2020-02-15 18:12:01
Daggupati V. Ramana, Karu Sudheer Kumar, Ealeswarapu Srujana, and Malapaka Chandrasekharam
Introduction
Ester, amide and amino groups are widely present in bioactive molecules and their incorporation into an organic molecule warrants multistep synthesis sequence. However, functionaliza- tion of inexpensive glycine ester or amide would be an effective strategy and represent important building blocks for drug de- velopment and natural product synthesis. Alkylation of arenes and heteroarenes is a challenging task in organic chemistry and Friedel-Crafts reaction is known to be the most suitable choice.[1] Traditionally alkyl halides and alcohols have been used as alkylating agents and particularly benzyl halides, benzyl alcohols and styrenes are suitable electrophiles for the genera- tion of 1,1-diarylalkanes. New developments in the Friedel- Crafts alkylation and their efficacy in aqueous media have been recently reviewed.[2] Double Friedel-Crafts alkylation of indoles with non-symmetrical divinyl ketones was achieved for the syn- thesis of complex tricyclic indoles.[3] Though double Friedel- Crafts alkylation provides easy entry to gem-diaryl alkyl com- pounds,[4] reports on glycine esters as alkylating agents in Frie- del-Crafts reactions are not very common. Several reports ap- peared on the α-C-H functionalization of glycine esters/amides using various nucleophiles in presence of metal or metal-free catalysts in organic solvents.[5] Congde Huo reported copper- catalyzed oxidative arylation of glycine derivatives with indoles in THF/H2O.[6] The same group also reported a very interesting triarylaminium salt-promoted synthesis of bisindolyl acetic esters from glycine ester derivatives where the scope of the nucleophile is limited to indole and industrially unacceptable DCM solvent was used.[7]
We have previously demonstrated the copper-catalyzed α- arylation,[8] amidation/imidation[9] of N-arylglycine esters under mild conditions. Tetrahydroquinolines (THQs) belong to a class of heterocycles of high pharmacological value and in general organic reactions in water as solvent attracted tremendous in- terest over other organic solvents due to its green and environ- mentally benign nature.[10] Therefore an efficient method for the synthesis of α-diaryl alkyl compounds from aryl/heteroaryl nucleophiles promoted by green solvents is highly warranted. In continuation of our interest in C-H functionalizations involv- ing the most abundant, less expensive, non-toxic iron and cop- per catalysts and water-mediated reactions,[11] we herein report a double Friedel-Crafts alkylation reaction of glycine ester on heteroaryl (tetrahydroquinolines, 2-meTHQ and indoles) and aryl (N-methyl aniline) nucleophiles to generate gem-diaryl/het- eroarylacetic esters with less expensive, non-toxic copper cata- lyst and environmentally benign aqueous conditions.
Results and Discussion
Methyl 2-(p-tolylamino)acetate 1a and tetrahydroquinoline 2a were chosen as model substrates and the reaction was con- ducted in the presence of 10 mol-% CuCl in DCE solvent at room temperature for 24 h under open flask conditions, antici- pating the formation of tetrahydroquinolinyl glycine ester deriv- ative resulting from α-C-H functionalization. However the formation of methyl 2,2-bis(1,2,3,4-tetrahydroquinolin-6-yl)- acetate 3aa (10 %) was observed along with the expected prod- uct (R)-methyl 2-(1,2,3,4-tetrahydroquinolin-6-yl)-2-(p-tolyl- amino)acetate 3aa (25 %). This result instigated us to optimize the conditions for the unexpected double Friedel-Crafts alkyl- ation product 3aa (Table 1). Various solvents were tested for this reaction and water was found to be the most suitable sol- vent for the predominant formation of the double Friedel-Crafts alkylation product 3aa (entries 2–11, Table 1). The solvents DCE, ACN, DCM, THF and toluene at room temperature favored the formation of 3aa, whereas the reaction conducted at 100 °C in toluene solvent resulted in the exclusive formation of 3aa in 23 %. While in MeOH, the selectivity and the overall yields were not encouraging, the reaction is not efficient in p-xylene solvent even at elevated (120° C) reaction temperature. The reaction is not successful with the solvents, DMF and DMSO, and the start- ing materials remained unreacted even at 120 °C. Among the catalysts CuI, CuII and FeCl3 employed for the reaction, CuI pro- duced an improved yield of 3aa (entries 12–17, Table 1). The conditions were then optimized for the reaction temperature, time and the catalyst loading under water solvent and CuI cata- lyst conditions (entries 18–23, Table 1). In the absence of a cata- lyst, the reaction does not proceed (entry 24, Table 1). Under the optimized reaction conditions, stirring the reaction mixture with 10 mol-% of CuI catalyst in water solvent at 100 °C for 24 h, the desired product 3aa was obtained in 85 % yield.
With the optimised conditions in hand, we examined the substrate scope for the double Friedel-Crafts alkylation reaction of glycine esters/amide with different nucleophiles i.e., 2- meTHQ, substituted indoles and N-methyl aniline to generate diverse gem-di-aryl/-heteroarylacetic esters/amide. Different N- arylglycine ester derivatives (R = Me, Et, nBu, iPr, tBu and Bn) were treated with THQ under the optimised reaction conditions to produce the corresponding desired products in excellent yields (3aa–3fa, Table 2). The scope of the nucleophile was suc- cessfully extended to 2-meTHQ, N-methyl aniline and 1-, 5- and 6,7-disubstituted indole derivatives. The copper-catalyzed reac- tion proceeds smoothly with 2-meTHQ and N-arylglycine ester 1a resulting in the formation of the desired product 3ab in 65 % yield (Table 2). N-methyl aniline was treated with different N-arylglycine esters (R = Me, Bn) and the reactions proceeded well to afford the corresponding desired products in good yields (3ac, 3fc, Table 2). Various substituted indoles employed as nucleophiles, for the double Friedel-Crafts alkylation reaction protocol with N-arylglycine ester 1a, delivered the correspond- ing products in good to excellent yields (3ad–3ag, Table 2). Under the optimized reaction conditions, we also observed the formation of α-C-H functionalization products in some of the cases, in very low yields.
The double Friedel-Crafts alkylation reaction protocol also works for the glycine amide derivative, 1-(pyrrolidin-1-yl)-2-(p- tolylamino) ethanone using 2-meTHQ or indole as nucleophiles, and delivered the corresponding alkylated products in very good yields (Scheme 1).
To understand the mechanism for the double Friedel-Crafts alkylation reaction, we carried out the following experiments as depicted in the Scheme 2. When the reaction was carried out in the presence of radical scavenger BHT (20 mol-%) under the standard conditions, the absence of the formation of the dou- ble Friedel-Crafts product and α-C-H functionalization product indicates that the reaction follows a radical-mediated path. The reaction did not proceed in the absence of oxygen implying that molecular oxygen is essential for the reaction to proceed. The intermediacy of the α-C-H functionalization is confirmed by the formation of the double Friedel-Crafts alkylated product in 58 % yield from the reaction of 3aa (0.5 mmol) with THQ (1.0 mmol).
Based on previous literature reports and our recent investiga- tions on CuI-catalyzed α-C-H functionalizations adjacent to the nitrogen atom, we proposed a plausible mechanism for this double Friedel-Crafts alkylation reaction as shown in the Scheme 3. Initially, CuI in presence of atmospheric oxygen gen- erates CuIIOO radical, which might abstract hydrogen radical from glycine derivative 1 delivering radical intermediate A, fur- ther interconverts to the iminium intermediate B. Nucleophile attack on intermediate B resulted in the α-C-H functionalization product 3. Then a second oxidation occurred on 3 compound to form another iminium intermediate C, followed by nucleo- phile addition resulted in the intermediate D. Finally, the double Friedel-Crafts alkylated product produced from the intermedi- ate D, by the loss of aniline.
Conclusions
In conclusion, we have successfully demonstrated a double Frie- del-Crafts reaction protocol of N-arylglycine esters/amide with heteroaryl (tetrahydroquinolines, 2-meTHQ and indoles) and aryl (N-methyl aniline) nucleophiles. Under the reaction condi- tions both the α-C-H functionalization and gem-diaryl/hetero- arylacetic esters/amide could be generated, however in the present investigation, the reaction conditions are optimized for double Friedel-Crafts products in excellent yields. The reaction is carried out with a less expensive, non-toxic copper catalyst in aqueous and open flask conditions. A single electron transfer (SET) mechanism was proposed for the reaction.
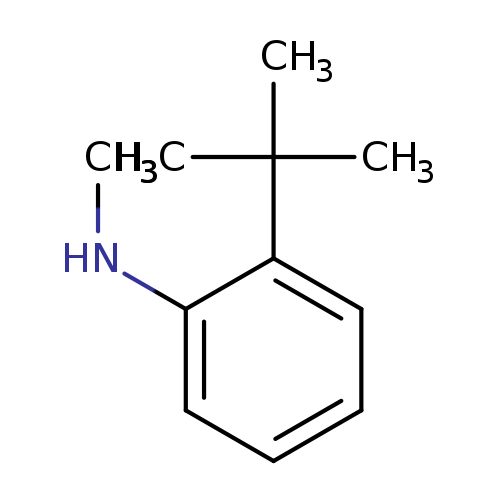
2-tert-Butyl-n-methylanilineCatalog No.:AA0096WP CAS No.:109932-97-0 MDL No.:MFCD11934511 MF:C11H17N MW:163.2594 |
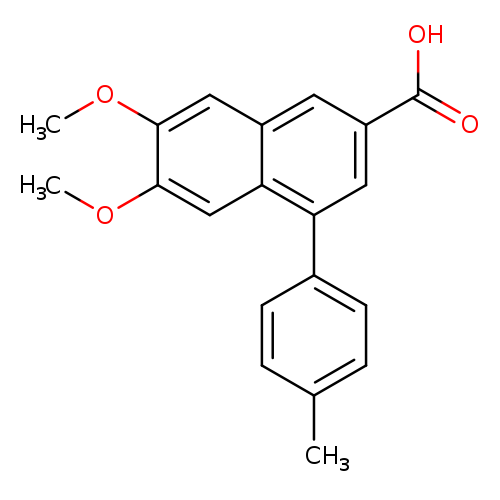
6,7-Dimethoxy-4-(4-methylphenyl)naphthalene-2-carboxylic acidCatalog No.:AA00HBKE CAS No.:109933-59-7 MDL No.:MFCD03212115 MF:C20H18O4 MW:322.3545 |
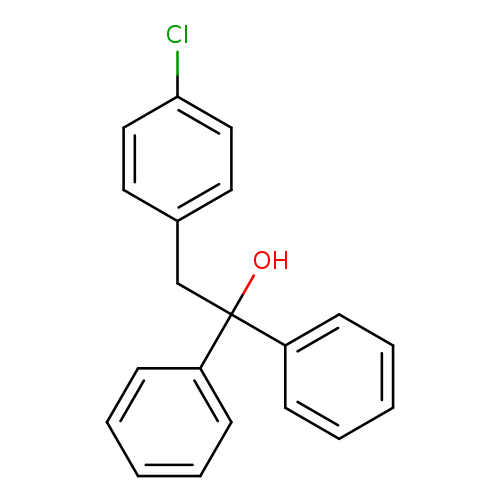
2-(4-Chlorophenyl)-1,1-diphenylethanolCatalog No.:AA0084L3 CAS No.:109936-21-2 MDL No.:MFCD00017507 MF:C20H17ClO MW:308.8014 |
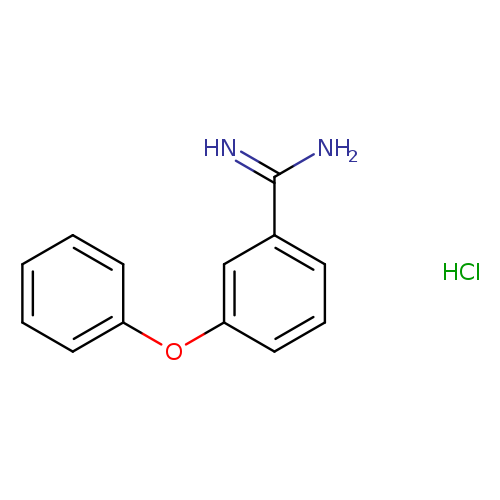
3-phenoxybenzene-1-carboximidamide hydrochlorideCatalog No.:AA01A7GI CAS No.:109941-13-1 MDL No.:MFCD14705700 MF:C13H13ClN2O MW:248.7081 |
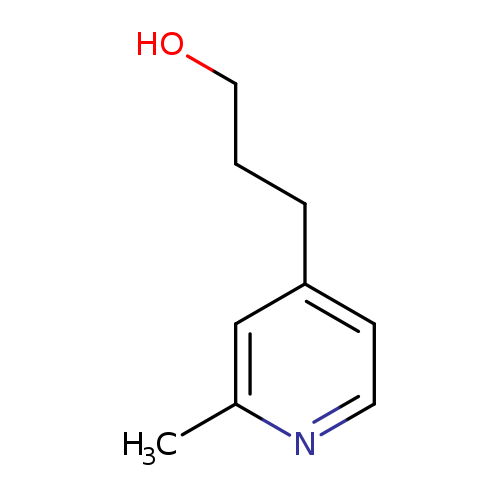
3-(2-Methylpyridin-4-yl)propan-1-olCatalog No.:AA00NZAV CAS No.:109942-70-3 MDL No.:MFCD06637505 MF:C9H13NO MW:151.2056 |
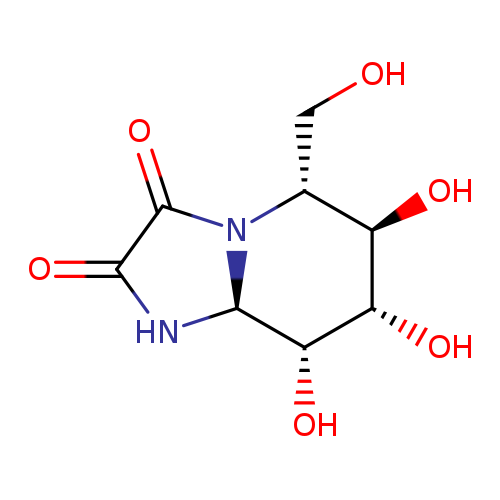
Imidazo[1,2-a]pyridine-2,3-dione,hexahydro-6,7,8-trihydroxy-5-(hydroxymethyl)-, (5R,6R,7S,8R,8aS)-Catalog No.:AA01CBZ9 CAS No.:109944-15-2 MDL No.:MFCD00270017 MF:C8H12N2O6 MW:232.1907 |
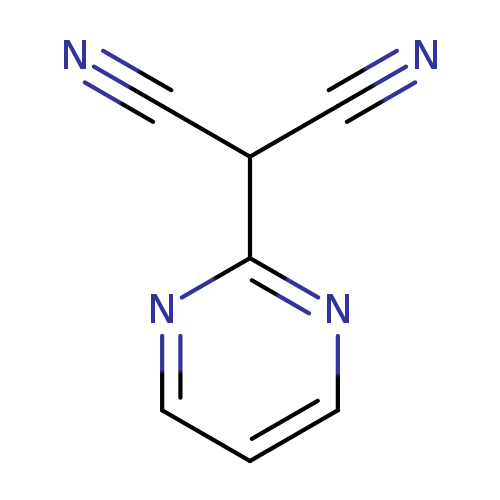
2-(Pyrimidin-2-yl)malononitrileCatalog No.:AA01B7Z4 CAS No.:1099471-49-4 MDL No.:MFCD28145608 MF:C7H4N4 MW:144.1335 |
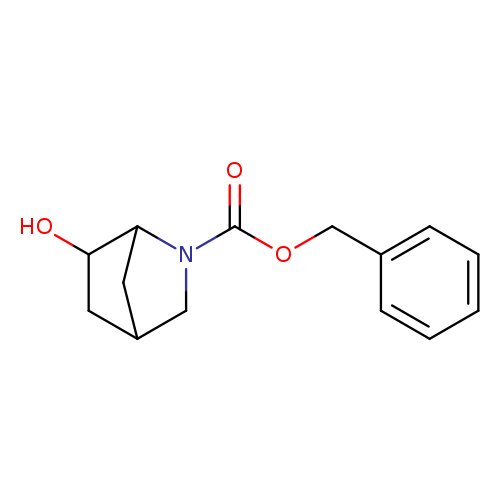
(1S,4S,6R)-tert-Butyl 6-hydroxy-2-azabicyclo[2.2.1]heptane-2-carboxylateCatalog No.:AA0096TJ CAS No.:1099570-25-8 MDL No.:MFCD30480541 MF:C14H17NO3 MW:247.2897 |
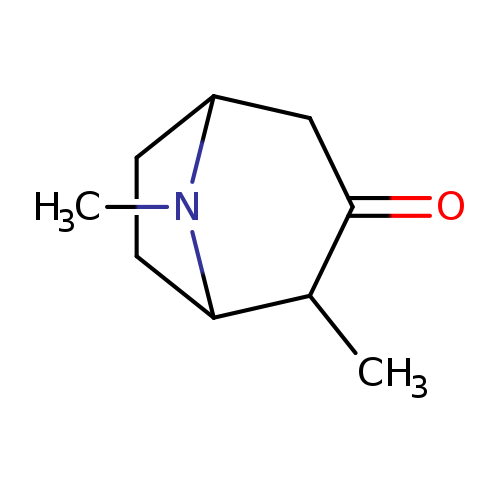
2,8-dimethyl-8-azabicyclo[3.2.1]octan-3-oneCatalog No.:AA01BRTN CAS No.:1099571-64-8 MDL No.:MFCD24738374 MF:C9H15NO MW:153.2215 |
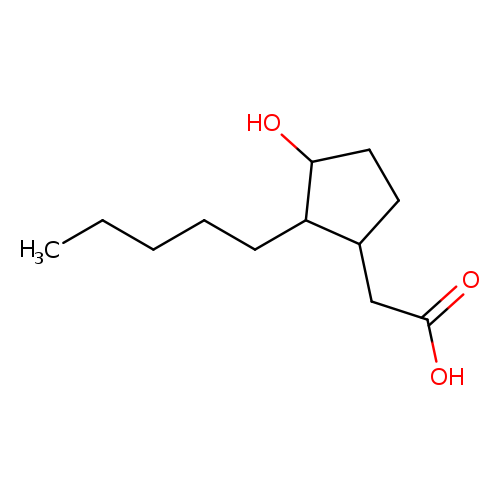
(3-Hydroxy-2-pentylcyclopentyl)acetic acidCatalog No.:AA00HBKJ CAS No.:109959-44-6 MDL No.:MFCD24483167 MF:C12H22O3 MW:214.3013 |
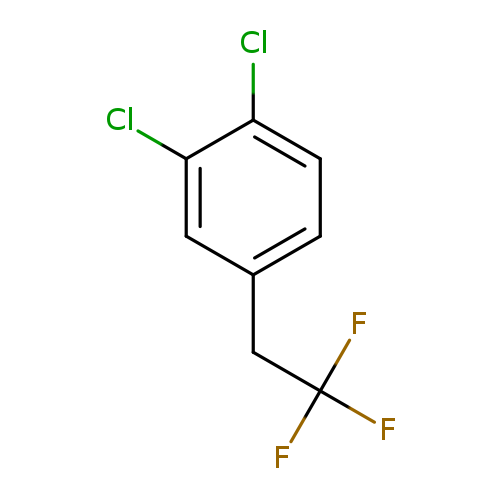
1,2-Dichloro-4-(2,2,2-trifluoroethyl)benzeneCatalog No.:AA01EQNV CAS No.:1099597-16-6 MDL No.:MFCD11226582 MF:C8H5Cl2F3 MW:229.0265 |
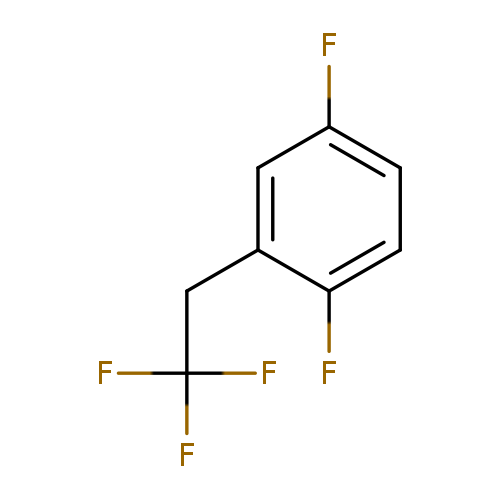
1,4-difluoro-2-(2,2,2-trifluoroethyl)benzeneCatalog No.:AA00MYZR CAS No.:1099597-19-9 MDL No.:MFCD11226583 MF:C8H5F5 MW:196.1173 |
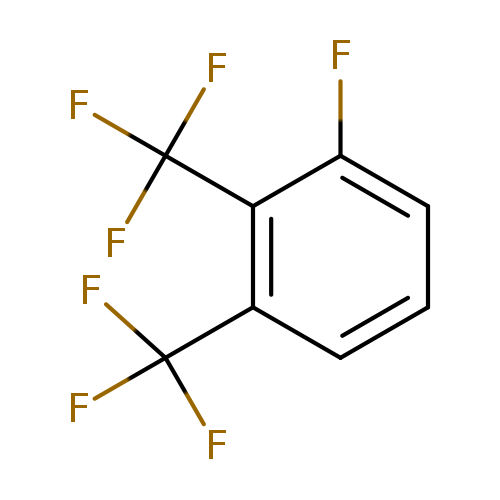
1-Fluoro-2,3-bis-(trifluoromethyl)benzeneCatalog No.:AA01FCST CAS No.:1099597-20-2 MDL No.:MFCD11226555 MF:C8H3F7 MW:232.0982 |
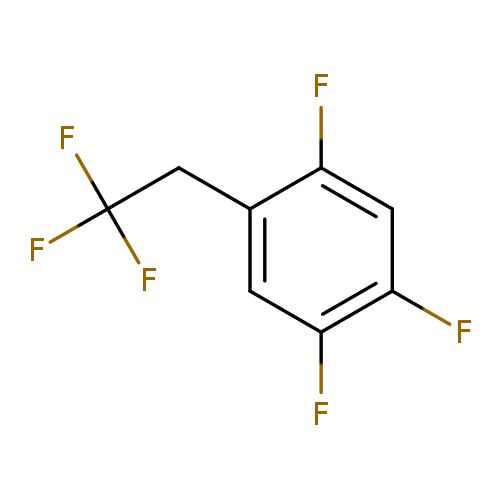
1,2,4-Trifluoro-5-(2,2,2-trifluoroethyl)-benzeneCatalog No.:AA01DO0M CAS No.:1099597-26-8 MDL No.:MFCD11226557 MF:C8H4F6 MW:214.1078 |
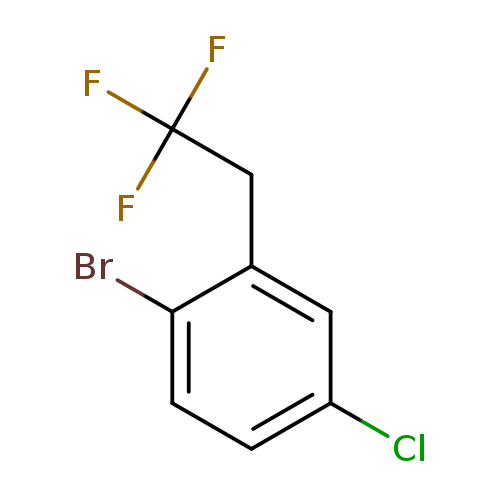
1-Bromo-4-chloro-2-(2,2,2-trifluoroethyl)benzeneCatalog No.:AA01FOAP CAS No.:1099597-30-4 MDL No.:MFCD11226573 MF:C8H5BrClF3 MW:273.4775 |
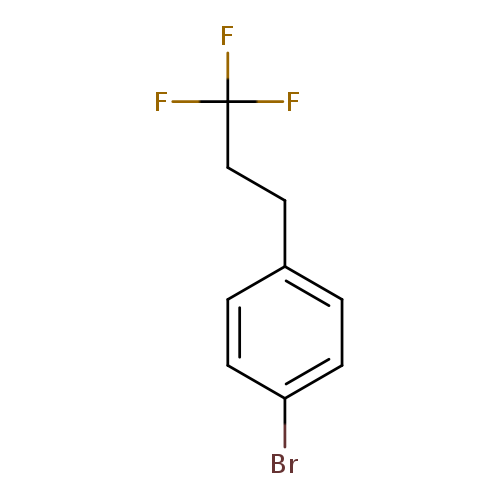
1-Bromo-4-(3,3,3-trifluoropropyl)benzeneCatalog No.:AA019DV9 CAS No.:1099597-31-5 MDL No.:MFCD11226575 MF:C9H8BrF3 MW:253.0590 |
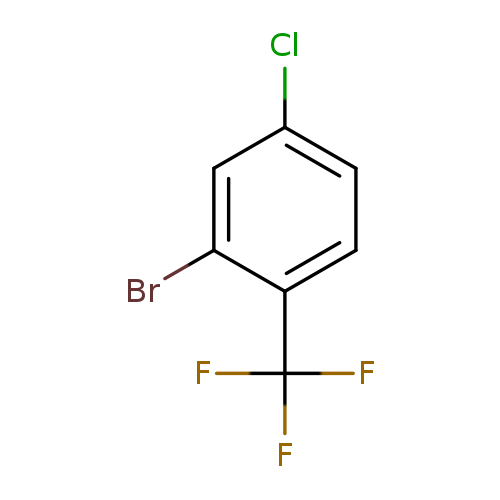
2-Bromo-4-chloro-1-(trifluoromethyl)benzeneCatalog No.:AA007EBU CAS No.:1099597-32-6 MDL No.:MFCD09839109 MF:C7H3BrClF3 MW:259.4509 |
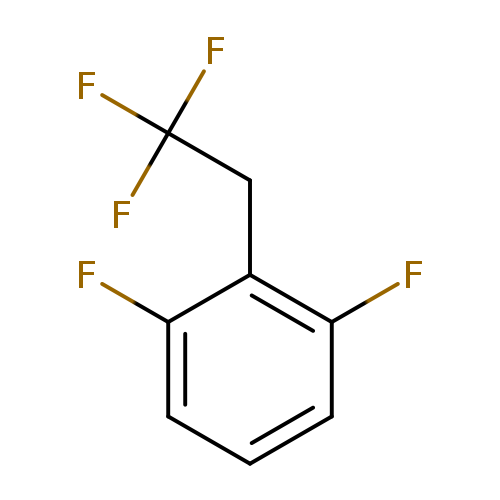
1,3-Difluoro-2-(2,2,2-trifluoroethyl)benzeneCatalog No.:AA01EQNW CAS No.:1099597-33-7 MDL No.:MFCD11226576 MF:C8H5F5 MW:196.1173 |
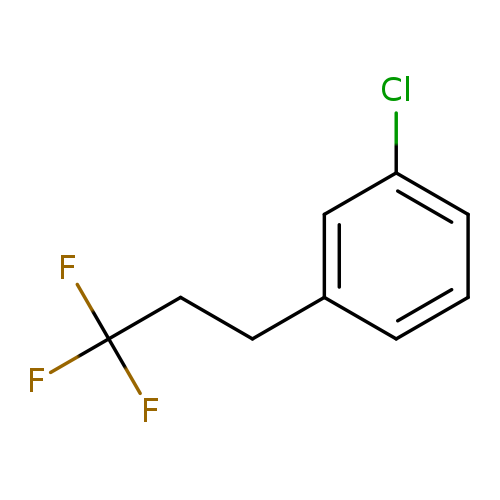
1-chloro-3-(3,3,3-trifluoropropyl)benzeneCatalog No.:AA00OMFQ CAS No.:1099597-34-8 MDL No.:MFCD11226577 MF:C9H8ClF3 MW:208.6080 |
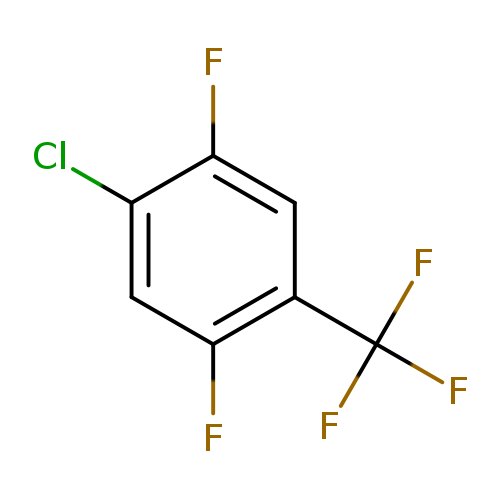
4-Chloro-2,5-difluorobenzotrifluorideCatalog No.:AA0090DW CAS No.:1099597-35-9 MDL No.:MFCD11226542 MF:C7H2ClF5 MW:216.5358 |
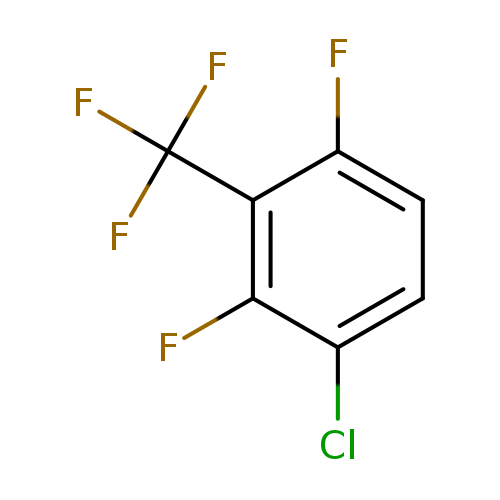
3-Chloro-2,6-difluorobenzotrifluorideCatalog No.:AA0090QO CAS No.:1099597-36-0 MDL No.:MFCD11226546 MF:C7H2ClF5 MW:216.5358 |
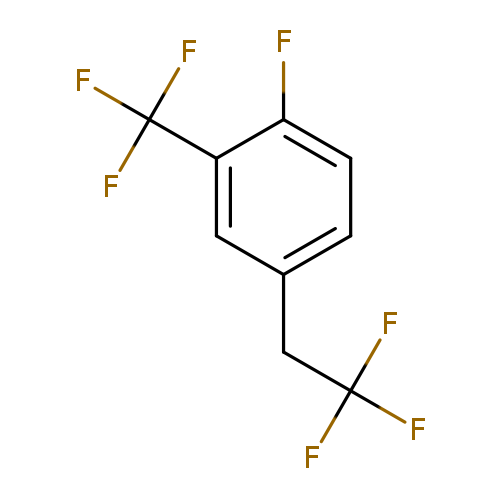
1-Fluoro-4-(2,2,2-trifluoroethyl)-2-(trifluoromethyl)benzeneCatalog No.:AA01EQNX CAS No.:1099597-38-2 MDL No.:MFCD11226547 MF:C9H5F7 MW:246.1248 |
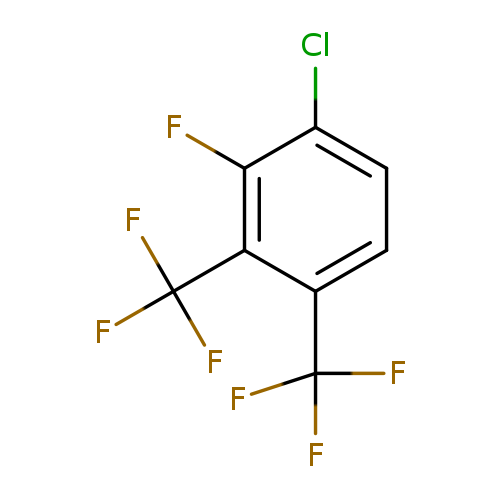
4-Chloro-3-fluoro-1,2-bis(trifluoromethyl)benzeneCatalog No.:AA01F93D CAS No.:1099597-43-9 MDL No.:MFCD11226663 MF:C8H2ClF7 MW:266.5433 |
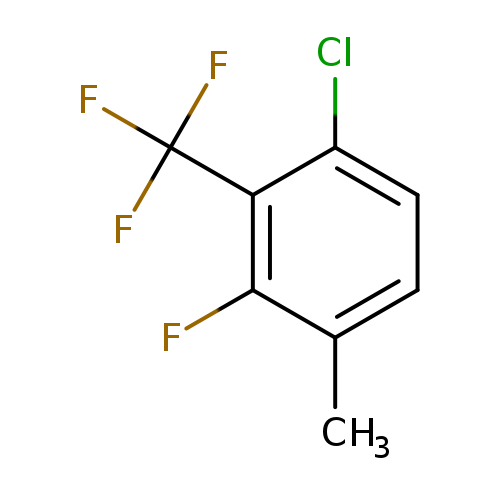
6-Chloro-2-fluoro-3-methylbenzotrifluorideCatalog No.:AA01FB2O CAS No.:1099597-48-4 MDL No.:MFCD11226667 MF:C8H5ClF4 MW:212.5719 |
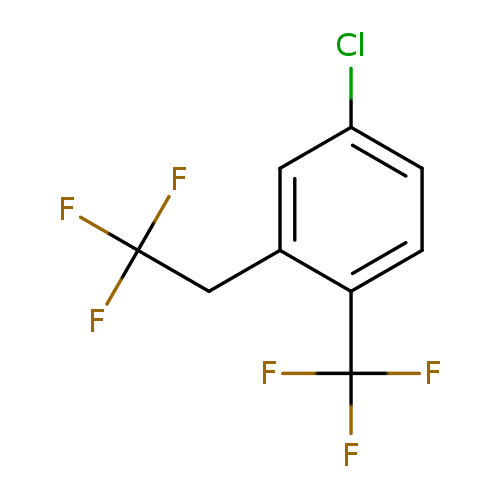
4-Chloro-2-(2,2,2-trifluoroethyl)-1-(trifluoromethyl)benzeneCatalog No.:AA01FOAV CAS No.:1099597-50-8 MDL No.:MFCD11226668 MF:C9H5ClF6 MW:262.5794 |
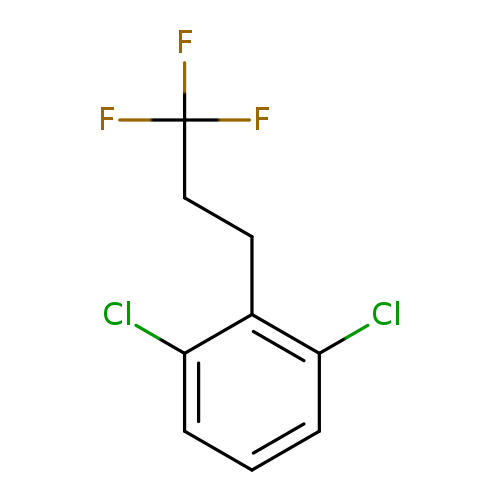
1,3-Dichloro-2-(3,3,3-trifluoropropyl)benzeneCatalog No.:AA01FOAW CAS No.:1099597-55-3 MDL No.:MFCD11226648 MF:C9H7Cl2F3 MW:243.0531 |
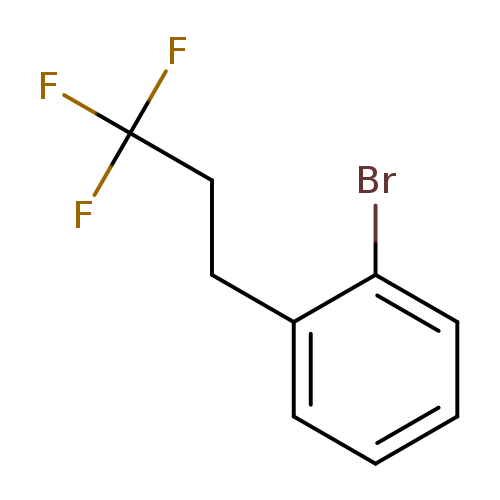
1-Bromo-2-(3,3,3-trifluoropropyl)benzeneCatalog No.:AA0090GX CAS No.:1099597-58-6 MDL No.:MFCD11226673 MF:C9H8BrF3 MW:253.0590 |
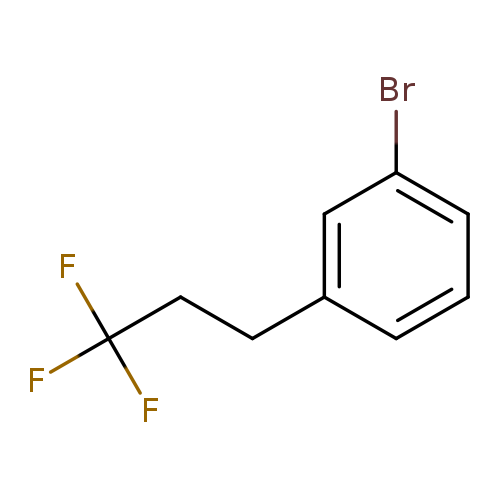
1-Bromo-3-(3,3,3-trifluoropropyl)benzeneCatalog No.:AA01FOAX CAS No.:1099597-60-0 MDL No.:MFCD11226672 MF:C9H8BrF3 MW:253.0590 |
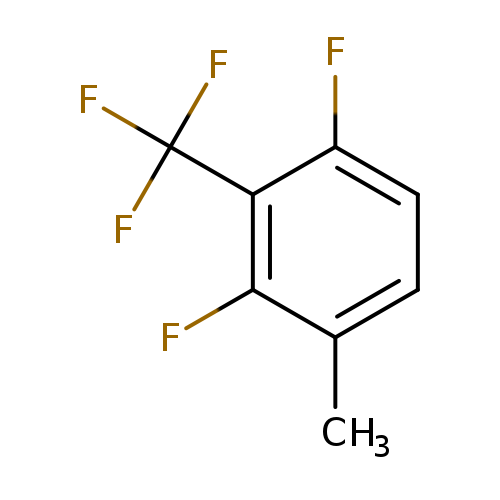
2,6-Difluoro-3-methylbenzotrifluorideCatalog No.:AA01FCSU CAS No.:1099597-62-2 MDL No.:MFCD11226679 MF:C8H5F5 MW:196.1173 |
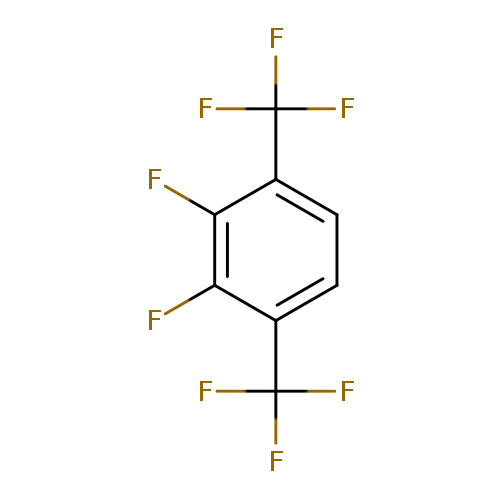
2,3-Difluoro-1,4-bis-(trifluoromethyl)benzeneCatalog No.:AA01FOAY CAS No.:1099597-67-7 MDL No.:MFCD11226683 MF:C8H2F8 MW:250.0887 |
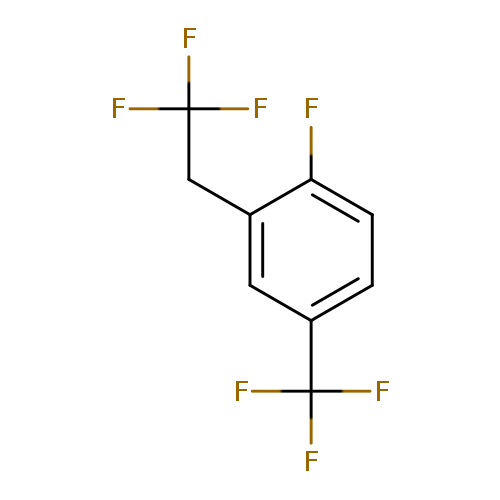
1-Fluoro-2-(2,2,2-trifluoroethyl)-4-(trifluoromethyl)benzeneCatalog No.:AA01FOAZ CAS No.:1099597-68-8 MDL No.:MFCD11226656 MF:C9H5F7 MW:246.1248 |
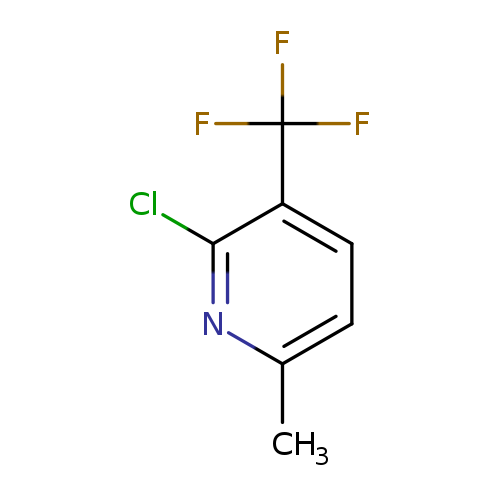
2-Chloro-6-methyl-3-(trifluoromethyl)pyridineCatalog No.:AA0090PL CAS No.:1099597-74-6 MDL No.:MFCD11226678 MF:C7H5ClF3N MW:195.5695 |
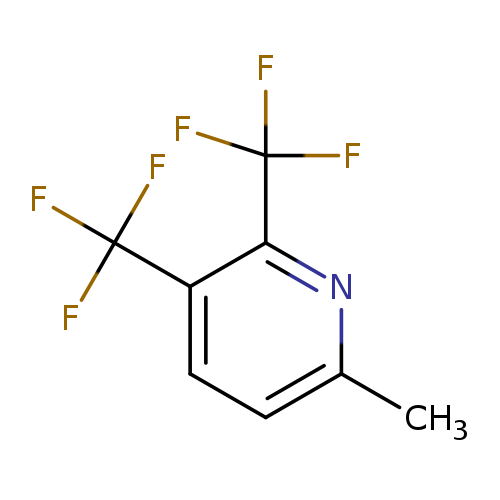
6-Methyl-2,3-bis-(trifluoromethyl)pyridineCatalog No.:AA01FOB2 CAS No.:1099597-76-8 MDL No.:MFCD11226677 MF:C8H5F6N MW:229.1224 |
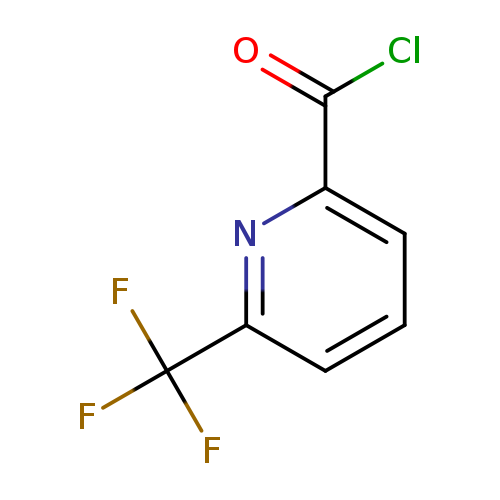
6-(Trifluoromethyl)pyridine-2-carbonyl chlorideCatalog No.:AA008U1Y CAS No.:1099597-77-9 MDL No.:MFCD11226641 MF:C7H3ClF3NO MW:209.5530 |
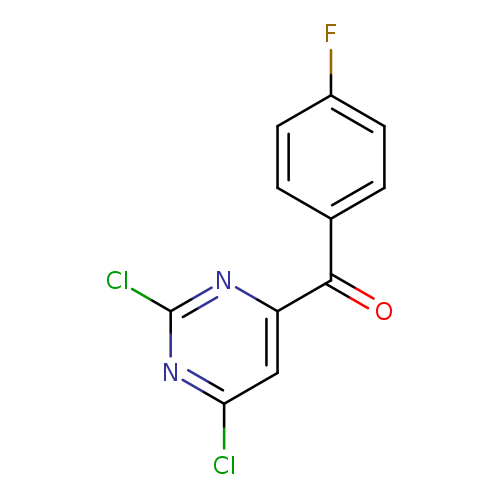
(2,6-Dichloropyrimidin-4-yl)-(4-fluorophenyl)methanoneCatalog No.:AA0084KV CAS No.:1099597-81-5 MDL No.:MFCD11100517 MF:C11H5Cl2FN2O MW:271.0746 |
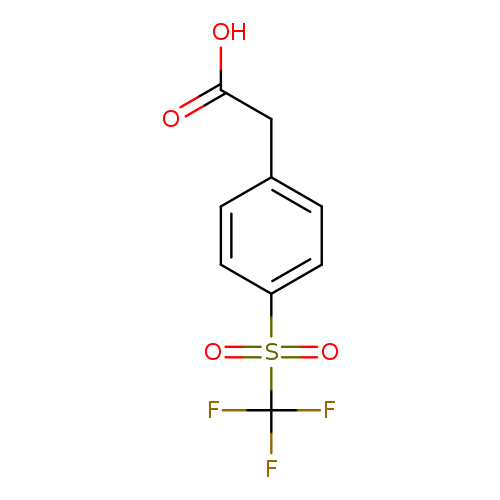
4-(Trifluoromethylsulfony)phenylacetic acidCatalog No.:AA007W3T CAS No.:1099597-82-6 MDL No.:MFCD04973012 MF:C9H7F3O4S MW:268.2097 |
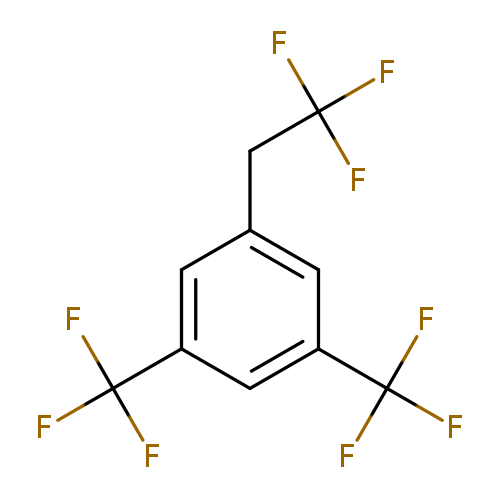
1-(2,2,2-Trifluoro-ethyl)-3,5-bis(trifluoromethyl)benzeneCatalog No.:AA0090F1 CAS No.:1099597-83-7 MDL No.:MFCD11100519 MF:C10H5F9 MW:296.1323 |
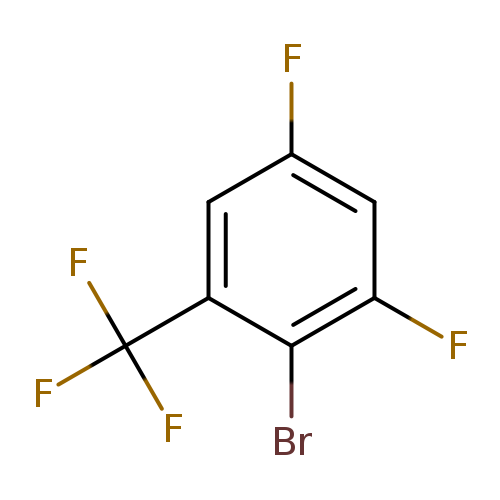
2-Bromo-1,5-difluoro-3-(trifluoromethyl)benzeneCatalog No.:AA01DZIM CAS No.:1099597-86-0 MDL No.:MFCD11100523 MF:C7H2BrF5 MW:260.9868 |
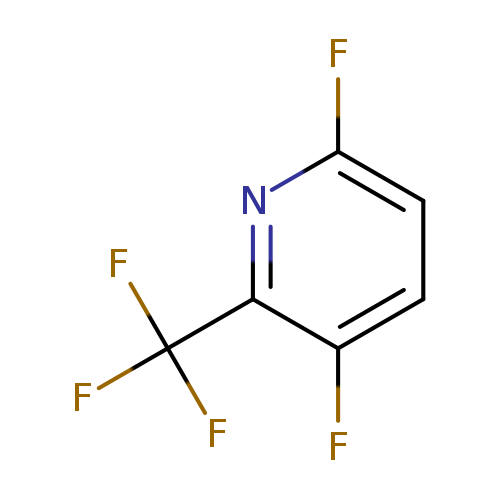
3,6-Difluoro-2-trifluoromethylpyridineCatalog No.:AA009NYI CAS No.:1099597-92-8 MDL No.:MFCD10699122 MF:C6H2F5N MW:183.0788 |
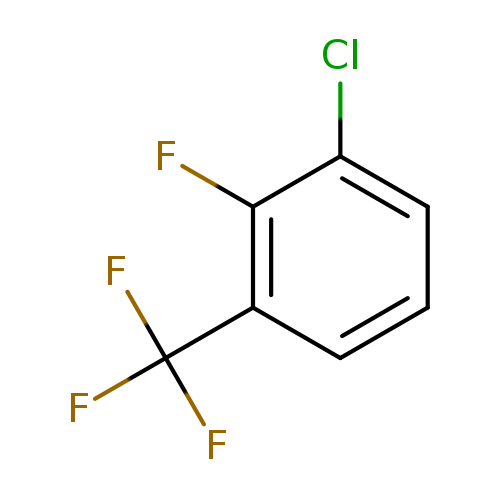
3-Chloro-2-fluorobenzotrifluorideCatalog No.:AA008X9P CAS No.:1099597-93-9 MDL No.:MFCD11100532 MF:C7H3ClF4 MW:198.5453 |
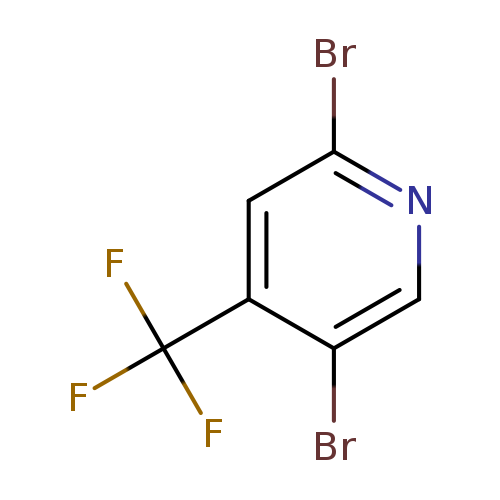
2,5-Dibromo-4-(trifluoromethyl)pyridineCatalog No.:AA008WJU CAS No.:1099597-94-0 MDL No.:MFCD10699123 MF:C6H2Br2F3N MW:304.8900 |
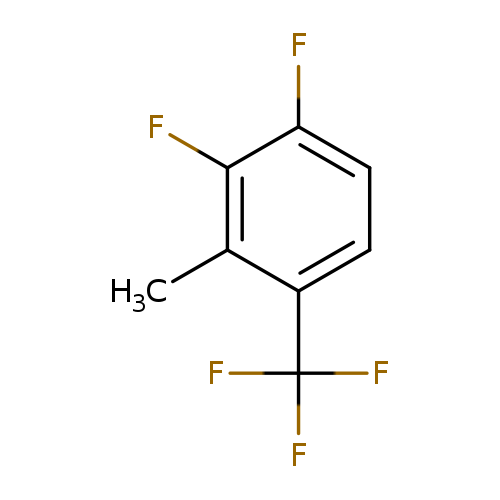
3,4-Difluoro-2-methylbenzotrifluorideCatalog No.:AA01FOAH CAS No.:1099597-95-1 MDL No.:MFCD11100534 MF:C8H5F5 MW:196.1173 |
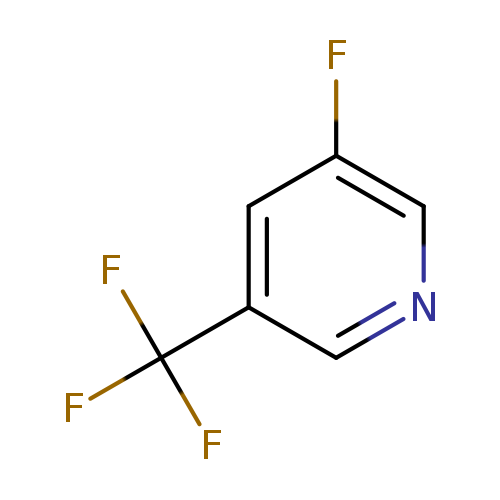
3-Fluoro-5-(trifluoromethyl)pyridineCatalog No.:AA00HBKK CAS No.:1099597-96-2 MDL No.:MFCD11100535 MF:C6H3F4N MW:165.0883 |
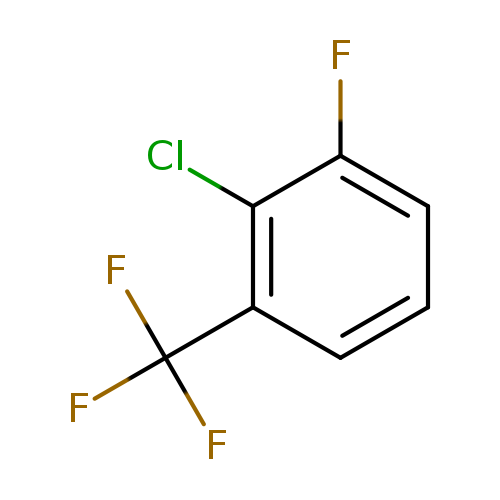
2-Chloro-3-fluorobenzotrifluorideCatalog No.:AA01FE84 CAS No.:1099597-97-3 MDL No.:MFCD11100537 MF:C7H3ClF4 MW:198.5453 |
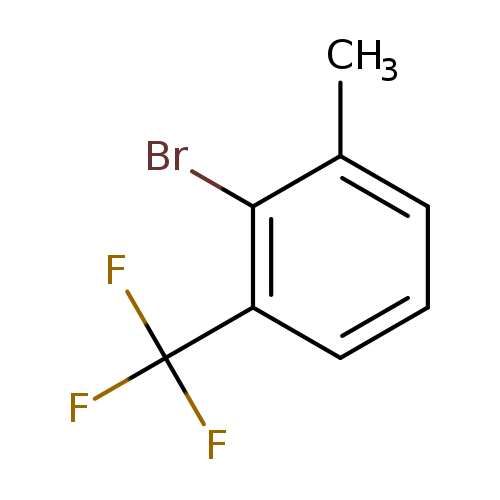
2-Bromo-1-methyl-3-(trifluoromethyl)benzeneCatalog No.:AA0095BG CAS No.:1099597-98-4 MDL No.:MFCD11100539 MF:C8H6BrF3 MW:239.0324 |
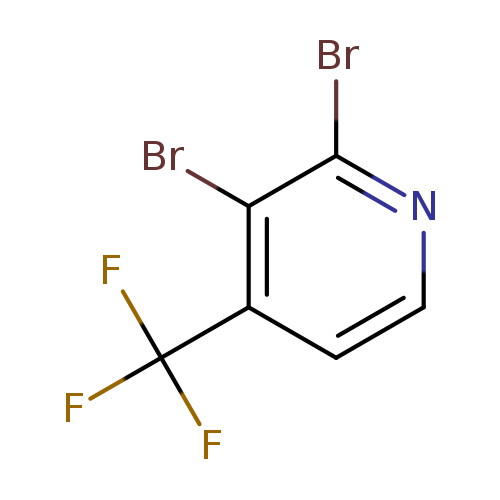
2,3-Dibromo-4-(trifluoromethyl)pyridineCatalog No.:AA0090OQ CAS No.:1099598-01-2 MDL No.:MFCD11100541 MF:C6H2Br2F3N MW:304.8900 |
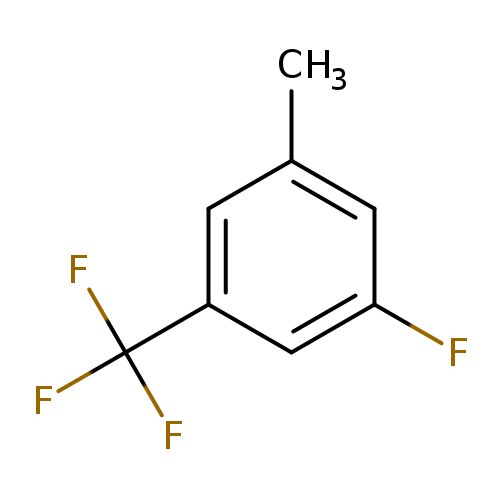
3-Fluoro-5-methylbenzotrifluorideCatalog No.:AA0090RZ CAS No.:1099598-02-3 MDL No.:MFCD11100542 MF:C8H6F4 MW:178.1269 |
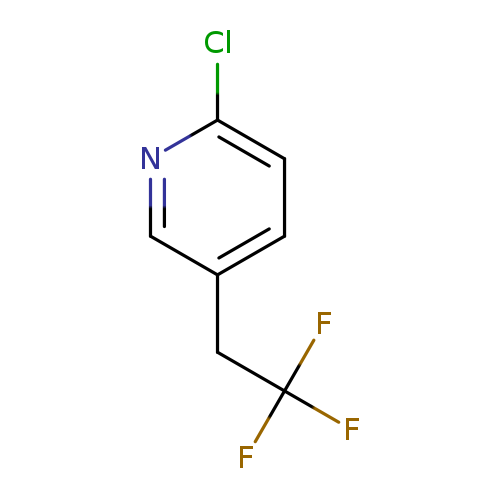
2-Chloro-5-(2,2,2-trifluoroethyl)pyridineCatalog No.:AA019CJC CAS No.:1099598-08-9 MDL No.:MFCD11100550 MF:C7H5ClF3N MW:195.5695 |
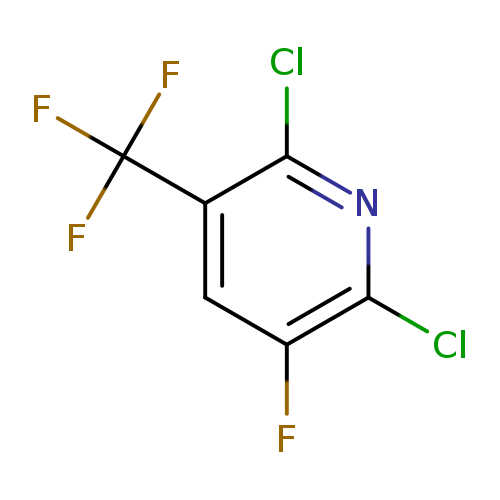
2,6-Dichloro-3-fluoro-5-(trifluoromethyl)pyridineCatalog No.:AA00HBKL CAS No.:1099598-11-4 MDL No.:MFCD11100554 MF:C6HCl2F4N MW:233.9785 |
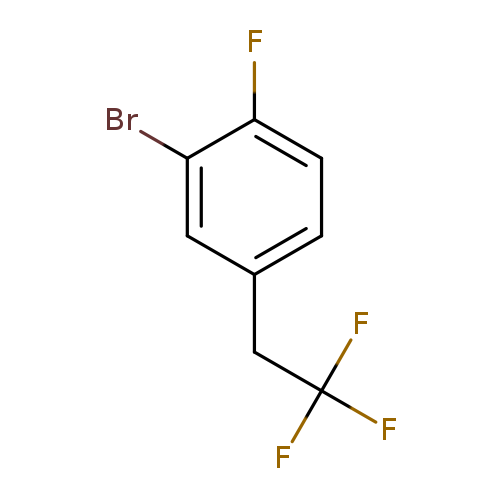
2-Bromo-1-fluoro-4-(2,2,2-trifluoroethyl)benzeneCatalog No.:AA01FOAJ CAS No.:1099598-18-1 MDL No.:MFCD11226531 MF:C8H5BrF4 MW:257.0229 |
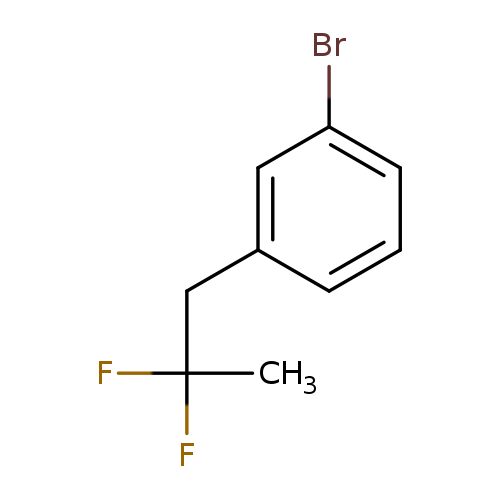
1-Bromo-3-(2,2-difluoropropyl)benzeneCatalog No.:AA01FOAK CAS No.:1099598-19-2 MDL No.:MFCD11226516 MF:C9H9BrF2 MW:235.0686 |
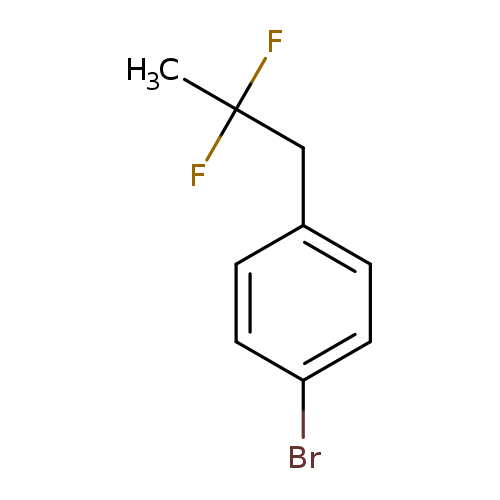
1-Bromo-4-(2,2-difluoropropyl)benzeneCatalog No.:AA01FOAL CAS No.:1099598-20-5 MDL No.:MFCD11226517 MF:C9H9BrF2 MW:235.0686 |
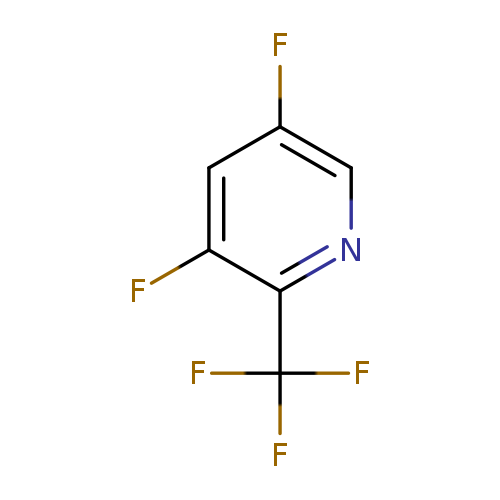
3,5-Difluoro-2-(trifluoromethyl)pyridineCatalog No.:AA008VEP CAS No.:1099598-21-6 MDL No.:MFCD11226519 MF:C6H2F5N MW:183.0788 |
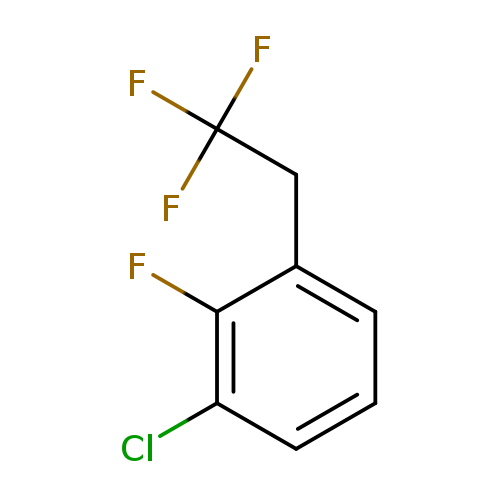
1-Chloro-2-fluoro-3-(2,2,2-trifluoroethyl)benzeneCatalog No.:AA01FOD4 CAS No.:1099598-23-8 MDL No.:MFCD11226522 MF:C8H5ClF4 MW:212.5719 |
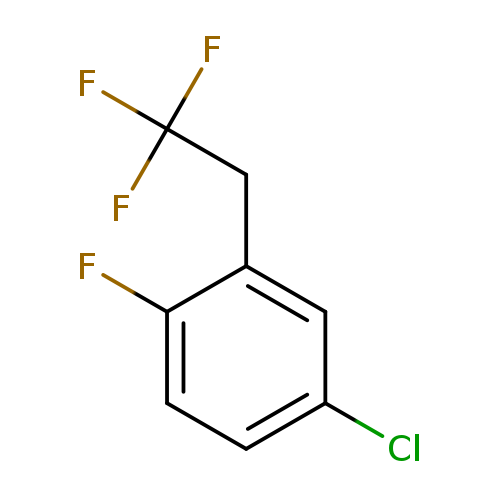
4-Chloro-1-fluoro-2-(2,2,2-trifluoroethyl)benzeneCatalog No.:AA01FOAM CAS No.:1099598-24-9 MDL No.:MFCD11226523 MF:C8H5ClF4 MW:212.5719 |
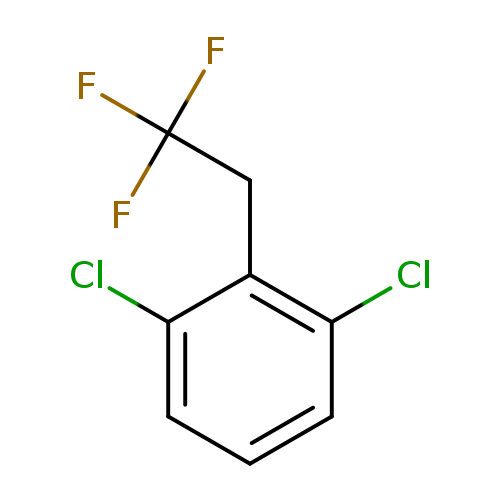
1,3-Dichloro-2-(2,2,2-trifluoroethyl)benzeneCatalog No.:AA01EQNY CAS No.:1099598-25-0 MDL No.:MFCD11226581 MF:C8H5Cl2F3 MW:229.0265 |
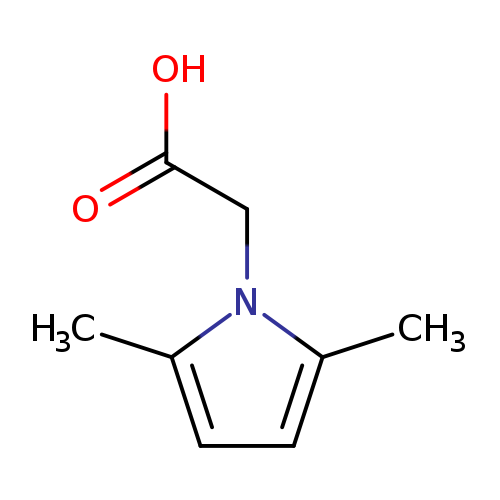
(2,5-Dimethyl-1h-pyrrol-1-yl)acetic acidCatalog No.:AA008VDV CAS No.:109960-17-0 MDL No.:MFCD09029000 MF:C8H11NO2 MW:153.1784 |
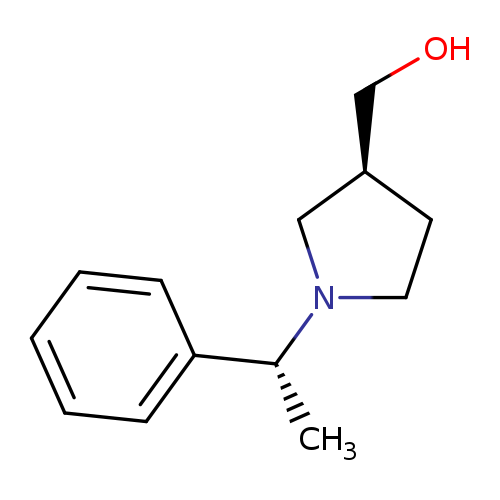
((S)-1-((R)-1-Phenylethyl)pyrrolidin-3-yl)methanolCatalog No.:AA003CG8 CAS No.:109960-55-6 MDL No.:MFCD12828252 MF:C13H19NO MW:205.2961 |
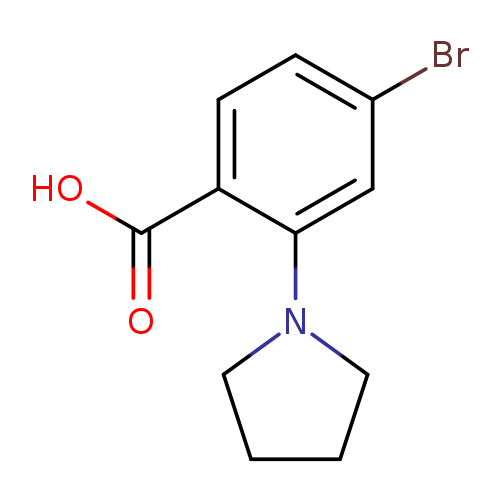
4-Bromo-2-pyrrolidinobenzoic acidCatalog No.:AA003KUQ CAS No.:1099609-12-7 MDL No.:MFCD11651817 MF:C11H12BrNO2 MW:270.1225 |
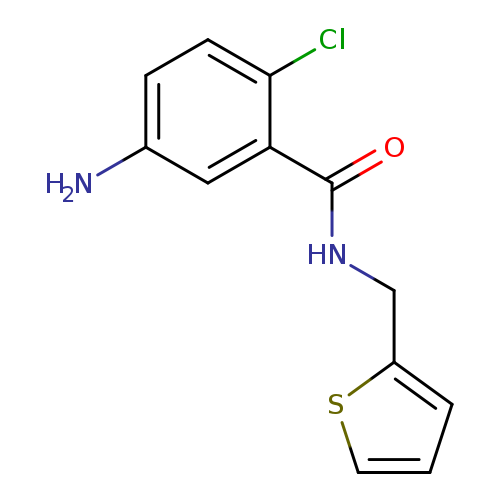
5-amino-2-chloro-N-(thiophen-2-ylmethyl)benzamideCatalog No.:AA019YNL CAS No.:1099610-86-2 MDL No.:MFCD11650815 MF:C12H11ClN2OS MW:266.7465 |
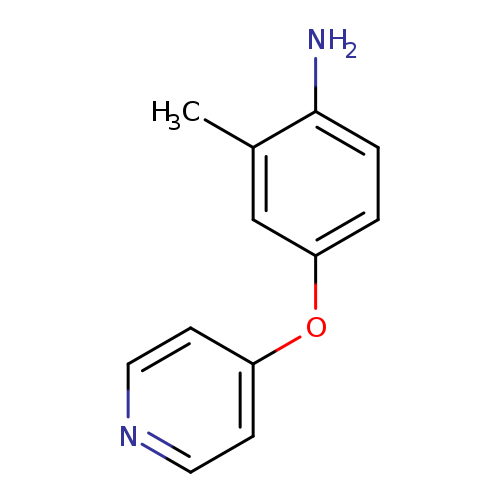
2-METHYL-4-(PYRIDIN-4-YLOXY)ANILINECatalog No.:AA01EJB7 CAS No.:1099611-74-1 MDL No.:MFCD11650937 MF:C12H12N2O MW:200.2365 |
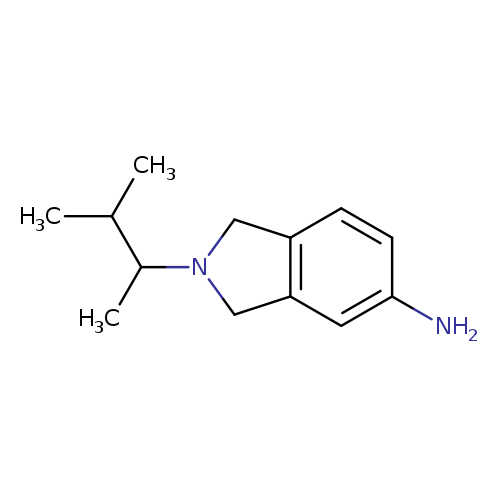
2-(3-methylbutan-2-yl)-2,3-dihydro-1H-isoindol-5-amineCatalog No.:AA01A98Z CAS No.:1099612-87-9 MDL No.:MFCD11655022 MF:C13H20N2 MW:204.3113 |
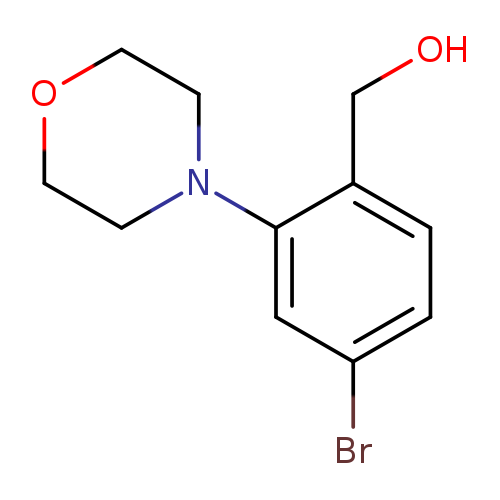
[4-bromo-2-(morpholin-4-yl)phenyl]methanolCatalog No.:AA01BCR1 CAS No.:1099619-96-1 MDL No.:MFCD11651958 MF:C11H14BrNO2 MW:272.1384 |
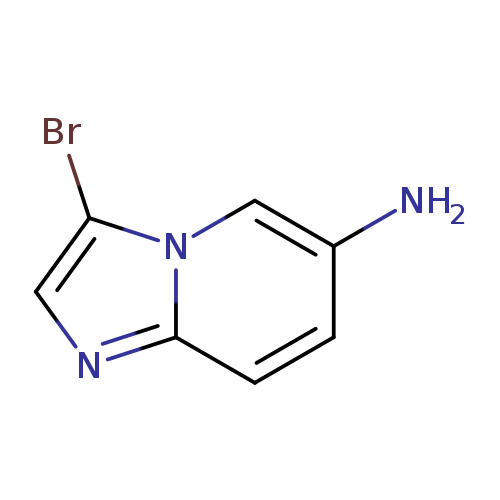
6-Amino-3-bromo-imidazo[1,2-a]pyridineCatalog No.:AA003MZQ CAS No.:1099621-14-3 MDL No.:MFCD11655113 MF:C7H6BrN3 MW:212.0466 |
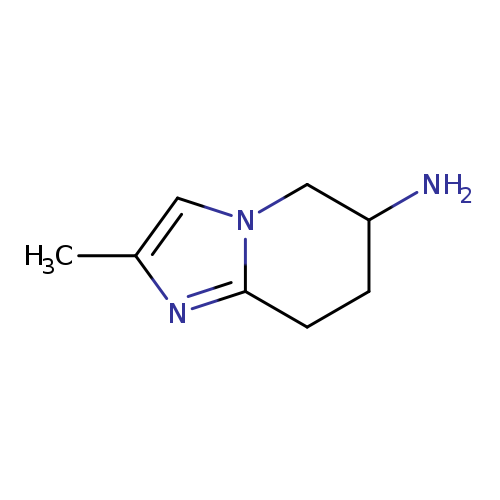
2-methyl-5H,6H,7H,8H-imidazo[1,2-a]pyridin-6-amineCatalog No.:AA01A8V2 CAS No.:1099621-16-5 MDL No.:MFCD11655119 MF:C8H13N3 MW:151.2089 |
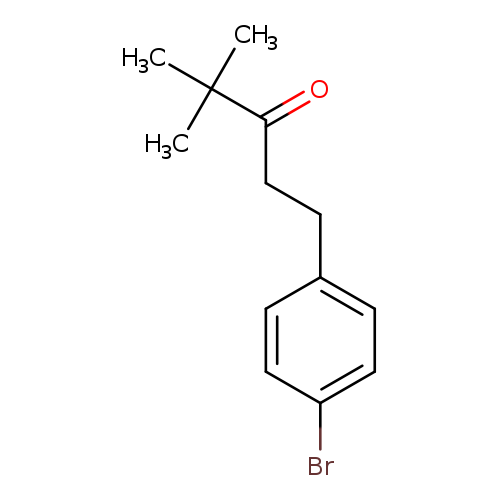
1-(4-bromophenyl)-4,4-dimethylpentan-3-oneCatalog No.:AA01A930 CAS No.:1099621-38-1 MDL No.:MFCD11655203 MF:C13H17BrO MW:269.1775 |