2020-02-27 09:26:59
Priscilla Maciel Quatrin, Daiane Flores Dalla Lana, Luana Candice Genz Bazana, Luis Fla´vio Souza de Oliveira, Mario Lettieri Teixeira,d Edilma Elaine Silva, William Lopes, Roˆmulo Faria Santos Canto, Gustavo Pozza Silveiraae and Alexandre Meneghello Fuentefria
1.Introduction
In 1993, 2-((phenylsulfinyl)methyl)-3-(phenylthio)-1H-indole was identified as a potent HIV reverse transcriptase inhibitor.9 Since then, several libraries of 3-thio-indole derivatives have been synthesized as new anti-retroviral agents10,11 and as strong poxvirus inhibitors.12 Also, arylthioindoles, such as 5-bromo-3-((3,4,5- trimethoxyphenyl)thio)-1H-indole (1) (Fig. 1), have been proposed as anti-tubulin inhibitors to be used as new antitumor drugs.
Commercially available drugs that are first prescribed to treat cancer, including 5-fluorouracil, gallium compounds, and mito- mycin C, are being repurposed as antibacterials.15 With these points in mind, we demonstrate that a library of 3-calcogenyl-indoles 3 (Fig. 1) presents good activities against Staphylococcus aureus isolates.16 Similarly, anticancer drugs are being repurposed as new antifungals. For instance, the geldanamycin derivative 17-AAG, which is an Hsp90 inhibitor, dramatically improves the fluconazole activity in a Galleria mellonella model of systemic candidiasis.17
Organoselenium compounds have several chemical and pharmacological applications18–22 due to their unique mechanisms of action including broad antimicrobial activity.23 For instance, diphenyl diselenide and its analogues showed growth-inhibition and fungistatic activity against filamentous fungi and pathogenic Candida spp.24 2,20-Dithienyl diselenide demonstrated fungistatic activity toward C. albicans.25 Ebselen analogues presented activity against C. albicans and filamentous fungi.26–28 Selenocyanates have a vast range of biological applications and can be prepared using different methodologies.29 Efficient preparation of 3-selenocyanate- indoles has been reported in the literature by the use of diverse selenocyanate species.
Recently, a library of allylic-selenocyanates, such as 3, was prepared by us as new agents to combat Fusarium spp. involved in human infections.35 Based on these previous studies and with our ongoing program seeking new molecules with anti- microbial properties16,35–37 that can be developed as new leads for future in vivo studies, we decided to prepare a collection of seven 3-selenocyanate-indoles 4 (Fig. 1) and screen them against Candida spp. and dermatophytes. Therefore, this work discloses the results of these screening tests and toxicological studies (cytotoxicity, genotoxicity, mutagenicity, and allergeni- city) to the best hit identified. The results are shown in the following sections.
2.Results and discussion
Seven 3-selenocyanate-indoles, 4a–g (Table 1), were synthesized in one chemical step by an electrophilic aromatic substitution of the respective indole with electrophilic selenocyanate species. Triselenium dicyanide (TSD), which can be easily prepared in situ from malononitrile and selenium dioxide in dimethyl- sulfoxide, was chosen as the electrophilic selenium reagent.32 So, the indoles reacted with TSD in dimethylsulfoxide at room temperature to yield the target indole selenocyanides in excel- lent yields (78–99%, ESI†). In a total, five new compounds, 4c–g, were prepared.
It has been identified that a halogen atom at the 5-position of the indole ring of 3-arylthioindoles results in a reduction of the free energy associated with the binding of the compounds to tubulin, which decreases their cytotoxicity.13 It is known that several compounds presenting anti-cancer activity also demonstrate antimicrobial capacity.15,17,38–40 Preliminary results of our group demonstrated that 3-chalcogenyl-indoles are active toward Gram- positive bacteria (Fig. 1). The lead compound in these screening tests presents a 5-bromoindole moiety.16 Similarly, this work shows the 3-selenocyanate-indoles 4a and 4b as lead compounds for further broad-spectrum antifungal drug development toward Candida spp. and dermatophytes of the genera Trichophyton and Microsporum. These fungi are typical dermatomycosis agents. Noteworthy, compound 4a also has a 5-bromoindole moiety as observed in the previous studies with different targets. The presence of iodine (4c) or other electron withdrawing groups such as CO2CH3 (4d) at the fifth position of the indole ring was deleterious to the activities (Table 1).
The lead compounds 4a and 4b were screened against a panel of 25 Candida strains and 15 filamentous fungi. These compounds presented a broad spectrum fungicidal profile against both genera tested and geometric means of 4.1 and 6.0 mg mL—1 toward Candida spp. and 1.2 and 2.2 mg mL—1 against Trichophyton and Microsporum (Table 2). These results are similar to the ones obtained using FCZ (2.5 mg mL—1), which is the prescribed drug in the treatment of candidiasis.41 Meanwhile, the selenocyanates 4a and 4b were 50–100 times less active than TBF (0.02 mg mL—1) against dermatophytes. However, since resistance to these micro- organisms is continuously observed,42–44 4a and 4b might be an alternative for the reference drug (TBF), especially considering that they do not share the same mechanism of action as that of TBF. The MIC of anidulafungin against yeasts and MEC for filamentous fungi increased significantly in the presence of sorbitol after 7 and 8 days, respectively, as an indication of its fungal cell wall activity. This was not observed for 3-selenocyanate-indoles 4a and 4b, since their MICs did not change (Table S1, ESI†). Similarly, ergo- sterol (100, 150, 200, and 250 mg mL—1) was added into the culture medium to verify if the compounds have action on the fungal cell
membrane. Amphotericin B was used as the control. The MICs also did not vary for the selenocyanate-indoles 4a and 4b (Table S2, ESI†). The ergosterol and sorbitol assays indicated that the broad- spectrum antifungal mechanism of action of compounds 4a and 4b are not related to interactions with the membrane and fungal cell wall chemicals. Hence, scanning electron microscopy (SEM) allowed us to visualize the effects of the 3-selenocyanate- indole 4a on the morphology and amount of C. albicans cells after in vitro treatment. A considerable reduction in the number of blastoconidia, hyphae, and pseudohyphae was evidenced by SEM images after exposure to 4a (Fig. 4). The fungicidal activity of this compound may be related to this effect. The formation of hyphae and pseudohyphae by yeast is considered an important mechanism of pathogenicity, which facilitates tissue invasion, colonization, and infection of mucous membranes.45,46 There-
fore, this information might be clinically relevant.
The fungal cells were exposed for 4, 12, 24, and 48 h (C. albicans) (Fig. 3A and C) and 4b (Fig. 3B and D) at concentrations MIC/2, MIC, MICx2, and MICx4 in a time-kill assay. Compound 4a presented a fungicidal profile (a reduction of 499.9% in log10 relative to the initial inoculum) against C. albicans (ATCC 18804) at MICx2 and MICx4 after 4 h of treatment. Meanwhile, at MIC/2 and MIC, microbial inhibition was observed after 12 h of experiment followed by cell proliferation equivalent to the positive control (Fig. 3A). A fungicidal effect was also observed toward T. rubrum at all concentrations over the initial 8 h of experiment (Fig. 3C). This complete inhibition of their growth in the early hours demonstrates an excellent fungicidal effect and could be further related to a possible high level of clinical efficacy. The time-kill assay demonstrated that 4b exhibits a fungistatic effect (a reduction of o99.9% in log10 in relation to the initial inoculum) against C. albicans (ATCC 18804) at MICx2 and MICx4 after 12 and 24 h of treatment, respectively. However, a significant reduction in the activity after 12 to 24 h of treatment followed by cell proliferation can be observed (Fig. 3B). This effect is noted with azole antifungals. These drugs do not completely eliminate fungal cells but inhibit their growth. The fungistatic mechanism usually works in conjunction with the immune system of the host, killing pathogenic or opportunistic microorganisms. However, this mechanism of action might be a major concern in immunocompromised, patients leading to a persistent infection.47 Meanwhile, the selenocyanate-indole 4b presented a fungicidal profile against T. rubrum (45) at MIC and MICx2 after 24 h and at MICx4 after 8 h of treatment. Cell proliferation is observed at MIC and MICx2 after 8 h of treatment, which would suggest the need for a new therapeutic dose. A dose-dependent effect was noted in the first few hours of the experiment with T. rubrum, which required a new therapeutic dose for complete microbial inhibition, as noticed at MIC and MICx2 concentrations after 24 h of treatment (Fig. 3D).
Selenocyanate-indoles 4a and 4b (32 mg mL—1) were not cytotoxic to human leukocyte cells, since no statistical differences were observed compared to phosphate-buffered saline (PBS, negative control). Bleomycin (BLE, positive control) reduced the cell viability to approximately 20% (Fig. 4A). Similarly, significant micronucleus frequency, as well as apoptotic and necrotic processes for compounds 4a and 4b (32 mg mL—1), was not noticed, inferring a non-mutagenic potential (Fig. 4B). However, the comet assay showed that 4a and 4b (32 mg mL—1) caused significant DNA damage (Fig. 4C).
As disclosed by Pukalskien˙e et al.,48 the divergence in comet and micronucleus results can be explained by the fact that proliferating leukocytes were used for the micronucleus assay. Leukocyte cells have higher repair capacity than cells in the comet’s G0 phase (resting cells). Therefore, the DNA damage observed in the comet assay can be repaired during the micro- nucleus experiment.49 Since the nuclei of the fungal and mammalian cells have similarities, it is believed that the targets of the compounds are the nucleic acids. Further experiments might be conducted to confirm this hypothesis.
Most imidazole antifungals present cell toxicity or bioavail- ability problems and are formulated for topical use.50 Ketoconazole is cytotoxic and presents a possible mechanism of mitochondrial dysfunction in liver cells, compromising mitochondrial DNA synthesis, resulting in induced apoptosis.51 Thus, even drugs demonstrating cellular toxicity for systemic use are prescribed for topical administration. In addition, in the present study, the toxicity of the selenocyanate-indoles 4a and 4b was evaluated at 32 mg mL—1 and the MICs (geometric mean) determined were in the range of 1.2 to 6.0 mg mL—1. Thus, DNA damage is being observed at higher concentrations of the compounds compared to the MICs.
The selenocyanate-indoles 4a and 4b (32 mg mL—1) are found to be non-irritant (IS = 2.19 and 3.01, respectively) through the HET-CAM assay (Fig. S15, ESI†). The chorioallantoic membrane is highly vascularized and responds to injuries caused by processes, such as inflammation, similar to that observed in the conjunctival tissue of rabbit eyes.52 This is an indication that formulations for topical administration might be developed without further mucous irritation.
Synergistic effects as a consequence of coinfection of Candida spp. with Staphylococcus have been demonstrated by invitro and ex vivo54 studies. It is assumed that Gram-positive bacteria adhere or bind to the hyphae of Candida spp. For instance, it has been shown that C. albicans can transport S. aureus into tissues disseminating the infection in an oral co-colonization model. Therefore, co-infection of Candida spp. and S. aureus results in a more serious infection than that caused by each microorganism individually.54 3-Selenocyanate-indoles 4a and 4b were also screened against S. aureus (ATCC 25923) and the MICs determined were 8 mg mL—1 (see the ESI†), indicating that the compounds might have antimicrobial capacities, avoiding this kind of co-infection.
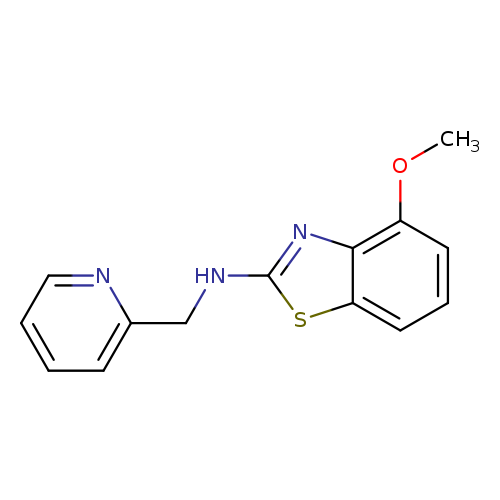
4-Methoxy-N-(pyridin-2-ylmethyl)-1,3-benzothiazol-2-amineCatalog No.:AA01ARLW CAS No.:1105195-04-7 MDL No.:MFCD11986751 MF:C14H13N3OS MW:271.3375 |
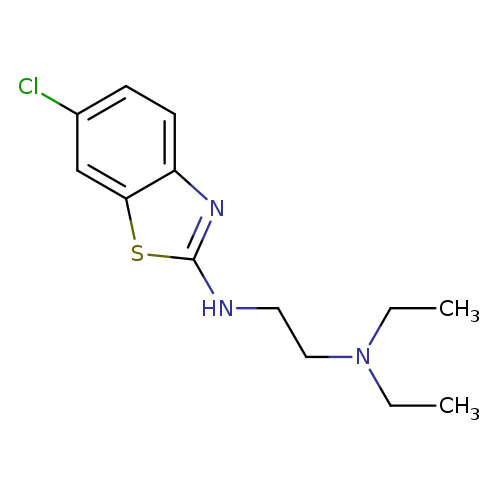
N'-(6-Chloro-1,3-benzothiazol-2-yl)-N,N-diethylethane-1,2-diamineCatalog No.:AA01ARJA CAS No.:1105195-05-8 MDL No.:MFCD11986980 MF:C13H18ClN3S MW:283.8201 |
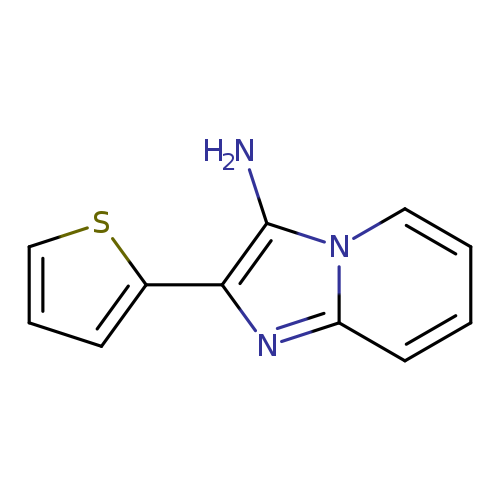
2-thien-2-ylimidazo[1,2-a]pyridin-3-amineCatalog No.:AA00J5IQ CAS No.:1105195-06-9 MDL No.:MFCD15732273 MF:C11H9N3S MW:215.2743 |
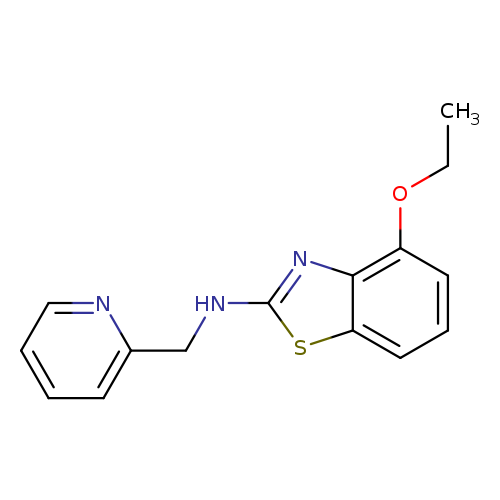
4-Ethoxy-N-(pyridin-2-ylmethyl)-1,3-benzothiazol-2-amineCatalog No.:AA01ARLX CAS No.:1105195-08-1 MDL No.:MFCD11986752 MF:C15H15N3OS MW:285.3641 |
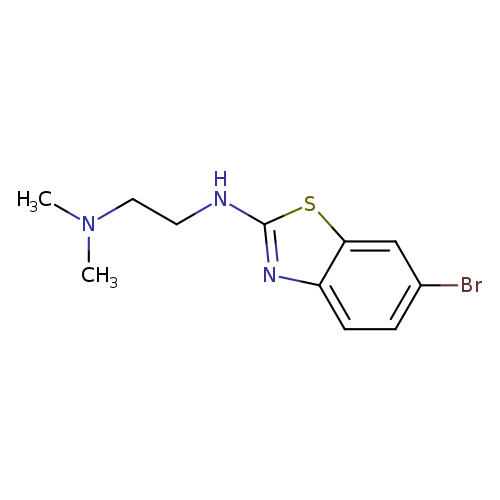
N'-(6-Bromo-1,3-benzothiazol-2-yl)-N,N-dimethylethane-1,2-diamineCatalog No.:AA01ARJC CAS No.:1105195-13-8 MDL No.:MFCD11986982 MF:C11H14BrN3S MW:300.2180 |
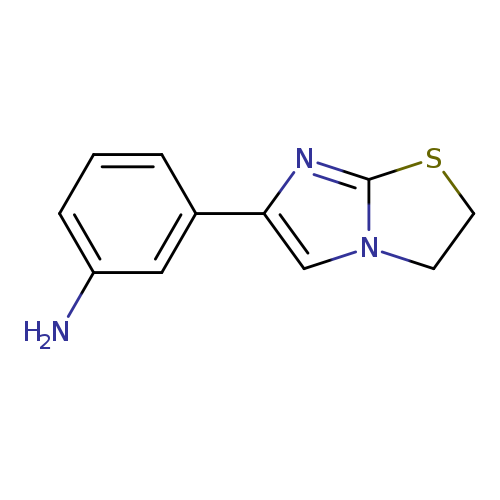
[3-(2,3-Dihydroimidazo[2,1-(b)][1,3]thiazol-6-yl)phenyl]amineCatalog No.:AA01AQ8Z CAS No.:1105195-14-9 MDL No.:MFCD16630775 MF:C11H11N3S MW:217.2901 |
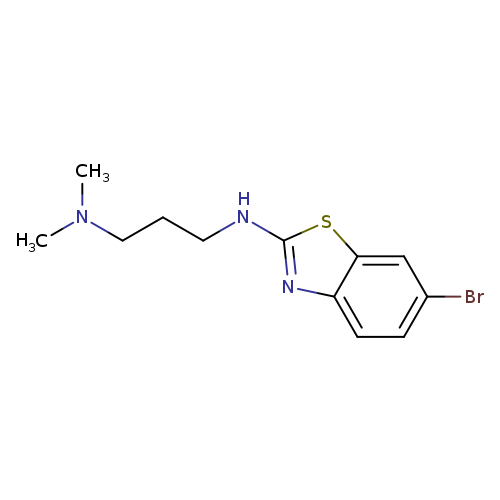
N'-(6-Bromo-1,3-benzothiazol-2-yl)-N,N-dimethylpropane-1,3-diamineCatalog No.:AA01ARJD CAS No.:1105195-16-1 MDL No.:MFCD11986983 MF:C12H16BrN3S MW:314.2445 |
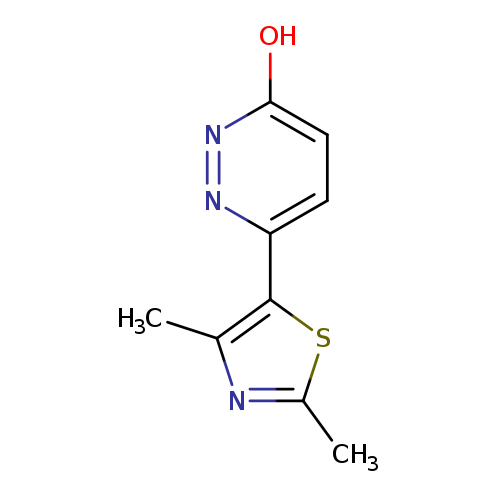
6-(2,4-Dimethyl-1,3-thiazol-5-yl)pyridazin-3-olCatalog No.:AA01AQGA CAS No.:1105195-22-9 MDL No.:MFCD16631566 MF:C9H9N3OS MW:207.2523 |
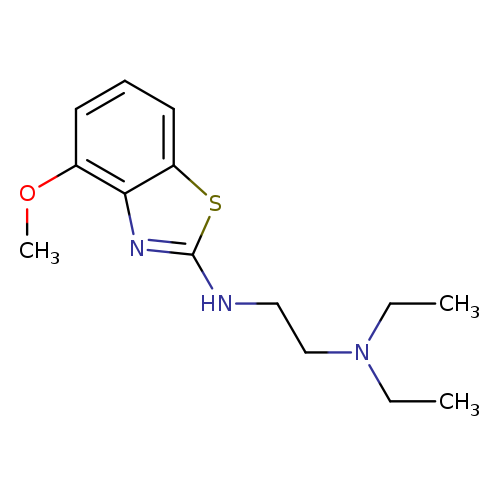
N,N-Diethyl-N'-(4-methoxy-1,3-benzothiazol-2-yl)ethane-1,2-diamineCatalog No.:AA01ARJE CAS No.:1105195-27-4 MDL No.:MFCD11986984 MF:C14H21N3OS MW:279.4010 |
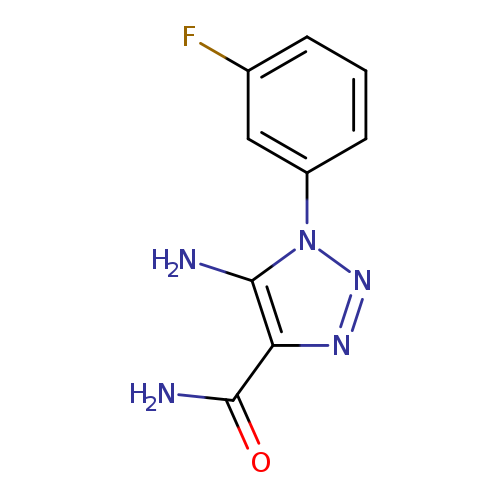
5-AMINO-1-(3-FLUOROPHENYL)-1H-1,2,3-TRIAZOLE-4-CARBOXAMIDECatalog No.:AA01AQE7 CAS No.:1105195-29-6 MDL No.:MFCD16653064 MF:C9H8FN5O MW:221.1911 |
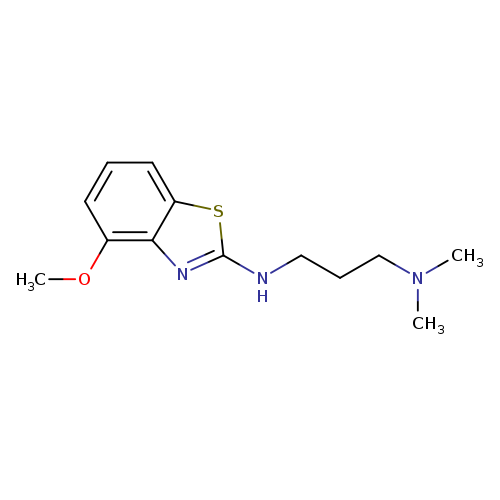
N'-(4-METHOXY-1,3-BENZOTHIAZOL-2-YL)-N,N-DIMETHYLPROPANE-1,3-DIAMINECatalog No.:AA01AQOF CAS No.:1105195-30-9 MDL No.:MFCD16653384 MF:C13H19N3OS MW:265.3745 |
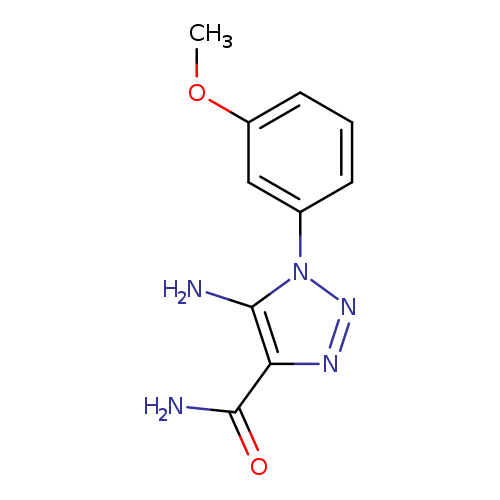
5-AMINO-1-(3-METHOXYPHENYL)-1H-1,2,3-TRIAZOLE-4-CARBOXAMIDECatalog No.:AA01AQFJ CAS No.:1105195-32-1 MDL No.:MFCD16653067 MF:C10H11N5O2 MW:233.2266 |
3-PIPERAZIN-1-YL-6-PYRIDIN-4-YLPYRIDAZINECatalog No.:AA01AQPU CAS No.:1105195-37-6 MDL No.:MFCD16652720 MF:C13H15N5 MW:241.2917 |
N'-(4-Chloro-1,3-benzothiazol-2-yl)-N,N-dimethylethane-1,2-diamineCatalog No.:AA01ARK3 CAS No.:1105195-39-8 MDL No.:MFCD11986987 MF:C11H14ClN3S MW:255.7670 |
3-Piperazin-1-yl-6-(2-thienyl)pyridazineCatalog No.:AA00JSR9 CAS No.:1105195-40-1 MDL No.:MFCD07386385 MF:C12H14N4S MW:246.3314 |
5-Methoxy-1-methylpyrido[2,3-d]pyrimidine-2,4(1H,3H)-dioneCatalog No.:AA01AQJ8 CAS No.:1105195-59-2 MDL No.:MFCD16631649 MF:C9H9N3O3 MW:207.1861 |
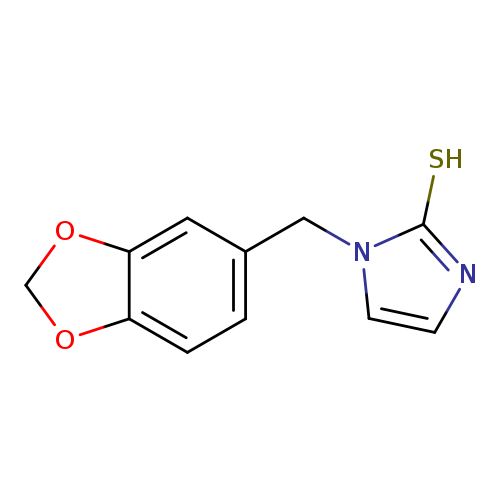
1-(1,3-Benzodioxol-5-ylmethyl)-1H-imidazole-2-thiolCatalog No.:AA01FLZI CAS No.:1105195-60-5 MDL No.:MFCD11986805 MF:C11H10N2O2S MW:234.2743 |
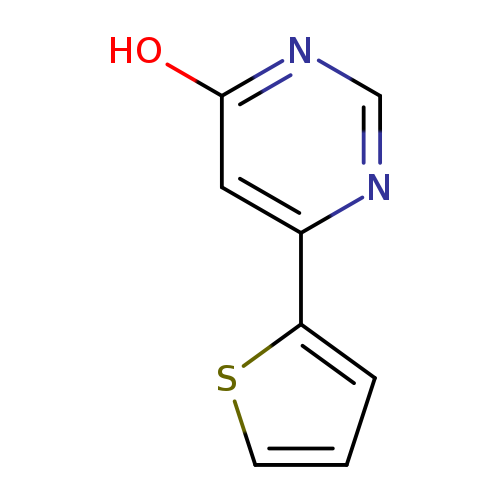
6-(2-Thienyl)-4(3H)-pyrimidinoneCatalog No.:AA01DZJ6 CAS No.:1105195-61-6 MDL No.:MFCD28152366 MF:C8H6N2OS MW:178.2110 |
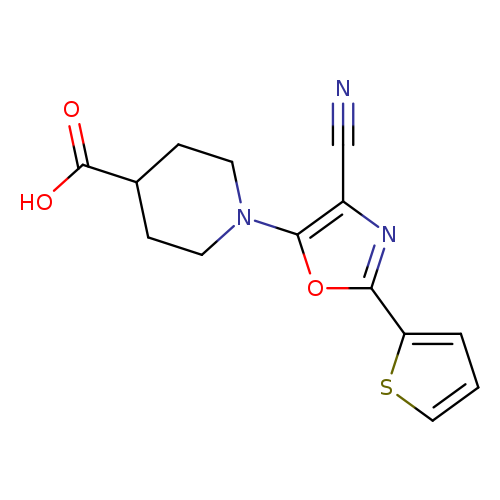
1-[4-Cyano-2-(2-thienyl)-1,3-oxazol-5-yl]piperidine-4-carboxylic acidCatalog No.:AA01ARHK CAS No.:1105195-76-3 MDL No.:MFCD11518871 MF:C14H13N3O3S MW:303.3363 |
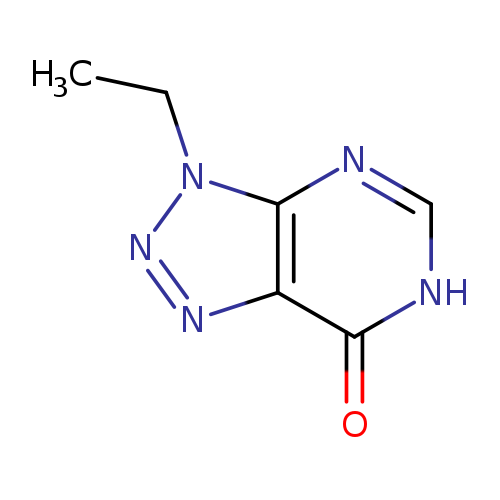
3-ETHYL-3,6-DIHYDRO-7H-[1,2,3]TRIAZOLO[4,5-D]PYRIMIDIN-7-ONECatalog No.:AA01AQLG CAS No.:1105195-78-5 MDL No.:MFCD16653085 MF:C6H7N5O MW:165.1527 |
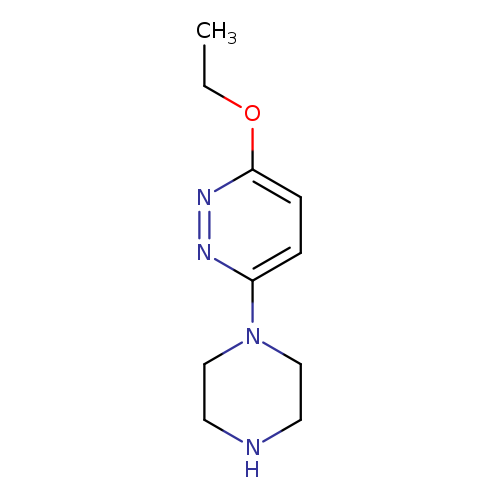
3-ethoxy-6-(piperazin-1-yl)pyridazineCatalog No.:AA01AROW CAS No.:1105195-92-3 MDL No.:MFCD11986518 MF:C10H16N4O MW:208.2602 |
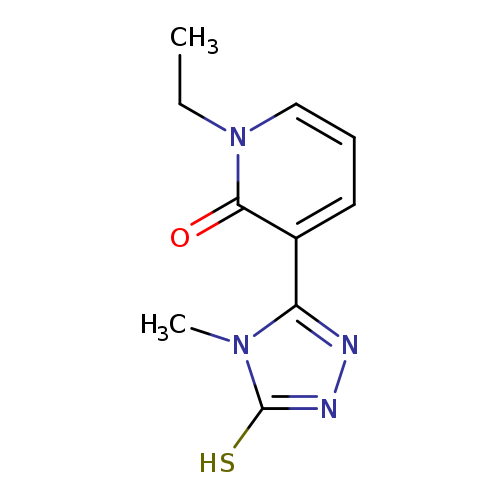
1-Ethyl-3-(5-mercapto-4-methyl-4h-1,2,4-triazol-3-yl)pyridin-2(1h)-oneCatalog No.:AA01FN16 CAS No.:1105196-08-4 MDL No.:MFCD16652893 MF:C10H12N4OS MW:236.2935 |
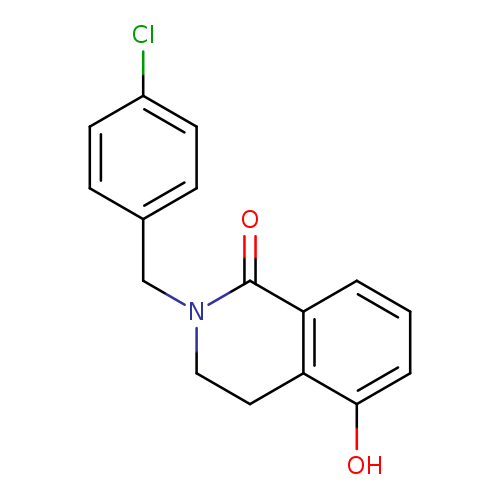
2-(4-CHLOROBENZYL)-5-HYDROXY-3,4-DIHYDROISOQUINOLIN-1(2H)-ONECatalog No.:AA01ARQR CAS No.:1105196-16-4 MDL No.:MFCD11986532 MF:C16H14ClNO2 MW:287.7409 |
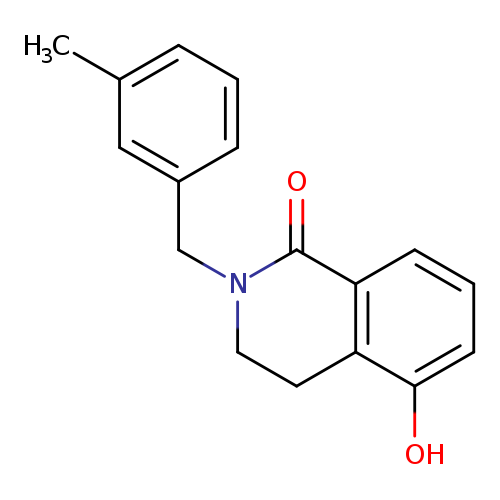
5-HYDROXY-2-(3-METHYLBENZYL)-3,4-DIHYDROISOQUINOLIN-1(2H)-ONECatalog No.:AA01ARQS CAS No.:1105196-17-5 MDL No.:MFCD11986533 MF:C17H17NO2 MW:267.3224 |
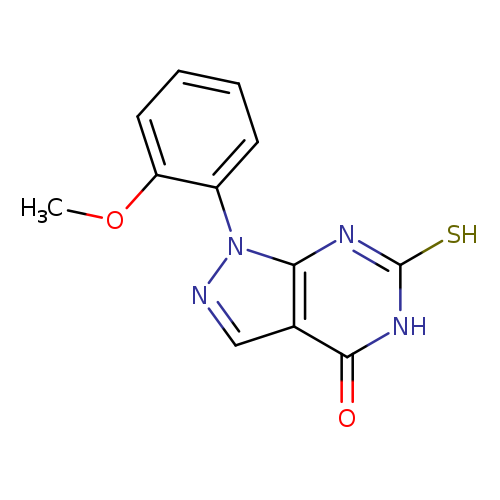
6-Mercapto-1-(2-methoxyphenyl)-1,5-dihydro-4h-pyrazolo[3,4-d]pyrimidin-4-oneCatalog No.:AA01ARQU CAS No.:1105196-23-3 MDL No.:MFCD11986535 MF:C12H10N4O2S MW:274.2984 |
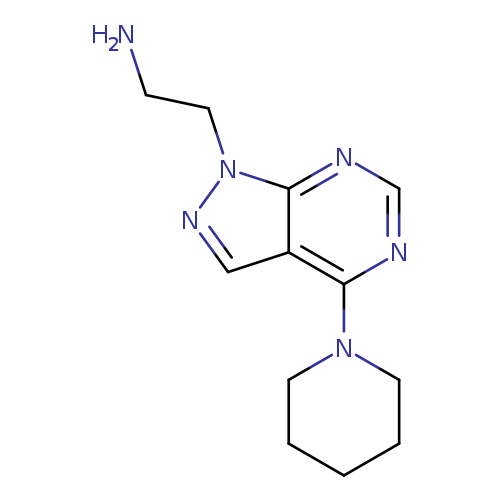
[2-(4-Piperidin-1-yl-1H-pyrazolo[3,4-d]pyrimidin-1-yl)ethyl]amineCatalog No.:AA01AQDZ CAS No.:1105196-28-8 MDL No.:MFCD16652895 MF:C12H18N6 MW:246.3115 |
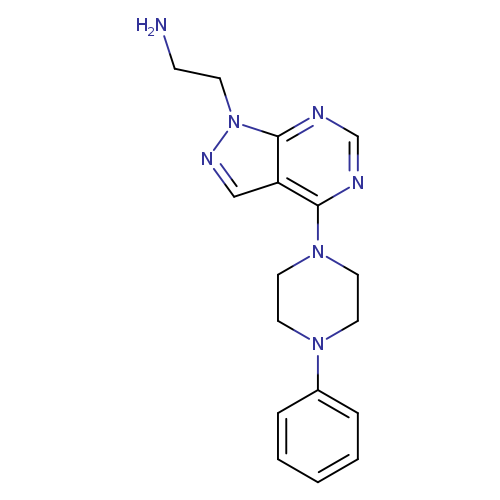
"2-[4-(4-phenylpiperazin-1-yl)-1H-pyrazolo[3,4-d]pyrimidin-1-yl]ethan-1-amine"Catalog No.:AA01FMGO CAS No.:1105196-33-5 MDL No.:MFCD11986539 MF:C17H21N7 MW:323.3955 |
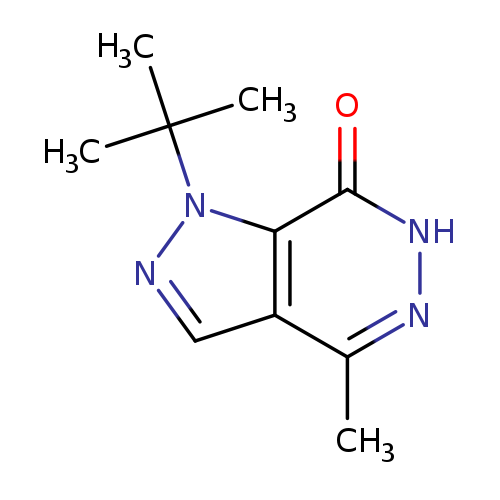
1-tert-Butyl-4-methyl-1,6-dihydro-7h-pyrazolo[3,4-d]pyridazin-7-oneCatalog No.:AA01ARS8 CAS No.:1105196-44-8 MDL No.:MFCD11986544 MF:C10H14N4O MW:206.2444 |
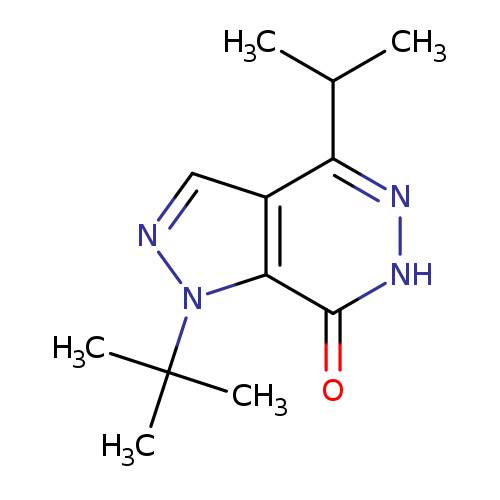
1-tert-Butyl-4-(propan-2-yl)-1h,6h,7h-pyrazolo[3,4-d]pyridazin-7-oneCatalog No.:AA01ART2 CAS No.:1105196-46-0 MDL No.:MFCD11986545 MF:C12H18N4O MW:234.2975 |
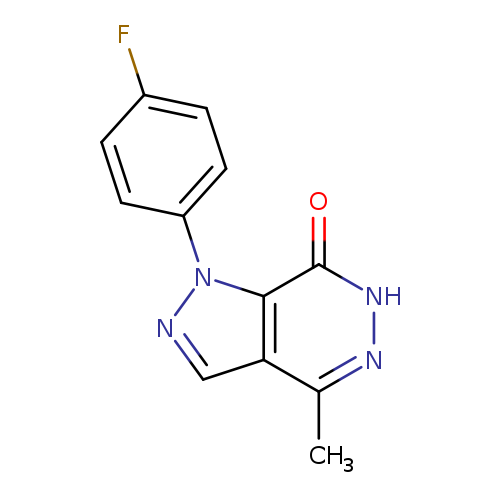
1-(4-Fluorophenyl)-4-methyl-1,6-dihydro-7h-pyrazolo[3,4-d]pyridazin-7-oneCatalog No.:AA01ART3 CAS No.:1105196-48-2 MDL No.:MFCD11986546 MF:C12H9FN4O MW:244.2245 |
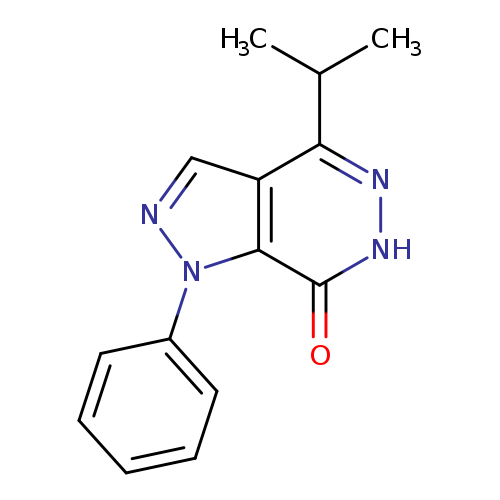
1-phenyl-4-(propan-2-yl)-1H,6H,7H-pyrazolo[3,4-d]pyridazin-7-oneCatalog No.:AA01ART4 CAS No.:1105196-52-8 MDL No.:MFCD11986547 MF:C14H14N4O MW:254.2872 |
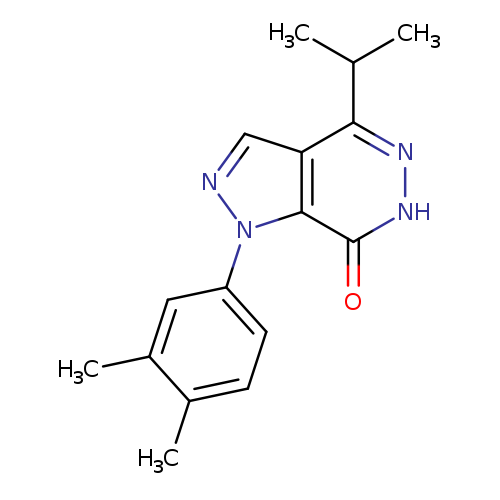
1-(3,4-Dimethylphenyl)-4-isopropyl-1,6-dihydro-7h-pyrazolo[3,4-d]pyridazin-7-oneCatalog No.:AA01ARTY CAS No.:1105196-58-4 MDL No.:MFCD11986549 MF:C16H18N4O MW:282.3403 |
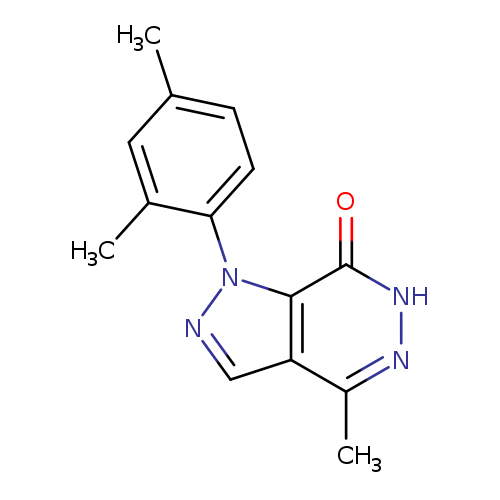
1-(2,4-DIMETHYLPHENYL)-4-METHYL-1,6-DIHYDRO-7H-PYRAZOLO[3,4-D]PYRIDAZIN-7+Catalog No.:AA01ARU0 CAS No.:1105196-61-9 MDL No.:MFCD11986551 MF:C14H14N4O MW:254.2872 |
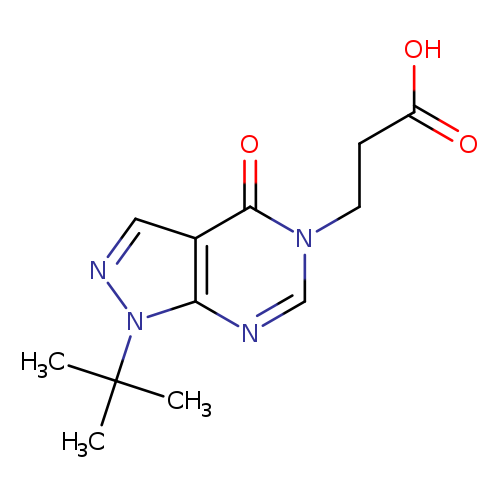
3-(1-TERT-BUTYL-4-OXO-1,4-DIHYDRO-5H-PYRAZOLO[3,4-D]PYRIMIDIN-5-YL)PROPAN+Catalog No.:AA01ARIU CAS No.:1105196-70-0 MDL No.:MFCD11986554 MF:C12H16N4O3 MW:264.2804 |
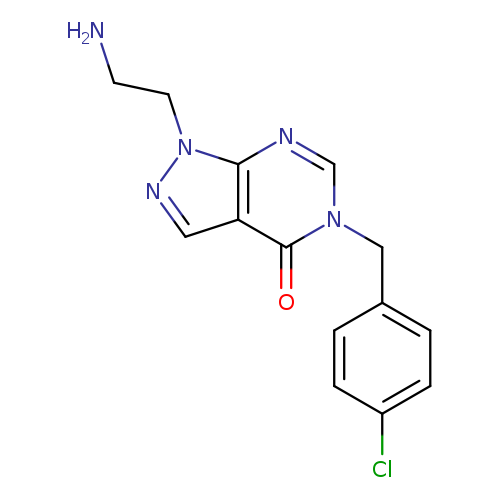
"1-(2-aminoethyl)-5-[(4-chlorophenyl)methyl]-1H,4H,5H-pyrazolo[3,4-d]pyrimidin-4-one"Catalog No.:AA01FMF4 CAS No.:1105196-80-2 MDL No.:MFCD11986557 MF:C14H14ClN5O MW:303.7469 |
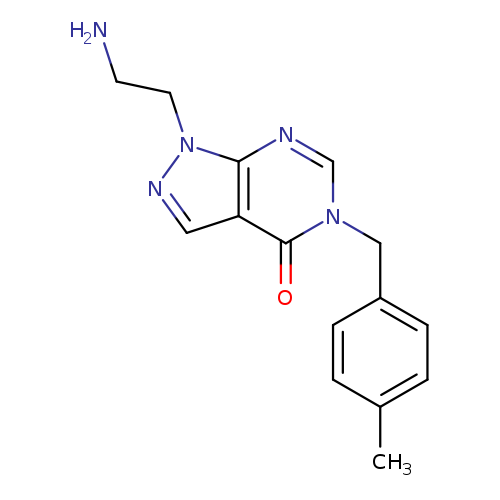
1-(2-AMINOETHYL)-5-(4-METHYLBENZYL)-1,5-DIHYDRO-4H-PYRAZOLO[3,4-D]PYRIMID+Catalog No.:AA01ARJL CAS No.:1105196-83-5 MDL No.:MFCD11986558 MF:C15H17N5O MW:283.3284 |
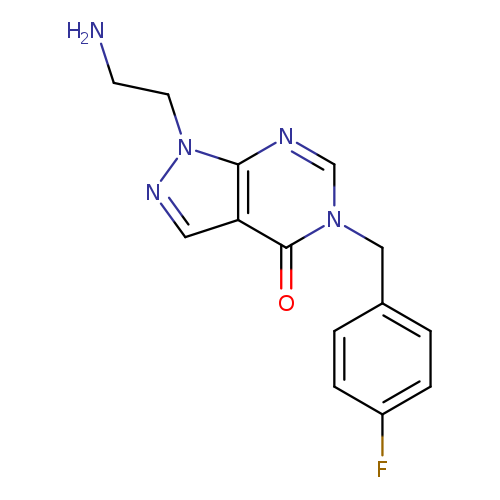
1-(2-Aminoethyl)-5-(4-fluorobenzyl)-1,5-dihydro-4h-pyrazolo[3,4-d]pyrimidin-4-oneCatalog No.:AA01ARJM CAS No.:1105196-86-8 MDL No.:MFCD11986559 MF:C14H14FN5O MW:287.2923 |
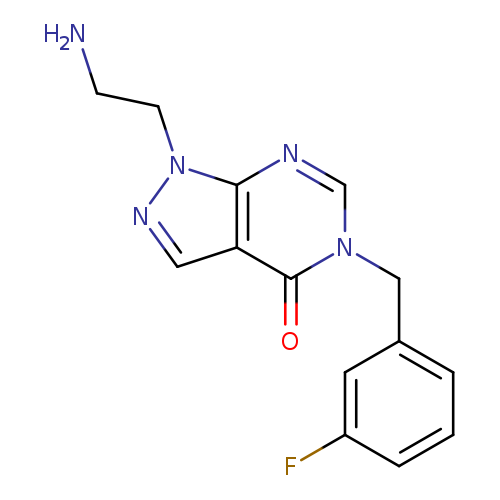
1-(2-Aminoethyl)-5-(3-fluorobenzyl)-1,5-dihydro-4h-pyrazolo[3,4-d]pyrimidin-4-oneCatalog No.:AA01ARJN CAS No.:1105196-92-6 MDL No.:MFCD11986561 MF:C14H14FN5O MW:287.2923 |
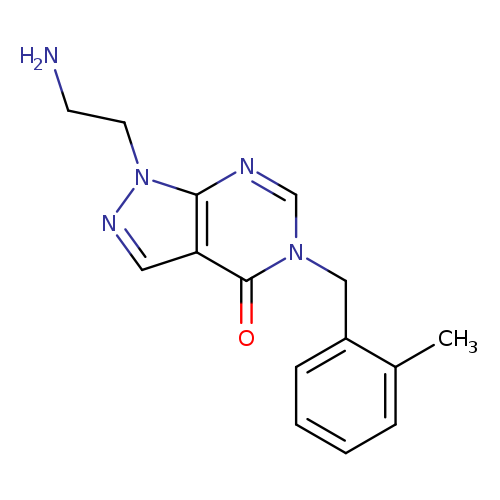
1-(2-Aminoethyl)-5-(2-methylbenzyl)-1,5-dihydro-4h-pyrazolo[3,4-d]pyrimidin-4-oneCatalog No.:AA01ARK9 CAS No.:1105196-98-2 MDL No.:MFCD11986563 MF:C15H17N5O MW:283.3284 |
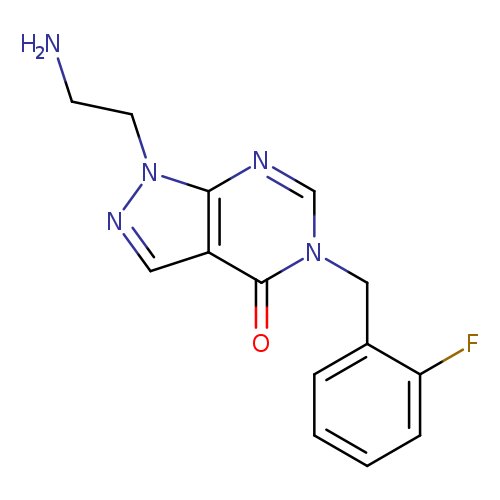
1-(2-Aminoethyl)-5-(2-fluorobenzyl)-1,5-dihydro-4h-pyrazolo[3,4-d]pyrimidin-4-oneCatalog No.:AA01ARKA CAS No.:1105197-02-1 MDL No.:MFCD11986564 MF:C14H14FN5O MW:287.2923 |
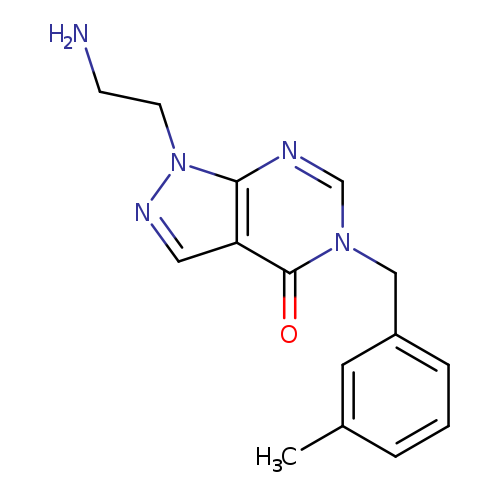
1-(2-AMINOETHYL)-5-(3-METHYLBENZYL)-1,5-DIHYDRO-4H-PYRAZOLO[3,4-D]PYRIMID+Catalog No.:AA01ARKC CAS No.:1105197-08-7 MDL No.:MFCD11986566 MF:C15H17N5O MW:283.3284 |
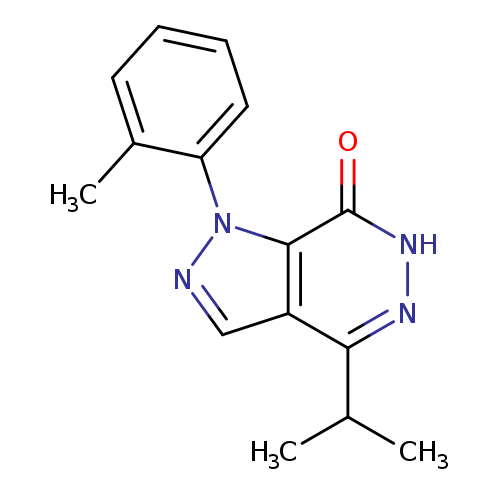
4-Isopropyl-1-(2-methylphenyl)-1,6-dihydro-7h-pyrazolo[3,4-d]pyridazin-7-oneCatalog No.:AA01ARKX CAS No.:1105197-18-9 MDL No.:MFCD11986569 MF:C15H16N4O MW:268.3137 |
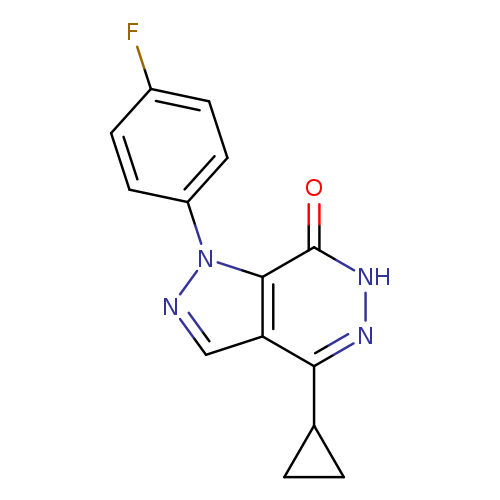
4-CYCLOPROPYL-1-(4-FLUOROPHENYL)-1,6-DIHYDRO-7H-PYRAZOLO[3,4-D]PYRIDAZIN-+Catalog No.:AA01ARKY CAS No.:1105197-21-4 MDL No.:MFCD11986570 MF:C14H11FN4O MW:270.2617 |
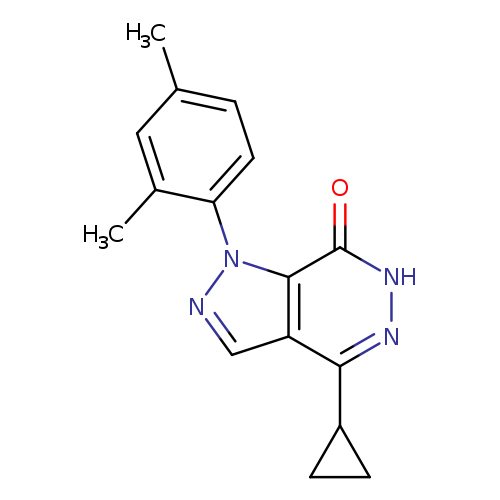
4-CYCLOPROPYL-1-(2,4-DIMETHYLPHENYL)-1,6-DIHYDRO-7H-PYRAZOLO[3,4-D]PYRIDA+Catalog No.:AA01ARKZ CAS No.:1105197-25-8 MDL No.:MFCD11986571 MF:C16H16N4O MW:280.3244 |
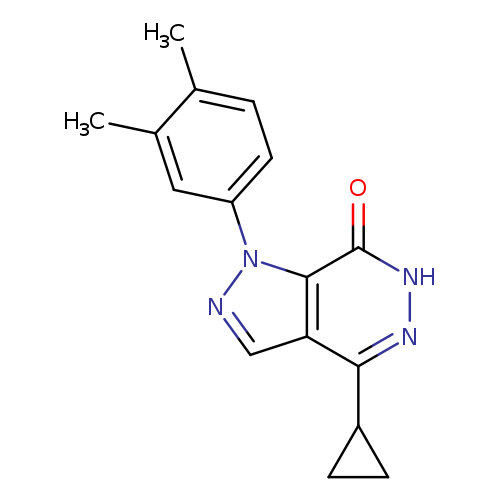
4-CYCLOPROPYL-1-(3,4-DIMETHYLPHENYL)-1,6-DIHYDRO-7H-PYRAZOLO[3,4-D]PYRIDA+Catalog No.:AA01ARLQ CAS No.:1105197-29-2 MDL No.:MFCD11986572 MF:C16H16N4O MW:280.3244 |
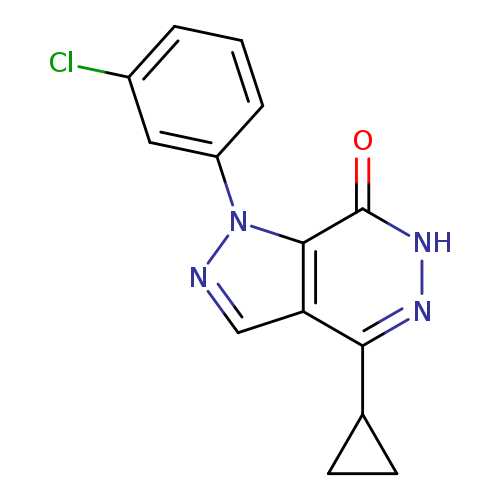
1-(3-CHLOROPHENYL)-4-CYCLOPROPYL-1,6-DIHYDRO-7H-PYRAZOLO[3,4-D]PYRIDAZIN-+Catalog No.:AA01ARLR CAS No.:1105197-33-8 MDL No.:MFCD11986573 MF:C14H11ClN4O MW:286.7163 |
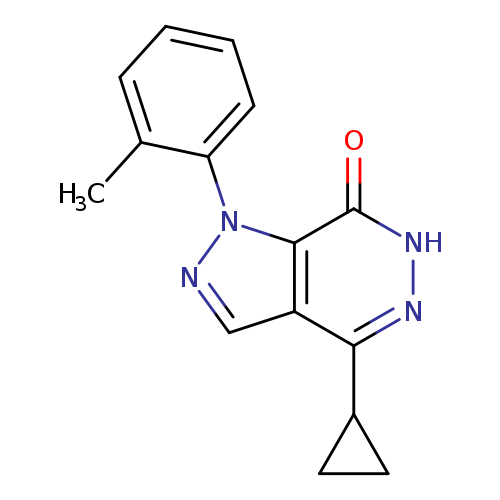
4-CYCLOPROPYL-1-(2-METHYLPHENYL)-1,6-DIHYDRO-7H-PYRAZOLO[3,4-D]PYRIDAZIN-+Catalog No.:AA01ARLS CAS No.:1105197-37-2 MDL No.:MFCD11986574 MF:C15H14N4O MW:266.2979 |
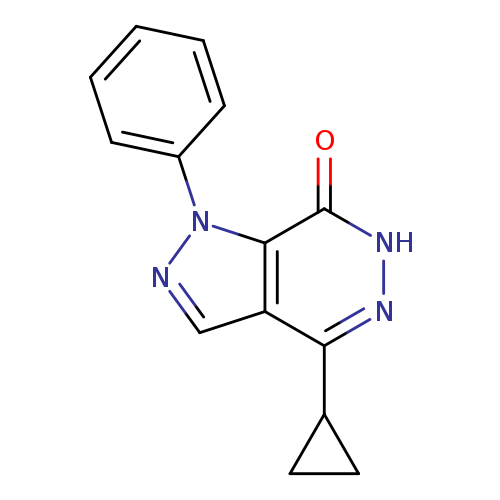
4-cyclopropyl-1-phenyl-1H,6H,7H-pyrazolo[3,4-d]pyridazin-7-oneCatalog No.:AA01ARLT CAS No.:1105197-41-8 MDL No.:MFCD11986575 MF:C14H12N4O MW:252.2713 |
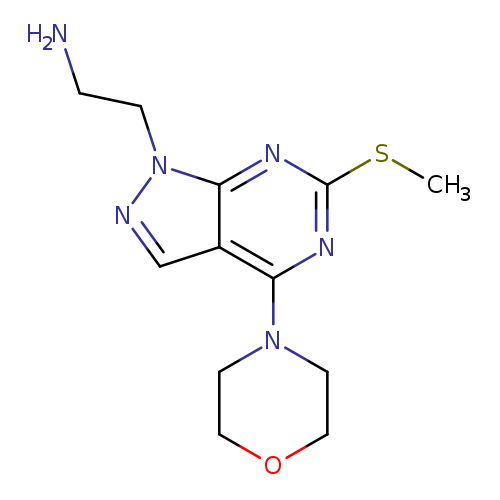
(2-[6-(METHYLTHIO)-4-MORPHOLIN-4-YL-1H-PYRAZOLO[3,4-D]PYRIMIDIN-1-YL]ETHY+Catalog No.:AA01AQF8 CAS No.:1105197-45-2 MDL No.:MFCD16652896 MF:C12H18N6OS MW:294.3759 |
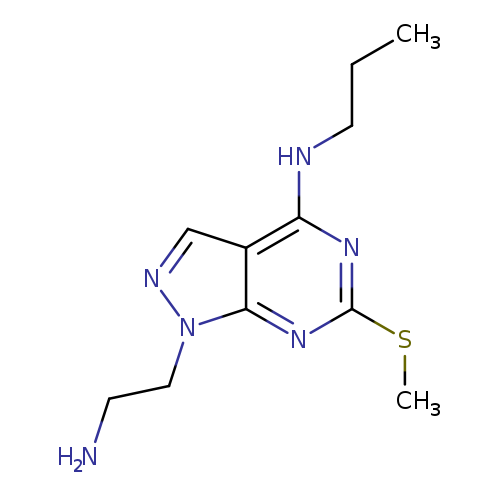
1-(2-AMINOETHYL)-6-(METHYLTHIO)-N-PROPYL-1H-PYRAZOLO[3,4-D]PYRIMIDIN-4-AM+Catalog No.:AA01ARMK CAS No.:1105197-49-6 MDL No.:MFCD11986576 MF:C11H18N6S MW:266.3658 |
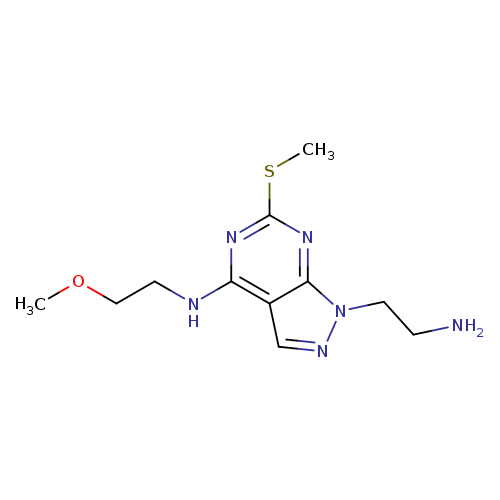
1-(2-AMINOETHYL)-N-(2-METHOXYETHYL)-6-(METHYLTHIO)-1H-PYRAZOLO[3,4-D]PYRI+Catalog No.:AA01ARML CAS No.:1105197-53-2 MDL No.:MFCD11986577 MF:C11H18N6OS MW:282.3652 |
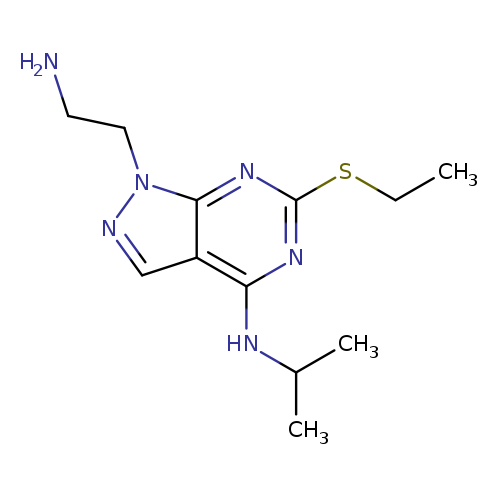
1-(2-Aminoethyl)-6-(ethylthio)-n-isopropyl-1h-pyrazolo[3,4-d]pyrimidin-4-amineCatalog No.:AA01ARND CAS No.:1105197-82-7 MDL No.:MFCD11986583 MF:C12H20N6S MW:280.3924 |
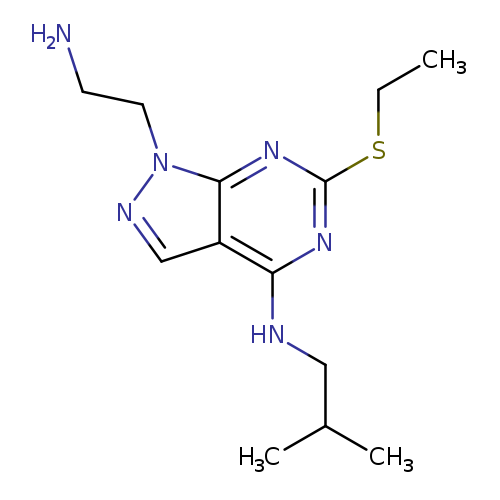
1-(2-AMINOETHYL)-6-(ETHYLTHIO)-N-ISOBUTYL-1H-PYRAZOLO[3,4-D]PYRIMIDIN-4-A+Catalog No.:AA01ARNE CAS No.:1105197-87-2 MDL No.:MFCD11986584 MF:C13H22N6S MW:294.4190 |
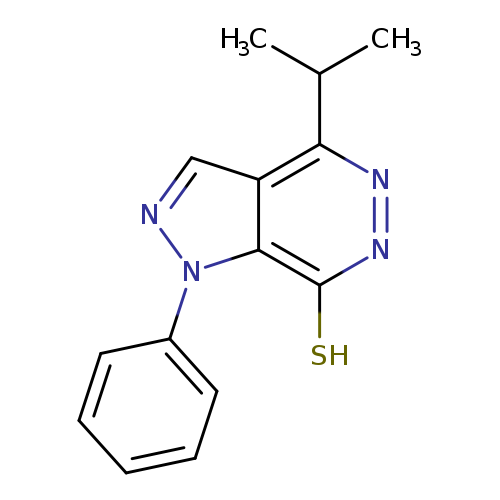
4-Isopropyl-1-phenyl-1h-pyrazolo[3,4-d]pyridazine-7-thiolCatalog No.:AA01ARNG CAS No.:1105197-96-3 MDL No.:MFCD11986586 MF:C14H14N4S MW:270.3528 |
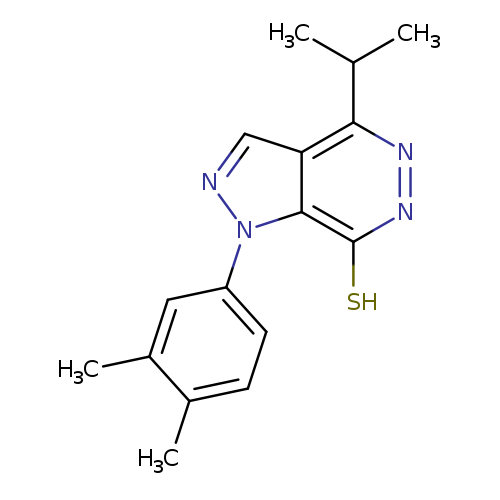
1-(3,4-DIMETHYLPHENYL)-4-ISOPROPYL-1H-PYRAZOLO[3,4-D]PYRIDAZINE-7-THIOLCatalog No.:AA01AQF9 CAS No.:1105198-01-3 MDL No.:MFCD16652898 MF:C16H18N4S MW:298.4059 |
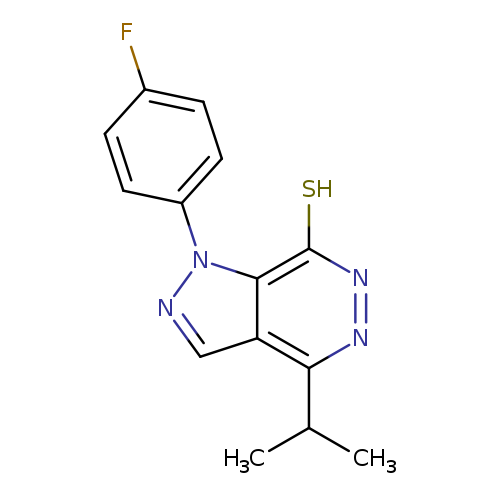
1-(4-FLUOROPHENYL)-4-ISOPROPYL-1H-PYRAZOLO[3,4-D]PYRIDAZINE-7-THIOLCatalog No.:AA01ARO7 CAS No.:1105198-06-8 MDL No.:MFCD11986587 MF:C14H13FN4S MW:288.3432 |
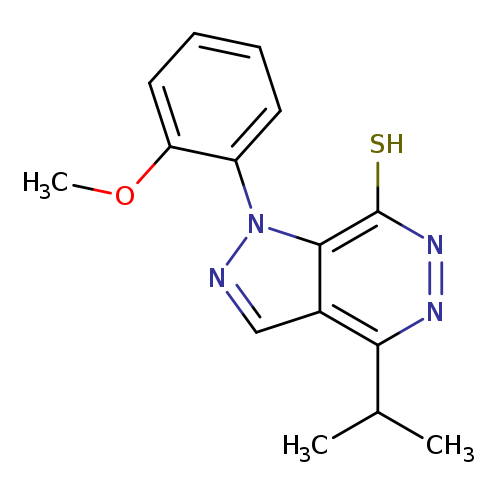
4-ISOPROPYL-1-(2-METHOXYPHENYL)-1H-PYRAZOLO[3,4-D]PYRIDAZINE-7-THIOLCatalog No.:AA01ARO8 CAS No.:1105198-11-5 MDL No.:MFCD11986588 MF:C15H16N4OS MW:300.3787 |
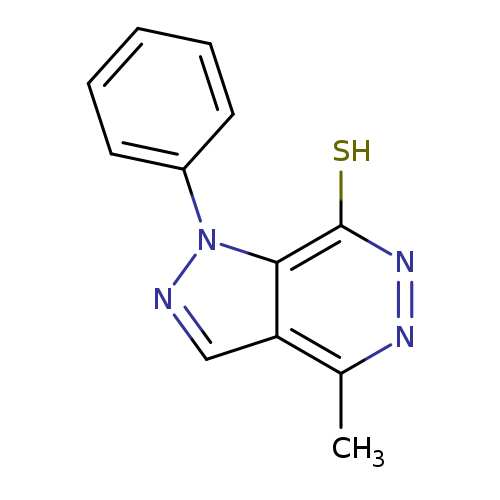
4-Methyl-1-phenyl-1h-pyrazolo[3,4-d]pyridazine-7-thiolCatalog No.:AA01ARO9 CAS No.:1105198-16-0 MDL No.:MFCD11986589 MF:C12H10N4S MW:242.2996 |
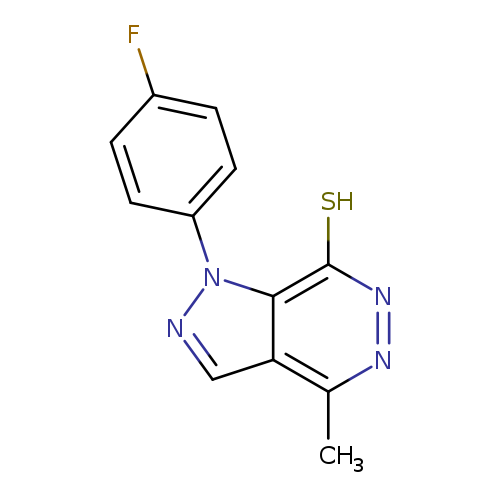
1-(4-FLUOROPHENYL)-4-METHYL-1H-PYRAZOLO[3,4-D]PYRIDAZINE-7-THIOLCatalog No.:AA01AROA CAS No.:1105198-21-7 MDL No.:MFCD11986590 MF:C12H9FN4S MW:260.2901 |
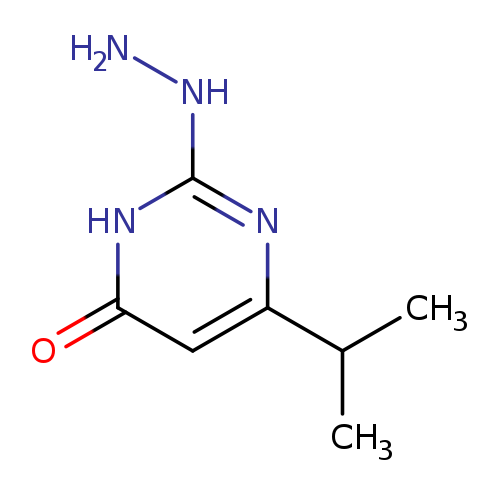
2-Hydrazino-6-isopropylpyrimidin-4(3H)-oneCatalog No.:AA01AQJ9 CAS No.:1105198-36-4 MDL No.:MFCD16631650 MF:C7H12N4O MW:168.1964 |
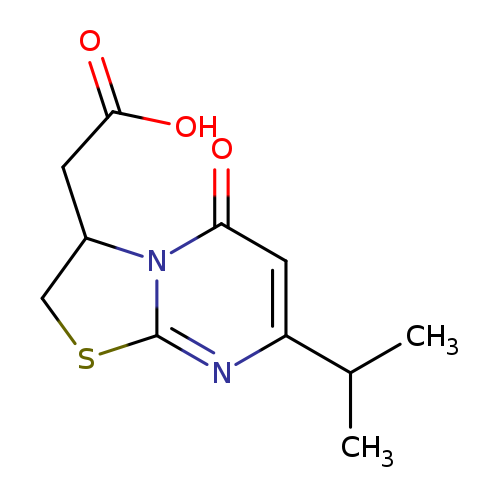
(7-ISOPROPYL-5-OXO-2,3-DIHYDRO-5H-[1,3]THIAZOLO[3,2-A]PYRIMIDIN-3-YL)ACET+Catalog No.:AA01ARPE CAS No.:1105198-41-1 MDL No.:MFCD11986597 MF:C11H14N2O3S MW:254.3055 |
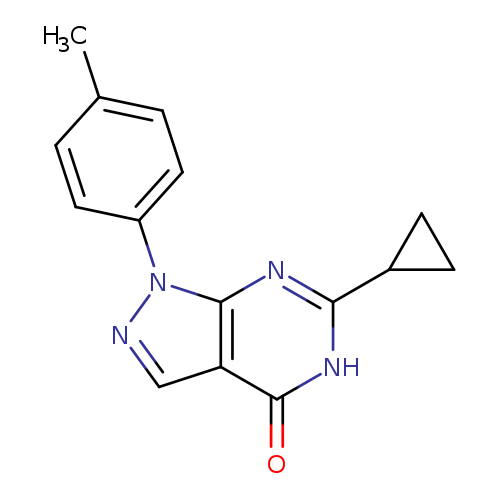
6-CYCLOPROPYL-1-(4-METHYLPHENYL)-1,5-DIHYDRO-4H-PYRAZOLO[3,4-D]PYRIMIDIN-+Catalog No.:AA01ARPF CAS No.:1105198-46-6 MDL No.:MFCD11986602 MF:C15H14N4O MW:266.2979 |
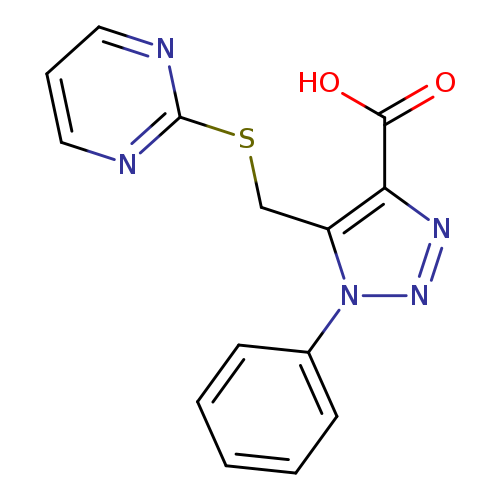
1-Phenyl-5-((pyrimidin-2-ylthio)methyl)-1H-1,2,3-triazole-4-carboxylic acidCatalog No.:AA01FMJ7 CAS No.:1105198-52-4 MDL No.:MFCD11986624 MF:C14H11N5O2S MW:313.3344 |
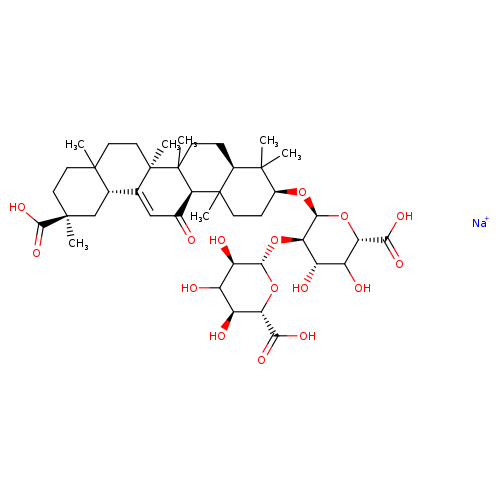
GLYCYRRHIZICACIDMONOSODIUMSALTCatalog No.:AA009OVB CAS No.:11052-19-0 MDL No.:MFCD03938959 MF:C42H62NaO16+ MW:845.9219 |
OligomycinCCatalog No.:AA01DZJ7 CAS No.:11052-72-5 MDL No.:MFCD00043231 MF:C45H74O10 MW:775.0631 |
Ibu-deoxycytidineCatalog No.:AA0083LA CAS No.:110522-75-3 MDL No.:MFCD00079383 MF:C13H19N3O5 MW:297.3071 |
2'-Deoxy-5'-O-DMT-N6-phenoxyacetyl-D-adenosineCatalog No.:AA008YJ9 CAS No.:110522-82-2 MDL No.:MFCD00058546 MF:C39H37N5O7 MW:687.7404 |
N4-(4-Methoxyphenyl)-n6-(pyridin-2-ylmethyl)-1h-pyrazolo[3,4-d]pyrimidine-4,6-diamineCatalog No.:AA01AS8Y CAS No.:1105224-27-8 MDL No.:MFCD12009136 MF:C18H17N7O MW:347.3739 |
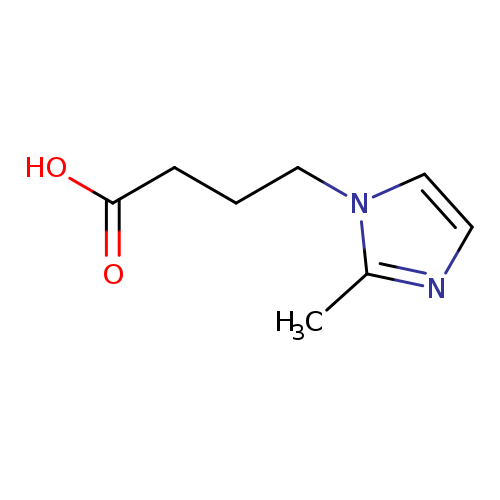
4-(2-Methyl-1H-imidazol-1-yl)butanoic acidCatalog No.:AA007CO6 CAS No.:110525-54-7 MDL No.:MFCD01721737 MF:C8H12N2O2 MW:168.1931 |
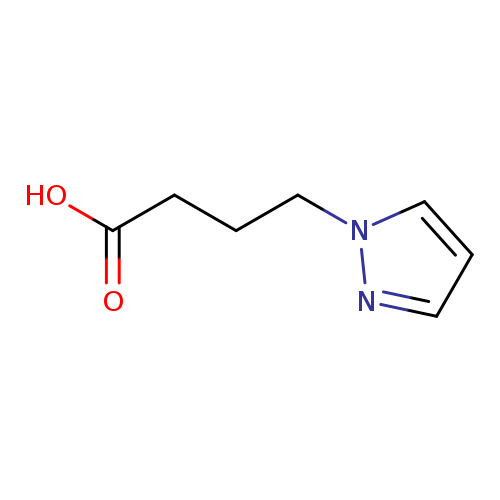
4-(1H-Pyrazol-1-yl)butanoic acidCatalog No.:AA0083L7 CAS No.:110525-56-9 MDL No.:MFCD01693645 MF:C7H10N2O2 MW:154.1665 |
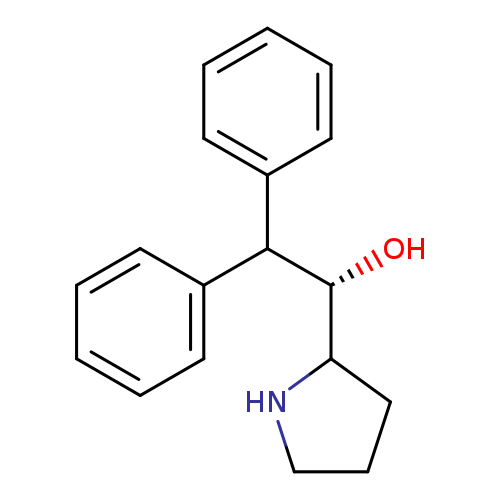
(S)-(+)-2-[Hydroxy(diphenyl)methyl]-1-methylpyrrolidineCatalog No.:AA003CDF CAS No.:110529-22-1 MDL No.:MFCD00145245 MF:C18H21NO MW:267.3654 |
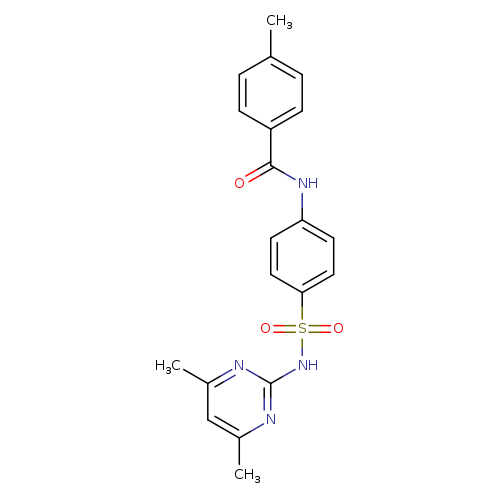
N-{4-[(4,6-Dimethylpyrimidin-2-yl)sulfamoyl]phenyl}-4-methylbenzamideCatalog No.:AA01F4YG CAS No.:110534-79-7 MDL No.:MFCD00863108 MF:C20H20N4O3S MW:396.4628 |
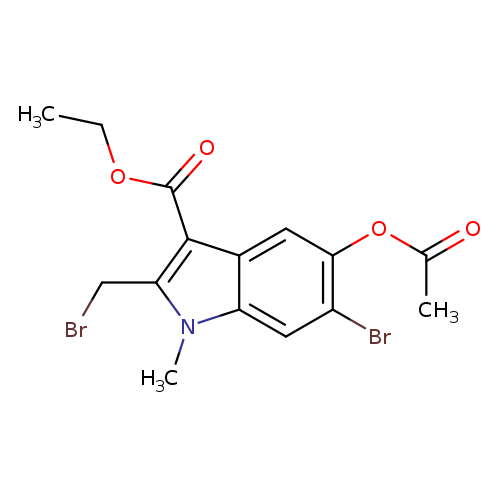
Ethyl 5-acetoxy-6-bromo-2-(bromomethyl)-1-methyl-1h-indole-3-carboxylateCatalog No.:AA003Q97 CAS No.:110543-98-1 MDL No.:MFCD00407019 MF:C15H15Br2NO4 MW:433.0919 |
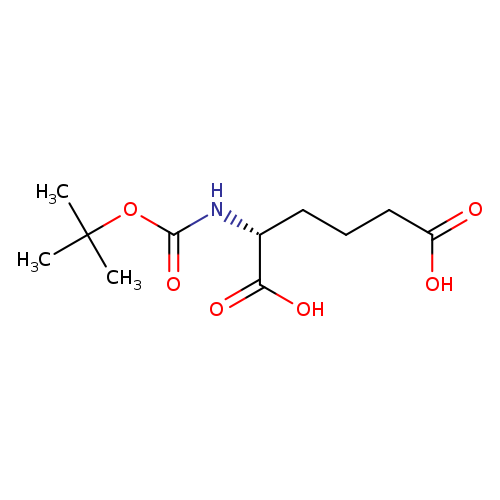
Boc-d-2-aminoadipic acidCatalog No.:AA007CO0 CAS No.:110544-97-3 MDL No.:MFCD00797554 MF:C11H19NO6 MW:261.2717 |
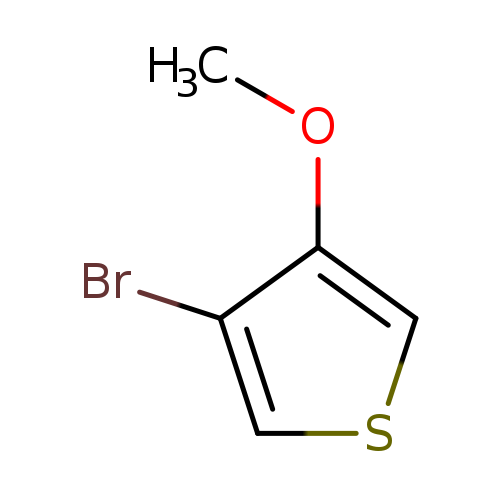
3-bromo-4-methoxythiopheneCatalog No.:AA01FMVY CAS No.:110545-69-2 MDL No.:MFCD25955496 MF:C5H5BrOS MW:193.0616 |
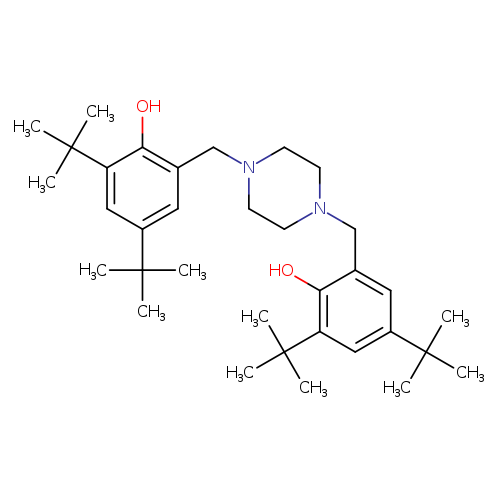
2,4-di-tert-butyl-6-({4-[(3,5-di-tert-butyl-2-hydroxyphenyl)methyl]piperazin-1-yl}methyl)phenolCatalog No.:AA00907O CAS No.:110546-20-8 MDL No.:MFCD14156144 MF:C34H54N2O2 MW:522.8048 |

3-Quinolinecarboxylic acid,6,7,8-trifluoro-1,4-dihydro-1-(2-hydroxy-1-methylethyl)-4-oxo-, ethylester, (R)-Catalog No.:AA01CCLL CAS No.:110548-05-5 MDL No.:MFCD30186682 MF:C15H14F3NO4 MW:329.2712 |
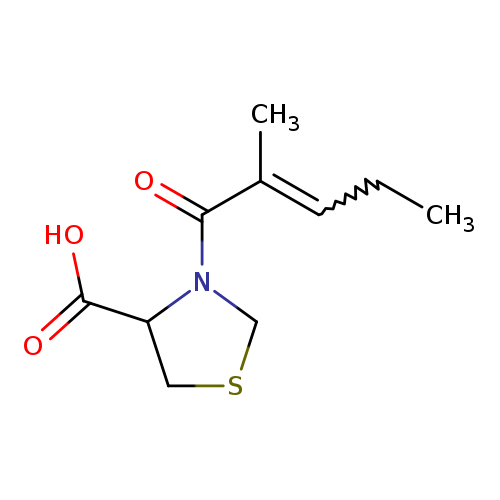
3-(2-methylpent-2-enoyl)-1,3-thiazolidine-4-carboxylic acidCatalog No.:AA01DU8S CAS No.:1105507-65-0 MDL No.:MFCD09932700 MF:C10H15NO3S MW:229.2960 |

Anthra[2,3-b]furan-7-carboxylic acid,5,10-dihydro-4,8,11-trimethoxy-6-methyl-5,10-dioxo-, methyl esterCatalog No.:AA01CBSN CAS No.:110551-55-8 MDL No.:MFCD30741516 MF: MW: |
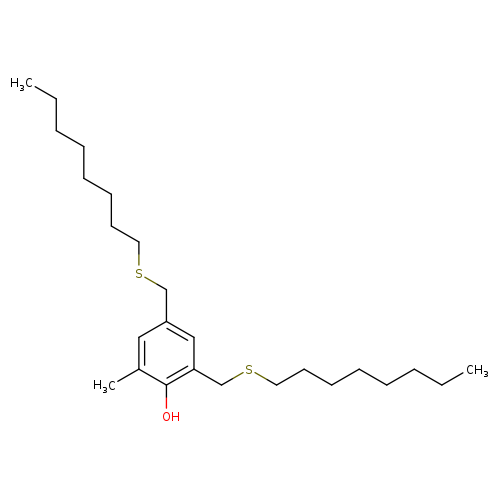
2-Methyl-4,6-bis(octylsulfanylmethyl)phenolCatalog No.:AA00HBNY CAS No.:110553-27-0 MDL No.:MFCD00134699 MF:C25H44OS2 MW:424.7463 |
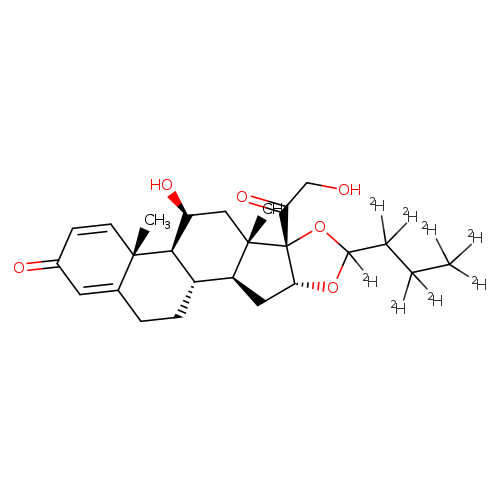
Budesonide-d8Catalog No.:AA01CC9N CAS No.:1105542-94-6 MDL No.:MFCD07369194 MF:C25H26D8O6 MW:438.5832 |
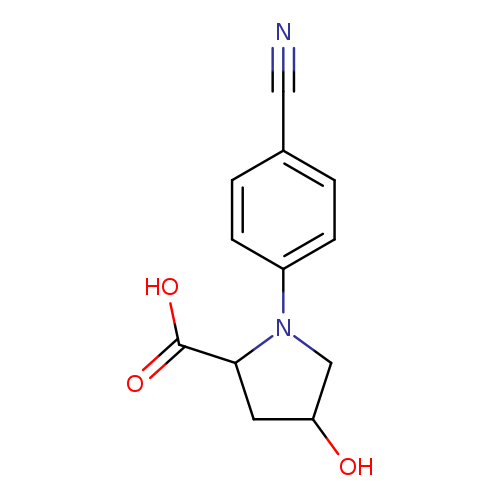
1-(4-cyanophenyl)-4-hydroxypyrrolidine-2-carboxylic acidCatalog No.:AA01E7UT CAS No.:1105547-86-1 MDL No.:MFCD11135473 MF:C12H12N2O3 MW:232.2353 |
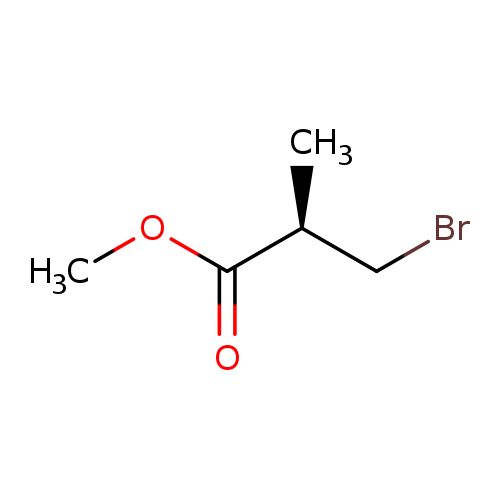
(R)-(+)-3-Bromoisobutyric acid methyl esterCatalog No.:AA003RG6 CAS No.:110556-33-7 MDL No.:MFCD00010639 MF:C5H9BrO2 MW:181.0278 |
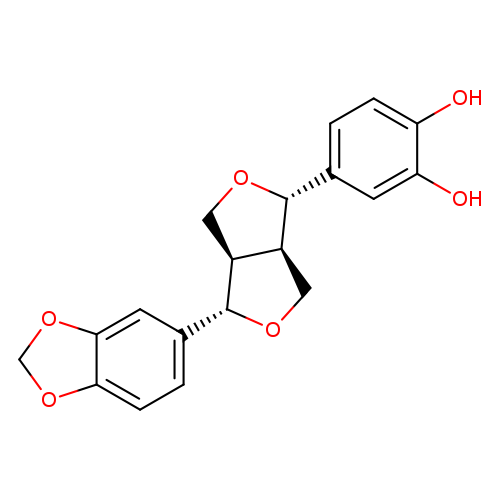
epi-SesaMin MonocatecholCatalog No.:AA008VTC CAS No.:1105568-81-7 MDL No.: MF:C19H18O6 MW:342.3426 |