2020-02-10 17:14:13
1. Marine Microorganism Sources
Marine microorganisms, one of the main sources of natural pigments, are often isolated from algae, sponges, mangroves, and sediments. These pigments are mainly divided into indole derivatives (quinones and violacein), alkaloids (prodiginines and tambjamines), polyenes, macrolides, peptides, and terpe- noids.[4] Importantly, these compounds present different bio- logical properties.
1.1 Prodiginines
Bacteria as an important source of red pigments was first seen when prodigiosin compounds were first isolated from Serratia marcescens. The common aromatic chemical structure of these pigmented compounds was first named prodiginine by Gerber.[69] Prodiginines mainly includes compounds similar in chemical structures to compounds (37–39). A novel marine bacterium strain, Vibrio gazogenes, effectively produced prodiginine type pigments, including the typical prodigiosins compound 37 and 38 as well as two new prodiginines (40– 41).[70] These two new pigments had maximum absorption peaks at 533 nm and these colourants could dye wool, silk and synthetic fabrics such as polyester and polyacrylic and showed antibacterial properties against Escherichia coli and Staph- ylococcus aureus bacteria. Li Houjin et al. isolated two kinds of prodigiosin, prodigiosin A (42) and new derivative dimethyllinosin B (43) from the bacterial Pseudomonas sp. isolated from the surface of the sea water in Daya Bay, Guangdong province.[71] A marine bacterium (Serratia protea- macula 657) was screened out from 251 marine sponge- associated bacteria originated from San Juan Island and a prodigiosin-like pigment (44) with highly antitumor activity was isolated,[5] showing strong inhibition of HeLa and MGC803 cell lines. A gram-negative, red-pigment-producing marine bacterial strain (Zooshikella rubidus S1-1), was isolated from tidal flat sediment in Yellow Sea, Korea[73] with two red pigments (45–46) being extracted. These pigments are both prodigiosins and showed antimicrobial activity against Bacillus subtilis, Escheria coli, Salmonella serovar Typhimu- rium, Staphylococcus aureus, and Candida albicans. Interest- ingly they also both have anticancer activity to two human melanoma cell lines, SKMEL-28 and A375P.
1.2 Carotenoids
Carotenoids as terpenoids are also abundant in marine microorganisms. Astaxanthin (33) can also be isolated from marine microorgannisms such as being produced by the marine bacterium, Paracoccus haeundaensis.
Two novel Carotenoids (47–48) were isolated from marine bacteria (Bacillus indicus HU36).[75] The maximum UV-VIS absorption values of the two carotenoids were 454 nm and 468 nm respectively. The carotenoids and phenolic antioxi- dants displayed synergistic activities in the inhibition of linoleic acid peroxidation induced by heme iron, but not by free iron.
1.3 Violacein
The violet pigment violacein is an indole derivative, predom- inantly isolated from bacteria of the genus Chromobacte- rium.[76] Violacein has a variety of biological activities, including antiviral, antibacterial, antiulcerogenic, antileishma- nial, and anticancer properties.[77–80] Hamilton and Austin first isolated violacein from marine bacteria (Chromobacterium marinum).[81] A marine bacterium (Pseudomonas sp.) was isolated from the surface of the sea water of Daya Bay in the south China sea.[82] The bacterium can produce the violacein Blue-1 (49). A series of anti-tumour activity experimental results showed that Blu-1 had a strong inhibition on tumour cell growth. The IC50 of against MCG803 and BEL7402 were 4.6 and 6.8 ug/ml respectively. Hideki Kobayashi et al. have purified a new violet pigment derived from Shewanella violacea DSS12 isolated from the deep-sea sediment of the Ryukyu Trench at a depth of 5,110 m.[83] The absorption maximum of this violet pigment (50) in THF was 616 nm and 636 nm in chloroform. The violet pigment of DSS12 was very stable and showed no antibiotic activity to Escherichia coli, but this was dependent on low permeation into cells because of the crystals’ insolubility in water or hydrocarbons.
1.4 Phenazine Compounds
Phenazines are redox-active, small nitrogen-containing aro- matic compounds produced by a diverse range of bacterial genera. Maskey et al. reported the isolation of two yellow pigments from the marine Pseudonocardia sp. B6273, a member of the Actinomycetes. Structural investigations identi- fied the two pigments as novel phenazostatin D(51) and phenazostatin B(52), and found to be inactive against the tested microorganisms, and methyl saphenate, a known phenazine antibiotic.[84] Phenazostatin B possessed a positive optical rotation at 365 nm. Li et al. also reported seven new oxidized and reduced phenazine-type pigments, dermacozines A–G (53–59) extracted from strains MT1.1 and MT1.2. All pigment molecules had the maximum ultraviolet absorption at 520 nm.[85] The bacteria were isolated from Mariana Trench sediment at a depth of 10898 m. Dermacozines F(58) and G(59) exhibited moderate cytotoxic activity against leukaemia cell line K562 with IC50 values of 9 and 7 mM, respectively, while the highest radical scavenger activity was observed for dermacozine C (50) with an IC50 value of 8.4 mM. The novel 5, 10-dihydrophencomycin methyl ester (60) and phencomycin(61) were isolated from an unidentified marine Streptomyces sp..[86] Compound (60) shows weak antibiotic activity against Escherichia coli and Bacillus subtilis.
1.5 Tambjamines
Tambjamines are alkaloids always isolated from various marine organisms like bryozoans, nudibranchs, and asci- dians.[87–88] A yellow pigment (62) was isolated from Pseudoal- teromonas tunicata and was identified as a new member of the tambjamine class of compounds.[89] P. tunicata has the highest and broadest range of biological activities which linked to the production of the yellow pigment.[90] David M. Pinkerton et al conducted a series of biological activity evaluation experi- ments on compond (62) showing antibacterial, antifungal effects and cytotoxic activity. The IC50 against human cancer cell lines HL-60 (promyelocytic leukaemia cells), MDA-MB-
435 (derived from the M14 melanoma cell line), HCT-8 (ileocecal colorectal adenocarcinoma cell line), and SF-295 (glioblastoma cell line) as well as PBM cells are 0.69, 1.11, 0.98, 0.83, 0.64 mg/ml, respectively.
1.6 Quinones
Quinone derivatives range in colour from yellow to red, exhibit antiviral, anti-infection, antimicrobial, insecticidal, and anticancer activities, and have many commercial applications as natural and artificial dyes and pigments.[92] Two new anthracycline antibiotics, designated as himalomycin A (63) and B (64), were isolated from the culture broth of the marine Streptomyces sp. isolate B6921, derived from sandy sediment of a coastal site of Mauritius (Indian Ocean).[93] These two compounds are liposoluble yellow solid, with their maximum absorbance in MeOH at 254, 290, 441 and 256, 292, 434 nm, respectively. At concentrations of 50 mg/disk, compound 63 and 64 both exhibited strong antibacterial activity against Bacillus subtilis, Streptomyces viridochromogenes, Staphylo- coccus aureus and Escherichia coli.
Two novel pigmented antitumor, antibiotics, ellowish brown liposoluble solid chinikomycin A (65) and red lip- osoluble solid chinikomycin B (66) were isolated from a marine Streptomyces sp. Strain M045.[94] Chinikomycins A(65) and B (66) both exhibited moderate antitumor activity, Chinikomycins A (65) being significantly more potent. It selectively inhibited proliferation in cell lines of mammary cancer (MAXF 401NL, IC50 = 2.41 mg/mL), melanoma (MEXF 462NL, IC50 = 4.15 mg/mL) and renal cancer (RXF 944 L, IC50 = 4.02 mg/mL). The Chinikomycins B (66) showed selective antitumor activity against the mammary cancer cell line MAXF 401NL (IC50 = 3.04 mg/mL).
1.7 Azaphilones
Azaphilones are a family of fungal pigments characterized by a highly oxygenated pyrano-quinone bicyclic core. The coloured azaphilone derivatives are produced by species of ascomyceteous and basidiomyceteous fungi, including the genera Penicillium, Aspergillus, Chaetomium, Talaomyces, Emericella, Epicoccum, Pestalotiopsis, Phomopsis, Monascus and Hypoxylon.[95] One yellow new azaphilone (67), along with six known analogues (68–73), were isolated from the fungus Penicillium 303#, which was collected from sea water of Zhanjiang Mangrove National Nature Reserve in Guang- dong Province, China, in 2014. Evaluation of cytotoxicity of these compounds found that compound 69 showed potent cytotoxic activity with its IC50 values of 7.13 mM against MDA-MB-435.[96] Moreover, Weiyi Wang et al. isolated four new azaphilones, chaephilone C (74), chaetoviridides A–C (75–77) from a Chaetomium sp. strain NA-S01-R1 isolated from the seawater sample at a depth of 4050 m in the West Pacific Ocean in 2017.[95] Except chaephilone C is yellow, the other three compounds are all red. Through antibacterial assay and cytotoxicity assay found compounds 74, 76 and 77 displayed similar anti-MRSA activities in comparison to chloramphenicol and compounds 74 and 76 demonstrated relatively stronger cytotoxic activities than the other com- pounds against HeLa cell. In addition, compound 75 showed the most potent cytotoxic activities towards Hep G2 cell with IC50 below 5 mM.
1.8 Other Pigments from Marine Bacteria
Rui He et al. constructed a metagenomic library of the Japanese marine sponge discodermia calyx and screened out two clones producing porphyrins (78) as red pigments. The pigment has the UV absorption maximum at 405 nm.[97] The ability of porphyrins to generate reactive oxygen species had been utilized in cancer therapy. In addition, vitamin B12 composed of a porphyrin skeleton has been produced by a biotechnological process using bacteria.
A yellow pigment (79) was isolated from marine bacterial strain Vibrio sp.,[98] isolated from water samples of the Arabian Sea. This pigment has antioxidant activity and shows significant inhibition of Gram-positive organisms (MIC=31.25 to 62.5 mg/ml) in the attribute biological properties against pathogenic bacteria. Scytonemin (80) is a yellowgreen pigment isolated from aquatic cyanobacteria and its maximum UV absorption at 370 nm.[99] Scytonemin is an important UV- radiation protective synthesised biomolecule. It protects bacteria by preventing about 85–90 % of all UV-light from entering through the cell membrane.[100] Grossart H P et al. extracted a blue pigment named Glaukothalin from Two g- Proteobacteria strains, isolated from the German Wadden Sea and the Øresund, Denmark, respectively. Glaukothalin is readily soluble in pyridine or HMPT, moderately soluble in DMSO, DMF or CHCl3, and insoluble in acetone, methanol, water (acidic or alkaline), benzene or cyclohexane. The UV spectrum of glaukothalin in CHCl3 exhibits characteristic absorption maxima at 636 nm (log e = 4.51), 582 nm (sh), 286 nm (sh), 241 nm. The molecular formula of Glaukothalin is C34H56N4O4, but the structure of this compound has not been reported so far.[101] AM13,1 strain, which was identified to belong to the Cytophaga/Flexibacteria cluster of North Sea bacteria, was found to produce yellow liposoluble tryptanthrin (81). This pigment is anti-fungal and anti-microbial and was found to show an antibacterial activity against Bacillus subtilis and demonstrated its hitherto unknown activity against dermatophytes. Its absorption maximum in MeOH were 248, 252, 277, 311, 328 nm and vis lmax at 387 nm.
2. Future Prospects & Biotechnology
Although marine pigment sources are very extensive, some pigments are far from reaching the goal of mass production, especially those from marine animals. Use of microorganisms have advantages over marine animal sources due to simple culture conditions and rapid propagation; therefore, the pig- ment can be produced in high quantity in a short time by fermentation. In recent decades, scientists have made break- throughs in pigment production using genetic engineering and microbial fermentation. A novel gene involved in ketocom- pound biosynthesis, designated as crtW, was isolated from the marine bacteria Agrobacterium aurantiacum and Alcaligenes PC-1. When this gene was introduced into Escherichia coli, that accumulated b-carotene due to the Erwinia carotenogenic genes, the E. coli transformants synthesized canthaxanthin, one of ketocarotenoids.[103]
Carotenoids are the most omnipresent pigments in the oceans with important biological roles such as light capture and antioxidative activities and have made great achievements in biosynthesis. The early attempts led to the production of lycopene, b-carotene, and astaxanthin in Saccharomyces cerevisiae and Candida utilis by the expression of caroteno- genic enzymes from Pantoea ananatisi.[104–105] The lycopene synthetic pathway was engineered in Escherichia coli using the carotenoid genes (crtE, crtB and crtI) of Pantoea agglomerans and Pantoea ananatis. A two-fold higher lycopene production is obtained in E. coli by the expression of carotenogenic enzymes from P. agglomerans (27 mg/L) than from P. ananatis (12 mg/L).[106]
To improve the efficiency of carotenoid production, the biological system of the host organism also needs to be optimized. Direct efforts were focused on the modification of associated genes to these pathways. For example, deletion of pyruvate kinases PykFA can balance the availability of pyruvate and G3P for the MEP pathway, and increase lycopene production by 2.8-fold in E. coli.[107]
Astaxanthin, as previously mentioned, has multiple poten- tial uses due to its role as an antioxidant therefore it is under scrutiny by the biosynthetic industry. For example, an astaxanthin producing Saccharomyces cerevisiae strain was created by successively introducing the Haematococcus pluvialis b-carotenoid hydroxylase (crtZ) and ketolase (bkt) genes into a previously constructed b-carotene hyperproducer. Through codon optimization, gene copy number adjustment and iron cofactor supplementation led to significant increase in the astaxanthin production, reaching up to 4.7 mg/g DCW in the shake-flask cultures which is the highest astaxanthin content in Saccharomyces cerevisiae reported to date.[108]
Recently, the production of astaxanthin is mainly based on microbial fermentation, including yeast and algae. Interest- ingly, astaxanthin from yeast is 100 % right-handed (3R-3’R) with partial antioxidant activity, while astaxanthin from algae is 100 % left-handed (3S-3’S) with the strongest biological activity. Since 1990, Roche began a large-scale production of synthetic astaxanthin and practically fulfilled the world market for the pigment, estimated at 150–200 million dollars.
Due to harsh and extreme environments, marine organisms have developed unique adaptation mechanisms and metabolic pathways to survive. Most studies investigating marine organisms have shown that they have the excellent prospects in producing pigmented metabolites, which are found to have antibiotic, anticancer, and immunosuppressive activities. As these pigments have diverse and promising activities for different diseases, they can play an important role in both medical and agricultural research.
Overall, this review underscores the importance of finding new pigments in the vast oceans of the planet. These pigments have many bioactive properties and continue to provide promising avenues for basic science and applied biomedical research.
Acknowledgements
We acknowledged the grants supports from the National innovative and Entrepreneurship Training Program for College Students in China (201710338015), Agro-scientific Research in the Public Interest of Zhejiang province [grant number LGN18C190011], Science Foundation of Zhejiang Sci-Tech University [17042058-Y], 13th Five-year Plan Teaching Re- form Research in Higher Education of Zhejiang province [jg20180092] and Project for Jiaozhou Excellent Innovation Team [grant number 18-CX-1].We also acknowledged students of the Department of Development Technology of Marine Resources, College of Life Sciences, Zhejiang Sci-Tech University, Muhammad Ridwanur Rahman, Zengyi, Meng Fengbin, Ying Junjie, Ji Xiaofeng, Sun Shaokang, Li zhi, Song Jiaying, Meng Yuan, Wang Gaoyang, Zhang Shunli, Lei Yutong, Zhang Meiling, Yang Tianyong, Shi Xuhui, Huang Chaoyu, Ren Qingyu and Zhang Mengying, for their help in preparing this article.
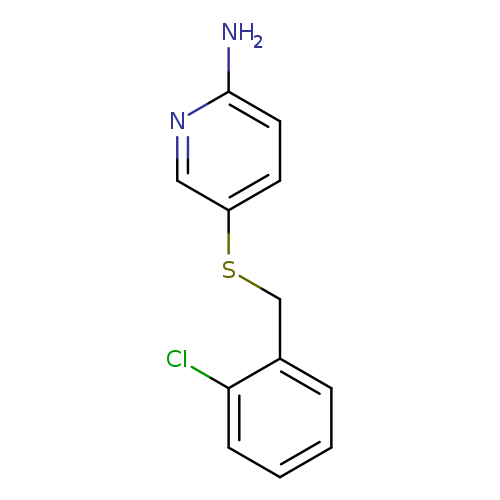
5-{[(2-chlorophenyl)methyl]sulfanyl}pyridin-2-amineCatalog No.:AA01ABOX CAS No.:1095503-57-3 MDL No.:MFCD11635963 MF:C12H11ClN2S MW:250.7471 |
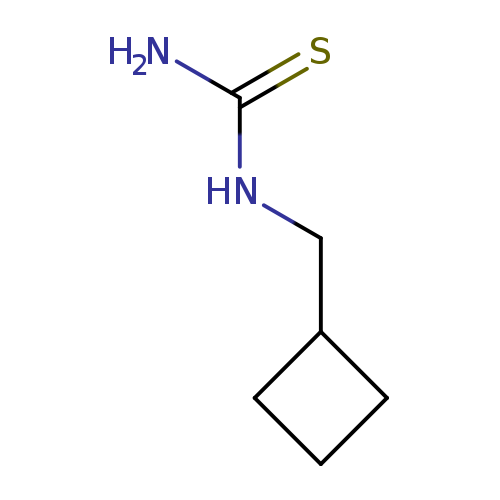
(Cyclobutylmethyl)thioureaCatalog No.:AA019X81 CAS No.:1095505-12-6 MDL No.:MFCD11634958 MF:C6H12N2S MW:144.2379 |
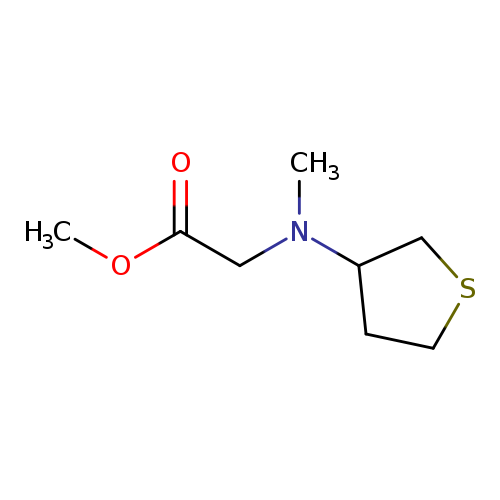
methyl 2-[methyl(thiolan-3-yl)amino]acetateCatalog No.:AA01A1JV CAS No.:1095511-68-4 MDL No.:MFCD11634240 MF:C8H15NO2S MW:189.2752 |
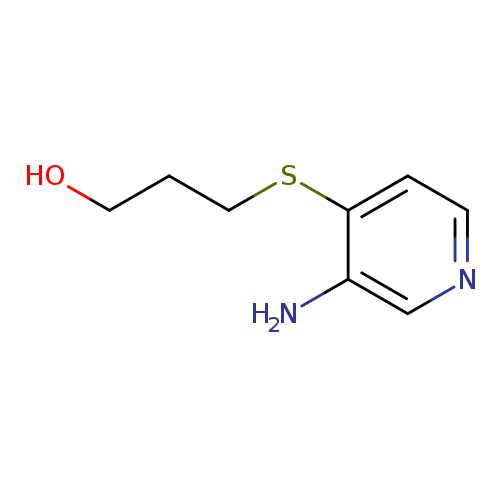
3-[(3-aminopyridin-4-yl)sulfanyl]propan-1-olCatalog No.:AA01AB4U CAS No.:1095514-79-6 MDL No.:MFCD11635271 MF:C8H12N2OS MW:184.2587 |
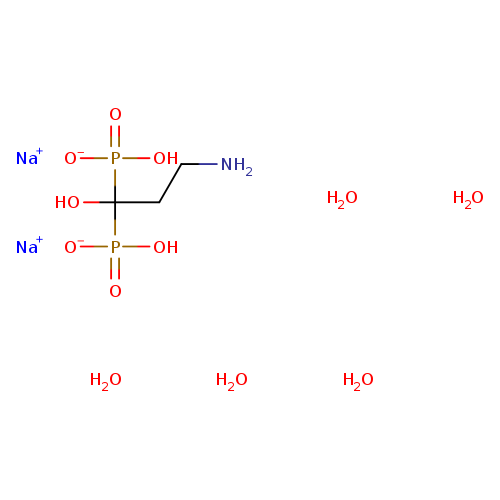
Pamidronate, Disodium SaltCatalog No.:AA007AEG CAS No.:109552-15-0 MDL No.:MFCD28127040 MF:C3H19NNa2O12P2 MW:369.1095 |
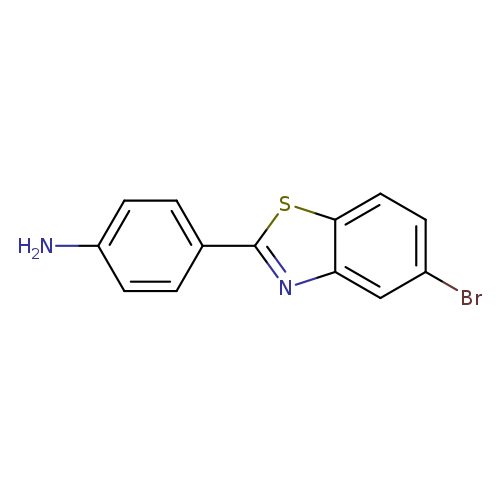
4-(5-Bromo-1,3-benzothiazol-2-yl)anilineCatalog No.:AA019ZVY CAS No.:1095528-03-2 MDL No.:MFCD11636975 MF:C13H9BrN2S MW:305.1930 |
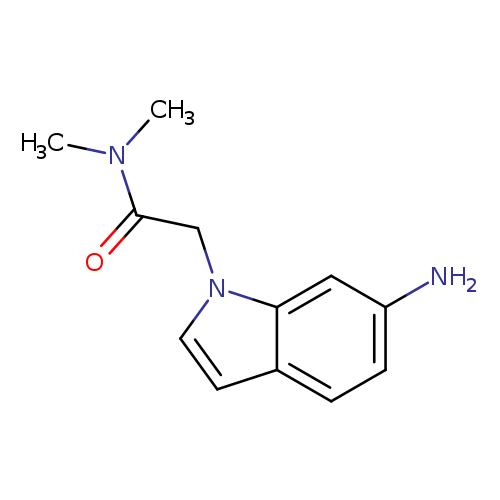
2-(6-amino-1H-indol-1-yl)-N,N-dimethylacetamideCatalog No.:AA019Q44 CAS No.:1095532-40-3 MDL No.:MFCD11638194 MF:C12H15N3O MW:217.2670 |
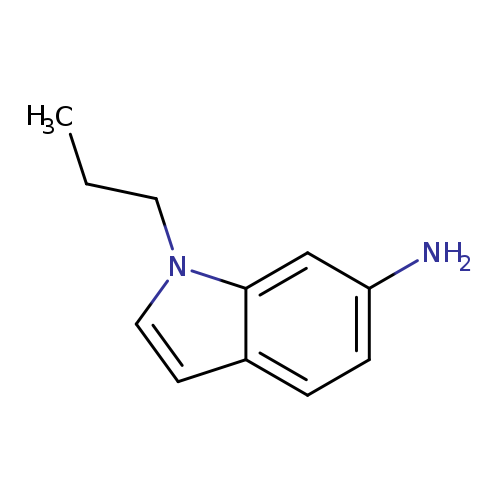
1-propyl-1H-indol-6-amineCatalog No.:AA01AJYX CAS No.:1095532-43-6 MDL No.:MFCD11638202 MF:C11H14N2 MW:174.2423 |
6-(Methylsulfonyl)-2-piperazin-1-yl-1,3-benzothiazoleCatalog No.:AA01ARJU CAS No.:1095538-90-1 MDL No.:MFCD11986741 MF:C12H15N3O2S2 MW:297.3964 |
6-bromo-1,3-dimethylindazoleCatalog No.:AA0095ZB CAS No.:1095539-84-6 MDL No.:MFCD12028626 MF:C9H9BrN2 MW:225.0852 |
1-Bromo-2-fluoro-4,5-dimethoxybenzeneCatalog No.:AA0095XF CAS No.:1095544-81-2 MDL No.:MFCD17237566 MF:C8H8BrFO2 MW:235.0503 |
4-Bromo-1-methanesulfonyl-2-methoxybenzeneCatalog No.:AA00945H CAS No.:1095544-87-8 MDL No.:MFCD22586698 MF:C8H9BrO3S MW:265.1243 |
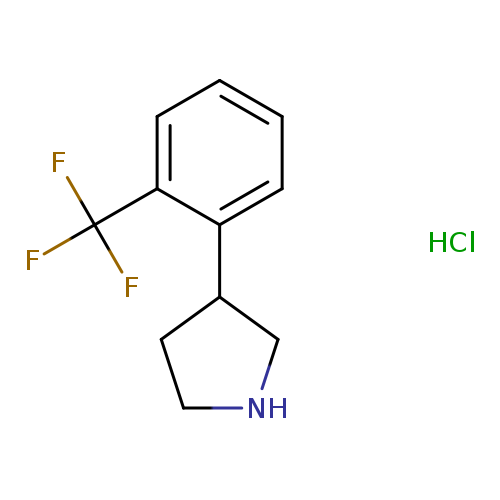
3-(2-(TRIFLUOROMETHYL)PHENYL)PYRROLIDINE HYDROCHLORIDECatalog No.:AA008RML CAS No.:1095545-09-7 MDL No.:MFCD03701256 MF:C11H13ClF3N MW:251.6758 |
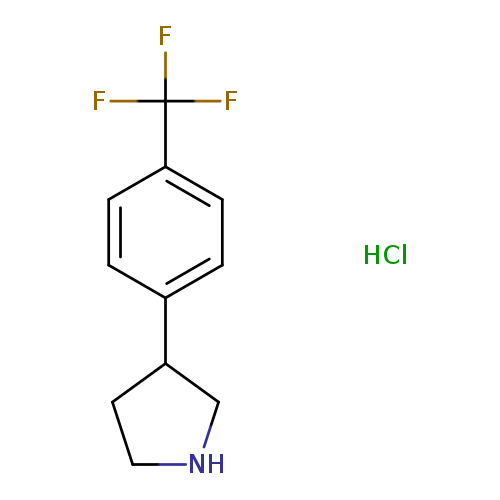
3-[4-(Trifluoromethyl)phenyl]pyrrolidine hydrochlorideCatalog No.:AA008RMU CAS No.:1095545-12-2 MDL No.:MFCD03701254 MF:C11H13ClF3N MW:251.6758 |
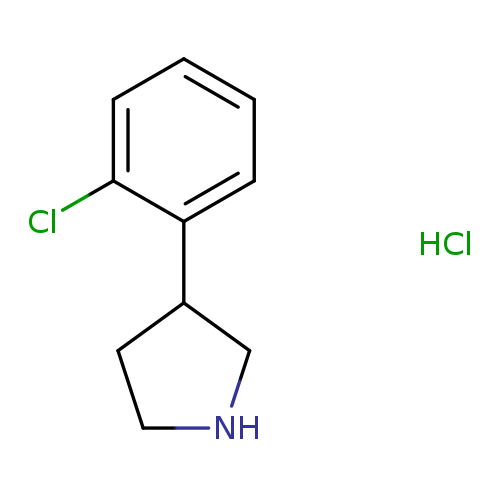
3-(2-Chlorophenyl)pyrrolidine, HClCatalog No.:AA008RMM CAS No.:1095545-14-4 MDL No.:MFCD03094760 MF:C10H13Cl2N MW:218.1229 |
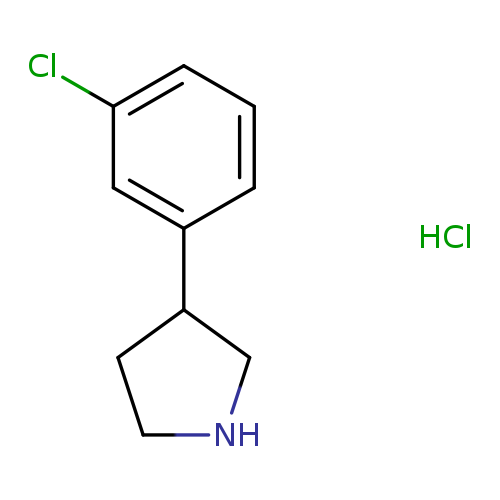
3-(3-Chlorophenyl)pyrrolidine hydrochlorideCatalog No.:AA008RMN CAS No.:1095545-16-6 MDL No.:MFCD03094759 MF:C10H13Cl2N MW:218.1229 |
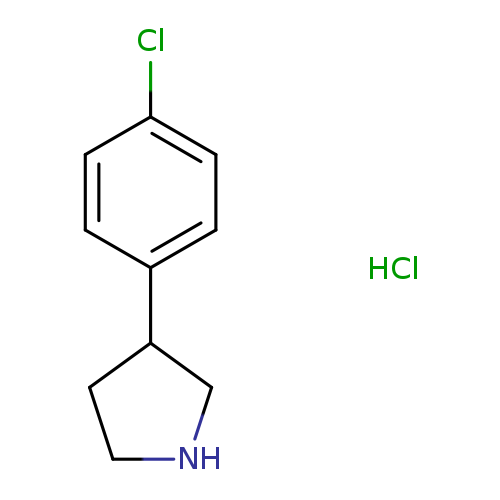
3-(4-chlorophenyl)pyrrolidine hydrochlorideCatalog No.:AA01AHCI CAS No.:1095545-18-8 MDL No.:MFCD03094758 MF:C10H13Cl2N MW:218.1229 |
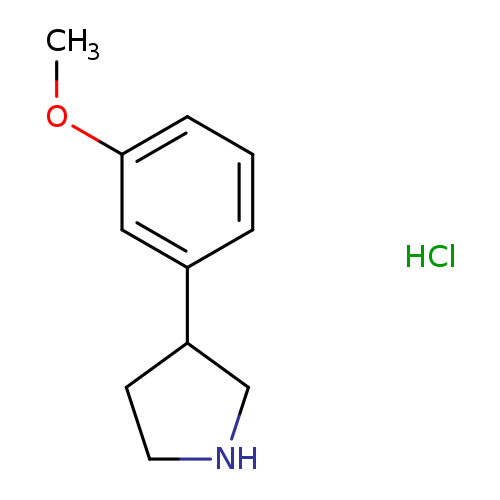
3-(3-Methoxyphenyl)pyrrolidine hydrochlorideCatalog No.:AA008RMQ CAS No.:1095545-66-6 MDL No.:MFCD03701257 MF:C11H16ClNO MW:213.7038 |
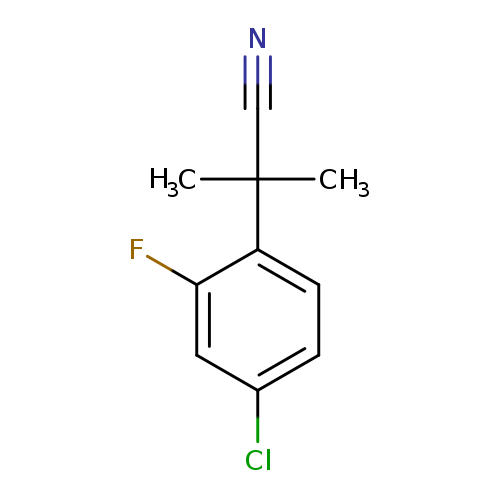
2-(4-chloro-2-fluorophenyl)-2-methylpropanenitrileCatalog No.:AA01BCN0 CAS No.:1095546-56-7 MDL No.:MFCD11036560 MF:C10H9ClFN MW:197.6366 |
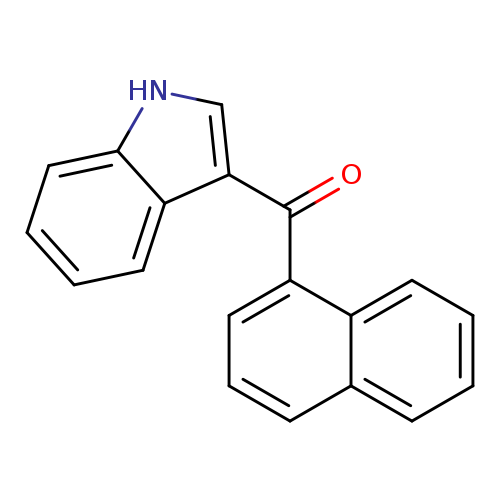
3-(1-Naphthoyl)indoleCatalog No.:AA008265 CAS No.:109555-87-5 MDL No.:MFCD12923093 MF:C19H13NO MW:271.3126 |
7-chloro-2-[4-(chloromethyl)phenyl]-1,3-benzothiazoleCatalog No.:AA019U03 CAS No.:1095568-30-1 MDL No.:MFCD11535106 MF:C14H9Cl2NS MW:294.1990 |
1-(2-methoxyethyl)-1H-indol-6-amineCatalog No.:AA01A3A3 CAS No.:1095573-80-0 MDL No.:MFCD11638170 MF:C11H14N2O MW:190.2417 |
5-{[1,1'-biphenyl]-4-yl}-1,2-oxazole-3-carboxylic acidCatalog No.:AA00JW15 CAS No.:109558-73-8 MDL No.:MFCD07377150 MF:C16H11NO3 MW:265.2634 |
Dehydrocurvularin, 10,11-Catalog No.:AA0094B1 CAS No.:1095588-70-7 MDL No.:MFCD09752720 MF:C16H18O5 MW:290.3111 |
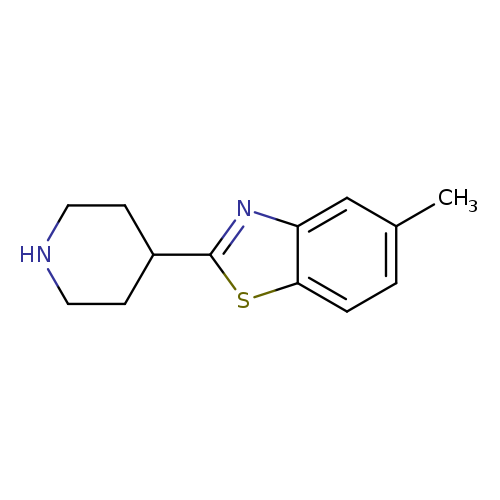
5-methyl-2-(piperidin-4-yl)-1,3-benzothiazoleCatalog No.:AA019WSW CAS No.:1095589-06-2 MDL No.:MFCD11636658 MF:C13H16N2S MW:232.3445 |
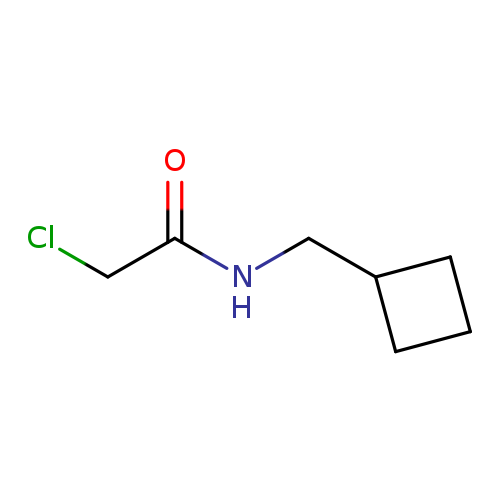
2-chloro-N-(cyclobutylmethyl)acetamideCatalog No.:AA01AGRE CAS No.:1095592-81-6 MDL No.:MFCD11634955 MF:C7H12ClNO MW:161.6293 |
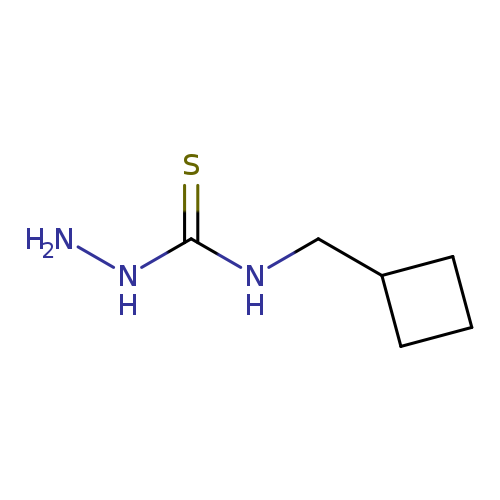
3-Amino-1-(cyclobutylmethyl)thioureaCatalog No.:AA01AA25 CAS No.:1095592-96-3 MDL No.:MFCD11635014 MF:C6H13N3S MW:159.2525 |
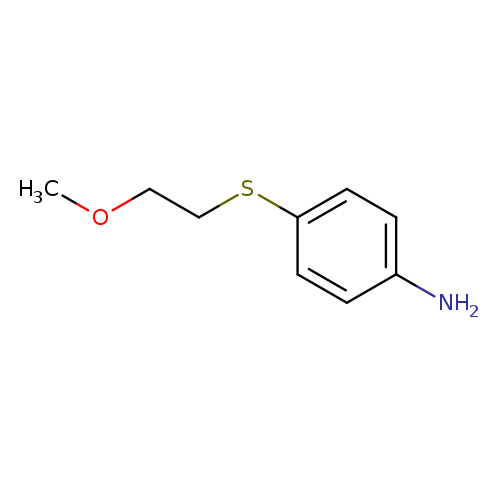
4-[(2-methoxyethyl)sulfanyl]anilineCatalog No.:AA01AHV1 CAS No.:1095599-55-5 MDL No.:MFCD11635172 MF:C9H13NOS MW:183.2706 |
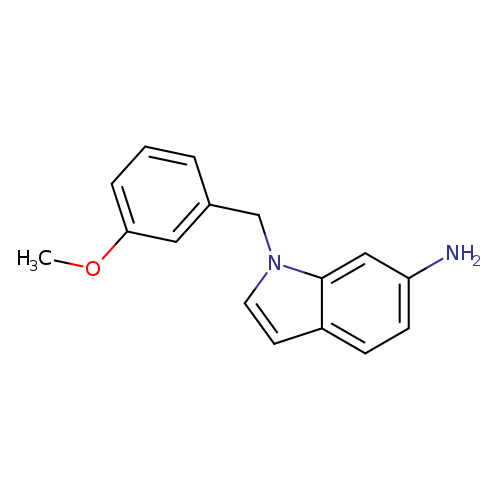
1-[(3-methoxyphenyl)methyl]-1H-indol-6-amineCatalog No.:AA01A3GY CAS No.:1095600-69-3 MDL No.:MFCD12665076 MF:C16H16N2O MW:252.3110 |
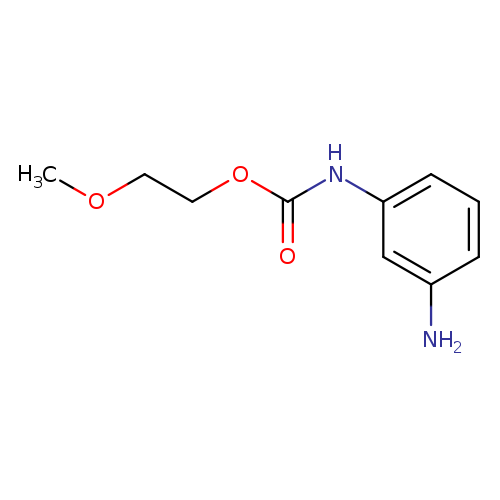
2-methoxyethyl N-(3-aminophenyl)carbamateCatalog No.:AA019XDS CAS No.:1095601-63-0 MDL No.:MFCD11638281 MF:C10H14N2O3 MW:210.2298 |
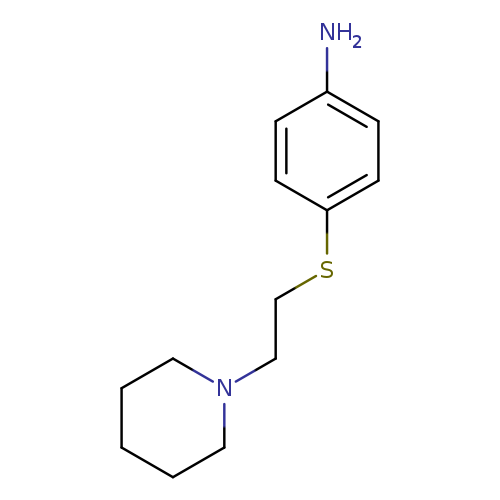
4-{[2-(piperidin-1-yl)ethyl]sulfanyl}anilineCatalog No.:AA01ALC0 CAS No.:1095603-02-3 MDL No.:MFCD11635200 MF:C13H20N2S MW:236.3763 |
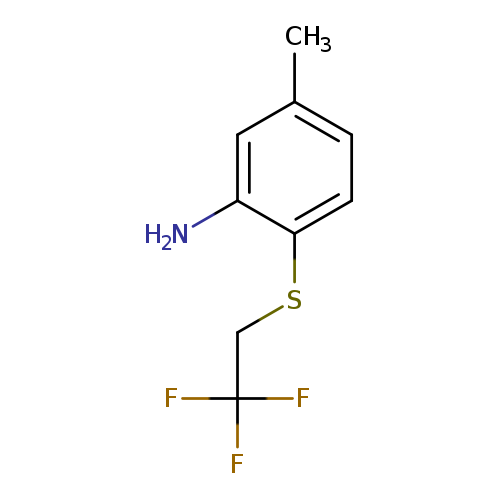
5-methyl-2-[(2,2,2-trifluoroethyl)sulfanyl]anilineCatalog No.:AA01AJO2 CAS No.:1095624-31-9 MDL No.:MFCD11636108 MF:C9H10F3NS MW:221.2426 |
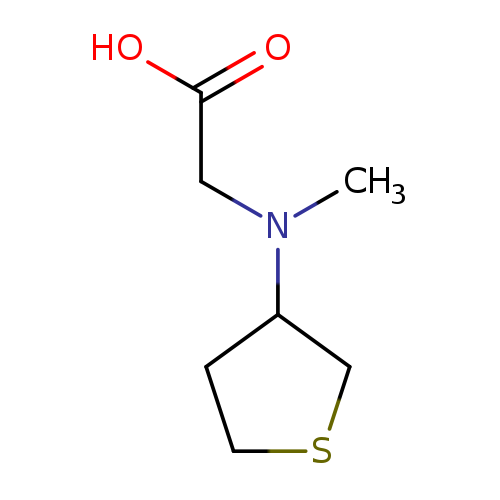
2-[methyl(thiolan-3-yl)amino]acetic acidCatalog No.:AA01A25Q CAS No.:1095626-10-0 MDL No.:MFCD11634233 MF:C7H13NO2S MW:175.2486 |
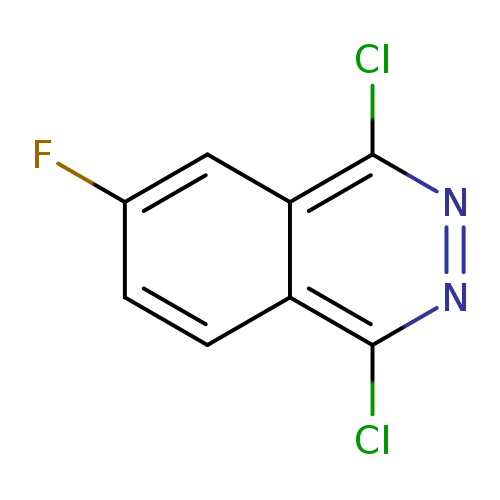
1,4-dichloro-6-fluorophthalazineCatalog No.:AA0096JL CAS No.:1095639-59-0 MDL No.:MFCD16037281 MF:C8H3Cl2FN2 MW:217.0272 |
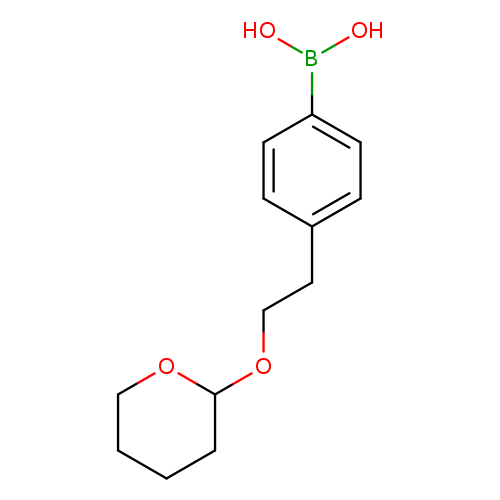
4-(2-O-Thp-hydroxy-ethyl)-phenyl-boronic acidCatalog No.:AA008RYB CAS No.:1095639-99-8 MDL No.:MFCD07369983 MF:C13H19BO4 MW:250.0986 |
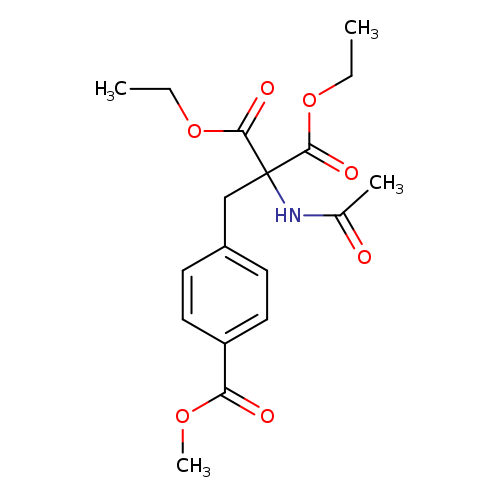
1,3-diethyl 2-acetamido-2-{[4-(methoxycarbonyl)phenyl]methyl}propanedioateCatalog No.:AA00INKJ CAS No.:109564-46-7 MDL No.:MFCD00214821 MF:C18H23NO7 MW:365.3777 |
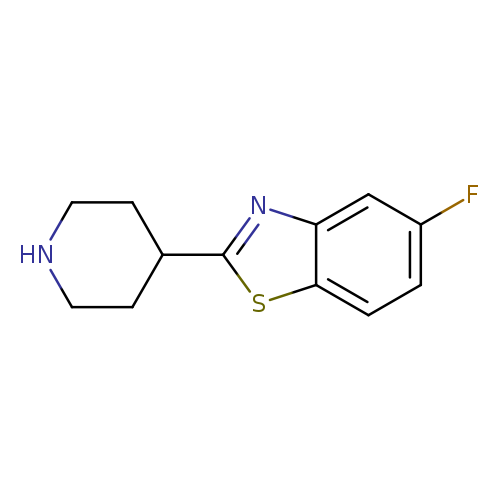
5-fluoro-2-(piperidin-4-yl)-1,3-benzothiazoleCatalog No.:AA019TP0 CAS No.:1095644-94-2 MDL No.:MFCD11636812 MF:C12H13FN2S MW:236.3084 |
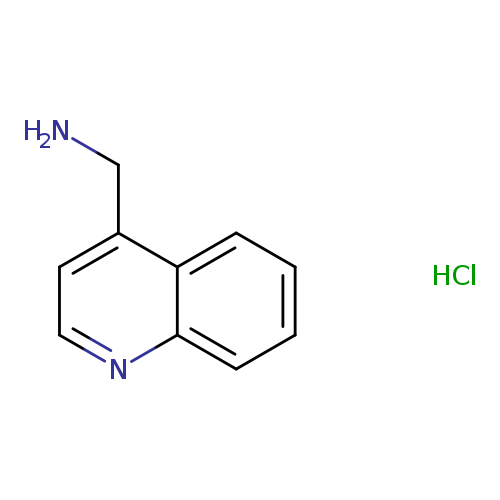
4-Aminomethylquinoline, HClCatalog No.:AA00388S CAS No.:1095661-17-8 MDL No.:MFCD05861486 MF:C10H11ClN2 MW:194.6607 |
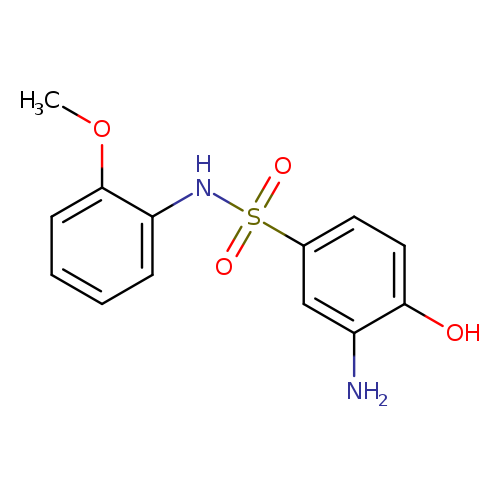
3-Amino-4-hydroxy-n-(2-methoxy-phenyl)-benzenesulfonamideCatalog No.:AA00HBGI CAS No.:109568-85-6 MDL No.:MFCD02704606 MF:C13H14N2O4S MW:294.3263 |
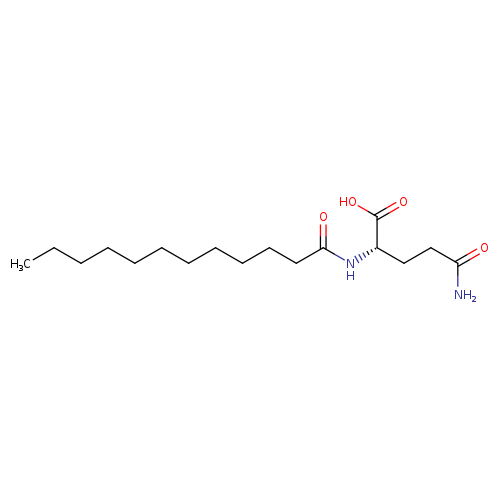
N-ALPHA-LAUROYL-L-GLUTAMINECatalog No.:AA008WI7 CAS No.:109570-04-9 MDL No.:MFCD00216729 MF:C17H32N2O4 MW:328.4470 |
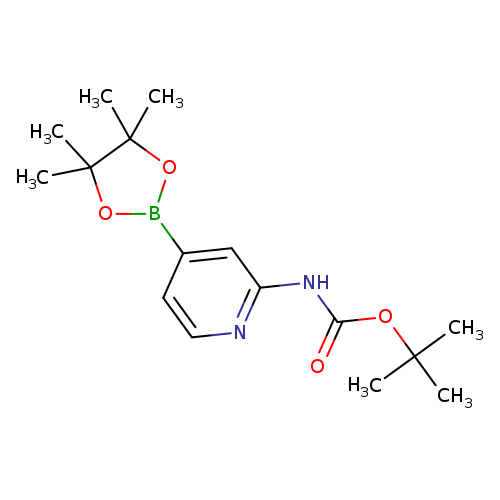
Boc-2-aminopyridine-4-boronic acid pinacol esterCatalog No.:AA008TXJ CAS No.:1095708-32-9 MDL No.:MFCD12032217 MF:C16H25BN2O4 MW:320.1917 |
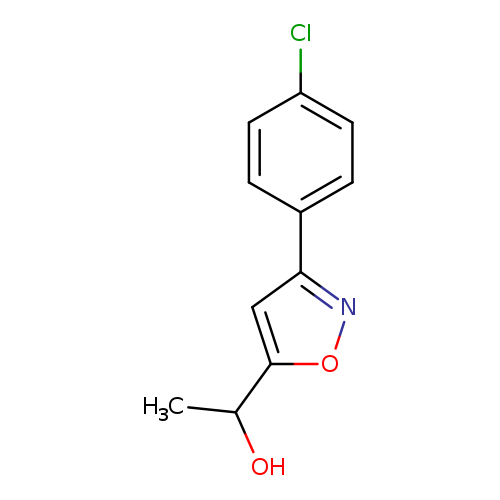
1-(3-(4-Chlorophenyl)isoxazol-5-yl)ethanolCatalog No.:AA0090FU CAS No.:109572-24-9 MDL No.:MFCD03425717 MF:C11H10ClNO2 MW:223.6556 |
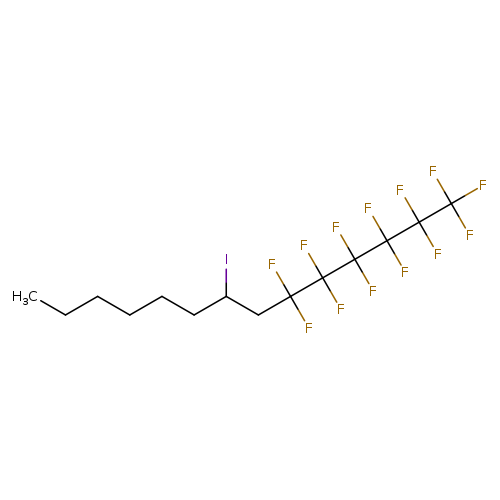
2-Iodo-1-(perfluorohexyl)octaneCatalog No.:AA009TO2 CAS No.:109574-84-7 MDL No.:MFCD16606065 MF:C14H16F13I MW:558.1606 |
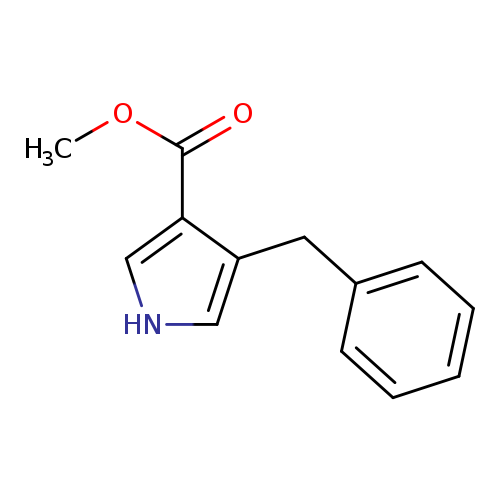
methyl 4-benzyl-1H-pyrrole-3-carboxylateCatalog No.:AA01EINU CAS No.:109578-39-4 MDL No.:MFCD31438850 MF:C13H13NO2 MW:215.2478 |
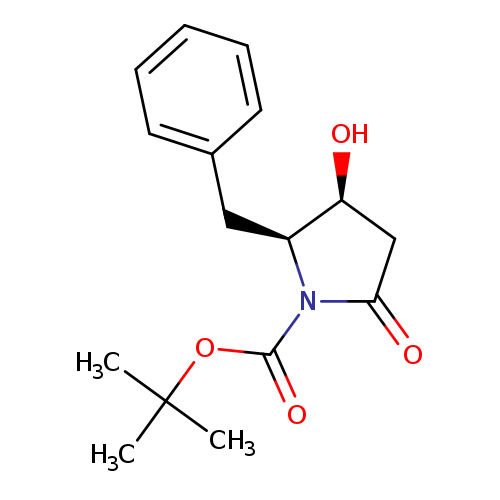
tert-Butyl (2s,3s)-2-benzyl-3-hydroxy-5-oxopyrrolidine-1-carboxylateCatalog No.:AA008V9C CAS No.:109579-10-4 MDL No.:MFCD12026858 MF:C16H21NO4 MW:291.3422 |
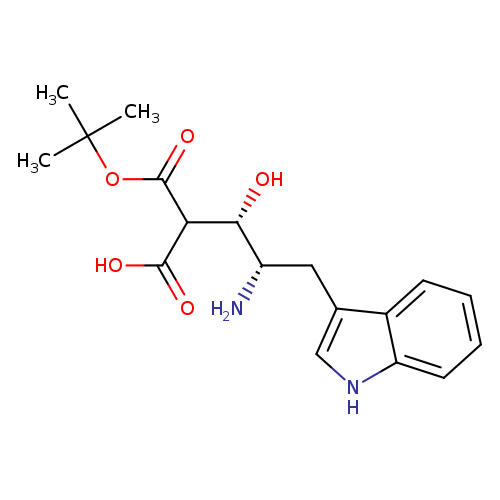
Boc-(3s,4s)-4-amino-3-hydroxy-5-(1h-indol-3-yl)-pentanoic acidCatalog No.:AA008260 CAS No.:109579-23-9 MDL No.:MFCD00800346 MF:C18H24N2O5 MW:348.3936 |
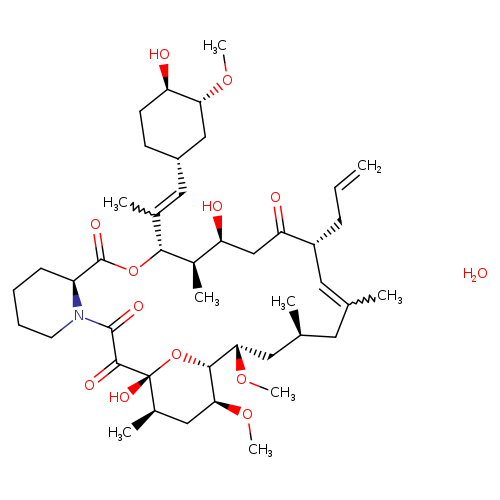
TacrolimusCatalog No.:AA0035JV CAS No.:109581-93-3 MDL No.:MFCD00869853 MF:C44H71NO13 MW:822.0334 |
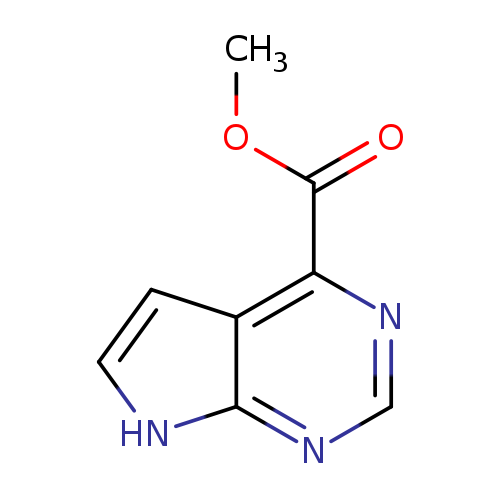
Methyl 7h-pyrrolo[2,3-d]pyrimidine-4-carboxylateCatalog No.:AA0039V5 CAS No.:1095822-17-5 MDL No.:MFCD17015873 MF:C8H7N3O2 MW:177.1601 |
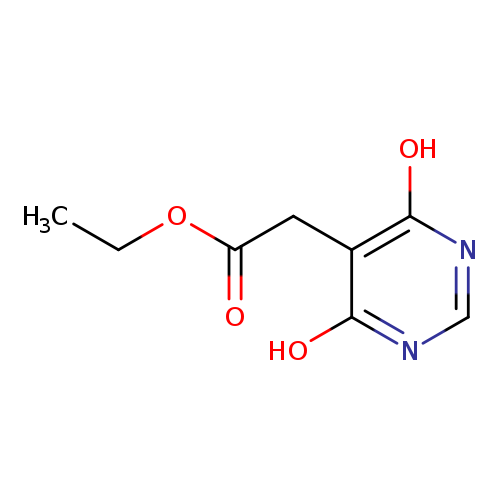
Ethyl 2-(4,6-Dihydroxy-5-pyrimidyl)acetateCatalog No.:AA00HBGL CAS No.:1095822-20-0 MDL No.:MFCD20527459 MF:C8H10N2O4 MW:198.1760 |
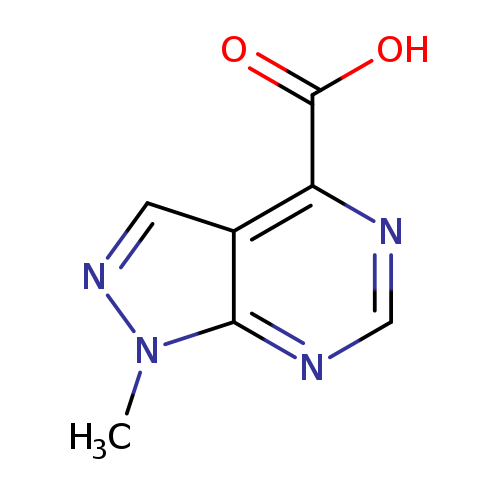
1-Methyl-1h-pyrazolo[3,4-d]pyrimidine-4-carboxylic acidCatalog No.:AA0091EL CAS No.:1095822-30-2 MDL No.:MFCD16619896 MF:C7H6N4O2 MW:178.1481 |
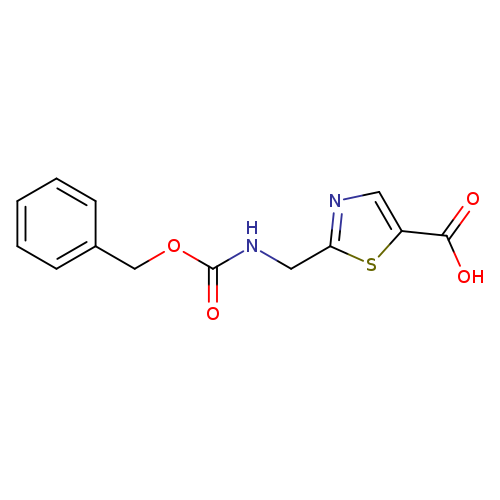
2-[1-(Cbz-amino)methyl]-5-thiazolecarboxylic acidCatalog No.:AA00999Z CAS No.:1095823-37-2 MDL No.:MFCD17214408 MF:C13H12N2O4S MW:292.3104 |
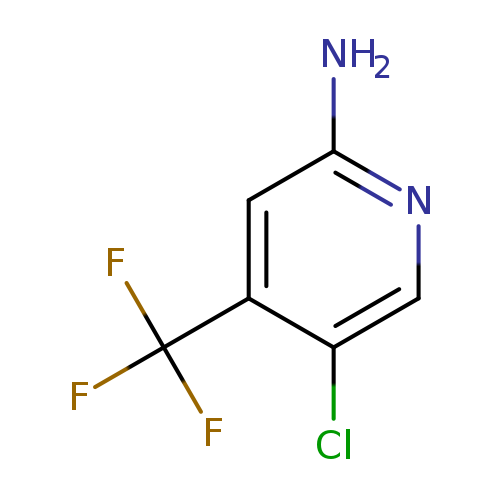
5-Chloro-4-(trifluoromethyl)pyridin-2-amineCatalog No.:AA00825Y CAS No.:1095823-39-4 MDL No.:MFCD18255015 MF:C6H4ClF3N2 MW:196.5576 |
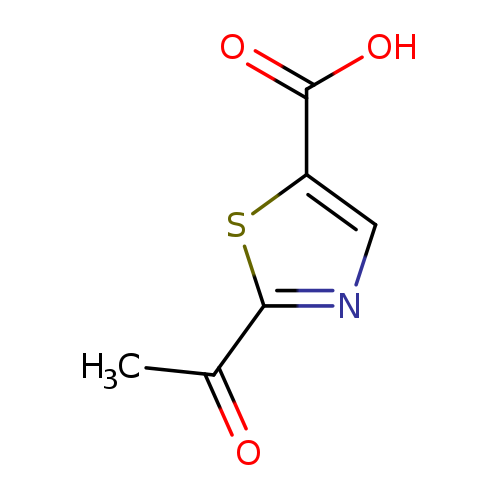
2-Acetylthiazole-5-carboxylic acidCatalog No.:AA008TA5 CAS No.:1095824-76-2 MDL No.:MFCD17169900 MF:C6H5NO3S MW:171.1738 |
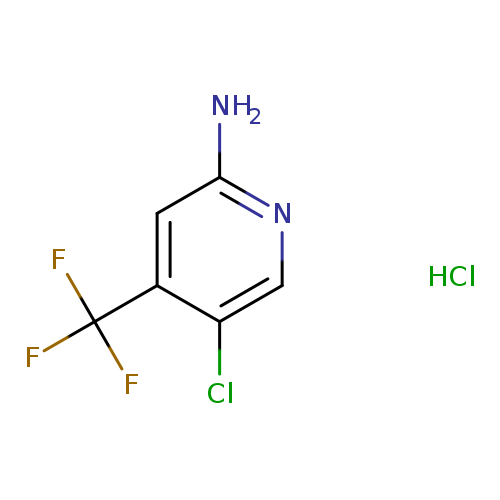
5-Chloro-4-(trifluoromethyl)pyridin-2-amine hydrochlorideCatalog No.:AA0093XE CAS No.:1095824-77-3 MDL No.:MFCD14706708 MF:C6H5Cl2F3N2 MW:233.0185 |

rac-(3R,5S)-1-[(tert-butoxy)carbonyl]-5-methylpiperidine-3-carboxylic acid, cisCatalog No.:AA01EKOW CAS No.:1095906-07-2 MDL No.: MF:C21H21NO4 MW:351.3957 |
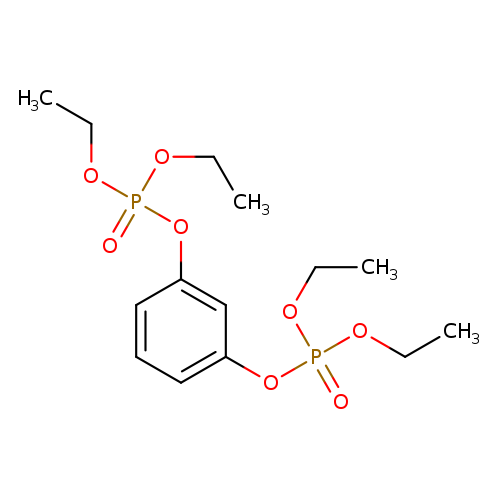
Phosphoric acid 3-(diethoxy-phosphoryloxy)-phenyl ester diethyl esterCatalog No.:AA00HBH2 CAS No.:109592-16-7 MDL No.:MFCD23726618 MF:C14H24O8P2 MW:382.2831 |
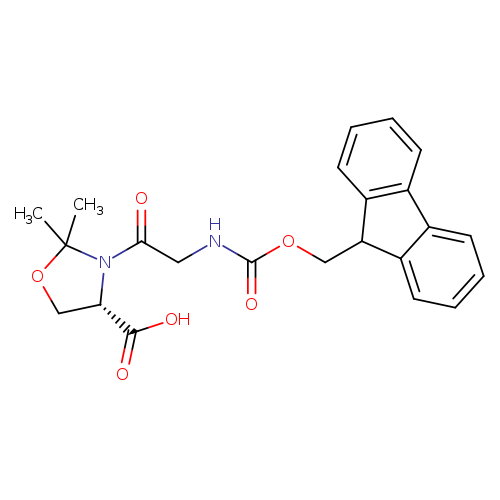
Fmoc-gly-ser(psime,mepro)-ohCatalog No.:AA008T1N CAS No.:1095952-22-9 MDL No.:MFCD18427718 MF:C23H24N2O6 MW:424.4465 |
N-[2-(2-AMINOPHENOXY)ETHYL]-N,N-DIETHYLAMINECatalog No.:AA00822X CAS No.:109598-74-5 MDL No.:MFCD07396661 MF:C12H20N2O MW:208.3000 |
6-chloro-5-nitropyridine-3-carbaldehydeCatalog No.:AA01B2OJ CAS No.:1095990-10-5 MDL No.:MFCD12547260 MF:C6H3ClN2O3 MW:186.5526 |
1-Benzyl-4-(phenylamino)piperidine-4-carboxamideCatalog No.:AA008V8H CAS No.:1096-03-3 MDL No.:MFCD00474726 MF:C19H23N3O MW:309.4054 |
Pregna-4,16-diene-3,20-dioneCatalog No.:AA003854 CAS No.:1096-38-4 MDL No.:MFCD00003651 MF:C21H28O2 MW:312.4458 |
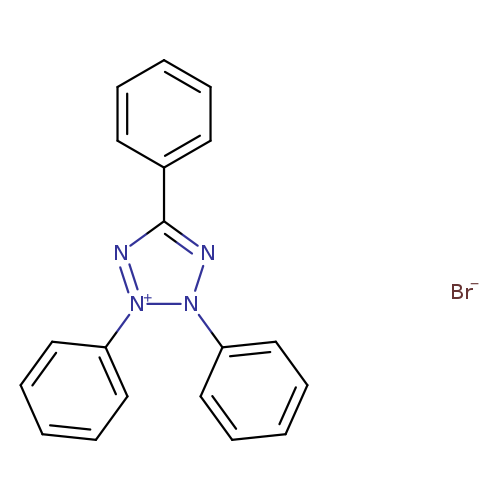
2,3,5-TRIPHENYLTETRAZOLIUM BROMIDECatalog No.:AA003FCU CAS No.:1096-80-6 MDL No.:MFCD00031797 MF:C19H15BrN4 MW:379.2532 |
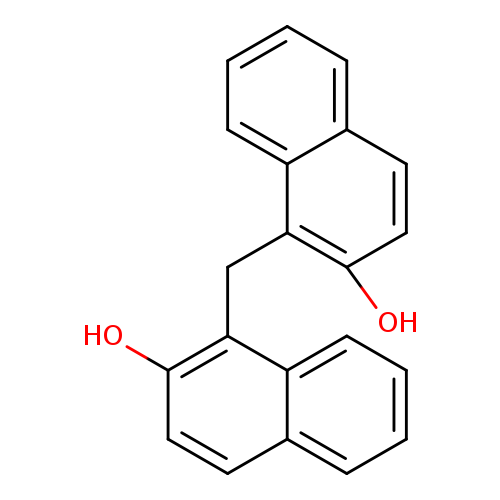
1,1'-Methylenedi-2-naphtholCatalog No.:AA003D6J CAS No.:1096-84-0 MDL No.:MFCD00046470 MF:C21H16O2 MW:300.3505 |
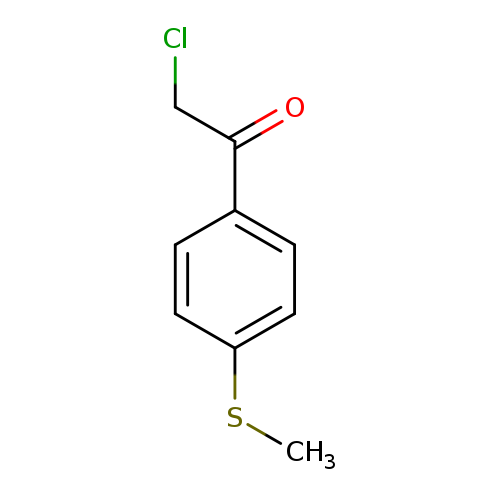
2-Chloro-1-(4-(methylthio)phenyl)ethanoneCatalog No.:AA019JXQ CAS No.:109607-24-1 MDL No.:MFCD06655176 MF:C9H9ClOS MW:200.6852 |
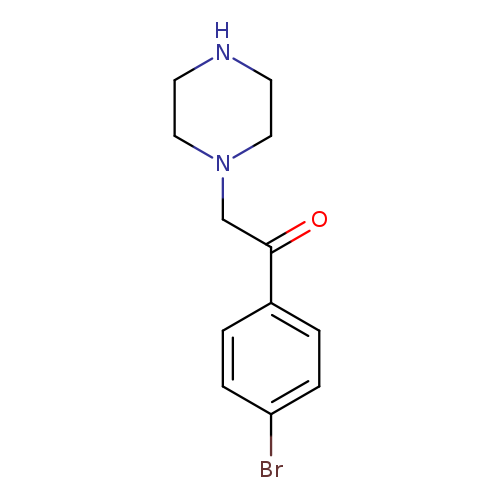
1-(4-Bromophenyl)-2-(piperazin-1-yl)ethanoneCatalog No.:AA007AE2 CAS No.:109607-56-9 MDL No.:MFCD06254940 MF:C12H15BrN2O MW:283.1643 |
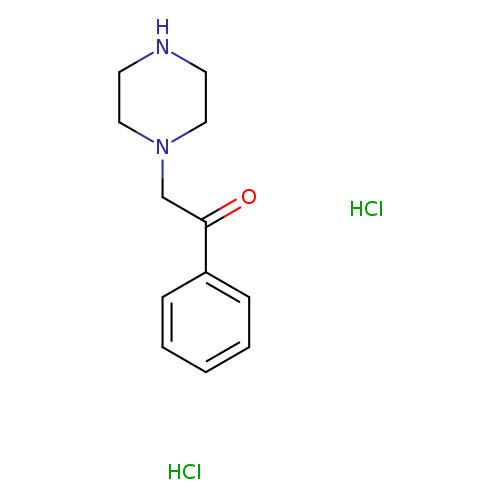
1-phenyl-2-(piperazin-1-yl)ethan-1-one dihydrochlorideCatalog No.:AA019PH4 CAS No.:109608-71-1 MDL No.:MFCD02671139 MF:C12H18Cl2N2O MW:277.1901 |
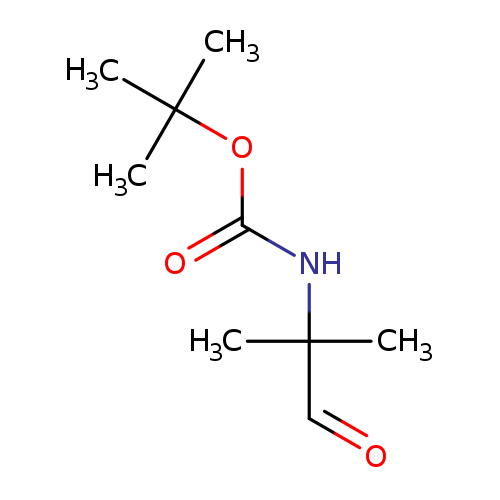
tert-Butyl (1,1-dimethyl-2-oxoethyl)carbamateCatalog No.:AA007SRV CAS No.:109608-77-7 MDL No.:MFCD09751786 MF:C9H17NO3 MW:187.2362 |
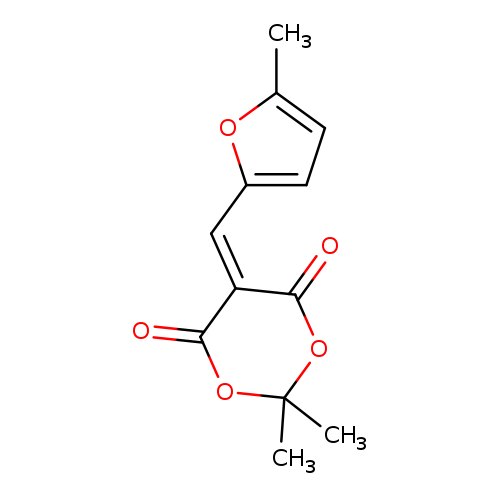
1,3-Dioxane-4,6-dione, 2,2-dimethyl-5-[(5-methyl-2-furanyl)methylene]-Catalog No.:AA0095JY CAS No.:109610-93-7 MDL No.:MFCD00473856 MF:C12H12O5 MW:236.2207 |
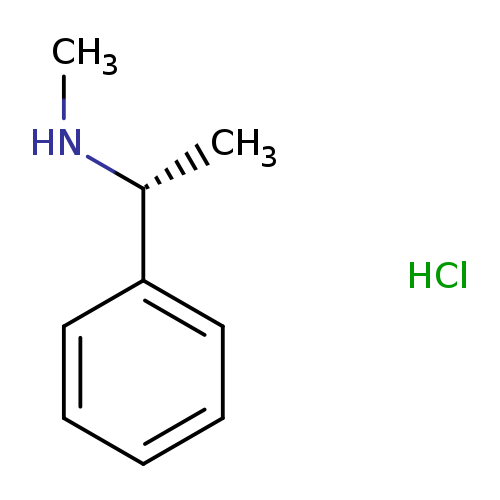
(R)-N-Methyl-1-phenylethanamine hydrochlorideCatalog No.:AA01FSII CAS No.:1096105-18-8 MDL No.:MFCD28138033 MF:C9H14ClN MW:171.6672 |
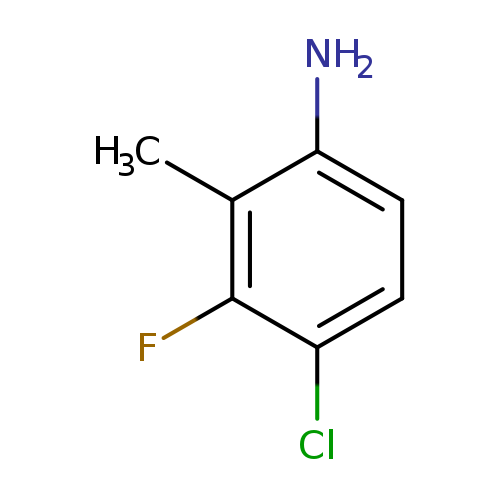
4-Chloro-3-fluoro-2-methylanilineCatalog No.:AA00HBH4 CAS No.:1096113-25-5 MDL No.:MFCD20726431 MF:C7H7ClFN MW:159.5886 |
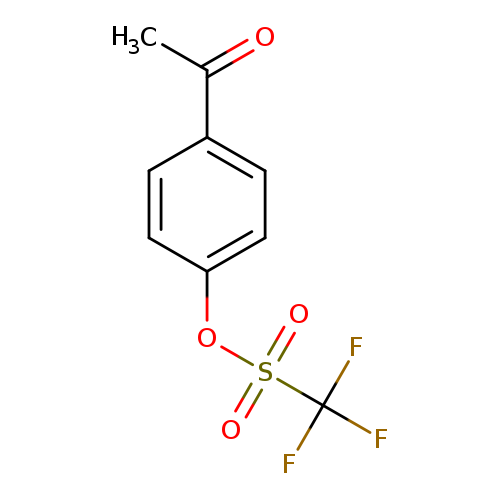
4-Acetylphenyl trifluoromethanesulfonateCatalog No.:AA003KK9 CAS No.:109613-00-5 MDL No.:MFCD00191706 MF:C9H7F3O4S MW:268.2097 |
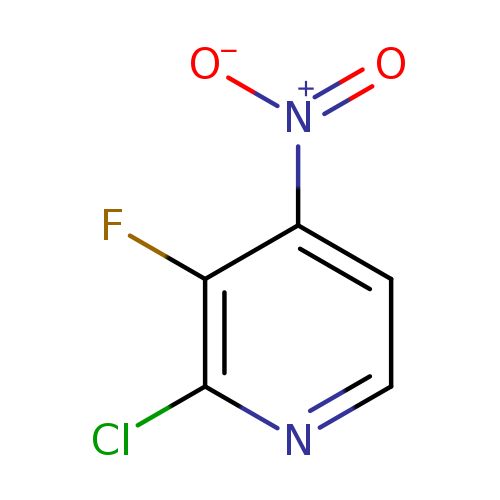
2-Chloro-3-fluoro-4-nitropyridineCatalog No.:AA00822Q CAS No.:109613-90-3 MDL No.:MFCD16251524 MF:C5H2ClFN2O2 MW:176.5330 |
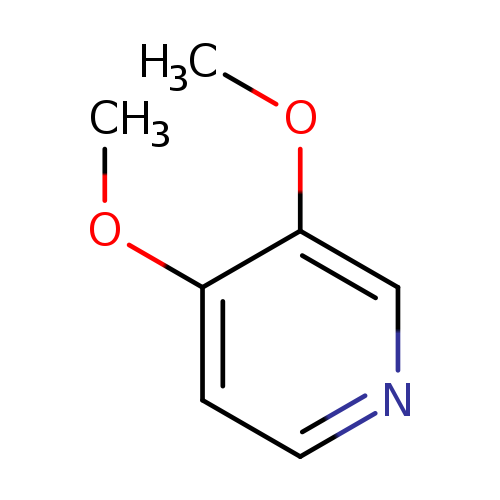
3,4-dimethoxypyridineCatalog No.:AA008Z03 CAS No.:109613-93-6 MDL No.:MFCD04037427 MF:C7H9NO2 MW:139.1519 |
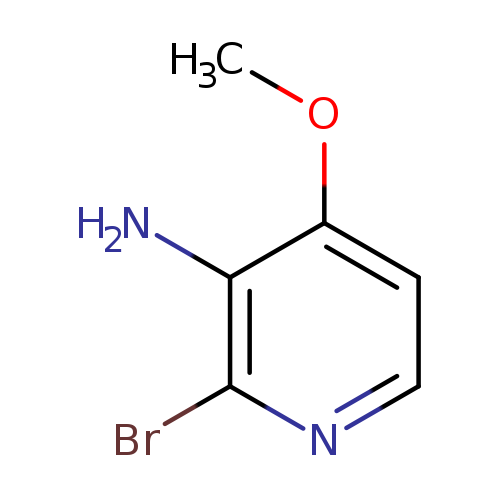
2-Bromo-4-methoxypyridin-3-amineCatalog No.:AA00822P CAS No.:109613-97-0 MDL No.:MFCD11044243 MF:C6H7BrN2O MW:203.0366 |
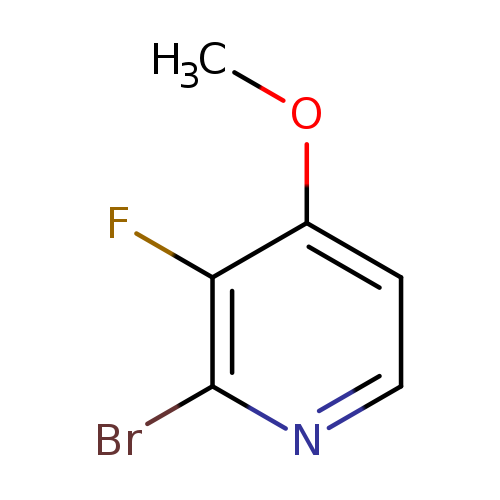
2-bromo-3-fluoro-4-methoxypyridineCatalog No.:AA008UOO CAS No.:109613-98-1 MDL No.:MFCD18257613 MF:C6H5BrFNO MW:206.0124 |
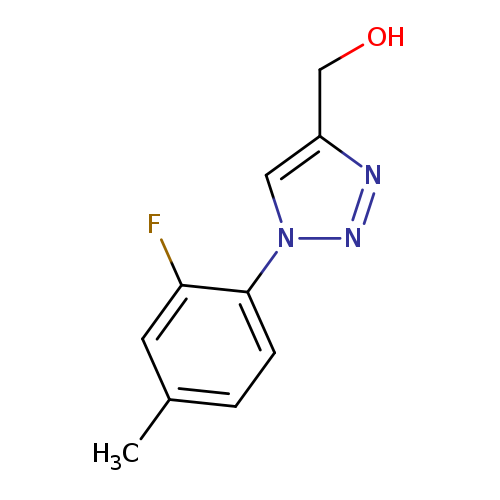
"[1-(2-fluoro-4-methylphenyl)-1H-1,2,3-triazol-4-yl]methanol"Catalog No.:AA01FM6F CAS No.:1096130-69-6 MDL No.:MFCD16747696 MF:C10H10FN3O MW:207.2043 |