2020-02-01 11:09:58
A. Sgueglia, A. Gentile, A. Frattarelli, G. Urbinati, M. A. Germanà & E. Caboni
Introduction
The European hazelnut, Corylus avellana L. (Betulaceae family), is of great economic importance and its nut production in Europe is third after walnut and almond (FAOstat 2016). Beside the commercial cultivars, the conservation of local cultivars is essential for breeders and other stakeholders (Boccacci et al. 2013; Bacchetta et al. 2015).
In vitro cultures represent an alternative means of ex situ conservation of plant genetic resources (Engelmann 2011). Such cultures can be established using few buds as initial material and have the added benefit of allowing the reintro- duction of ancient cultivars because they ensure rapid multiplication.
Corylus avellana is usually vegetatively propagated by suckers, although this method is known to spread diseases (Scortichini 2002). Micropropagation is a suitable tool for the production of true-to-type and disease free plants; in hazel- nut cultivars micropropagation has been the topic of several studies (Bassil et al. 1992; Yu and Reed 1995; Nas and Read 2001, 2004; Damiano et al. 2005; Latawa et al. 2016) and advanced statistical approaches have been also used to opti- mize the tissue culture medium for some genotypes (Hand and Reed 2014; Hand et al. 2014; Akin et al. 2017). Nevertheless, further optimization is needed for individ- ual cultivars.
Establishment of in vitro shoot cultures in hazelnut is a difficult step, particularly when explants are collected from adult plants grown in fields, due to the high fungal and bac- terial contamination and their poor attitude to the in vitro conditions (Yu and Reed 1995; Hand et al. 2016).
In plant tissue culture, the exogenous application of cyto- kinins (CKs) is essential for in vitro shoot proliferation. However, exposure of plantlets to unsuitable types or con- centrations of CKs can induce morphological abnormalities, such as hyperhydricity (Amoo et al. 2011). Topolins, a group of natural aromatic CKs (Strnad 1997; Tarkowsk´a et al. 2003) have been shown to reduce these problems in micropropa- gation of several species (Aremu et al. 2012; Amoo and Van Staden 2013; Gentile et al. 2014; Amoo et al. 2015), and to improve in vitro shoot multiplication and quality in Corylus colurna (Gentile et al. 2017).
Adventitious root formation starts with the establishment of root meristemoids (induction phase), followed by the for- mation of root primordia (initiation phase) and by root emer- gence (expression phase) (Druege et al. 2016). In micropropagation of several species, an exogenous applica- tion of auxins is necessary to reach the endogenous levels required for adventitious root induction (De Klerk et al. 1999).
The aim of this study was to define an efficient micro- propagation protocol for four local Italian hazelnut cultivars. We investigated two treatment times for the decontamin- ation of the buds in the establishment of in vitro cultures, compared two CKs, for shoot multiplication and two indole- 3-butyric acid (IBA) concentrations for rooting.
Materials and methods
Four Italian cultivars of C. avellana L., ‘Carrello’, ‘Ghirara’, ‘Minnulara’ and ‘Panottara’ (Baratta et al. 1994; Boccacci et al. 2013), were used. Shoot cultures were established in vitro using axillary buds excised from one-year-old-twigs from mother plants growing in a field collection located in Ucria (Messina, Sicily, Italy). Twig portions (about 2 cm) were soaked with LysoformVR soap (Uniliver Italia SpA, Italy) and washed under tap water for 2 h.
Decontamination was per- formed by immersion in 70% ethanol for 1 min, rinsing in sterile distilled water (sdw), soaking in commercial bleach solution (1% sodium hypochlorite), supplemented with a few drops of TweenVR 20, and rinsing with sdw. The explants were then immersed in 0.1% sodium merthiolate (C H HgNaO S; Carlo Erba, Italy) with a few drops of Tween 20 and rinsed in sdw three times. Two decontamination times were compared for sodium hypochlorite and sodium merthiolate (35 35 min and 40 40 min). Axillary buds were excised under a laminar flow hood and individually placed on a medium containing DKW (Driver and Kuniyuki 1984) salts, modified according to Mc Granahan et al. (1987), MS (Murashige and Skoog 1962) vitamins, 2 g L—1 charcoal, 20 g L—1 sucrose, 7 g L—1 agar (B&V, Italy) and supplemented with 0.29 lM GA3 and 4.4 lM N6-benzyladenine (BA). After 20 days, explant contamination, necrosis and overall survival were recorded and surviving explants were transferred into vessels (Magenta-Sigma, Italy) containing 50 mL of a basal medium (Gentile et al. 2017) with 4.4 lM BA, 0.049 lM IBA and 0.29 lM GA3. After three subcultures (30 days each) shoots, 30 mm in length, were transferred to the same medium supplemented with 8.2 lM metatopolin (mT) or 6.6 lM BA as CKs. At the end of the third subculture (30 days each), multiplication rate, calculated as the ratio of shoot final number to the initial number, and percentage of hyper- hydric shoots were recorded.
For rooting induction, in a first experiment, in vitro grown shoots of the two cultivars ‘Carrello’ and ‘Panottara’ were transferred to a rooting medium (RM) consisting in half strength MS macrosalts, full MS microsalts and organics, 20 g L—1 sucrose, 5.6 g L—1 agar (B&V, Italy), according to Gentile et al. (2017), and supplemented with 9.80 or 17.6 mM IBA for 7 days and then transferred to a hormone free RM with ver- miculite and agar (1:1 v/v). After 30 days, the percentage of explants producing roots and number of roots per rooted plant were recorded.
In a second rooting experiment shoots of the four culti- vars were induced to root on RM with 17.6 mM IBA for 7 days and then transferred to Jiffy-7VR Peat Pellets in the greenhouse, under a polyethylene tunnel with 90% humidity,
approximate temperature of 22 ○C day/10 ○C night and midday average PPF of 35 lmol m—2s—1. Shoots were trans- planted, after 30 days, to pots containing a mixture of 55% peat, 30% coconut peat and 15% vermiculite. Rooting and acclimatisation were evaluated four months after transfer of shoots from RM.
The pH of all the media was adjusted to 5.6 prior the add-provided by Osram fluora L58W/77 lamps (Germany), at
37.5 lmol m—2 s—1 photosynthetic photon flux (PPF) and 24 ± 1 ○C. All chemicals were from Sigma-Aldrich (Italy) except where differently reported.
A completely randomized design was used with results presented as means of the three independent replications ± standard error (SE). For each replication 10 shoots for estab- lishment and 15 for multiplication and rooting experiments were used. Since Shapiro–Wilk test indicated not normality of data distribution from decontamination experiments, Kruskal–Wallis and Mann–Whitney’s test (p 0.05) were used for data statistical analysis. For multiplication and rooting experiments data were subjected to a one-way analysis of variance (ANOVA) with mean separations by Tukey’s test (p ≤ 0.05). Past software was used for all the statistical analysis.
Results and discussion
Ex situ conservation based on in vitro culture can reduce costs and risks of loss of accessions maintained in open field due to abiotic and biotic factors (Engelmann 2011).
Hazelnuts are generally difficult to be initiated into culture due to microbial contaminants and decontamination techni- ques and juvenility of starting material significantly affected the success of obtaining viability of the explants (Yu and Reed 1995; Hand et al. 2016).
In our experiment axillary buds were excised from 1-year- old twigs of adult plants and 35 or 40 min of application of sodium hypochlorite and sodium merthiolate were chosen since a previous protocol, successfully applied to other Italian cultivars (Damiano et al. 2005), consisting in shorter application time of both these sterilizing agents, did not allow to obtain decontaminated viable explants. We observed a positive effect of the 40 þ 40 min treatment in reducing explant contamination in all the cultivars but it also induced an overall higher necrosis incidence and particularly in ‘Carrello’ (50%) and ‘Panottara’ (46.7%). The highest bud survival was obtained with the 40 þ 40 min treatment in ‘Minnulara’ (26.7%) and ‘Ghirara’ (20%) while the 35 þ 35 min treatment was more effective for ‘Carrello’ (33.3%) and ‘Panottara’ (23.3%) (Table 1; Figure 1(A)).
These chemical treatments allowed the establishment of aseptic cultures from axillary buds excised from 1-year-old twigs collected in the fields, thereby making unnecessary to root suckers and to grow plants in a greenhouse as a means to reduce initial contamination (Bacchetta et al. 2008; Hand et al. 2016).
Exogenous CKs application is generally necessary for in vitro shoot multiplication but type and concentration have to be optimized in relationship to the species to avoid the physiological disorders limiting micropropagation on a com- mercial scale (Amoo et al. 2011; Ivanova and Van Staden 2011). In several species mT improved multiplication rates and explant quality compared to BA (Aremu et al. 2012). Basal growth medium and mT concentration (8.2 mM) were chosen since previously successfully applied in C. colurna in vitro multiplication (Gentile et al. 2017) while 6.6 mM BA was shown to be suitable in ‘Ghirara’ and other Italian cultivars (Bacchetta et al. 2008).
Shoot multiplication, averaged across the four cultivars, was not significantly different between BA and mT, but was greater for BA in ‘Ghirara’ and ‘Minnulara’ (Table 2; Figure 1(B)). However, about 20% of the shoots in the BA treatment showed hyperhydricity, while those in mT treat- ment did not, consistent with the results in Prunus spp. (Gentile et al. 2014).
IBA is one of the most effective auxin for root induction, acting by itself and after conversion to indole-3-acetic acid (Fattorini et al. 2017). The response to exogenous application in woody species varies with concentration, application time and genotype (Caboni et al. 1997; De Klerk et al. 1999). While in vitro rooting protocols have been reported for ‘Tonda Gentile Romana’ and ‘Montebello’, still they must be optimized for other cultivars (Damiano et al. 2005; Bacchetta et al. 2008; Caboni et al. 2009).
In the in vitro rooting experiment with ‘Carrello’ and ‘Panottara’, adventitious rooting percentage and number of roots per explant was higher in shoots induced with 17.6 mM IBA (Figure 1(C) and 1(D)), than with 9.8 mM IBA (Table 3). The results confirm that IBA concentration applied influences adventitious rooting by promoting formation and develop- ment of lateral root primordia (Ludwig-Muller et al. 2005; Overvoorde et al. 2010).
Nas and Read (2004) showed ex vitro rooting with high
survival in hybrid hazelnut. Thus, in the second experiment, rooting was induced in vitro with 17.6 mM IBA and then the plantlets were transferred to ex vitro conditions, where more than 85% of shoots rooted and acclimatized (Table 4; Figure 1(E) and 1(F)), an improvement of the rooting response obtained by Bacchetta et al. (2008).
In conclusion, an efficient protocol was developed for the establishment of in vitro cultures of Sicilian hazelnut cultivars, their multiplication and rooting which will be useful for propagating and preserving these cultivars.
Acknowledgements
This work was supported by the Italian Agricultural Ministry (RGV-FAO Project). The authors wish to thank Prof. Basilio Baratta for providing the plant material. Thanks are also due to University of Palermo (Italy) for the ‘Mediterranean Fruit Crops’ Ph.D. course, attended by Dr. Alessandra Sgueglia.
Disclosure statement
No potential conflict of interest was reported by the authors.
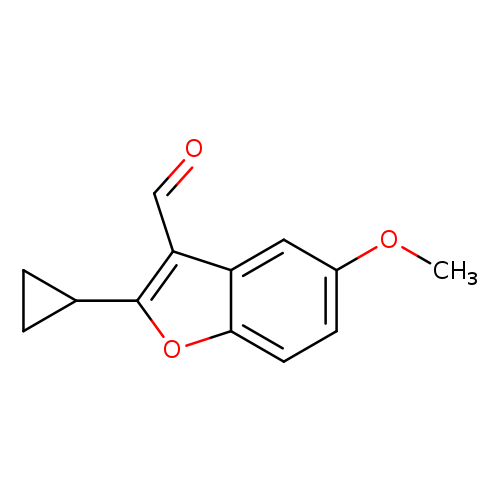
2-Cyclopropyl-5-methoxybenzofuran-3-carbaldehydeCatalog No.:AA00IRLL CAS No.:1092352-26-5 MDL No.:MFCD11553025 MF:C13H12O3 MW:216.2326 |
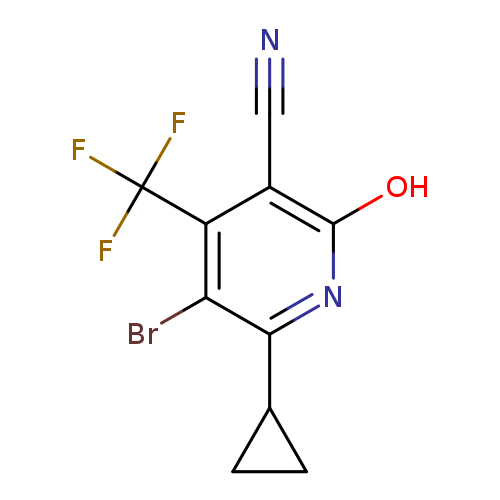
5-Bromo-6-cyclopropyl-2-oxo-4-(trifluoromethyl)-1,2-dihydropyridine-3-carbonitrileCatalog No.:AA00IS6O CAS No.:1092352-29-8 MDL No.:MFCD29044840 MF:C10H6BrF3N2O MW:307.0666 |
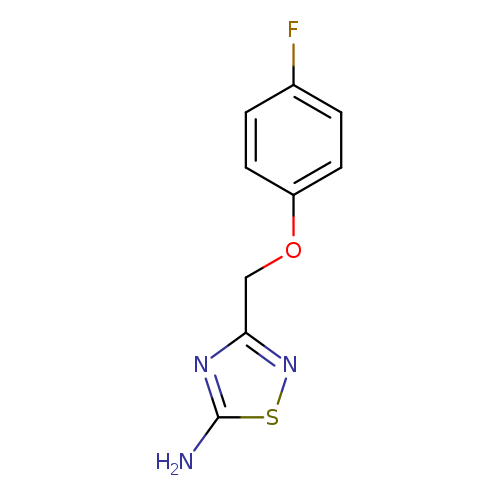
3-[(4-fluorophenoxy)methyl]-1,2,4-thiadiazol-5-amineCatalog No.:AA00IWLO CAS No.:1092352-32-3 MDL No.:MFCD00245317 MF:C9H8FN3OS MW:225.2427 |
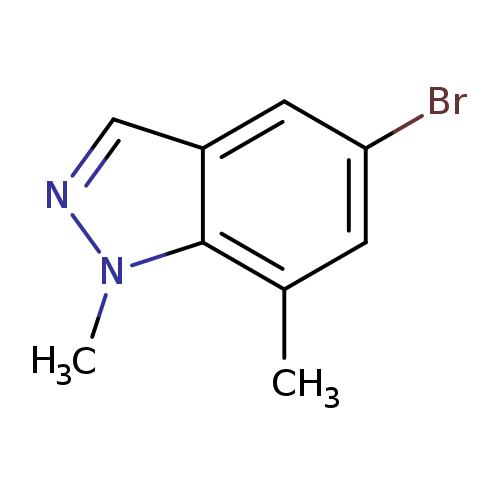
5-Bromo-1,7-dimethyl-1H-indazoleCatalog No.:AA008TSR CAS No.:1092352-34-5 MDL No.:MFCD11226595 MF:C9H9BrN2 MW:225.0852 |
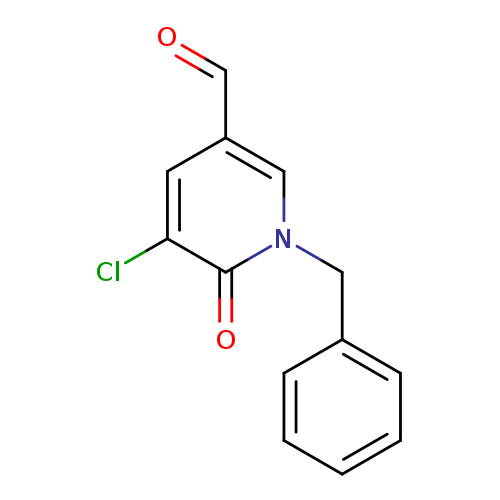
1-Benzyl-5-chloro-6-oxo-1,6-dihydropyridine-3-carbaldehydeCatalog No.:AA00ISJ3 CAS No.:1092352-35-6 MDL No.:MFCD11553029 MF:C13H10ClNO2 MW:247.6770 |
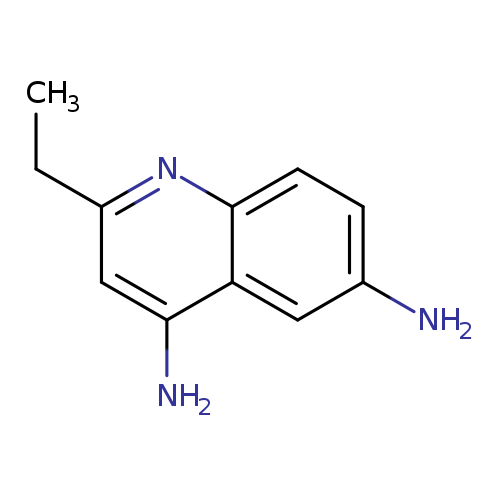
2-Ethylquinoline-4,6-diamineCatalog No.:AA01A4C9 CAS No.:1092352-36-7 MDL No.:MFCD13192505 MF:C11H13N3 MW:187.2410 |
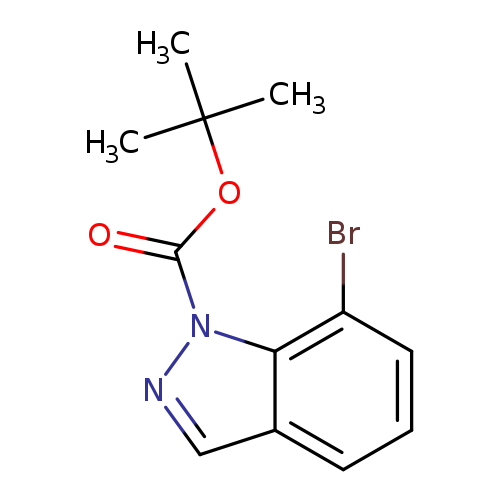
7-Bromoindazole-1-carboxylic acid tert-butyl esterCatalog No.:AA008U1C CAS No.:1092352-37-8 MDL No.:MFCD11505878 MF:C12H13BrN2O2 MW:297.1478 |
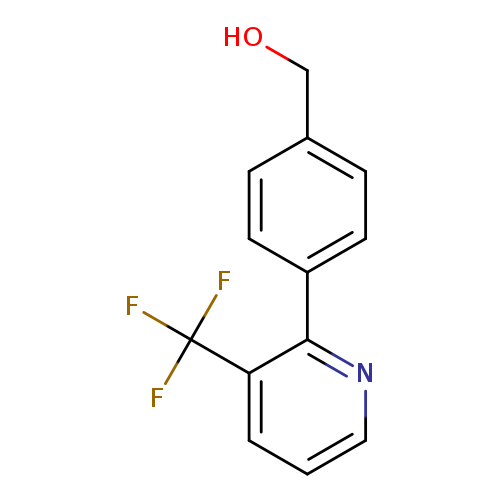
(4-(3-(Trifluoromethyl)pyridin-2-yl)phenyl)methanolCatalog No.:AA0090UY CAS No.:1092352-38-9 MDL No.:MFCD11501048 MF:C13H10F3NO MW:253.2198 |
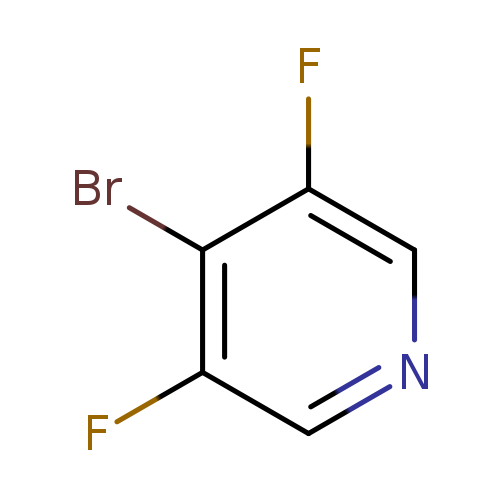
4-Bromo-3,5-difluoropyridineCatalog No.:AA007TBN CAS No.:1092352-40-3 MDL No.:MFCD11505879 MF:C5H2BrF2N MW:193.9769 |
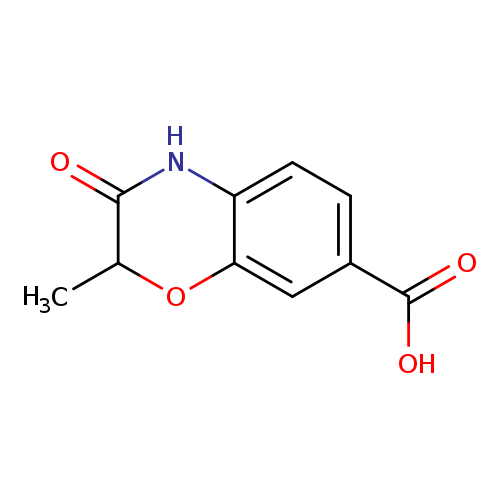
2-Methyl-3-oxo-3,4-dihydro-2H-benzo[b][1,4]oxazine-7-carboxylic acidCatalog No.:AA00IZKC CAS No.:1092352-41-4 MDL No.:MFCD11553035 MF:C10H9NO4 MW:207.1828 |
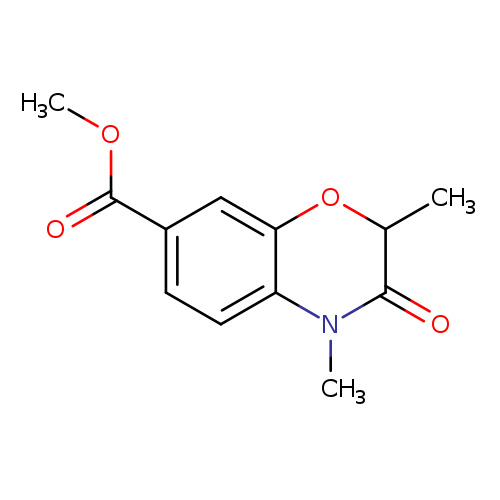
Methyl 2,4-dimethyl-3-oxo-3,4-dihydro-2H-benzo[b][1,4]oxazine-7-carboxylateCatalog No.:AA00IUJN CAS No.:1092352-44-7 MDL No.:MFCD11553036 MF:C12H13NO4 MW:235.2359 |
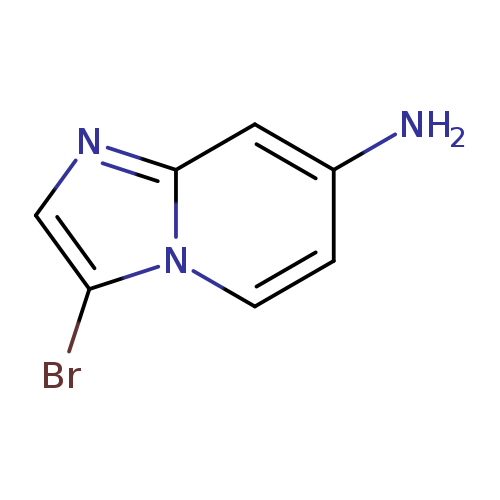
3-Bromoimidazo[1,2-a]pyridin-7-amineCatalog No.:AA008UFU CAS No.:1092352-46-9 MDL No.:MFCD11518906 MF:C7H6BrN3 MW:212.0466 |
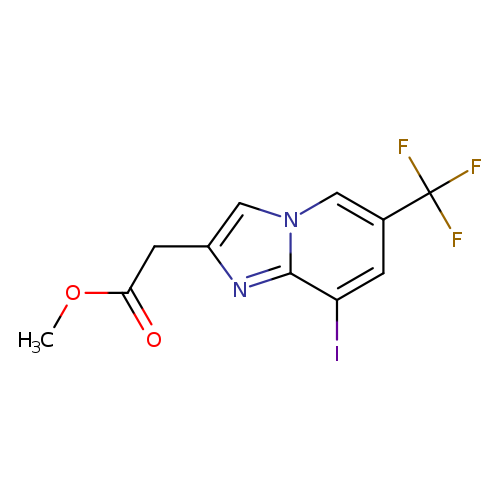
methyl 2-[8-iodo-6-(trifluoromethyl)imidazo[1,2-a]pyridin-2-yl]acetateCatalog No.:AA00HB9L CAS No.:1092352-47-0 MDL No.:MFCD11553037 MF:C11H8F3IN2O2 MW:384.0931 |
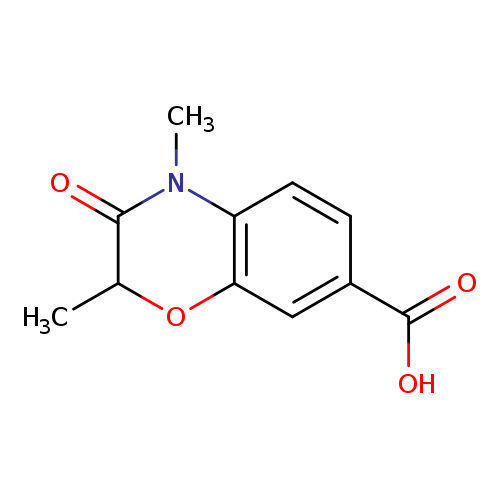
2,4-Dimethyl-3-oxo-3,4-dihydro-2H-benzo[b][1,4]oxazine-7-carboxylic acidCatalog No.:AA00IZKD CAS No.:1092352-50-5 MDL No.:MFCD11553039 MF:C11H11NO4 MW:221.2093 |
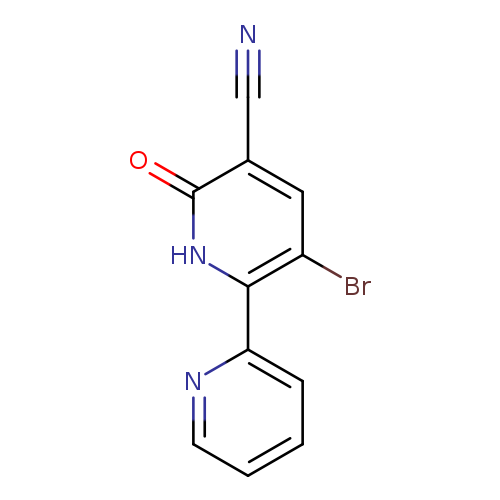
3-Bromo-6-oxo-1,6-dihydro-[2,2'-bipyridine]-5-carbonitrileCatalog No.:AA00IUJO CAS No.:1092352-53-8 MDL No.:MFCD11553040 MF:C11H6BrN3O MW:276.0888 |
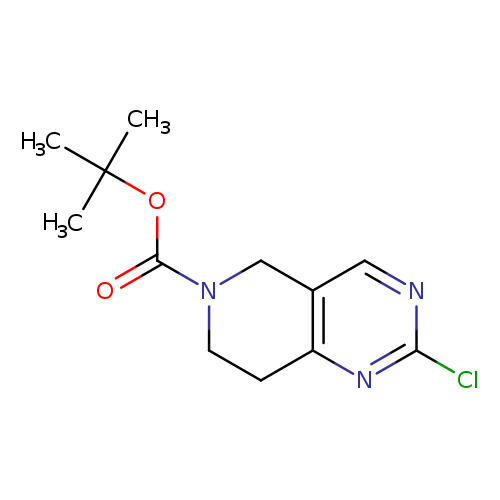
tert-Butyl 2-chloro-7,8-dihydropyrido[4,3-d]pyrimidine-6(5h)-carboxylateCatalog No.:AA0082MX CAS No.:1092352-55-0 MDL No.:MFCD11518980 MF:C12H16ClN3O2 MW:269.7273 |
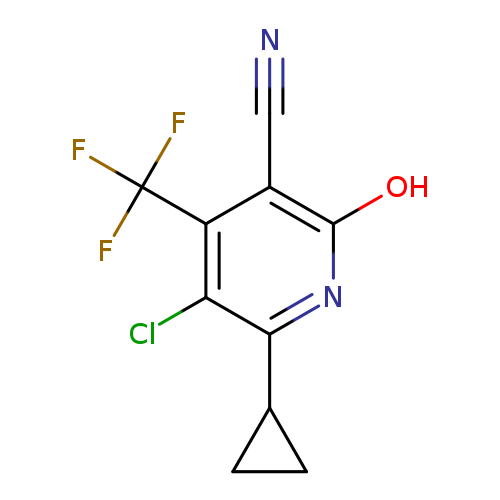
5-Chloro-6-cyclopropyl-2-hydroxy-4-(trifluoromethyl)nicotinonitrileCatalog No.:AA00IZMM CAS No.:1092352-56-1 MDL No.:MFCD11553041 MF:C10H6ClF3N2O MW:262.6156 |
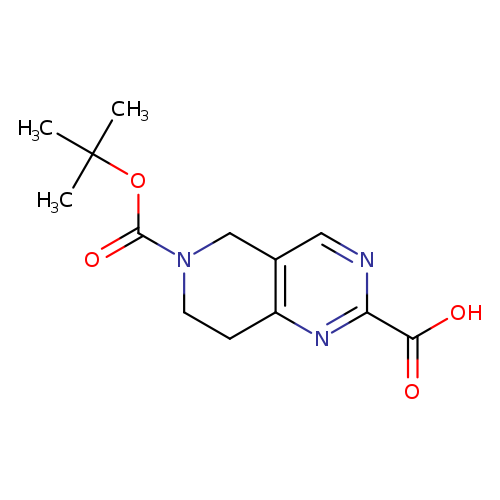
6-(tert-Butoxycarbonyl)-5,6,7,8-tetrahydropyrido[4,3-d]pyrimidine-2-carboxylic acidCatalog No.:AA007B8E CAS No.:1092352-58-3 MDL No.:MFCD11519389 MF:C13H17N3O4 MW:279.2918 |
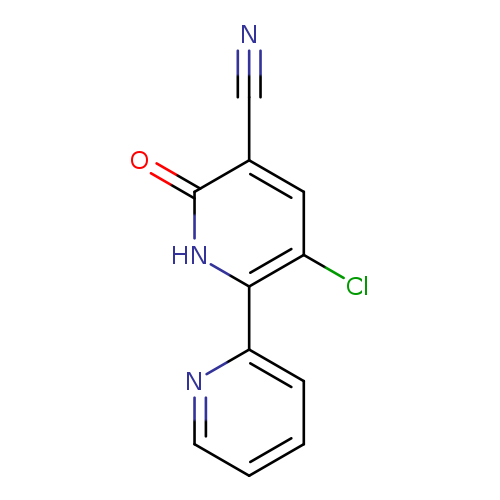
3-Chloro-6-oxo-1,6-dihydro-[2,2'-bipyridine]-5-carbonitrileCatalog No.:AA00IZMN CAS No.:1092352-59-4 MDL No.:MFCD11553042 MF:C11H6ClN3O MW:231.6378 |
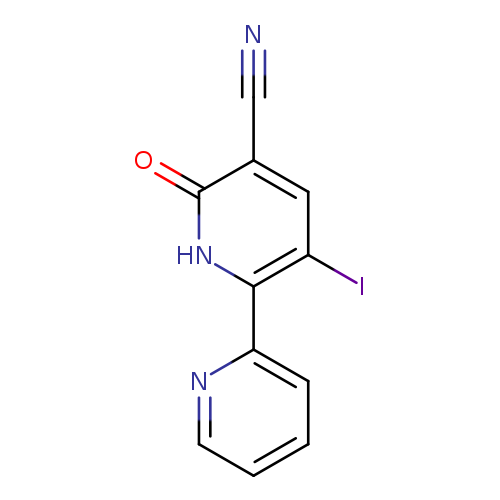
3-Iodo-6-oxo-1,6-dihydro-[2,2'-bipyridine]-5-carbonitrileCatalog No.:AA00ISQ8 CAS No.:1092352-61-8 MDL No.:MFCD11553043 MF:C11H6IN3O MW:323.0893 |
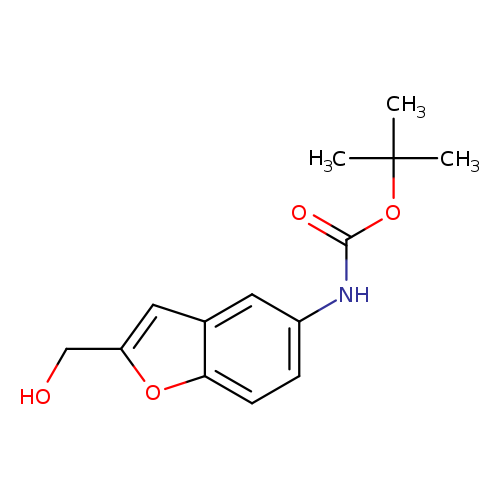
tert-Butyl (2-(hydroxymethyl)benzofuran-5-yl)carbamateCatalog No.:AA00IWS0 CAS No.:1092352-63-0 MDL No.:MFCD11553049 MF:C14H17NO4 MW:263.2891 |
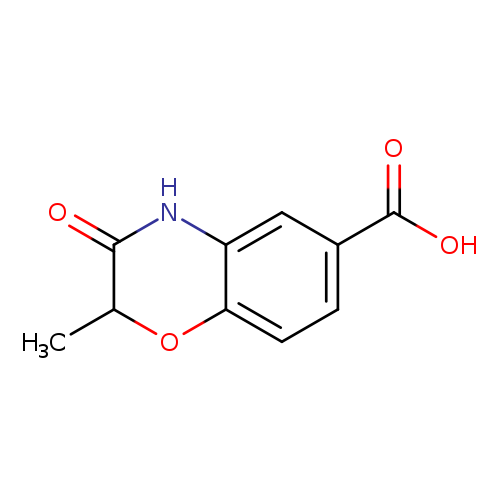
2-Methyl-3-oxo-3,4-dihydro-2H-benzo[b][1,4]oxazine-6-carboxylic acidCatalog No.:AA0090VG CAS No.:1092352-65-2 MDL No.:MFCD11207468 MF:C10H9NO4 MW:207.1828 |
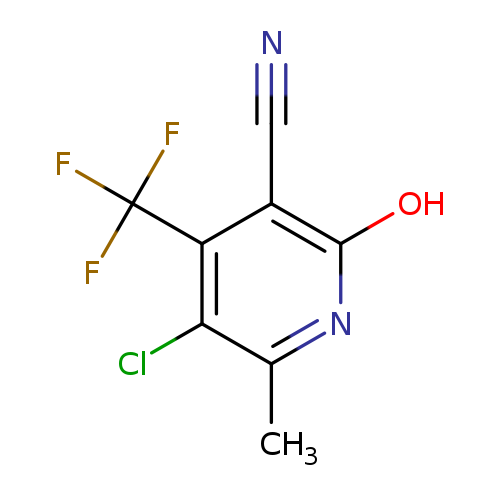
5-Chloro-2-hydroxy-6-methyl-4-(trifluoromethyl)nicotinonitrileCatalog No.:AA00HB9M CAS No.:1092352-68-5 MDL No.:MFCD11501054 MF:C8H4ClF3N2O MW:236.5784 |
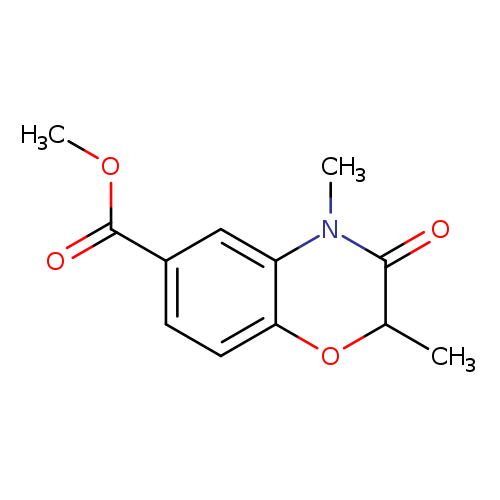
methyl 2,4-dimethyl-3-oxo-3,4-dihydro-2H-1,4-benzoxazine-6-carboxylateCatalog No.:AA00IWVH CAS No.:1092352-70-9 MDL No.:MFCD11553051 MF:C12H13NO4 MW:235.2359 |
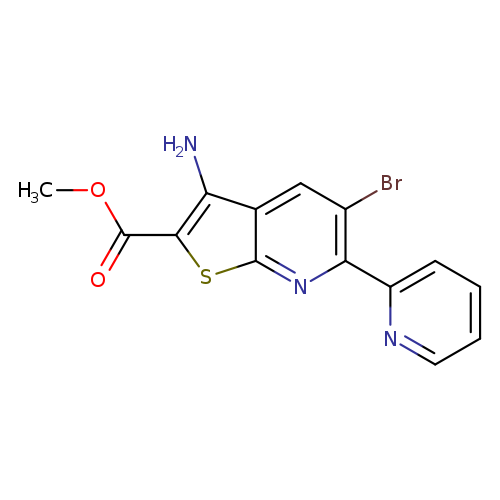
methyl 3-amino-5-bromo-6-(pyridin-2-yl)thieno[2,3-b]pyridine-2-carboxylateCatalog No.:AA00IWVI CAS No.:1092352-72-1 MDL No.:MFCD11553053 MF:C14H10BrN3O2S MW:364.2171 |
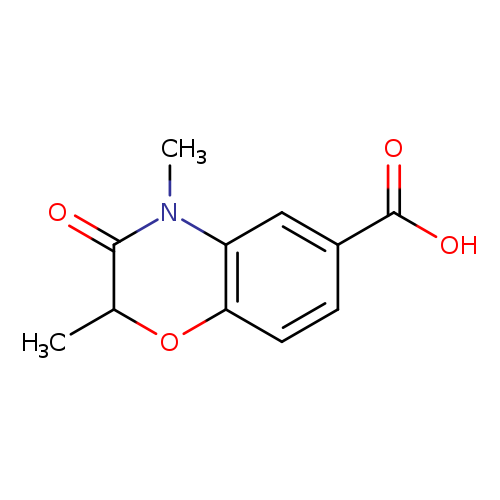
2,4-Dimethyl-3-oxo-3,4-dihydro-2H-benzo[b][1,4]oxazine-6-carboxylic acidCatalog No.:AA00ISW5 CAS No.:1092352-74-3 MDL No.:MFCD11553054 MF:C11H11NO4 MW:221.2093 |
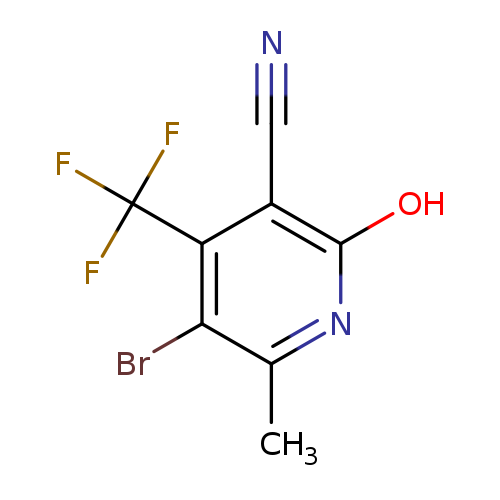
5-Bromo-2-hydroxy-6-methyl-4-(trifluoromethyl)nicotinonitrileCatalog No.:AA0090WA CAS No.:1092352-76-5 MDL No.:MFCD11501053 MF:C8H4BrF3N2O MW:281.0294 |
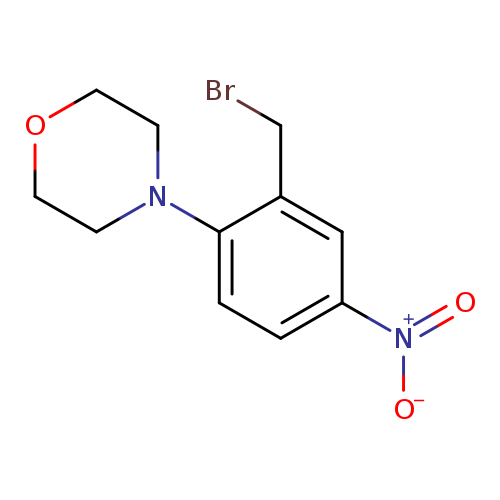
4-(2-(Bromomethyl)-4-nitrophenyl)morpholineCatalog No.:AA00IZO6 CAS No.:1092352-78-7 MDL No.:MFCD11553056 MF:C11H13BrN2O3 MW:301.1365 |
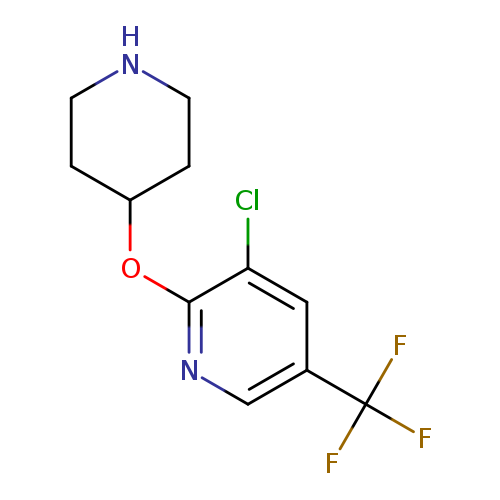
3-chloro-5-(trifluoromethyl)-2-pyridinyl 4-piperidinyl etherCatalog No.:AA0090VW CAS No.:1092352-80-1 MDL No.:MFCD11553058 MF:C11H12ClF3N2O MW:280.6740 |
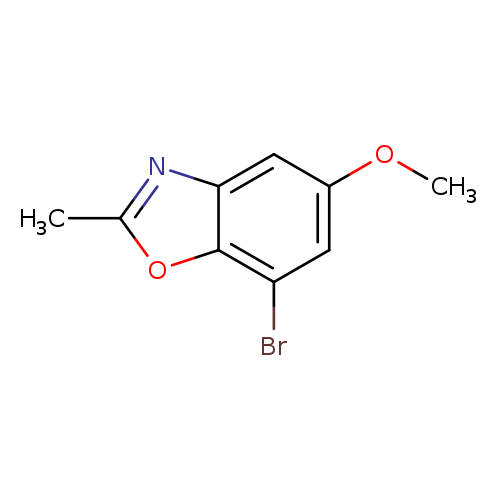
7-Bromo-5-methoxy-2-methylbenzo[d]oxazoleCatalog No.:AA00IZO7 CAS No.:1092352-82-3 MDL No.:MFCD11553059 MF:C9H8BrNO2 MW:242.0693 |
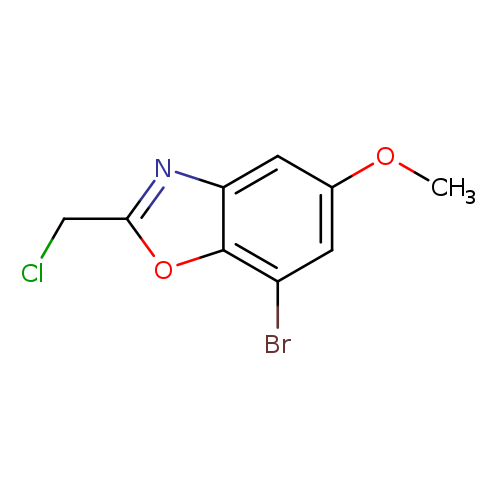
7-Bromo-2-(chloromethyl)-5-methoxybenzo[d]oxazoleCatalog No.:AA00IUR9 CAS No.:1092352-84-5 MDL No.:MFCD11553060 MF:C9H7BrClNO2 MW:276.5144 |
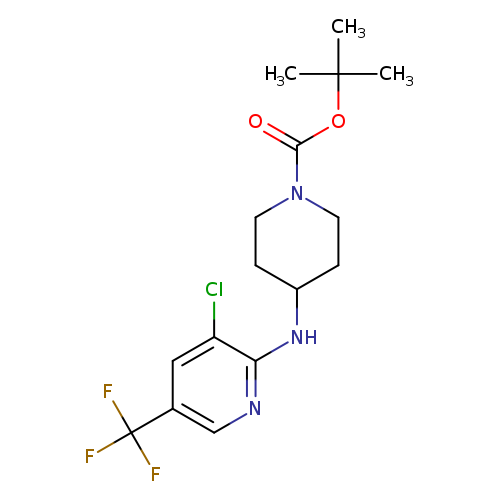
tert-Butyl 4-([3-chloro-5-(trifluoromethyl)-2-pyridinyl]amino)tetrahydro-1(2h)-pyridinecarboxylateCatalog No.:AA0090X8 CAS No.:1092352-86-7 MDL No.:MFCD11553061 MF:C16H21ClF3N3O2 MW:379.8050 |
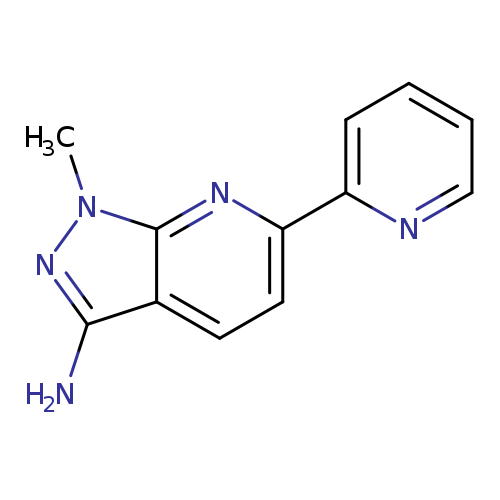
1-methyl-6-(pyridin-2-yl)-1H-pyrazolo[3,4-b]pyridin-3-amineCatalog No.:AA00IURA CAS No.:1092352-88-9 MDL No.:MFCD11553063 MF:C12H11N5 MW:225.2492 |
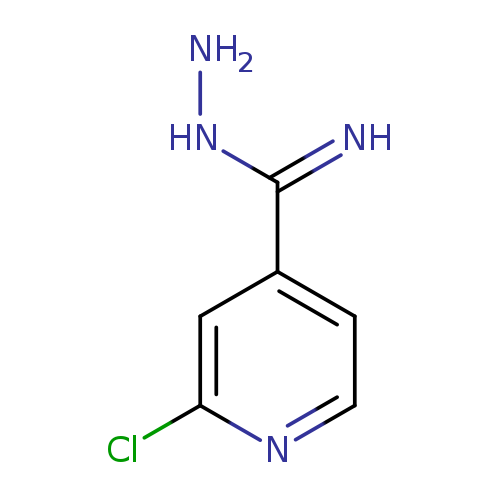
2-ChloroisonicotinimidohydrazideCatalog No.:AA00IZO8 CAS No.:1092352-90-3 MDL No.:MFCD11553064 MF:C6H7ClN4 MW:170.5996 |
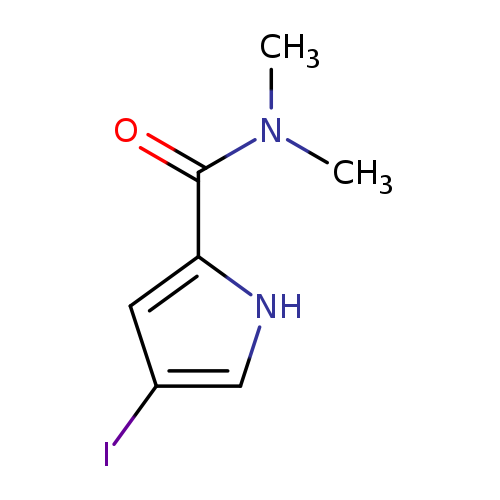
4-Iodo-N,N-dimethyl-1H-pyrrole-2-carboxamideCatalog No.:AA00IWWA CAS No.:1092352-92-5 MDL No.:MFCD11553066 MF:C7H9IN2O MW:264.0636 |
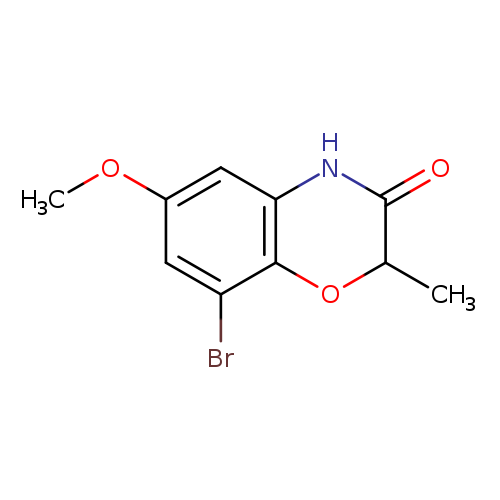
8-Bromo-6-methoxy-2-methyl-2H-benzo[b][1,4]oxazin-3(4H)-oneCatalog No.:AA00ISZ0 CAS No.:1092352-94-7 MDL No.:MFCD11553068 MF:C10H10BrNO3 MW:272.0953 |
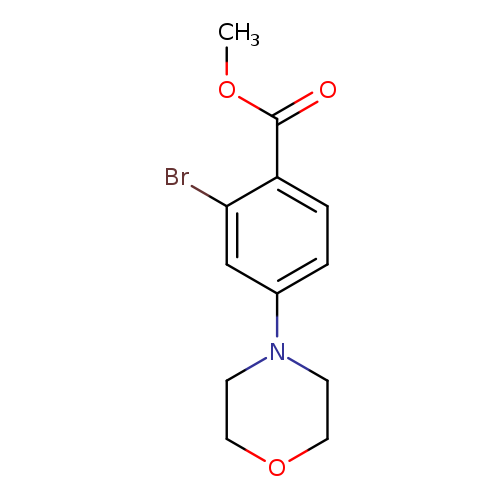
Methyl 2-bromo-4-morpholinobenzoateCatalog No.:AA00IWWC CAS No.:1092352-96-9 MDL No.:MFCD11553069 MF:C12H14BrNO3 MW:300.1485 |
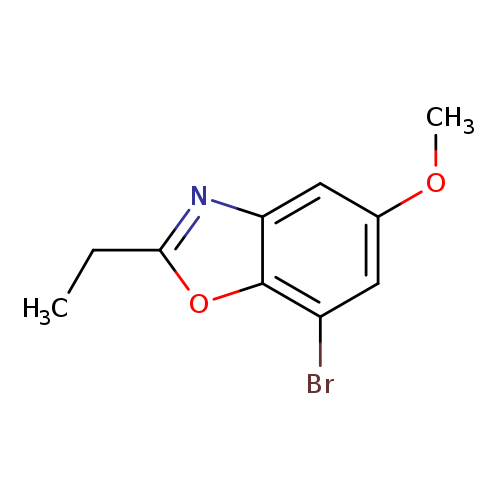
7-Bromo-2-ethyl-5-methoxybenzo[d]oxazoleCatalog No.:AA00ISZ3 CAS No.:1092352-98-1 MDL No.:MFCD11553073 MF:C10H10BrNO2 MW:256.0959 |
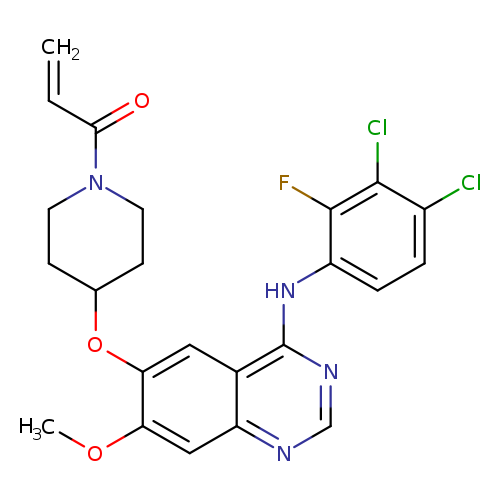
1-[4-[[4-[(3,4-Dichloro-2-fluorophenyl)amino]-7-methoxy-6-quinazolinyl]oxy]-1-piperidinyl]-2-propen-1-oneCatalog No.:AA0039IJ CAS No.:1092364-38-9 MDL No.:MFCD28044290 MF:C23H21Cl2FN4O3 MW:491.3422 |
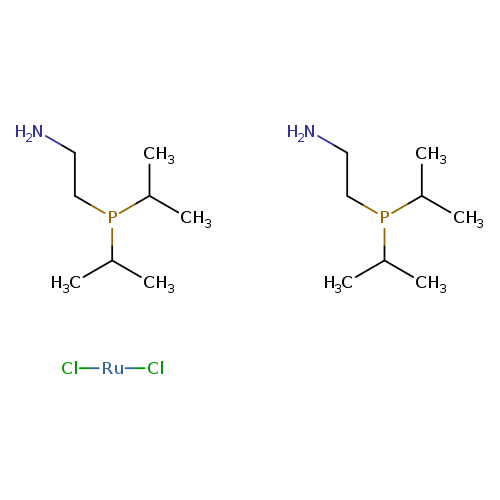
Dichlorobis[2-(di-i-propylphosphino)ethylamine]ruthenium(II)Catalog No.:AA003PAP CAS No.:1092372-90-1 MDL No.:MFCD07782005 MF:C16H40Cl2N2P2Ru MW:494.4257 |
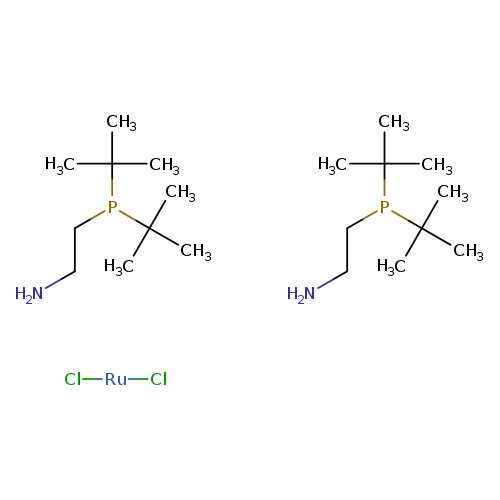
Dichlorobis[2-(di-t-butylphosphino)ethylamine]ruthenium(II)Catalog No.:AA008V9S CAS No.:1092372-91-2 MDL No.:MFCD07782003 MF:C20H48Cl2N2P2Ru MW:550.5320 |
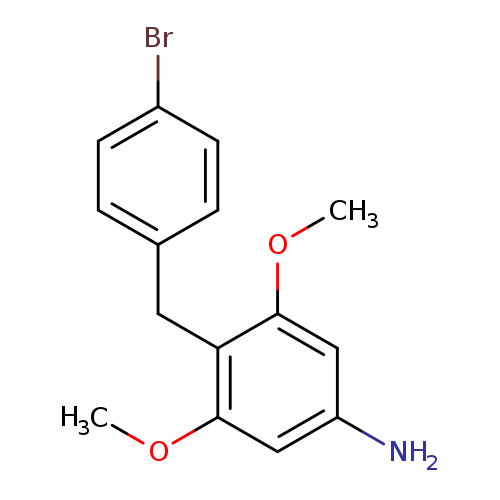
4-(4-Bromobenzyl)-3,5-dimethoxyanilineCatalog No.:AA0090VZ CAS No.:1092389-35-9 MDL No.:MFCD11841062 MF:C15H16BrNO2 MW:322.1970 |
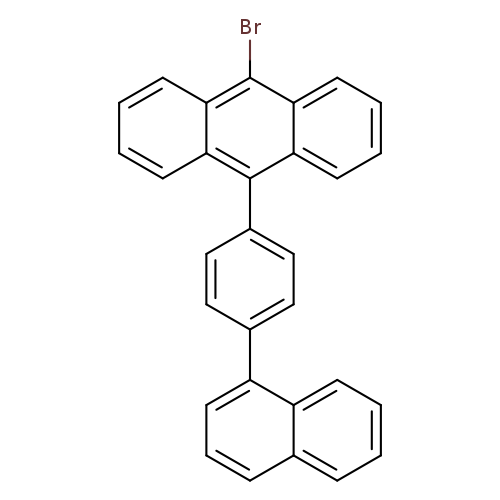
9-Bromo-10-[4-(1-naphthyl)phenyl]anthraceneCatalog No.:AA0091XH CAS No.:1092390-01-6 MDL No.:MFCD20486477 MF:C30H19Br MW:459.3759 |
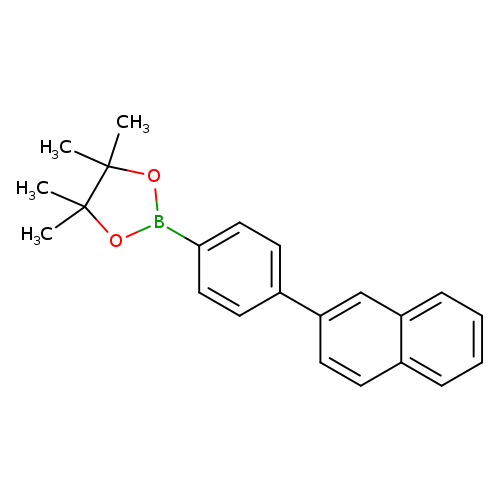
4-(Naphthalene-2-yl)phenylboronic acid pinacol esterCatalog No.:AA00395O CAS No.:1092390-02-7 MDL No.:MFCD09800562 MF:C22H23BO2 MW:330.2278 |
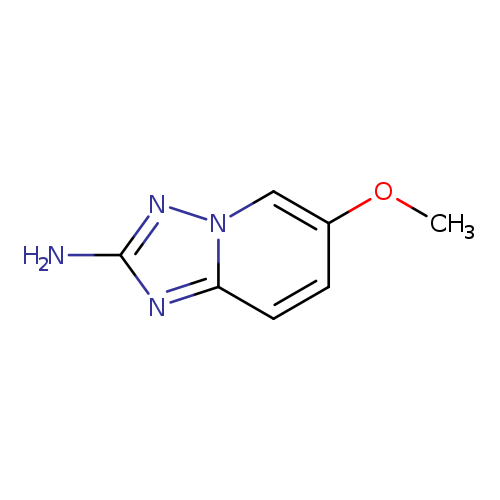
6-Methoxy-[1,2,4]triazolo[1,5-a]pyridin-2-amineCatalog No.:AA0039SS CAS No.:1092394-15-4 MDL No.:MFCD06761827 MF:C7H8N4O MW:164.1646 |
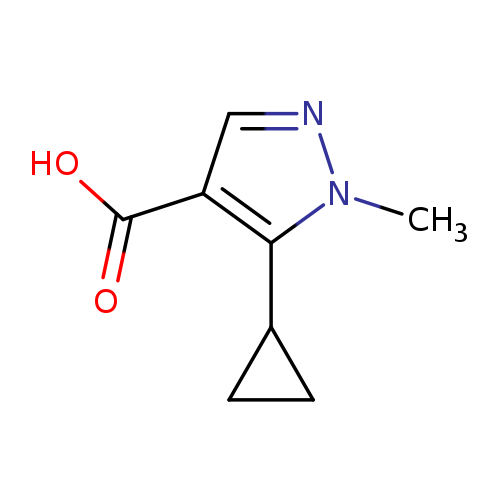
5-Cyclopropyl-1-methyl-1H-pyrazole-4-carboxylic acidCatalog No.:AA00JFXD CAS No.:1092394-30-3 MDL No.:MFCD11118807 MF:C8H10N2O2 MW:166.1772 |