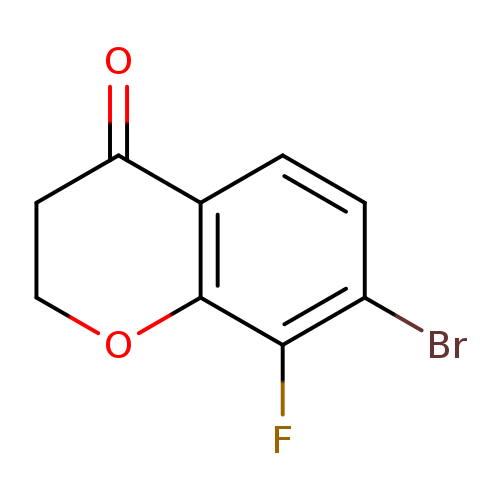
7-Bromo-8-fluorochroman-4-oneCatalog No.:AA01BVO0 CAS No.:1092350-77-0 MDL No.:MFCD11518510 MF:C9H6BrFO2 MW:245.0451 |
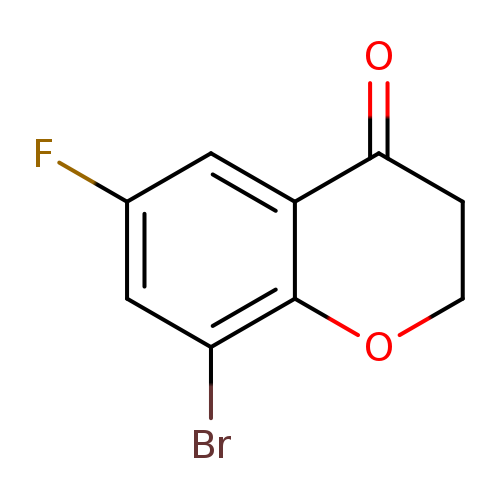
8-Bromo-6-fluorochroman-4-oneCatalog No.:AA009651 CAS No.:1092350-87-2 MDL No.:MFCD11104781 MF:C9H6BrFO2 MW:245.0451 |
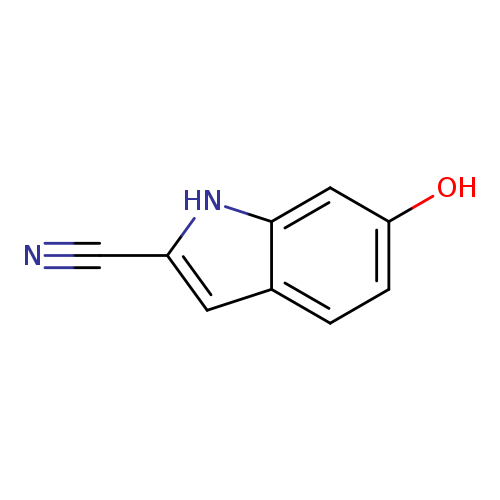
6-Hydroxy-1h-indole-2-carbonitrileCatalog No.:AA00HB9K CAS No.:1092350-96-3 MDL No.:MFCD13191940 MF:C9H6N2O MW:158.1567 |
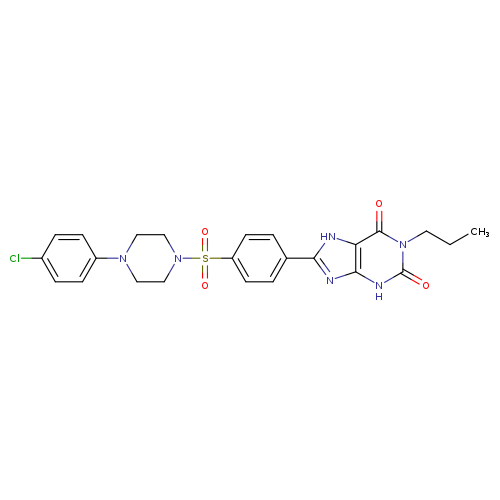
8-[4-[4-(4-Chlorophenzyl)piperazide-1-sulfonyl)phenyl]]-1-propylxanthineCatalog No.:AA008Y1N CAS No.:1092351-10-4 MDL No.:MFCD11519964 MF:C24H25ClN6O4S MW:529.0111 |
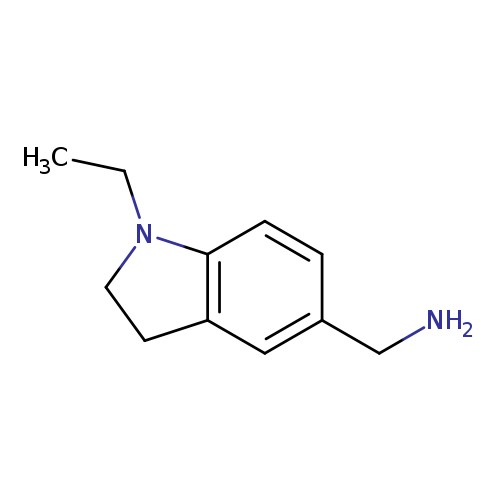
(1-ETHYL-2,3-DIHYDRO-1H-INDOL-5-YL)METHANAMINECatalog No.:AA01DUVZ CAS No.:1092351-24-0 MDL No.:MFCD13192105 MF:C11H16N2 MW:176.2581 |
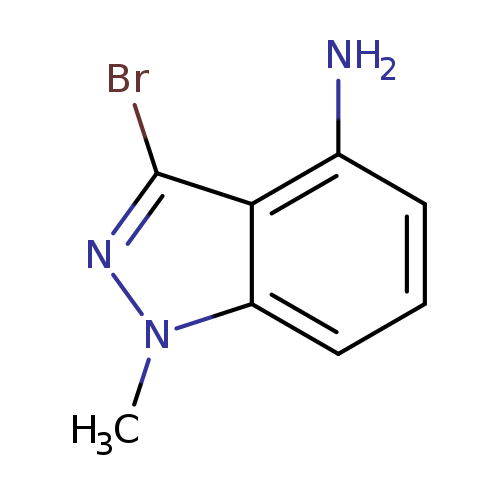
4-Amino-3-bromo-1-methylindazoleCatalog No.:AA008TXL CAS No.:1092351-47-7 MDL No.:MFCD11109355 MF:C8H8BrN3 MW:226.0732 |
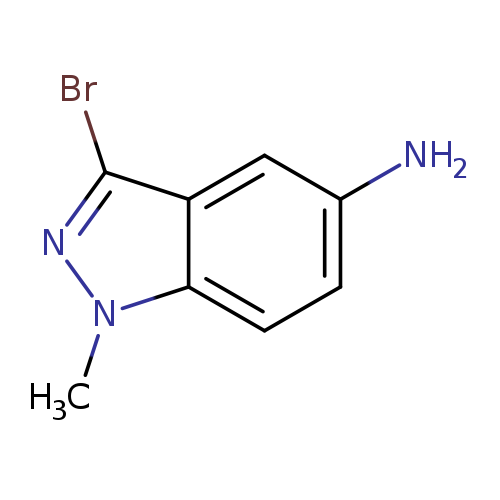
5-Amino-3-bromo-1-methylindazoleCatalog No.:AA008S0T CAS No.:1092351-49-9 MDL No.:MFCD11100026 MF:C8H8BrN3 MW:226.0732 |
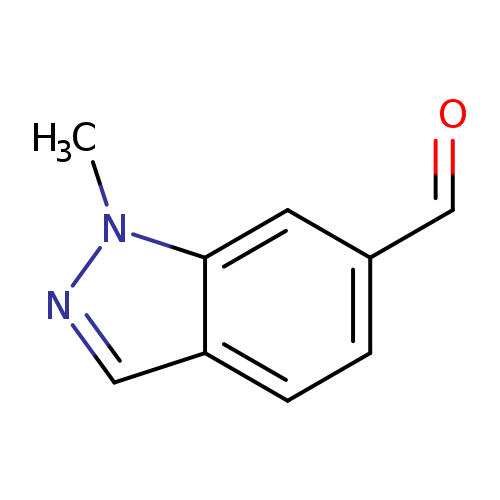
1-Methyl-1H-indazole-6-carbaldehydeCatalog No.:AA008TR6 CAS No.:1092351-51-3 MDL No.:MFCD11109366 MF:C9H8N2O MW:160.1726 |
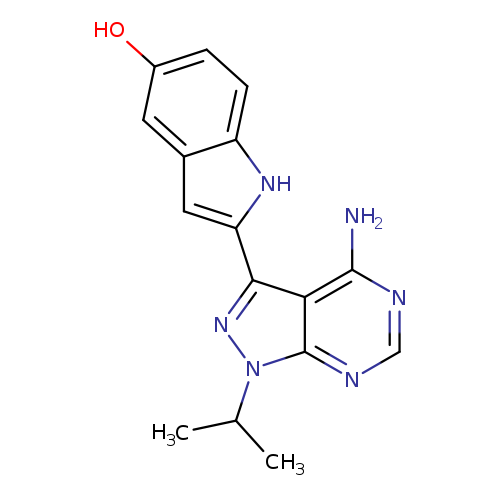
PP242Catalog No.:AA008SPK CAS No.:1092351-67-1 MDL No.:MFCD12196869 MF:C16H16N6O MW:308.3378 |
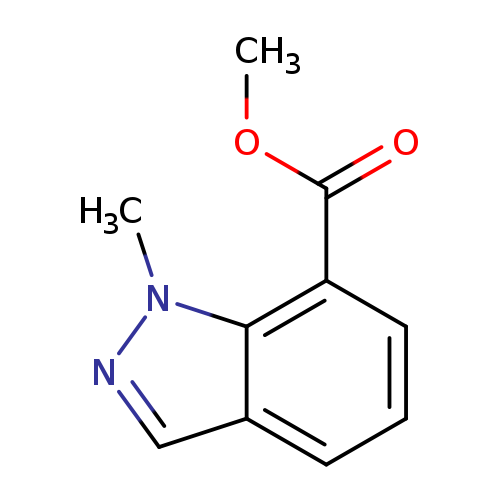
1-Methyl-1H-indazole-7-carboxylic acid methyl esterCatalog No.:AA008TQW CAS No.:1092351-84-2 MDL No.:MFCD11109404 MF:C10H10N2O2 MW:190.1986 |
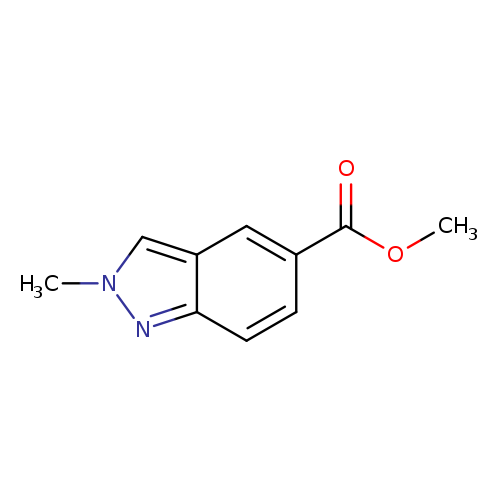
2-Methyl-2h-indazole-5-carboxylic acid methyl esterCatalog No.:AA008TV5 CAS No.:1092351-86-4 MDL No.:MFCD11109406 MF:C10H10N2O2 MW:190.1986 |
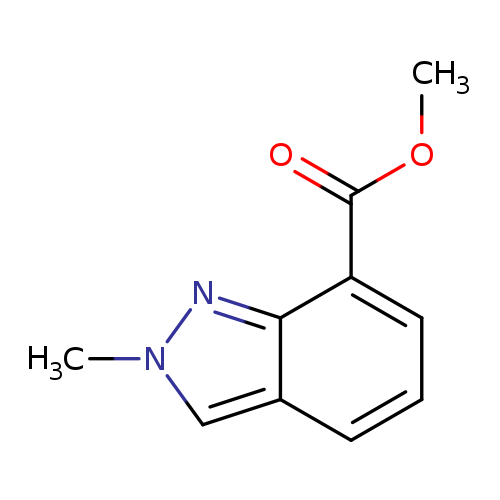
2-Methyl-2H-indazole-7-carboxylic acid methyl esterCatalog No.:AA008TVB CAS No.:1092351-88-6 MDL No.:MFCD11109408 MF:C10H10N2O2 MW:190.1986 |
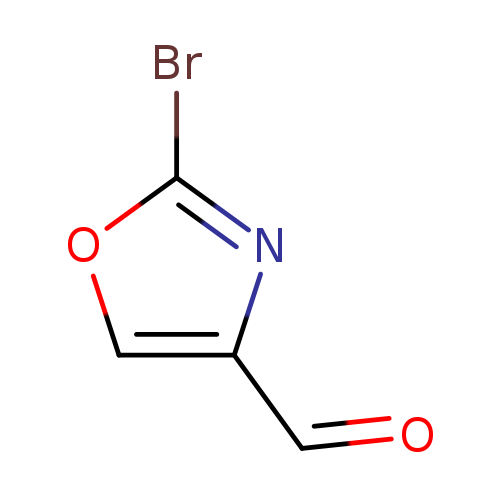
2-BroMooxazole-4-carbaldehydeCatalog No.:AA0094S9 CAS No.:1092351-90-0 MDL No.:MFCD11109412 MF:C4H2BrNO2 MW:175.9682 |
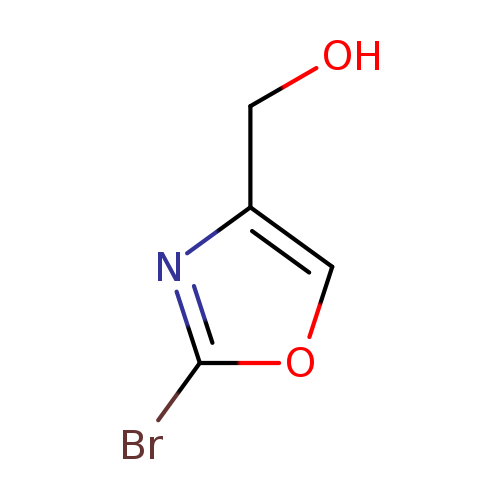
(2-Bromooxazol-4-yl)methanolCatalog No.:AA008UNG CAS No.:1092351-92-2 MDL No.:MFCD11109413 MF:C4H4BrNO2 MW:177.9841 |
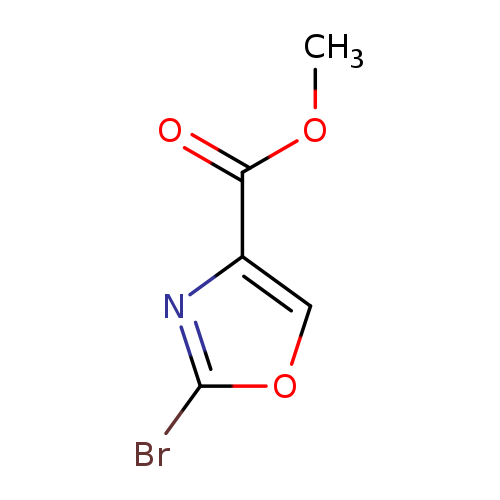
Methyl 2-bromo-4-oxazolecarboxylateCatalog No.:AA008YYE CAS No.:1092351-94-4 MDL No.:MFCD11109414 MF:C5H4BrNO3 MW:205.9942 |
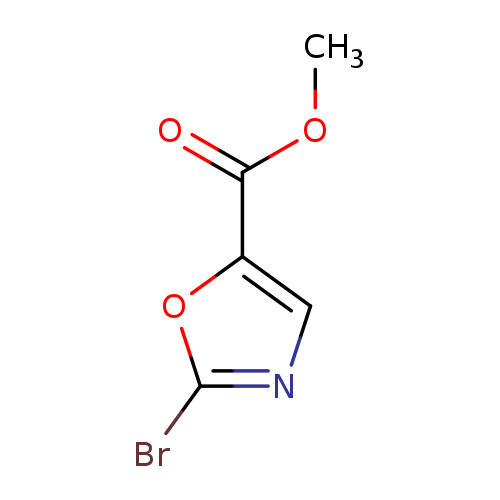
Methyl 2-bromooxazole-5-carboxylateCatalog No.:AA003A8E CAS No.:1092351-96-6 MDL No.:MFCD11109415 MF:C5H4BrNO3 MW:205.9942 |
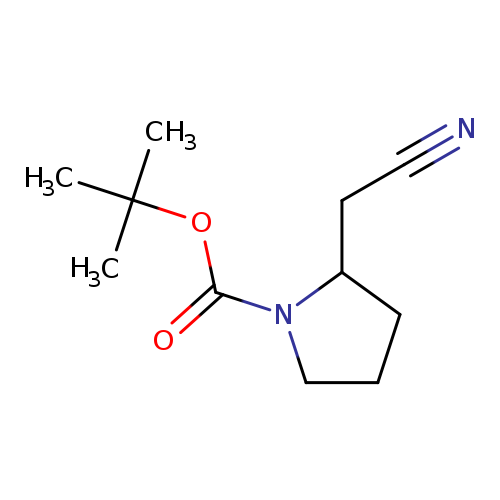
tert-Butyl 2-(cyanomethyl)pyrrolidine-1-carboxylateCatalog No.:AA009203 CAS No.:1092352-11-8 MDL No.:MFCD12912059 MF:C11H18N2O2 MW:210.2728 |
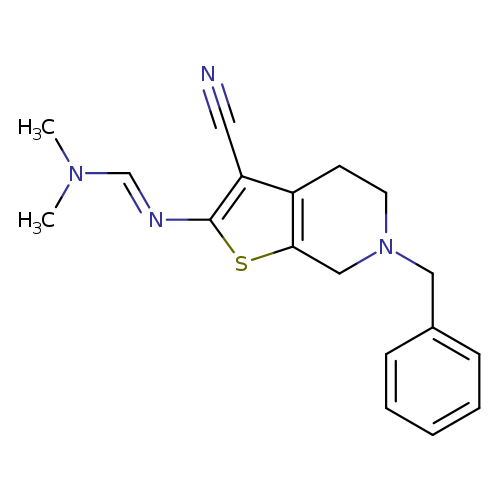
N'-(6-Benzyl-3-cyano-4,5,6,7-tetrahydrothieno[2,3-c]pyridin-2-yl)-N,N-dimethylformimidamideCatalog No.:AA00IPE7 CAS No.:1092352-17-4 MDL No.:MFCD11553021 MF:C18H20N4S MW:324.4432 |
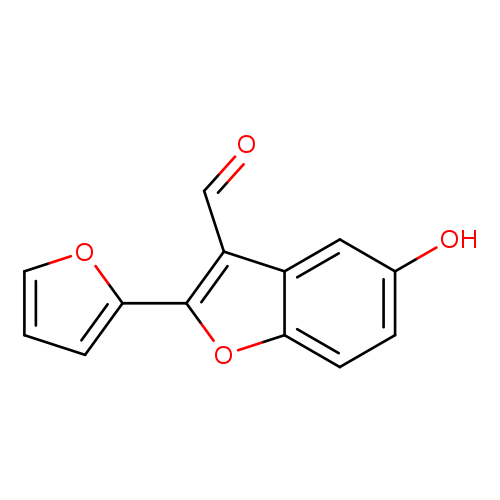
2-(Furan-2-yl)-5-hydroxybenzofuran-3-carbaldehydeCatalog No.:AA00IQ9N CAS No.:1092352-20-9 MDL No.:MFCD11553023 MF:C13H8O4 MW:228.2002 |
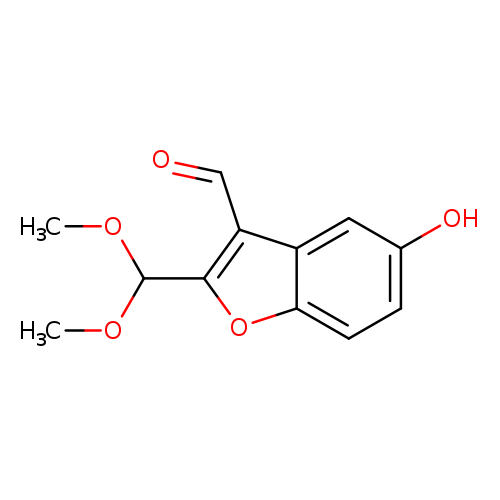
2-(Dimethoxymethyl)-5-hydroxybenzofuran-3-carbaldehydeCatalog No.:AA00IYKG CAS No.:1092352-23-2 MDL No.:MFCD11553024 MF:C12H12O5 MW:236.2207 |