2020-02-24 19:05:00
Maria Gessica Ciulla,a Stefan Zimmermanna,b and Kamal Kumar
Introduction
Cascade reactions rapidly enhance the structural complexity of a desired scaffold. Nature’s synthetic ability to create highly complex natural products endowed with interesting bioactivities has inspired organic chemists to take up challenging de novo total synthesis studies of natural products (NPs).1–3 However, the multistep and tedious total syntheses are often marred by low efficiency and offer a limited exploration of NP chemical space for bio- logical research. Chemists have therefore resorted to other more efficient synthetic designs and strategies leading to a wider NP-like chemical space for the discovery of bioactive small molecules.4–9 For instance, the synthesis of small mole- cules based on privileged scaffolds has garnered a great deal of attention from organic and medicinal chemists.
A large number of NPs embodying polycyclic indole scaffolds display interesting, potent and wide-ranging biologi- cal activities (Fig. 1).14–18 Consequently, in the last decade, a surge in synthesis endeavours targeting polycyclic indoles has been observed.19–27 In a multistep synthesis, even a single low yielding or stereoselectively dissatisfying reaction step can drastically reduce the overall efficiency of the whole process.28–30 A cascade or domino reaction is a chemical trans- formation wherein a number of reactions (at least two) happen in a sequential manner in the same reaction apparatus and the product of one reaction step is the substrate for the next sequential reaction step. Cascade reactions rapidly enhance the structural complexity of a desired scaffold. They are economically better choices for multistep synthesis as they increase manifold the efficiency of a synthetic strategy and avoid a number of purifications and reaction work-ups which may otherwise reduce the yield of the products (Fig. 2). Thus, it is not surprising that their development has taken major strides in recent years.
The aim of this review is to present recent and interesting cascade reaction based synthetic strategies leading to indole polycycles including some biologically intriguing and NP- inspired indole frameworks. There are already enlightening reviews published about the concept of cascade and domino reactions38–41 and, therefore, we will not attempt to explain these concepts here. Also, we have focused only on indole poly- cyclic small molecules, and cascade synthesis targeting poly- cyclic oxindoles42,43 is not covered in this review article.
The design of a cascade reaction-driven synthesis of a polycyclic indole or for that matter any complex small molecule needs to involve some key reactions which can either generate the whole of a scaffold or an important intermediate leading to the desired molecular architecture. For the synthesis of complex indoles, a number of chemical reactions can play a key role in a cascade reaction design. Such designs may employ either a simple indole substrate or other more basic precursors that generate the indole ring during a cascade reac- tion sequence in addition to further molecular complexity. The key reactions may include cycloaddition and/or cyclization reactions, transition metal catalysed coupling reactions, or C–H activation routes as well as coinage metal catalysed cascade rearrangements. In order to facilitate an understand- ing of these key reactions in the cascade synthesis of polycyclic indoles, transition metal free i.e. either uncatalysed or organo- catalysed reactions and transition metal-catalysed cascade reactions are discussed in the following in separate sections.
Transition metal-free cascade syntheses of indole polycycles
Cascade syntheses employing dipolar cycloaddition reaction as the key step
Cycloaddition reactions are among the most powerful tools in organic synthesis to generate complex small molecules. In particular, 1,3-dipolar cycloaddition reactions have emerged as immensely useful transformations that can be applied in cascade or domino reaction designs for rapidly building molecular complexity around a desired five-membered heterocycle.44–48 In a number of cases, dipolar cycloaddition reactions deliver products with high yields and in a regio- and stereocontrolled manner. In the following section, some cascade reaction designs making use of dipolar cycloaddition reactions for building indole polycycles are discussed.
DNA interacting heterocycles are generally sp2-carbon rich and flat in their structures. However, the positioning of hetero- atoms in these heterocycles plays a crucial role in ensuring DNA interaction.49 For generating similar heterocycles, Lauria et al. developed a domino reaction strategy to synthesize indolo-[2,3-e][1,2,3]-triazolo[1,5-a]-pyrimidine (6) from 3-azido- indoles (1, Scheme 1).50 Treatment of acetonitrile (2) with sodium metal generated anion 3, which entered into a dipolar cyclization with the azide moiety to form the intermediate 4. Under the same reaction conditions, the imine moiety in 4 iso- merized to enamine and formed the lactam by reacting with the ester moiety on C2 of the indole ring. Thus, domino reac- tions of azidoindoles and acetonitriles afforded the synthetic entry to annelated indolo[2,3-e][1,2,3]triazolo[1,5-a]pyr- imidines (6).
In docking studies to evaluate the ability of indolopyr- imidines 6, with DNA, 6a (R = Ph) was found to have a greater change in free energy of binding (−13.76 kcal mol−1) than the control actinomycin D (−10.37 kcal mol−1). Thus, indolotria- zolo-pyrimidine derivatives are capable of making stable com- plexes with DNA.
A highly useful and elegant cascade reaction strategy to build polycyclic heterocycles is through tandem and dipolar [4 + 2]–[3 + 2] cycloaddition reactions of nitroalkenes (such as oxadienes) with olefins.51 The nitronate products formed in the first cycloaddition reaction represent another 1,3-dipole that can be trapped with a number of dipolarophiles in a cascade reaction manner to deliver structurally complex indoles.52–54
Chataigner and Piettre envisaged that the chemoselectivity in cycloaddition reactions of 2-nitroindoles (7) with olefins will be directed by the LUMO of nitroindole (such as oxadiene) in the inverse electron-demand oxa-Diels–Alder reaction with electron-rich olefins and by the HOMO of nitronate intermedi- ate (11) in the next 1,3-dipolar cycloaddition reaction with elec- tron-poor olefins.55 The sufficiently different electron demands of the two sequential cycloadditions facilitated a selective domino process. However, establishing the right reac- tion conditions was not straightforward for this multicompo- nent [4 + 2]–[3 + 2] cascade reaction between nitroindole (7),
vinyl ethers (8) and acrylates (9).
It was observed that the cascade reaction worked at room temperature albeit under high pressure in tetrahydrofuran (THF). Interestingly, the [4 + 2] inverse electron-demand cyclo- addition was completely endo-selective and totally influenced the facial selectivity of the subsequent [3 + 2] cycloaddition reaction to set up the stereochemistry at the ring-junction. In two operations, a polycyclic diamine (12) featuring a quatern- ary stereocenter at the ring junction is efficiently generated (Scheme 2).
Boger’s group has explored and exploited the tandem intra- molecular Diels–Alder/1,3-dipolar cycloaddition reactions of 1,3,4-oxadiazoles in the synthesis of complex NPs and their analogues.56–58 Tryptamine derived 1,3,4-oxadiazoles (13, Scheme 3) supporting an olefin linker were employed in a cascade reaction sequence targeting the synthesis of vindoline based indole derivatives. The oxadiazoles (13) behave as elec- tron-deficient azadienes in the intramolecular Diels–Alder reaction forming the initial [4 + 2]-cycloadducts (14), which lose N2 to generate a carbonyl ylide (15). The latter further reacts with the proximal indole moiety in a 1,3-dipolar cyclo-addition reaction to deliver adduct 16 (Scheme 3).59 The reactions were most facile when initiated by an inverse electrondemand Diels–Alder reaction; however, the unactivated or elec- tron-deficient tethered dienophiles efficiently reacted to afford polycyclic indoles in a completely stereoselective fashion to form a single diastereomer of adducts (16). The cascade of cycloadditions constructed three new rings along with the for- mation of four new C–C bonds. In the cascade process, all six stereocenters characteristic of vindoline NP including three contiguous among the four quaternary centers were set in a single operation. The factors that controlled the stereo- selectivity involved the dienophile geometry as well as the exclusive indole endo-[3 + 2] cycloaddition reaction sterically directed to the α-face of the 1,3-dipole.
2-Chloro-3-[(4-fluorobenzyl)oxy]pyridineCatalog No.:AA01ARN0 CAS No.:1105190-73-5 MDL No.:MFCD11986849 MF:C12H9ClFNO MW:237.6574 |
(7-METHYL-5-OXO-2,3-DIHYDRO-5H-[1,3]THIAZOLO[3,2-A]PYRIMIDIN-3-YL)ACETIC +Catalog No.:AA01ARHN CAS No.:1105190-78-0 MDL No.:MFCD11518877 MF:C9H10N2O3S MW:226.2523 |
(6,7-DIMETHYL-5-OXO-2,3-DIHYDRO-5H-[1,3]THIAZOLO[3,2-A]PYRIMIDIN-3-YL)ACE+Catalog No.:AA01ARJJ CAS No.:1105190-84-8 MDL No.:MFCD11986422 MF:C10H12N2O3S MW:240.2789 |
(5-OXO-2,3,6,7,8,9-HEXAHYDRO-5H-[1,3]THIAZOLO[2,3-B]QUINAZOLIN-3-YL)ACETI+Catalog No.:AA01ARHO CAS No.:1105190-90-6 MDL No.:MFCD11518878 MF:C12H14N2O3S MW:266.3162 |
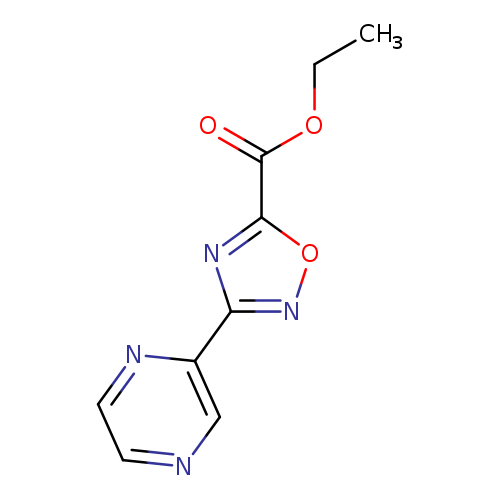
Ethyl 3-(pyrazin-2-yl)-1,2,4-oxadiazole-5-carboxylateCatalog No.:AA01AQ7F CAS No.:1105190-97-3 MDL No.:MFCD16653106 MF:C9H8N4O3 MW:220.1848 |
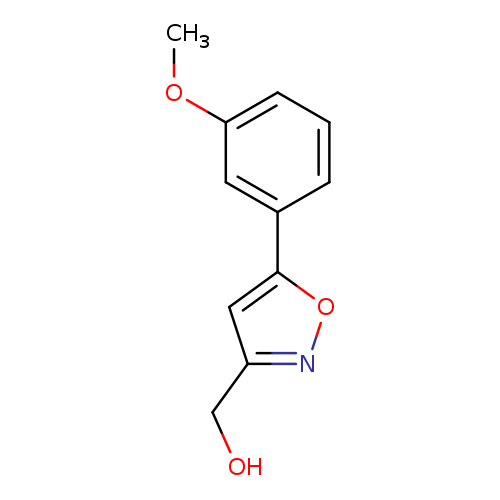
[5-(3-methoxyphenyl)isoxazol-3-yl]methanolCatalog No.:AA01FM65 CAS No.:1105191-02-3 MDL No.:MFCD11868818 MF:C11H11NO3 MW:205.2099 |
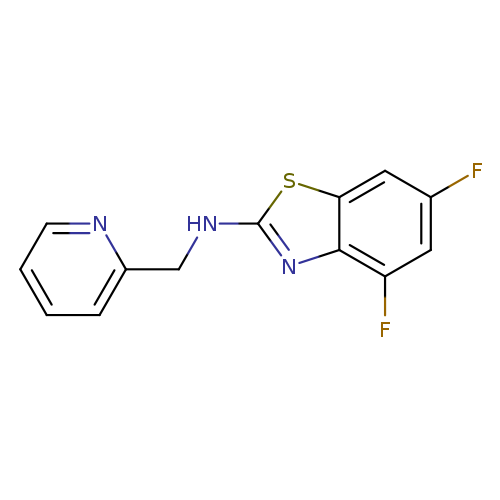
4,6-Difluoro-N-(pyridin-2-ylmethyl)-1,3-benzothiazol-2-amineCatalog No.:AA01ARLY CAS No.:1105191-11-4 MDL No.:MFCD11986753 MF:C13H9F2N3S MW:277.2925 |
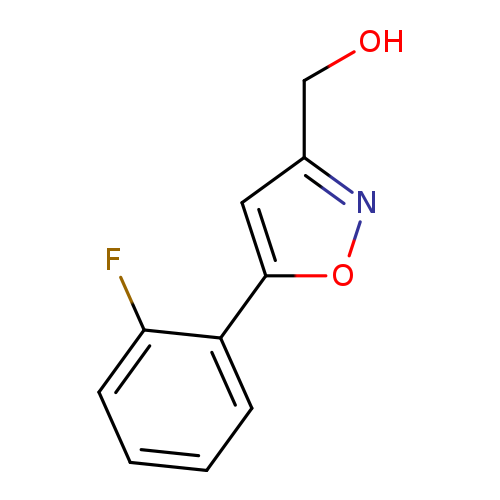
[5-(2-FLUOROPHENYL)ISOXAZOL-3-YL]METHANOLCatalog No.:AA01AR9Q CAS No.:1105191-15-8 MDL No.:MFCD09752375 MF:C10H8FNO2 MW:193.1744 |
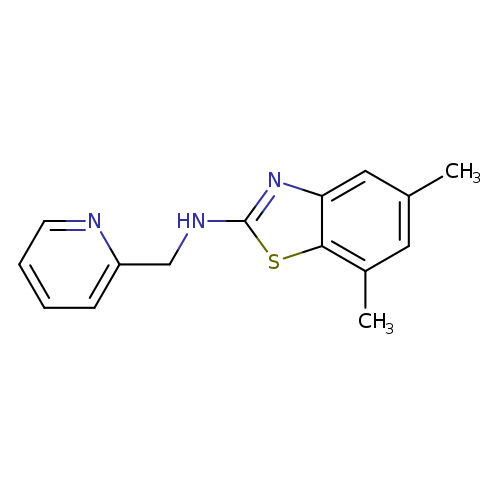
5,7-Dimethyl-N-(pyridin-2-ylmethyl)-1,3-benzothiazol-2-amineCatalog No.:AA01ARLZ CAS No.:1105191-18-1 MDL No.:MFCD09814357 MF:C15H15N3S MW:269.3647 |
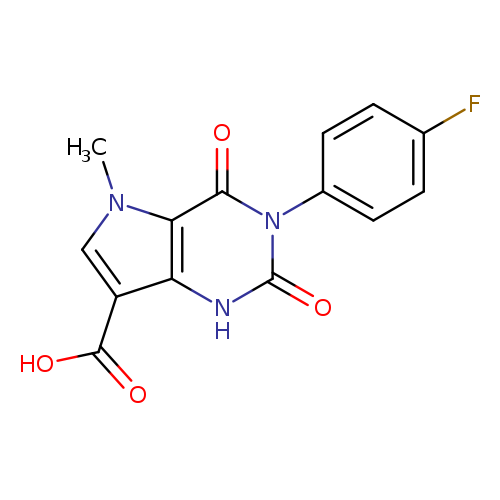
3-(4-FLUOROPHENYL)-5-METHYL-2,4-DIOXO-2,3,4,5-TETRAHYDRO-1H-PYRROLO[3,2-D+Catalog No.:AA01ARNH CAS No.:1105191-19-2 MDL No.:MFCD11986684 MF:C14H10FN3O4 MW:303.2453 |
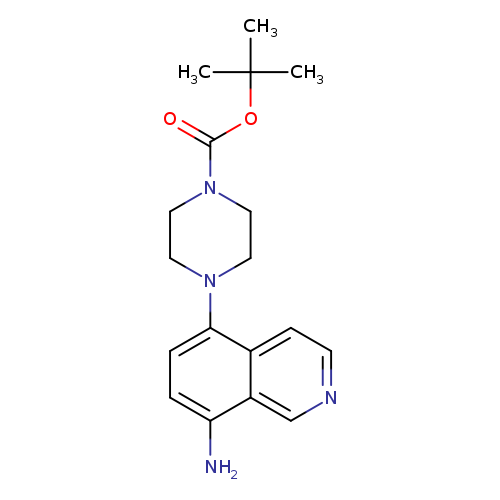
tert-Butyl 4-(8-amino-5-isoquinolinyl)-1-piperazinecarboxylateCatalog No.:AA01AQEV CAS No.:1105191-23-8 MDL No.:MFCD16631744 MF:C18H24N4O2 MW:328.4088 |
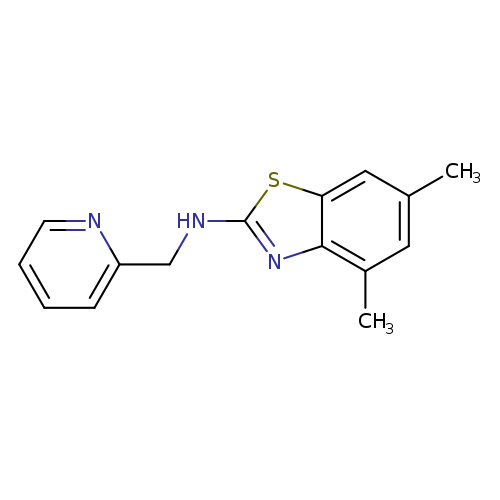
4,6-Dimethyl-N-(pyridin-2-ylmethyl)-1,3-benzothiazol-2-amineCatalog No.:AA01ARM0 CAS No.:1105191-24-9 MDL No.:MFCD11986755 MF:C15H15N3S MW:269.3647 |
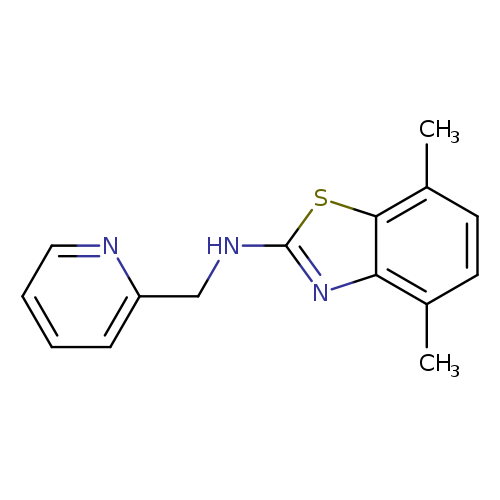
4,7-Dimethyl-n-(pyridin-2-ylmethyl)-1,3-benzothiazol-2-amineCatalog No.:AA01ARMS CAS No.:1105191-30-7 MDL No.:MFCD11986756 MF:C15H15N3S MW:269.3647 |
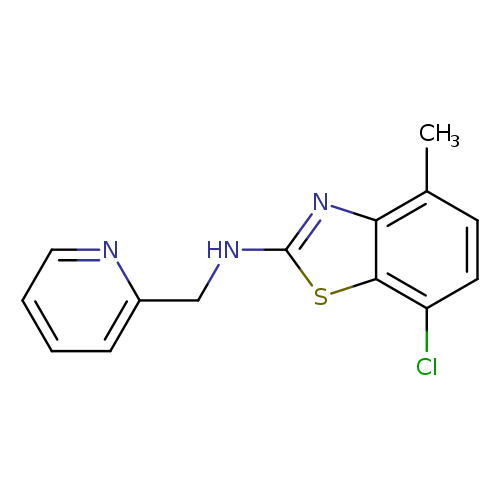
7-Chloro-4-methyl-N-(pyridin-2-ylmethyl)-1,3-benzothiazol-2-amineCatalog No.:AA01ARMT CAS No.:1105191-36-3 MDL No.:MFCD11986757 MF:C14H12ClN3S MW:289.7832 |
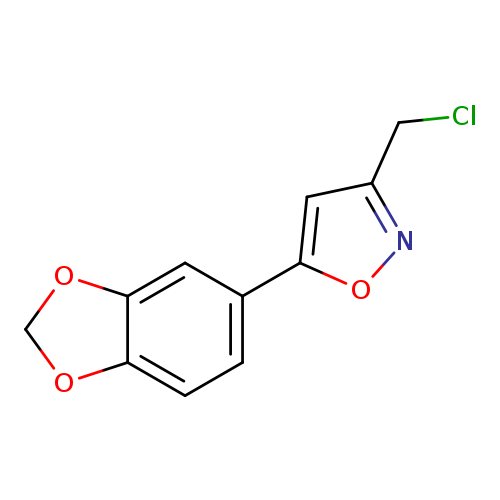
5-(1,3-Benzodioxol-5-yl)-3-(chloromethyl)isoxazoleCatalog No.:AA01ARKV CAS No.:1105191-39-6 MDL No.:MFCD11986428 MF:C11H8ClNO3 MW:237.6391 |
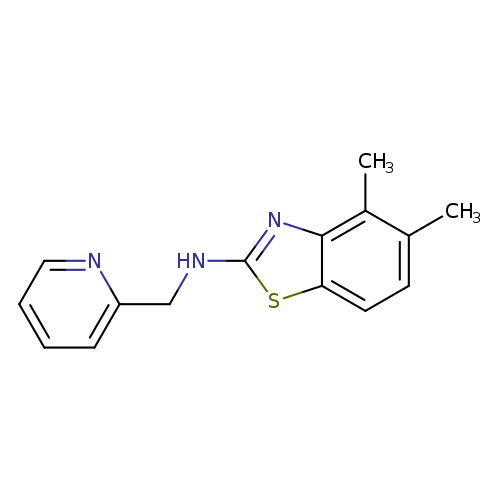
4,5-Dimethyl-N-(pyridin-2-ylmethyl)-1,3-benzothiazol-2-amineCatalog No.:AA01ARMU CAS No.:1105191-41-0 MDL No.:MFCD11986758 MF:C15H15N3S MW:269.3647 |
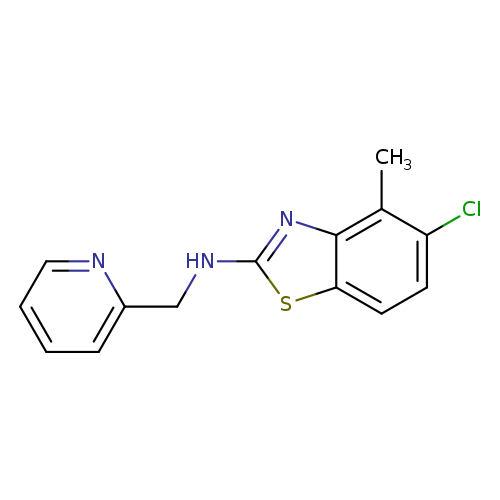
5-Chloro-4-methyl-N-(pyridin-2-ylmethyl)-1,3-benzothiazol-2-amineCatalog No.:AA01ARMV CAS No.:1105191-46-5 MDL No.:MFCD11986759 MF:C14H12ClN3S MW:289.7832 |
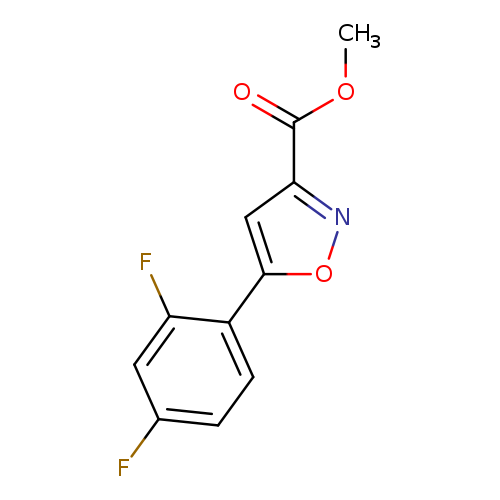
Methyl 5-(2,4-difluorophenyl)isoxazole-3-carboxylateCatalog No.:AA003RTJ CAS No.:1105191-49-8 MDL No.:MFCD11986431 MF:C11H7F2NO3 MW:239.1750 |
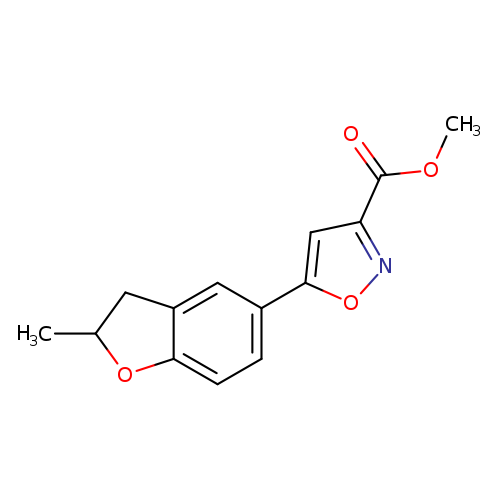
Methyl 5-(2-methyl-2,3-dihydro-1-benzofuran-5-yl)isoxazole-3-carboxylateCatalog No.:AA01ARLO CAS No.:1105191-54-5 MDL No.:MFCD11986433 MF:C14H13NO4 MW:259.2573 |
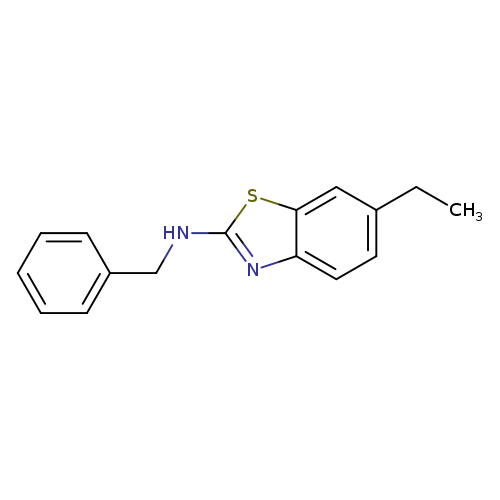
N-Benzyl-6-ethyl-1,3-benzothiazol-2-amineCatalog No.:AA01ARNL CAS No.:1105191-56-7 MDL No.:MFCD11986762 MF:C16H16N2S MW:268.3766 |
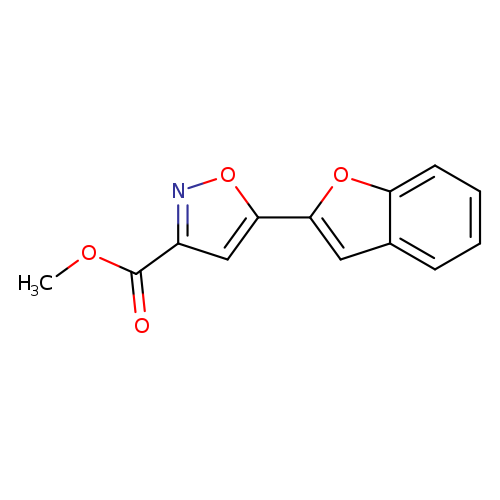
Methyl 5-(1-benzofuran-2-yl)isoxazole-3-carboxylateCatalog No.:AA01ARME CAS No.:1105191-59-0 MDL No.:MFCD11986434 MF:C13H9NO4 MW:243.2149 |
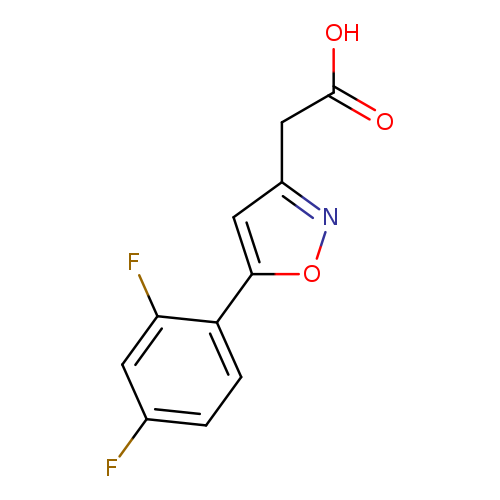
[5-(2,4-Difluorophenyl)isoxazol-3-yl]acetic acidCatalog No.:AA01FLP5 CAS No.:1105191-64-7 MDL No.:MFCD16652869 MF:C11H7F2NO3 MW:239.1750 |
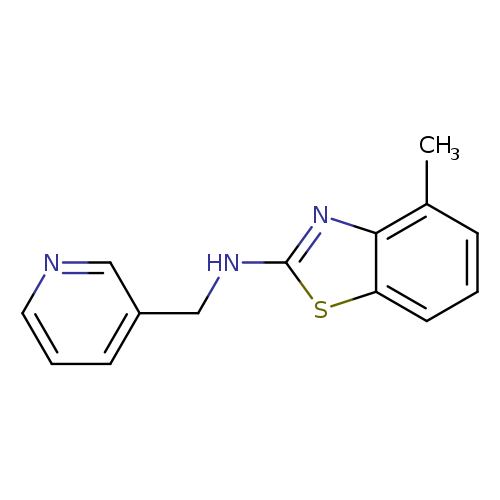
4-Methyl-N-(pyridin-3-ylmethyl)-1,3-benzothiazol-2-amineCatalog No.:AA01ARNO CAS No.:1105191-71-6 MDL No.:MFCD11986765 MF:C14H13N3S MW:255.3381 |
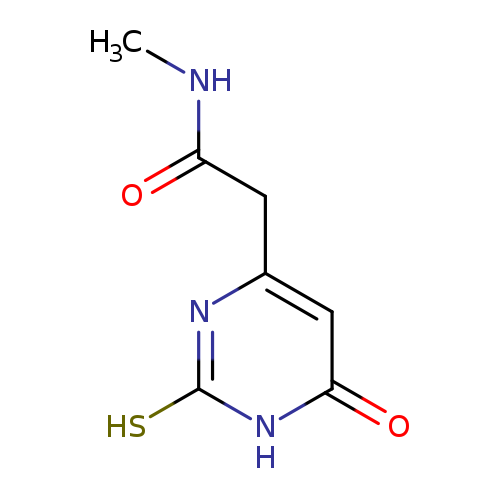
2-(2-MERCAPTO-6-OXO-1,6-DIHYDROPYRIMIDIN-4-YL)-N-METHYLACETAMIDECatalog No.:AA01ARK0 CAS No.:1105191-77-2 MDL No.:MFCD11986918 MF:C7H9N3O2S MW:199.2303 |
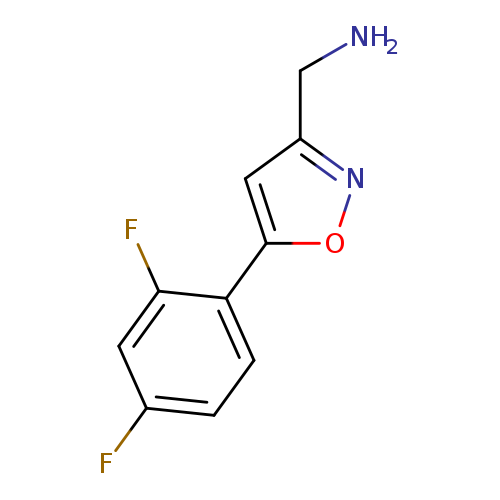
[5-(2,4-difluorophenyl)-1,2-oxazol-3-yl]methanamineCatalog No.:AA01ATC3 CAS No.:1105191-82-9 MDL No.:MFCD11986437 MF:C10H8F2N2O MW:210.1801 |
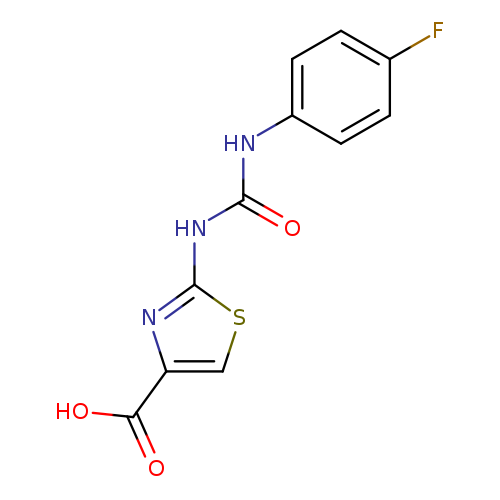
2-(([(4-Fluorophenyl)amino]carbonyl)amino)-1,3-thiazole-4-carboxylic acidCatalog No.:AA01ARPH CAS No.:1105191-83-0 MDL No.:MFCD11986871 MF:C11H8FN3O3S MW:281.2629 |
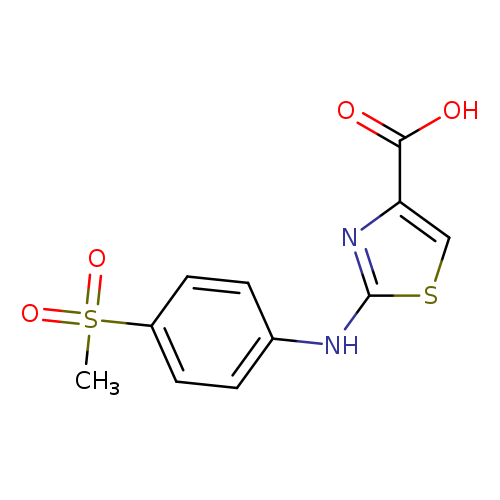
2-([4-(Methylsulfonyl)phenyl]amino)-1,3-thiazole-4-carboxylic acidCatalog No.:AA01ARPJ CAS No.:1105191-87-4 MDL No.:MFCD11986873 MF:C11H10N2O4S2 MW:298.3381 |
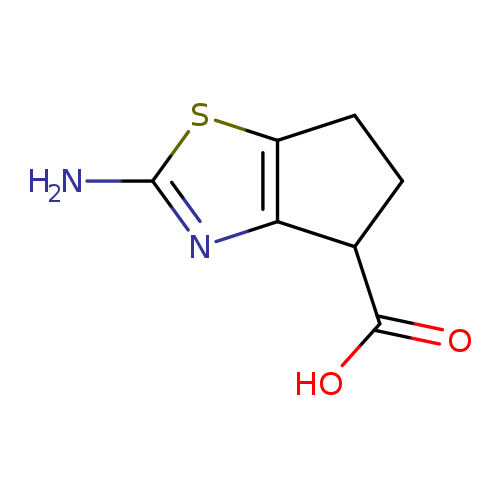
2-amino-4H,5H,6H-cyclopenta[d][1,3]thiazole-4-carboxylic acidCatalog No.:AA01ARID CAS No.:1105191-92-1 MDL No.:MFCD11909837 MF:C7H8N2O2S MW:184.2156 |
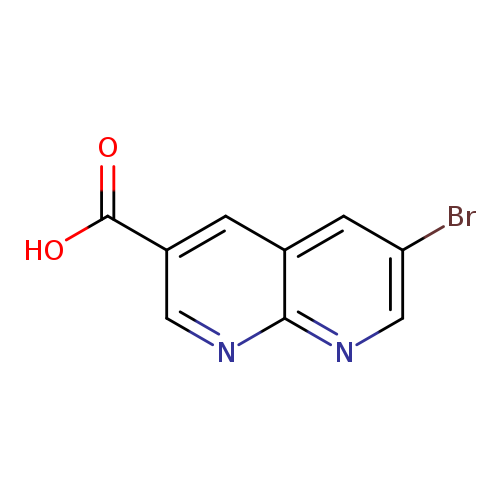
6-Bromo-1,8-naphthyridine-3-carboxylic acidCatalog No.:AA01AFZL CAS No.:1105191-97-6 MDL No.:MFCD16652617 MF:C9H5BrN2O2 MW:253.0522 |
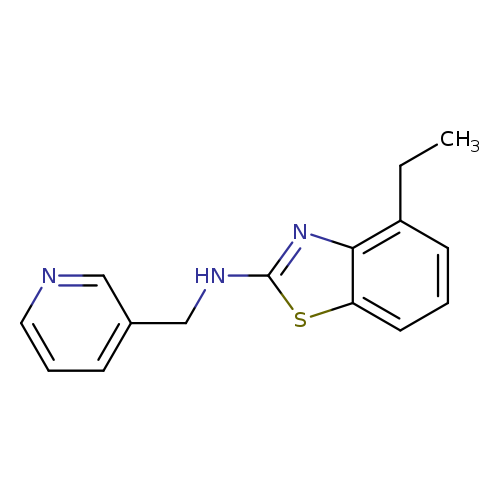
4-Ethyl-N-(pyridin-3-ylmethyl)-1,3-benzothiazol-2-amineCatalog No.:AA01AROF CAS No.:1105191-99-8 MDL No.:MFCD11986766 MF:C15H15N3S MW:269.3647 |
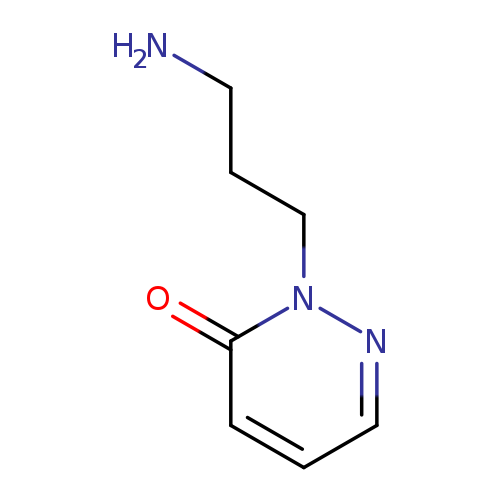
2-(3-Aminopropyl)pyridazin-3(2(h))-oneCatalog No.:AA01AQEQ CAS No.:1105192-02-6 MDL No.:MFCD16631561 MF:C7H11N3O MW:153.1817 |
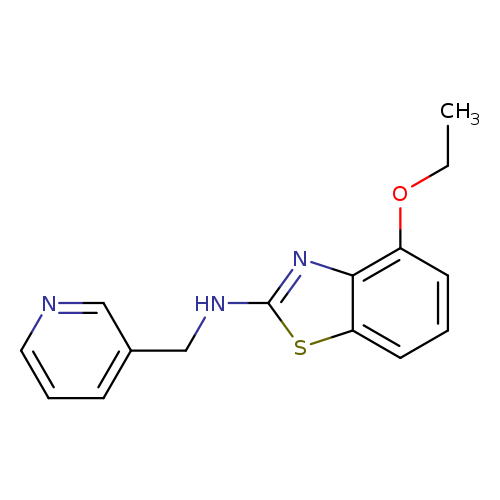
4-Ethoxy-n-(pyridin-3-ylmethyl)-1,3-benzothiazol-2-amineCatalog No.:AA01AROG CAS No.:1105192-04-8 MDL No.:MFCD11986767 MF:C15H15N3OS MW:285.3641 |
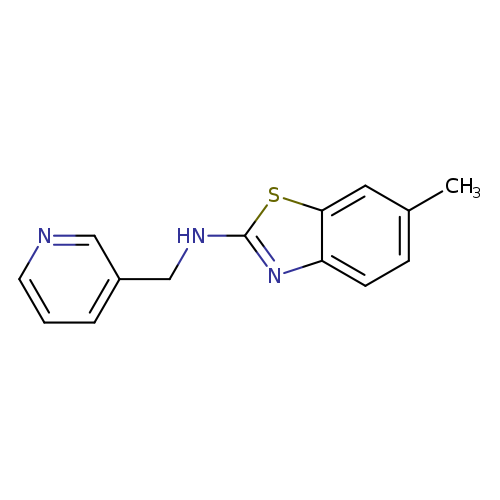
6-Methyl-n-(pyridin-3-ylmethyl)-1,3-benzothiazol-2-amineCatalog No.:AA01AROH CAS No.:1105192-10-6 MDL No.:MFCD11986768 MF:C14H13N3S MW:255.3381 |
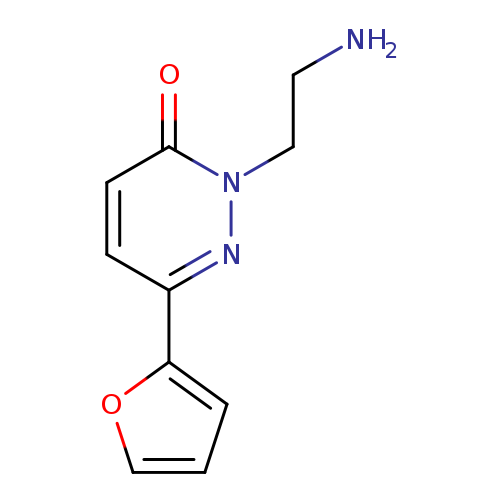
2-(2-AMINOETHYL)-6-(2-FURYL)PYRIDAZIN-3(2(H))-ONECatalog No.:AA01ARO2 CAS No.:1105192-13-9 MDL No.:MFCD11984634 MF:C10H11N3O2 MW:205.2132 |
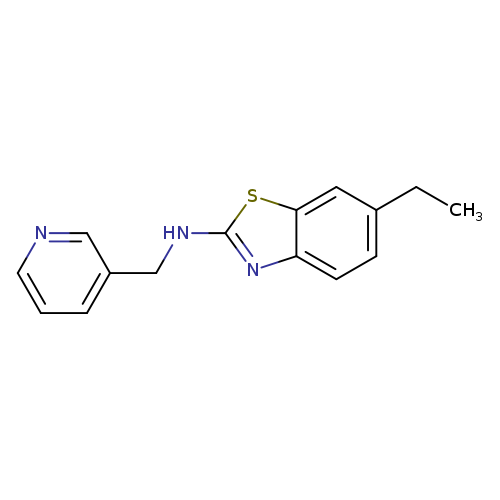
6-Ethyl-N-(pyridin-3-ylmethyl)-1,3-benzothiazol-2-amineCatalog No.:AA01AROI CAS No.:1105192-15-1 MDL No.:MFCD11986769 MF:C15H15N3S MW:269.3647 |
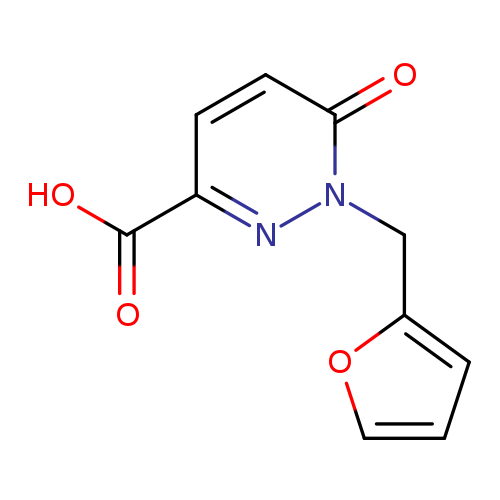
1-(2-Furylmethyl)-6-oxo-1,6-dihydropyridazine-3-carboxylic acidCatalog No.:AA01AQON CAS No.:1105192-25-3 MDL No.:MFCD16652637 MF:C10H8N2O4 MW:220.1815 |
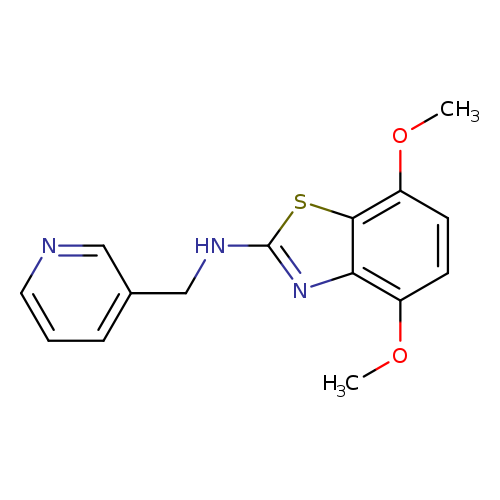
4,7-Dimethoxy-N-(pyridin-3-ylmethyl)benzo[d]thiazol-2-amineCatalog No.:AA01FLXP CAS No.:1105192-32-2 MDL No.:MFCD11986772 MF:C15H15N3O2S MW:301.3635 |
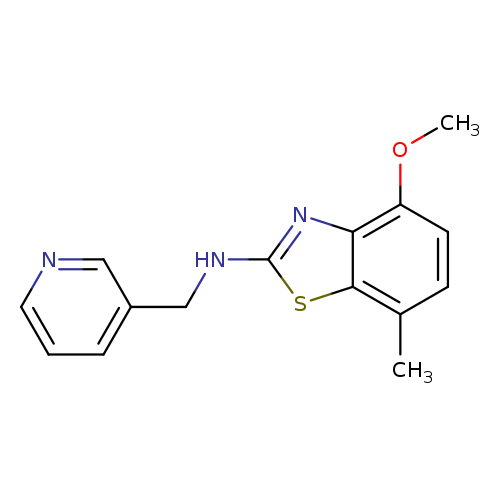
4-Methoxy-7-methyl-N-(pyridin-3-ylmethyl)-1,3-benzothiazol-2-amineCatalog No.:AA01ARP3 CAS No.:1105192-38-8 MDL No.:MFCD11986773 MF:C15H15N3OS MW:285.3641 |
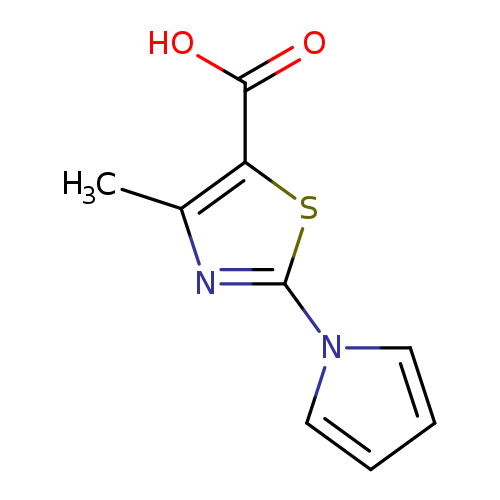
4-Methyl-2-(1h-pyrrol-1-yl)-1,3-thiazole-5-carboxylic acidCatalog No.:AA00HBNU CAS No.:1105192-39-9 MDL No.:MFCD11986883 MF:C9H8N2O2S MW:208.2370 |
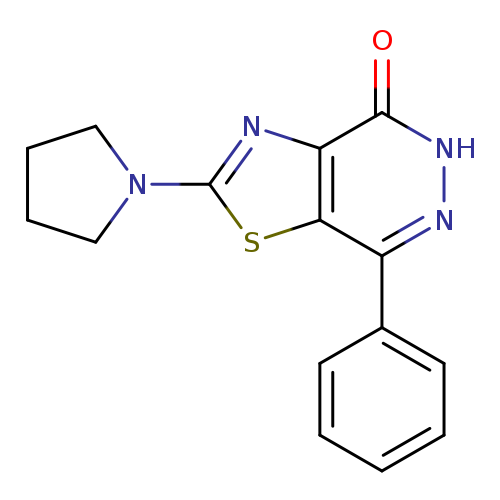
7-Phenyl-2-pyrrolidin-1-yl[1,3]thiazolo[4,5-d]pyridazin-4(5H)-oneCatalog No.:AA01ARQQ CAS No.:1105192-40-2 MDL No.:MFCD11986458 MF:C15H14N4OS MW:298.3629 |
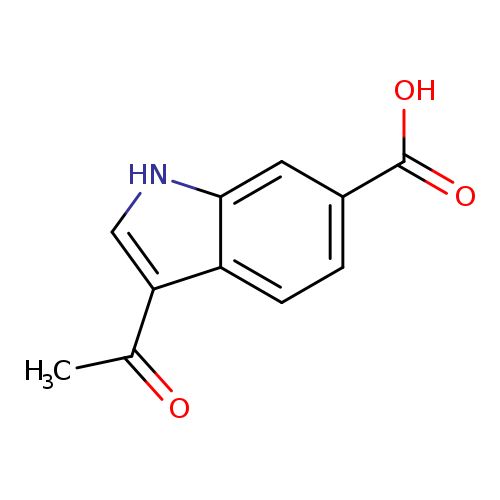
3-Acetyl-1h-indole-6-carboxylic acidCatalog No.:AA01AQD3 CAS No.:1105192-45-7 MDL No.:MFCD16653131 MF:C11H9NO3 MW:203.1941 |
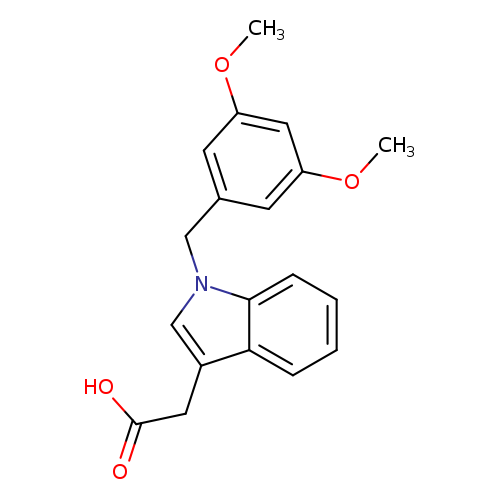
[1-(3,5-Dimethoxybenzyl)-1H-indol-3-yl]acetic acidCatalog No.:AA01ARRP CAS No.:1105192-51-5 MDL No.:MFCD11986884 MF:C19H19NO4 MW:325.3585 |
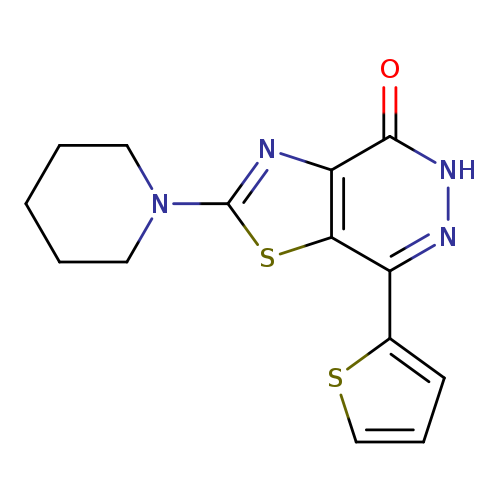
2-Piperidin-1-yl-7-(2-thienyl)[1,3]thiazolo[4,5-d]pyridazin-4(5H)-oneCatalog No.:AA01FML2 CAS No.:1105192-52-6 MDL No.:MFCD11986459 MF:C14H14N4OS2 MW:318.4172 |
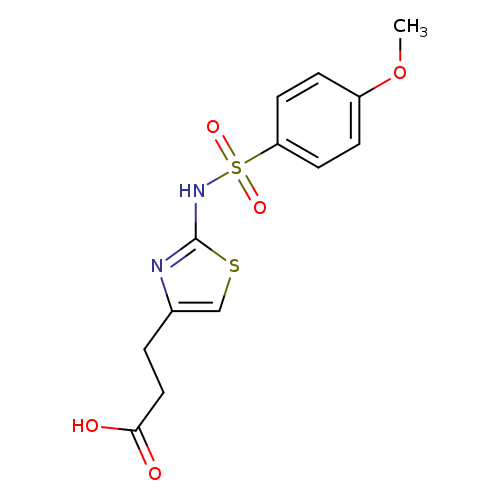
3-(2-([(4-METHOXYPHENYL)SULFONYL]AMINO)-1,3-THIAZOL-4-YL)PROPANOIC ACIDCatalog No.:AA01AQK3 CAS No.:1105192-54-8 MDL No.:MFCD16653154 MF:C13H14N2O5S2 MW:342.3907 |
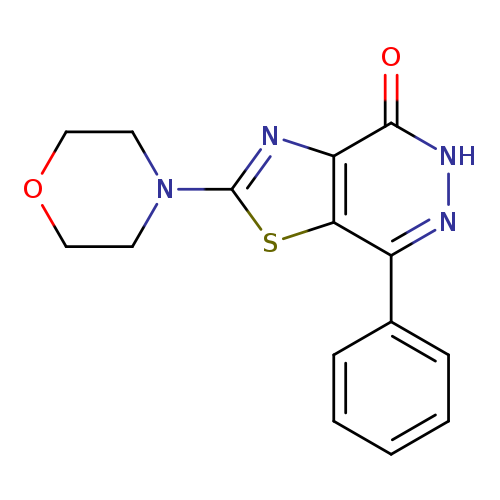
2-Morpholin-4-yl-7-phenyl[1,3]thiazolo[4,5-d]pyridazin-4(5H)-oneCatalog No.:AA01ARRE CAS No.:1105192-58-2 MDL No.:MFCD11986463 MF:C15H14N4O2S MW:314.3623 |
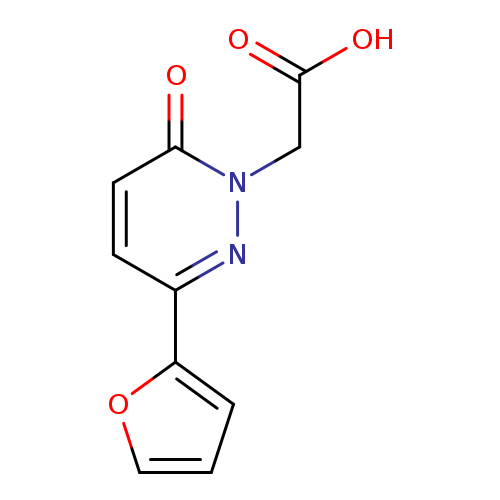
[3-(2-FURYL)-6-OXOPYRIDAZIN-1(6(H))-YL]ACETIC ACIDCatalog No.:AA01AQ6Q CAS No.:1105192-61-7 MDL No.:MFCD16652644 MF:C10H8N2O4 MW:220.1815 |
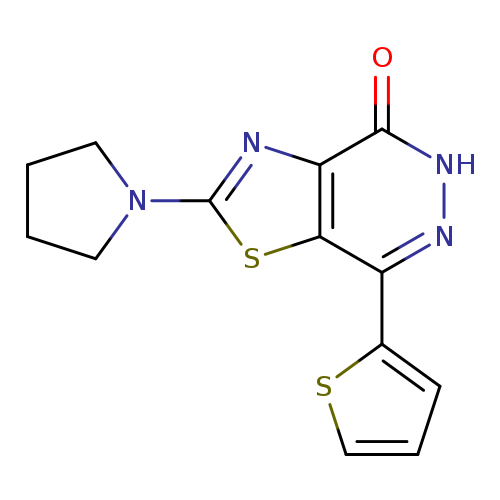
2-Pyrrolidin-1-yl-7-(2-thienyl)[1,3]thiazolo[4,5-d]pyridazin-4(5H)-oneCatalog No.:AA01ARS5 CAS No.:1105192-70-8 MDL No.:MFCD11986465 MF:C13H12N4OS2 MW:304.3906 |
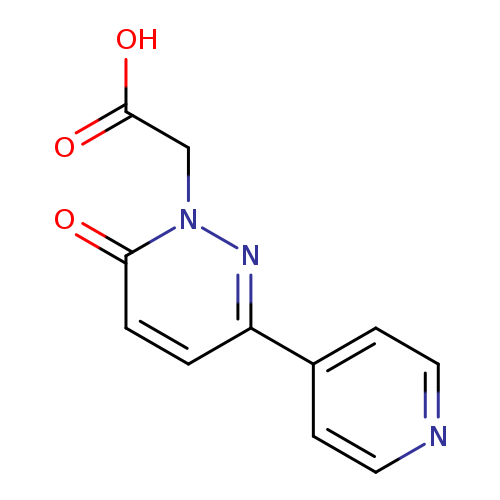
(6-OXO-3-PYRIDIN-4-YLPYRIDAZIN-1(6(H))-YL)ACETIC ACIDCatalog No.:AA01AQ6S CAS No.:1105192-73-1 MDL No.:MFCD16652646 MF:C11H9N3O3 MW:231.2075 |
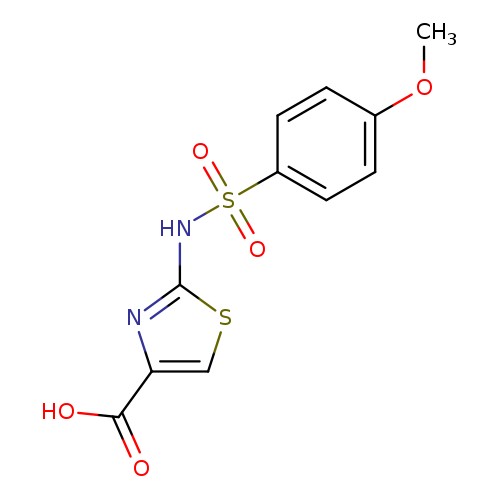
2-([(4-Methoxyphenyl)sulfonyl]amino)-1,3-thiazole-4-carboxylic acidCatalog No.:AA01ARRQ CAS No.:1105192-75-3 MDL No.:MFCD11986889 MF:C11H10N2O5S2 MW:314.3375 |
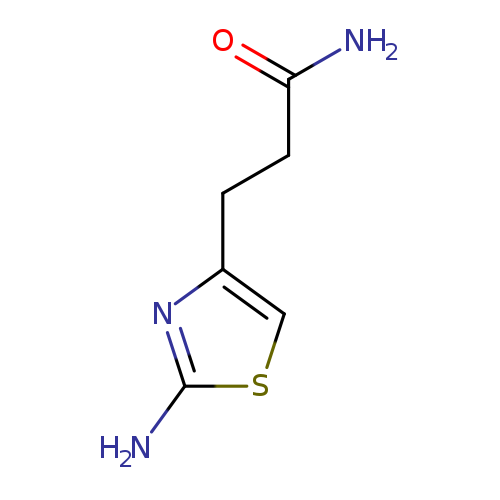
3-(2-amino-1,3-thiazol-4-yl)propanamideCatalog No.:AA01A1C7 CAS No.:1105192-78-6 MDL No.:MFCD22421819 MF:C6H9N3OS MW:171.2202 |
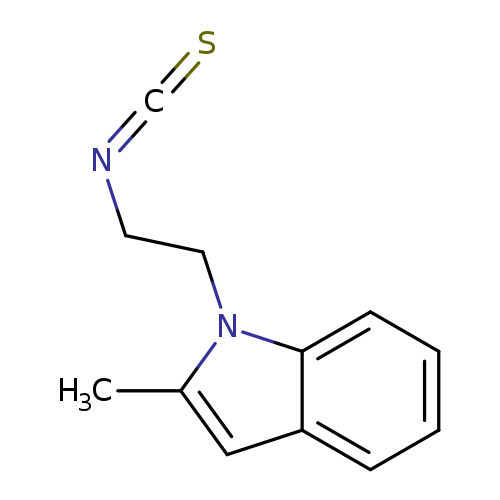
1-(2-Isothiocyanatoethyl)-2-methyl-1H-indoleCatalog No.:AA01AQGV CAS No.:1105192-89-9 MDL No.:MFCD16652904 MF:C12H12N2S MW:216.3021 |
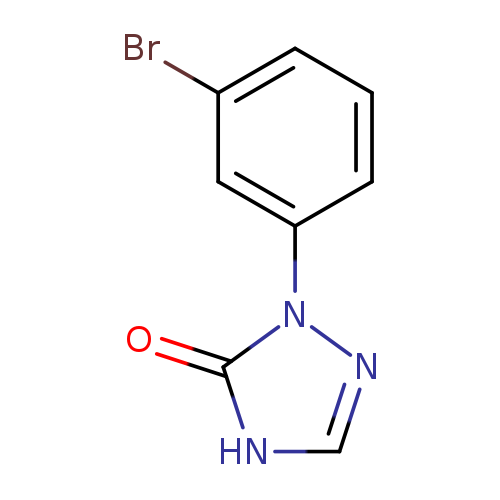
1-(3-bromophenyl)-4,5-dihydro-1H-1,2,4-triazol-5-oneCatalog No.:AA01AQMV CAS No.:1105193-10-9 MDL No.:MFCD16653163 MF:C8H6BrN3O MW:240.0567 |
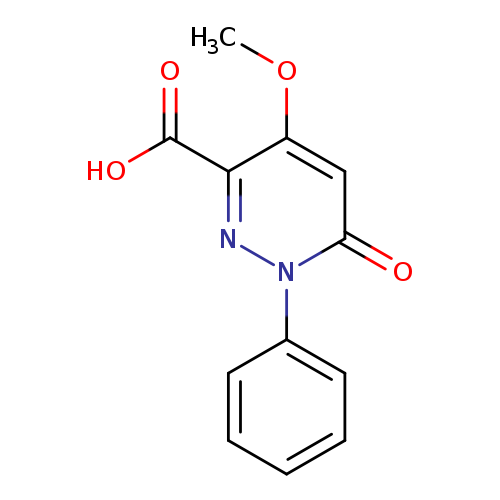
4-Methoxy-6-oxo-1-phenyl-1,6-dihydropyridazine-3-carboxylic acidCatalog No.:AA01AQ82 CAS No.:1105193-11-0 MDL No.:MFCD16652652 MF:C12H10N2O4 MW:246.2188 |
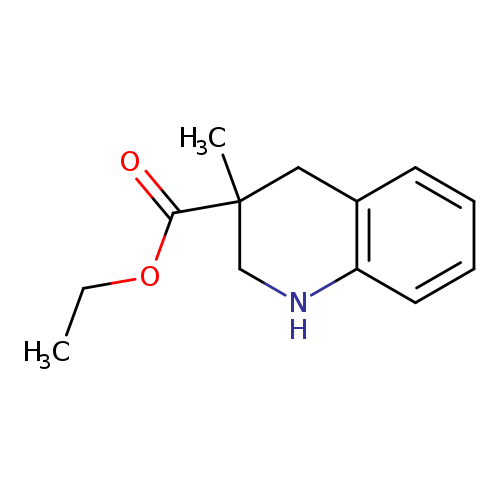
Ethyl 3-methyl-1,2,3,4-tetrahydro-3-quinolinecarboxylateCatalog No.:AA01AQNX CAS No.:1105193-12-1 MDL No.:MFCD16653016 MF:C13H17NO2 MW:219.2796 |
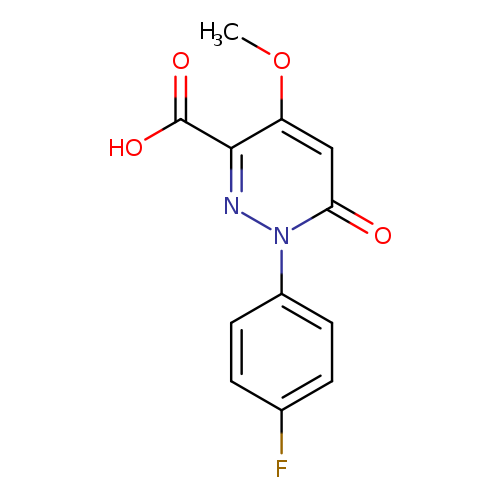
1-(4-fluorophenyl)-4-methoxy-6-oxo-1,6-dihydropyridazine-3-carboxylic acidCatalog No.:AA00J0O7 CAS No.:1105193-26-7 MDL No.:MFCD16652655 MF:C12H9FN2O4 MW:264.2093 |
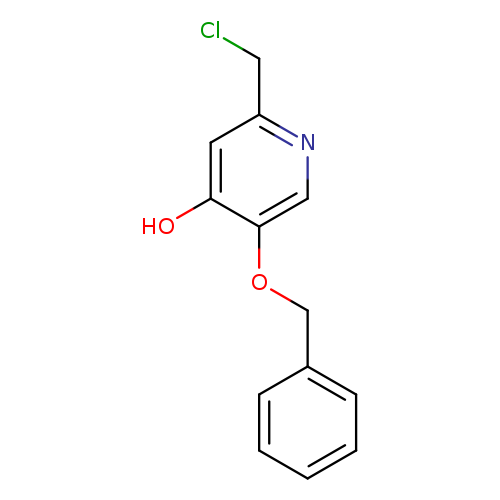
5-(benzyloxy)-2-(chloromethyl)pyridin-4-olCatalog No.:AA00IWIM CAS No.:1105193-30-3 MDL No.:MFCD12922672 MF:C13H12ClNO2 MW:249.6929 |
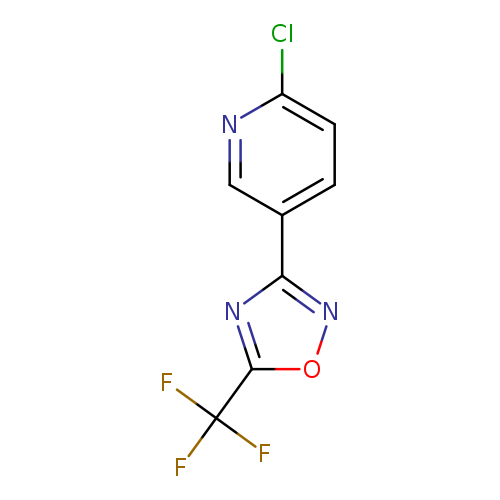
2-Chloro-5-[5-(trifluoromethyl)-1,2,4-oxadiazol-3-yl]pyridineCatalog No.:AA01AQQ9 CAS No.:1105193-32-5 MDL No.:MFCD16653022 MF:C8H3ClF3N3O MW:249.5771 |
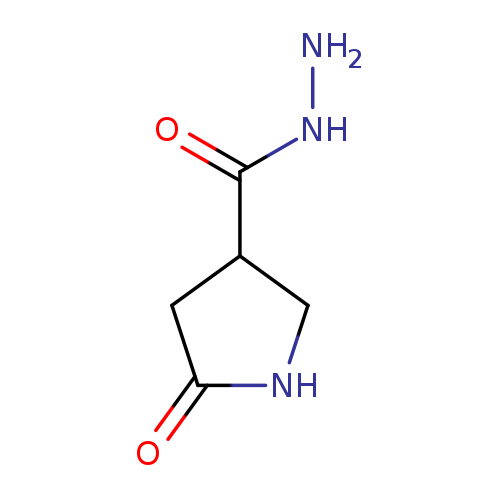
3-Pyrrolidinecarboxylic acid, 5-oxo-, hydrazideCatalog No.:AA00970S CAS No.:1105193-37-0 MDL No.:MFCD16653028 MF:C5H9N3O2 MW:143.1439 |