2020-03-04 10:19:08
Erfei Wang,[a] Jiawei Zhang,[b] Zhuoran Zhong,[a] Kaixuan Chen,[a] and Mao Chen*[a]
Dedicated to Professors Stephen L. Buchwald and John F. Hartwig for celebration of the receipt of the 2019 Wolf Prize in Chemistry
1.Introduction
Transition-metal-catalyzed cross-coupling reaction has become one of the most powerful transformations in organic synthesis for the carbon-carbon bond formation. For example, named reactions including Suzuki-Miyaura,[1] Sonogashira,[2] Heck,[3] Hiyama[4] and Stille[5] reactions have revolutionized avenues to access organic molecules. These homogeneous catalytic processes have been extensively used in both academic and industrial fields,[6] providing important compounds including pharmaceuticals,[7] agrochemicals,[8] natural products,[9] poly- mer materials[10] and so on.[11]
Given the increasing concerns of sustainable development and production costs (i.e., catalyst, purification), many efforts have been devoted to improve the recyclability of catalysts and/or reduce the amount of transition-metal catalysts.[12] Consequently, the development of transition-metal catalysis has been accompanied with the evolution and upgrading of ligands,[13] which has enabled homogeneous catalysis with decreased catalyst usages, improved activity and selectivity, as well as expanded substrate scopes.[14] However, the application of homogeneous catalytic processes could be influenced by concerns of transition-metal contaminations. In this regard, heterogeneous transition-metal-catalyzed cross-couplings based on polymeric, inorganic and other carrying materials have been developed to simplify the purification process and reduce the catalyst cost.[15] A combination of the high activity of homogeneous catalysis and the ease in post-process of heterogenous catalysis would be desirable for the cross- coupling reactions.
Recently, we demostrated a highly efficient and low- catalyst-loading Pd-catalyzed Suzuki Miyaura cross-coupling method with a polymeric monophosphine ligand, “WePhos”, enabling rapid catalsyt shuttling, simple catalyst seperation and continuous catalyst-recycling synthesis under biphasic conditions (Scheme 1).[16] However, when further efforts were taken to extend the application scope of WePhos in other C-C bond forming cross-couplings, lower reactivities were ob- served, even at higher catalyst loadings. In this work, inspired by the elegent design of biaryldialkyl phosphine ligands,[17] we developed a poly(ethylene glycol) (PEG) linked ortho-MeO- phenyldicyclohexylphosphine ligand, “MeO-WePhos”, which allows extending the efficient shuttling catalysis to Pdcatalyzed cross-couplings inlcuding Sonogashira, Heck, Hiya- ma and Stille reactions as regulated by temperature.
2. Results and Discussion
The thermoresponsive polymeric ligand 4 a MeO-WePhos5000 (number average molecular weight (Mn) of PEG is 5000 g/ mol) was synthesized at 79 % overall yield following the synthetic route summarized in Scheme 2. The structure of 4 a was analysed with matrix-assisted laser desorption/ionization- time of flight (MALDI-TOF) mass spectrometry. As shown in Figure 1a, a single set of peaks for phosphine ligand 4 a is observed in the MALDI-TOF mass spectrum, and the differ- ence value of two neighbouring peaks equals the molar mass of a single repeating unit (m/z = 44.05, Figure 1b) in PEG. The absolute value of m/z is in accord with the molecular weight calculated by the MALDI-TOF result of 4 a. In Figure 1c, the y-intercept of m/z versus the corresponding number of repeating units demonstrates that the molecular weight of the chain-end group in 4 a was consistent with the expected value. Measurements with proton nuclear magnetic resonance (1 H NMR), 31 P NMR ( 9.41 ppm) and size-exclusion chroma- tography (SEC) further confirm the precise chemical structure of the phosphine ligand MeO-WePhos5000 (Figures S1).
With the same synthetic route, the WePhos5000 4 b[16] and PPh3-PEG5000 4 c[18] were prepared at 83 % and 87 % overall yields, respectively. The structures of 4 b and 4 c were also confirmed by MALDI-TOF mass spectra (Figures S2c–S2d), NMR spectra and other measurements.
Sonogashira reaction is among the most useful methods to synthesize conjugated (hetero)arynes via the cross-coupling of terminal alkynes and aryl halides.[19] The polymeric ligands from 4 a to 4 c were employed in the Pd-catalyzed Sonogashira cross-coupling reaction of bromobenzene and phenylacetylene using potassium carbonate (K2CO3) as the base at 90 °C in water/toluene (v/v = 4/1). As shown in Figure 2, the reaction with 4 a provided the highest yield (99 %) in 6 h reaction time as determined by gas chromatography (GC) (90 % and 78 % for 4 b and 4 c, respectively). When the reaction was complete, the reaction mixture was cooled to room temperature. The Pd complex coordinated with MeO-WePhos5000 rapidly transferred into the aqueous layer, and the generated diphenylacetylene product was kept in the toluene layer. After a simple phase separation, the aqueous layer was collected and reused in the next reaction. The catalyst recycling process was repeated for 6 times in a same way without clear decrease in GC yields. The final aqueous layer was characterized with inductively coupled plasma-atomic emission spectrometry (ICP-AES) measurement, which confirmed that 98 % Pd was remained in the aqueous phase (Figure S3), highlighting that the catalyst recycling process could be efficiently and easily achieved using the catalyst shuttling approach.
We further investigated the Sonogashira reaction with a variety of (hetero)aryl halides and aromatic alkynes to generate substituted alkynes. In Scheme 3, all electrophiles underwent complete conversions with MeO-WePhos5000 and Pd at 90 °C in 6 h, giving target products with isolated yields of up to 99 %. Electron- deficient, electron-rich and electron- neutral aryl bromides were tolerant in this catalyst system, and could be successfully conducted with ortho-, meta-, and para- substituted (hetero)aryl compounds. Functional groups (i. e., acetyl (9)), and heterocycles (i. e., thiophene (10) and pyridine (12)) could be compatible to generate the corresponding products in good to excellent yields. In addition, the MeO- WePhos5000 4 a could also be employed to promote the Sonogashira cross-coupling with (hetero)aryl chlorides (13, 14).
The vinylation or arylation of alkenes via the Pd- catalyzed reaction was independently discovered by Heck and Mizoroki around 1970,[3b,c] and was widely used in organic synthesis for C C bond formation.[7–8,20] As shown in Scheme 4, MeO- WePhos5000 was investigated in the Pd-catalyzed Heck reac- tions, providing a variety of substituted alkenes under biphasic reaction conditions. Electrophiles of aryl and heteroaryl (i. e., thiophene (18) and pyridine (19, 22)) bromides could be successfully transformed into corresponding products in the presence of acrylates and styrene at high isolated yields with 0.1mol% Pd2(dba)3.
Organometallic reagents of organosilicon and organotin were employed as nucleophiles, respectively, in Hiyama and Stille cross-coupling reactions, delivering biaryl units in pharmaceutical, agrochemical and other areas.[7–8,21] Next, the polymeric ligand 4 a was employed in the Hiyama and Stille reactions as shown in Scheme 5. For both types of cross- couplings, aryl, fused aryl and heteroaryl electrophiles were investigated using either PhSi(OEt)3 or PhSn(Bu)3 as nucleo- philes, providing bi(hetero)aryl products in 86–96 % isolated yields with 0.1 mol% Pd (dba) . For all reactions investigated in this work, the reaction mixtures underwent rapid phase separation at room temperature after reaction, affording products in the organic phase and catalyst kept in the water phase, further facilitating catalyst recycling and separation.
3.Conclusion
In conclusion, we have developed a novel thermoresponsive polymeric ligand, MeO-WePhos, which promoted Pd-cata- lyzed Sonogashira, Heck, Hiyama and Stille reactions under biphasic conditions, facilitating the preparation of correspond- ing alkynes, alkenes or bi(hetero)aryls from a variety of electrophiles and nucleophiles. Importantly, the catalyst shut- tling behavior enabled by the LCST property of PEG has allowed convenient catalyst recycling by simply phase seperation. 98 % Pd was kept in the water phase after recycling the catalsyt solution for 6 times. Given the profound impact of transition-metal-catalyzed covalent bond formation and the increasing demand of sustainable chemistry, this work provides an alternative method to conduct cross-couplings with a shuttling catalyst. We believe that other innovative ligands and new catalytic modes could be developed by introducing the unique property of macromolecules into ligand design.
Acknowledgements
This research was finally supported by the National Natural Science Foundation of China (NSFC, no. 21704016), the start- up funding from Fudan University, and the National Program for Thousand Young Talents of China.
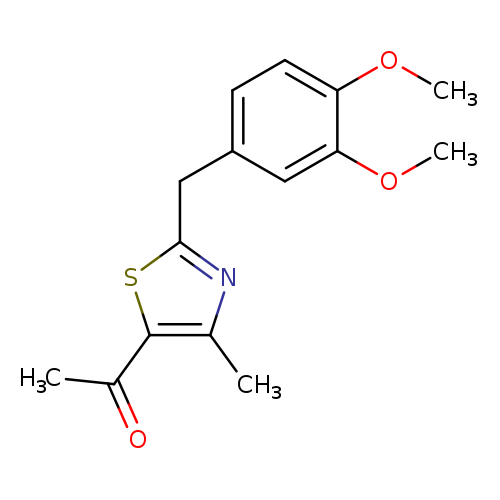
1-{2-[(3,4-dimethoxyphenyl)methyl]-4-methyl-1,3-thiazol-5-yl}ethan-1-oneCatalog No.:AA01ABG1 CAS No.:1110903-37-1 MDL No.:MFCD11802325 MF:C15H17NO3S MW:291.3654 |
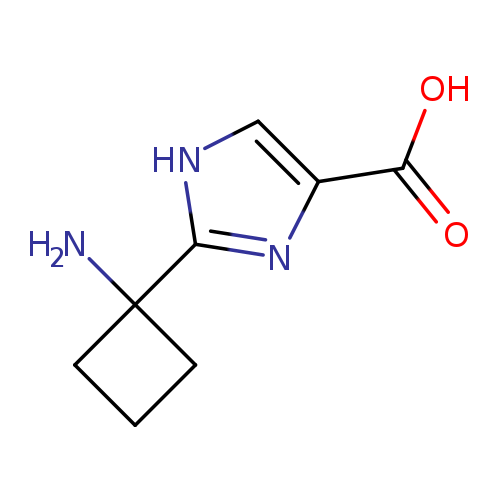
2-(1-aminocyclobutyl)-1H-imidazole-4-carboxylic acidCatalog No.:AA019NEC CAS No.:1110909-14-2 MDL No.:MFCD11505598 MF:C8H11N3O2 MW:181.1918 |
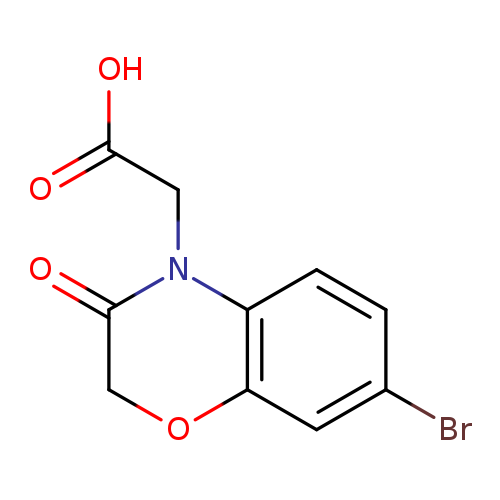
(7-Bromo-3-oxo-2,3-dihydro-4h-1,4-benzoxazin-4-yl)acetic acidCatalog No.:AA019N88 CAS No.:1110927-80-4 MDL No.:MFCD11505526 MF:C10H8BrNO4 MW:286.0788 |
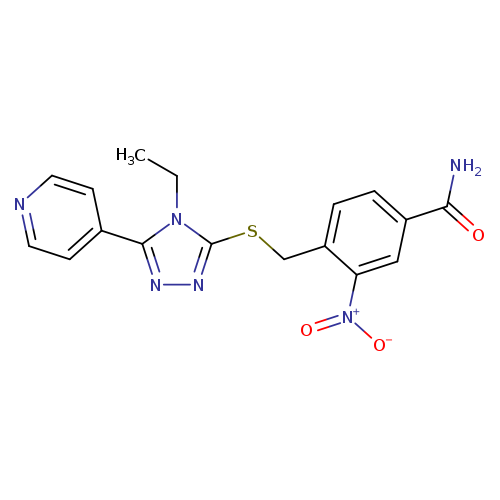
4-(([4-Ethyl-5-(pyridin-4-yl)-4h-1,2,4-triazol-3-yl]sulfanyl)methyl)-3-nitrobenzamideCatalog No.:AA01DUWH CAS No.:1110949-41-1 MDL No.:MFCD11772834 MF:C17H16N6O3S MW:384.4123 |
1-Methyl-1,2,3,4-tetrahydronaphthalen-1-amineCatalog No.:AA01A57T CAS No.:1110967-11-7 MDL No.:MFCD12067723 MF:C11H15N MW:161.2435 |
N-(1H-1,2,3-Benzotriazol-1-ylmethyl)pyridin-2-amineCatalog No.:AA01F8L9 CAS No.:111098-20-5 MDL No.:MFCD00444629 MF:C12H11N5 MW:225.2492 |
N-(1H-1,2,3-Benzotriazol-1-ylmethyl)-9H-purin-6-amineCatalog No.:AA01F82Z CAS No.:111098-24-9 MDL No.:MFCD00956577 MF:C12H10N8 MW:266.2614 |
1-[1-(Phenylsulfanyl)butyl]-1H-1,2,3-benzotriazoleCatalog No.:AA01EQOB CAS No.:111098-62-5 MDL No.:MFCD00958665 MF:C16H17N3S MW:283.3913 |
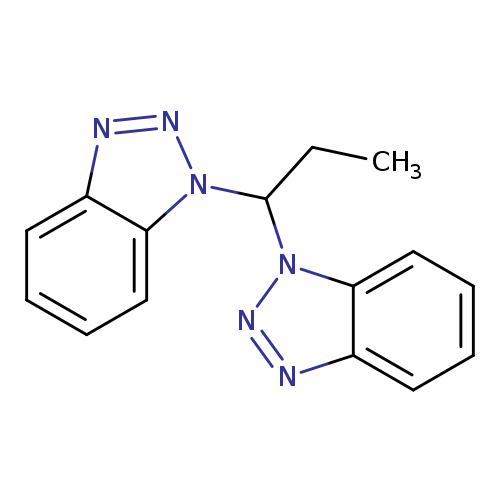
1-[1-(1H-1,2,3-Benzotriazol-1-yl)propyl]-1H-1,2,3-benzotriazoleCatalog No.:AA01EQOC CAS No.:111098-66-9 MDL No.:MFCD00958332 MF:C15H14N6 MW:278.3119 |
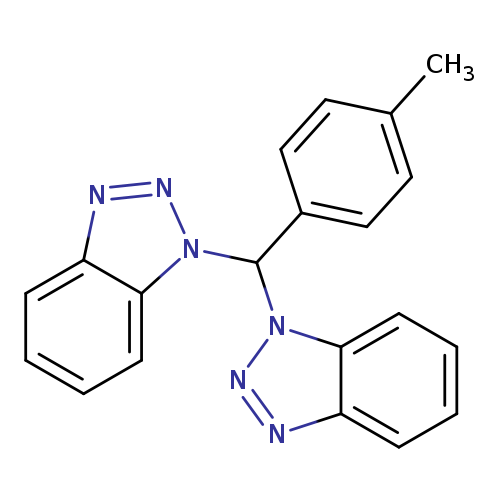
1-[1H-1,2,3-Benzotriazol-1-yl(4-methylphenyl)methyl]-1H-1,2,3-benzotriazoleCatalog No.:AA01EQOD CAS No.:111098-80-7 MDL No.:MFCD00835182 MF:C20H16N6 MW:340.3812 |
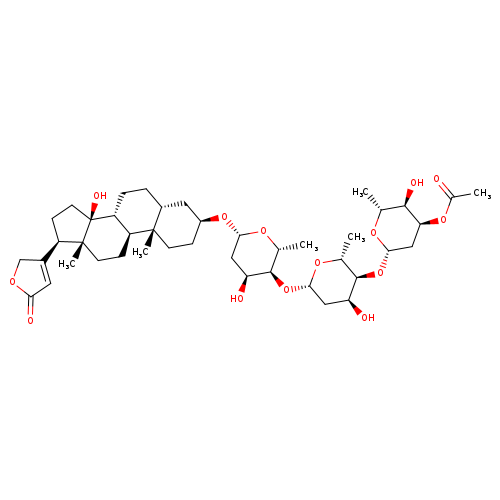
ACETYLDIGITOXINCatalog No.:AA008R34 CAS No.:1111-39-3 MDL No.:MFCD00151402 MF:C43H66O14 MW:806.9757 |
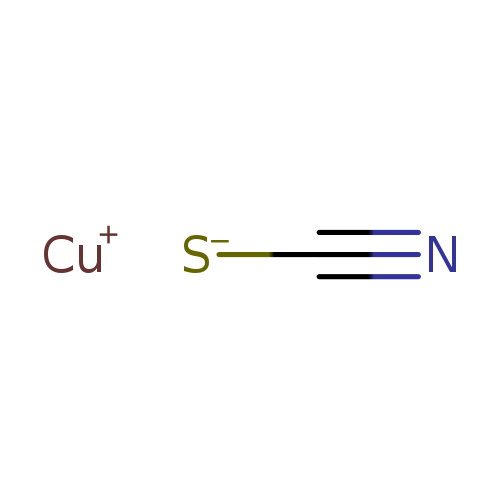
Cuprous thiocyanateCatalog No.:AA003OZ7 CAS No.:1111-67-7 MDL No.:MFCD00010980 MF:CCuNS MW:121.6284 |
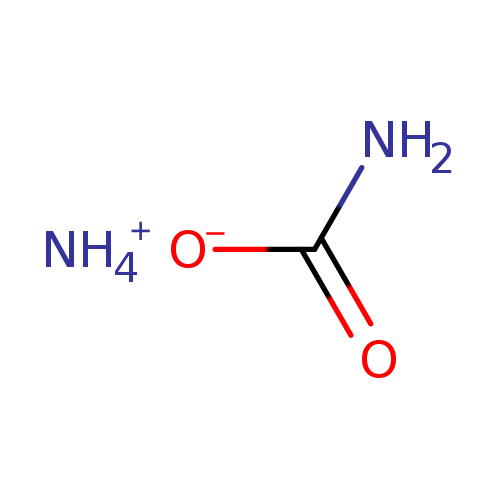
Ammonium carbamateCatalog No.:AA007BE1 CAS No.:1111-78-0 MDL No.:MFCD00013010 MF:CH6N2O2 MW:78.0705 |
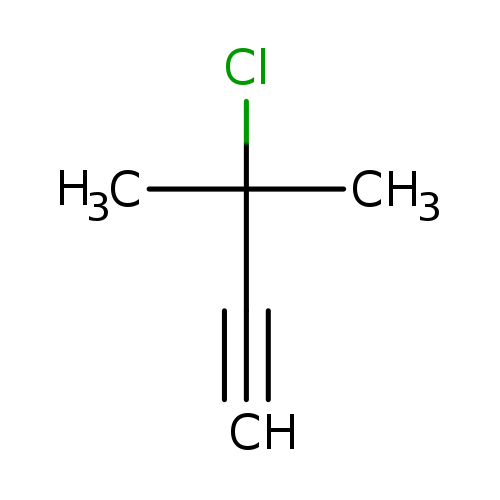
3-Chloro-3-methyl-1-butyneCatalog No.:AA0033KS CAS No.:1111-97-3 MDL No.:MFCD00190221 MF:C5H7Cl MW:102.5621 |
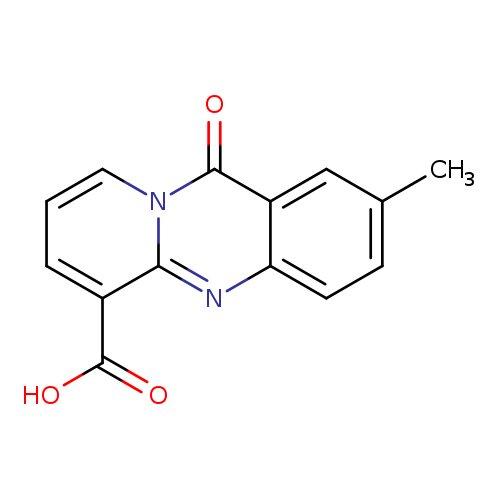
2-Methyl-11-oxo-11h-pyrido[2,1-b]quinazoline-6-carboxylic acidCatalog No.:AA00HBQC CAS No.:1111000-64-6 MDL No.:MFCD20754075 MF:C14H10N2O3 MW:254.2408 |
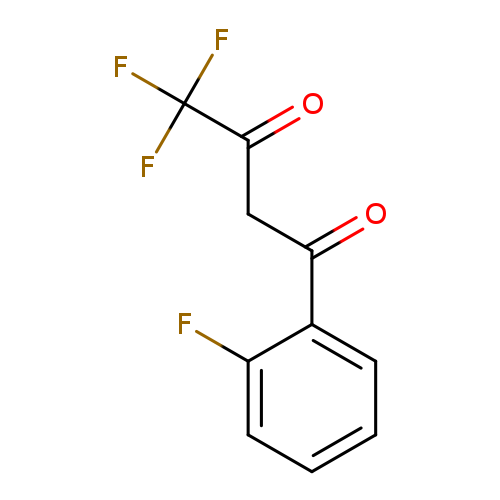
4,4,4-trifluoro-1-(2-fluorophenyl)butane-1,3-dioneCatalog No.:AA009TXT CAS No.:111102-82-0 MDL No.:MFCD03420699 MF:C10H6F4O2 MW:234.1471 |
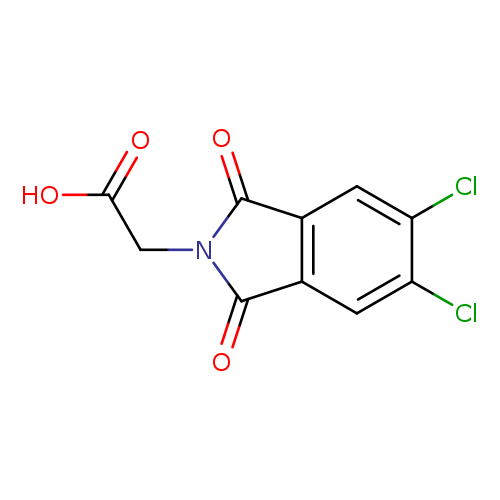
2-(5,6-Dichloro-1,3-dioxoisoindolin-2-yl)acetic acidCatalog No.:AA007BF9 CAS No.:111104-25-7 MDL No.:MFCD02189143 MF:C10H5Cl2NO4 MW:274.0570 |
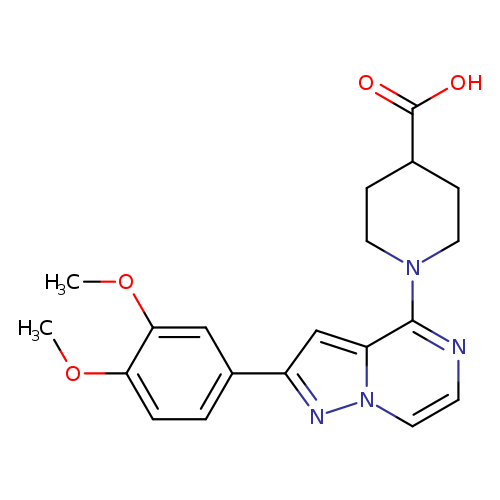
1-[2-(3,4-Dimethoxyphenyl)pyrazolo[1,5-a]pyrazin-4-yl]piperidine-4-carboxylic acidCatalog No.:AA00HBQF CAS No.:1111058-13-9 MDL No.:MFCD18201617 MF:C20H22N4O4 MW:382.4131 |
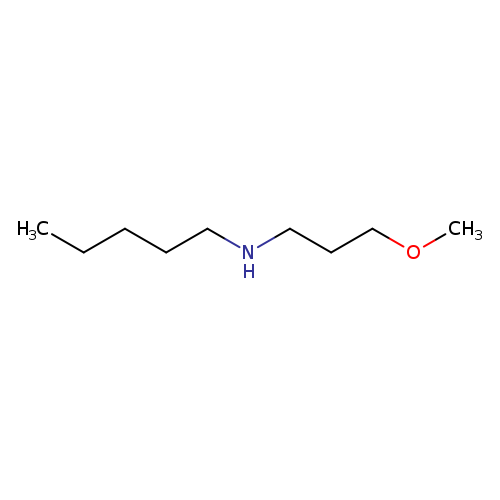
3-Methoxy propyl pentylaCatalog No.:AA008SBL CAS No.:111106-31-1 MDL No.:MFCD11161633 MF:C9H21NO MW:159.2691 |
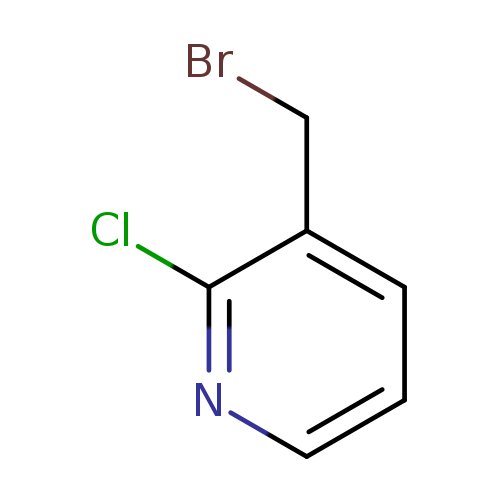
3-(Bromomethyl)-2-chloropyridineCatalog No.:AA003I49 CAS No.:111108-72-6 MDL No.:MFCD07368894 MF:C6H5BrClN MW:206.4676 |
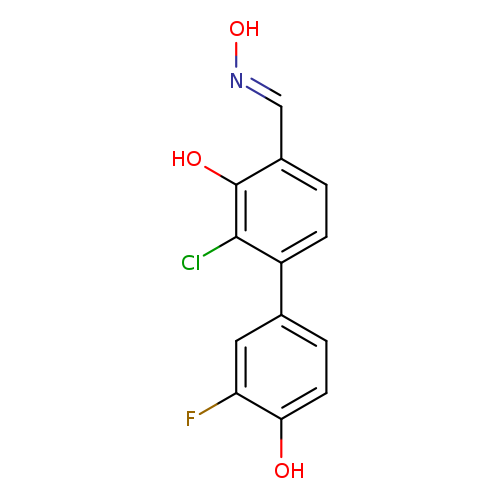
2-Chloro-3'-fluoro-3,4'-dihydroxy-[1,1-biphenyl]-4-carboxaldehydeoximeCatalog No.:AA008Y1Y CAS No.:1111084-78-6 MDL No.: MF:C13H9ClFNO3 MW:281.6669 |
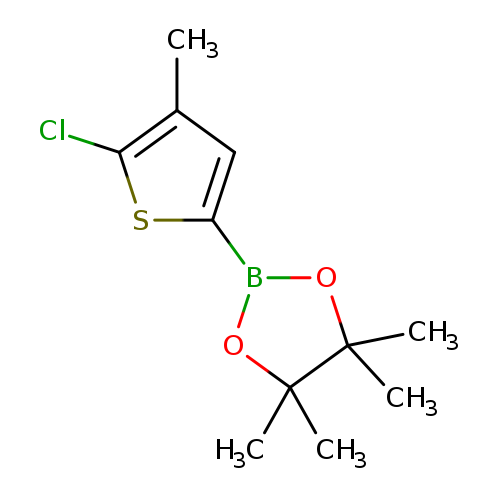
5-Chloro-4-methylthiophen-2-boronic acid, pinacol esterCatalog No.:AA00944R CAS No.:1111095-98-7 MDL No.:MFCD12405478 MF:C11H16BClO2S MW:258.5725 |
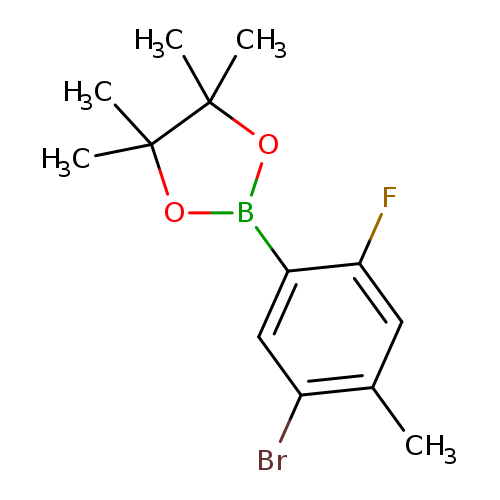
2-(5-Bromo-2-fluoro-4-methylphenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolaneCatalog No.:AA00HBQH CAS No.:1111096-03-7 MDL No.:MFCD12405354 MF:C13H17BBrFO2 MW:314.9863 |
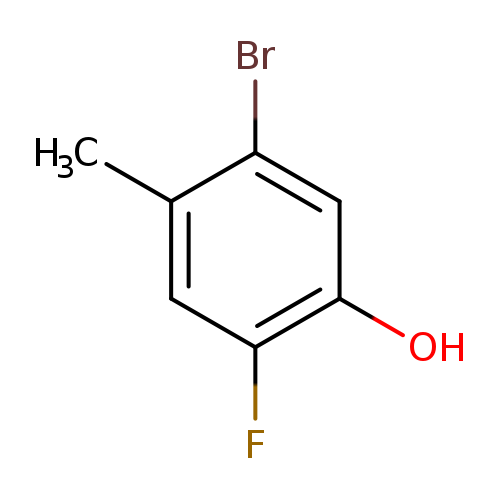
5-Bromo-2-fluoro-4-methylphenolCatalog No.:AA003ME7 CAS No.:1111096-04-8 MDL No.:MFCD12405415 MF:C7H6BrFO MW:205.0243 |
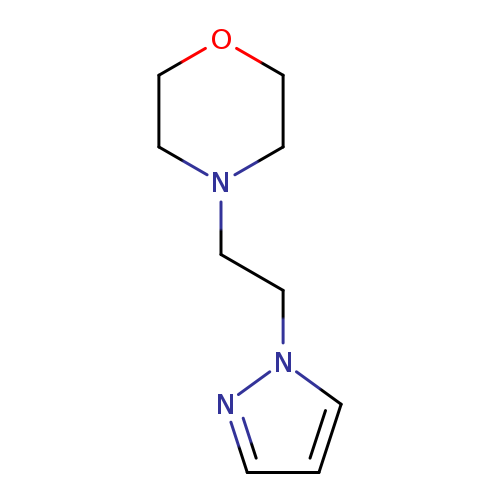
1-(2-Morpholinoethyl)pyrazoleCatalog No.:AA003CSL CAS No.:1111096-05-9 MDL No.:MFCD12405513 MF:C9H15N3O MW:181.2349 |
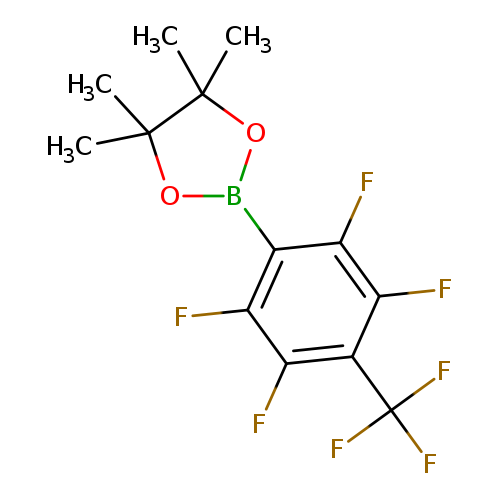
4,4,5,5-Tetramethyl-2-(2,3,5,6-tetrafluoro-4-(trifluoromethyl)phenyl)-1,3,2-dioxaborolaneCatalog No.:AA008SG3 CAS No.:1111096-06-0 MDL No.:MFCD12405355 MF:C13H12BF7O2 MW:344.0330 |
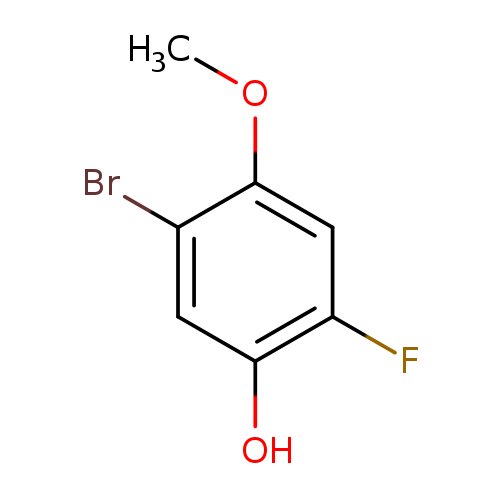
5-Bromo-2-fluoro-4-methoxyphenolCatalog No.:AA00HBQJ CAS No.:1111096-08-2 MDL No.:MFCD12405416 MF:C7H6BrFO2 MW:221.0237 |
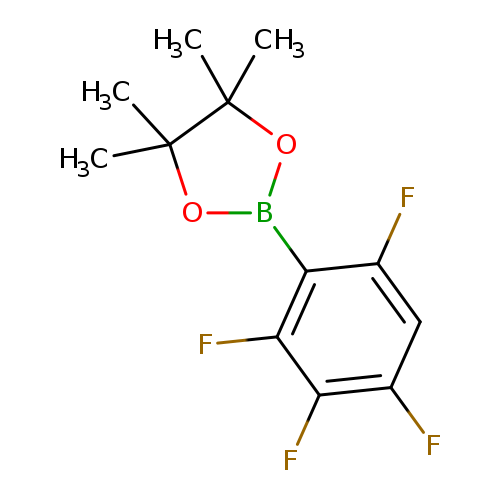
2,3,4,6-Tetrafluorophenylboronic acid pinacol esterCatalog No.:AA00HBQR CAS No.:1111096-18-4 MDL No.:MFCD12405358 MF:C12H13BF4O2 MW:276.0350 |
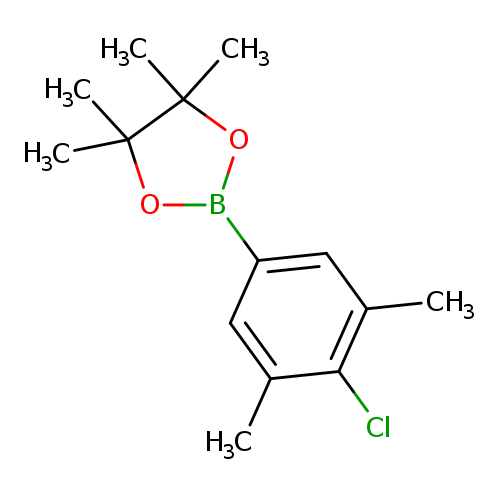
4-Chloro-3,5-dimethylphenylboronic acid, pinacol esterCatalog No.:AA0037WB CAS No.:1111096-20-8 MDL No.:MFCD12405360 MF:C14H20BClO2 MW:266.5714 |
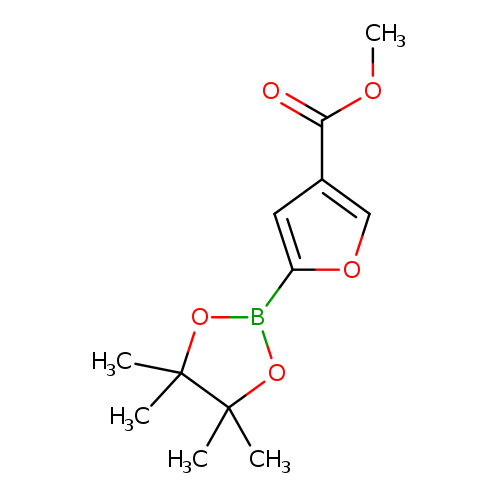
4-(Methoxycarbonyl)furan-2-boronic acid, pinacol esterCatalog No.:AA003K63 CAS No.:1111096-29-7 MDL No.:MFCD12407248 MF:C12H17BO5 MW:252.0714 |
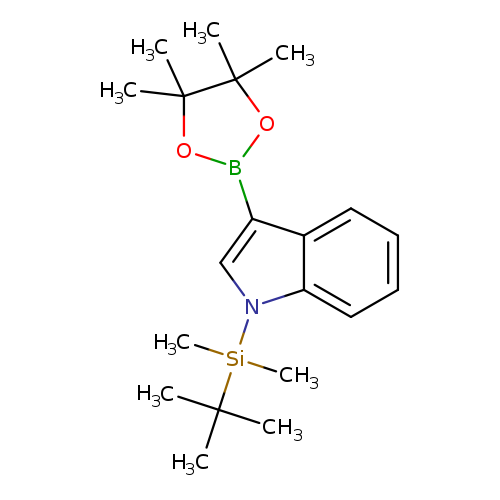
1-(t-Butyldimethylsilyl)indole-3-boronic acid pinacol esterCatalog No.:AA0099CY CAS No.:1111096-51-5 MDL No.:MFCD10698499 MF:C20H32BNO2Si MW:357.3701 |
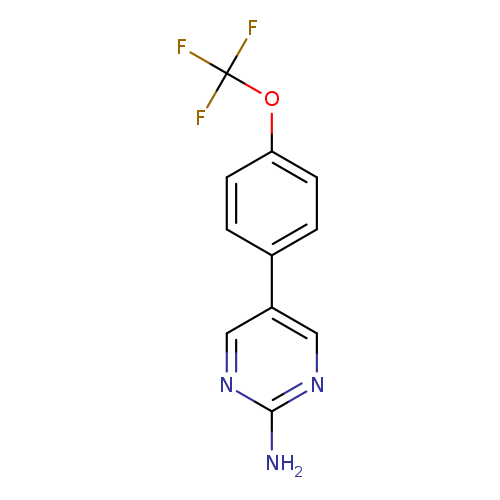
5-(4-(Trifluoromethoxy)phenyl)pyrimidin-2-amineCatalog No.:AA01FP6L CAS No.:1111105-03-3 MDL No.:MFCD11877104 MF:C11H8F3N3O MW:255.1959 |
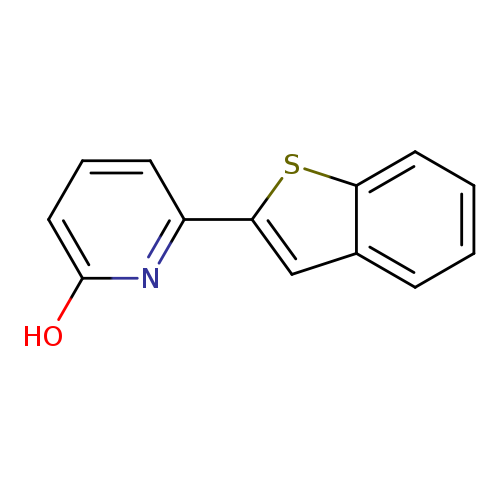
6-[Benzo(b)thiophen-2-yl]-2-hydroxypyridineCatalog No.:AA0093Z0 CAS No.:1111105-57-7 MDL No.:MFCD11876406 MF:C13H9NOS MW:227.2817 |
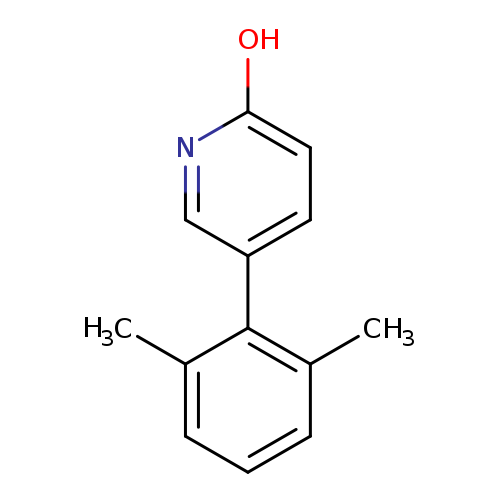
5-(2,6-dimethylphenyl)pyridin-2-olCatalog No.:AA01EMG3 CAS No.:1111106-00-3 MDL No.:MFCD11876443 MF:C13H13NO MW:199.2484 |
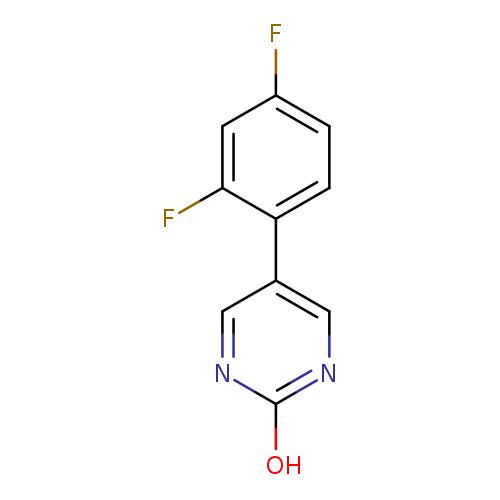
5-(2,4-Difluorophenyl)-2-hydroxypyrimidineCatalog No.:AA009475 CAS No.:1111107-95-9 MDL No.:MFCD11876802 MF:C10H6F2N2O MW:208.1642 |
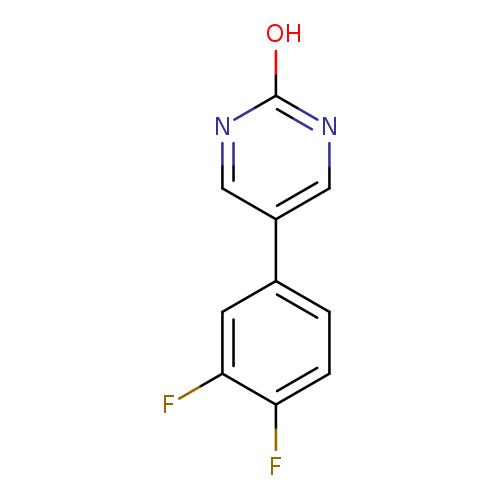
5-(3,4-Difluorophenyl)-2-hydroxypyrimidineCatalog No.:AA0082TN CAS No.:1111108-31-6 MDL No.:MFCD11876836 MF:C10H6F2N2O MW:208.1642 |
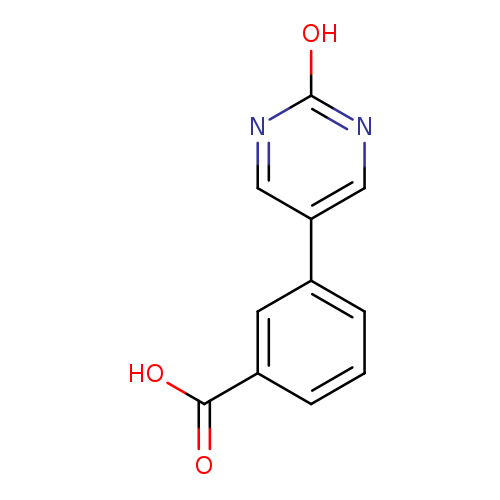
5-(3-Carboxyphenyl)-2-hydroxypyrimidineCatalog No.:AA00HBUH CAS No.:1111108-95-2 MDL No.:MFCD11876892 MF:C11H8N2O3 MW:216.1928 |
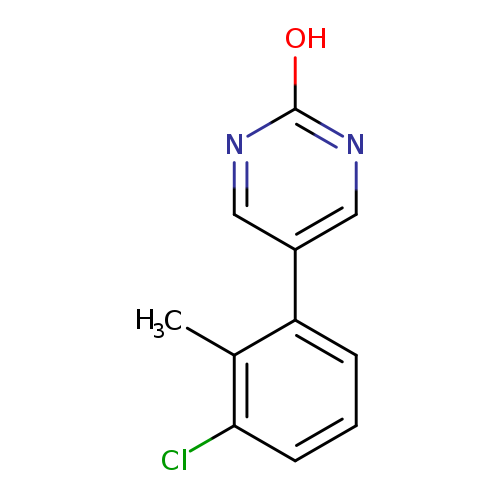
5-(3-Chloro-2-methylphenyl)-2-hydroxypyrimidineCatalog No.:AA0093ZL CAS No.:1111109-06-8 MDL No.:MFCD11876901 MF:C11H9ClN2O MW:220.6550 |
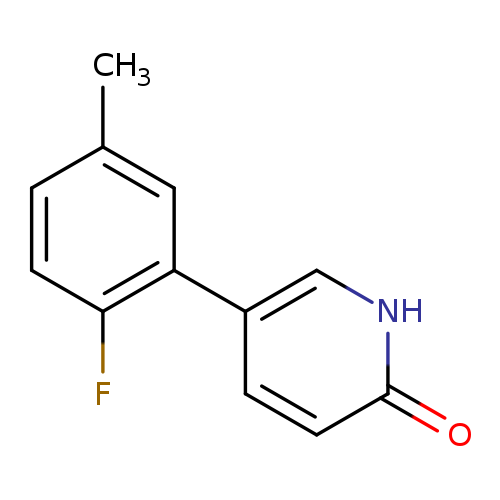
5-(2-Fluoro-5-methylphenyl)-2-hydroxypyridineCatalog No.:AA00HBV2 CAS No.:1111109-59-1 MDL No.:MFCD11876519 MF:C12H10FNO MW:203.2123 |
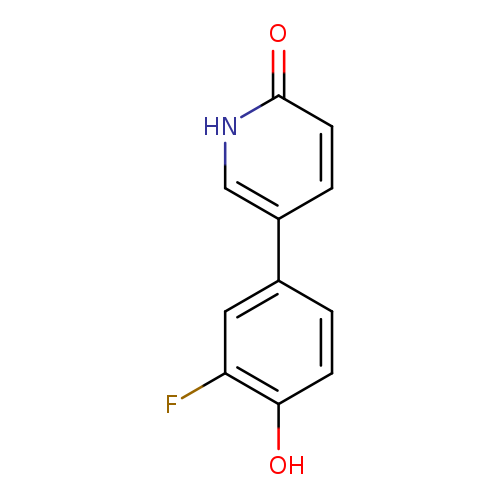
5-(3-Fluoro-4-hydroxyphenyl)-2-hydroxypyridineCatalog No.:AA00HBVD CAS No.:1111109-87-5 MDL No.:MFCD11876545 MF:C11H8FNO2 MW:205.1851 |
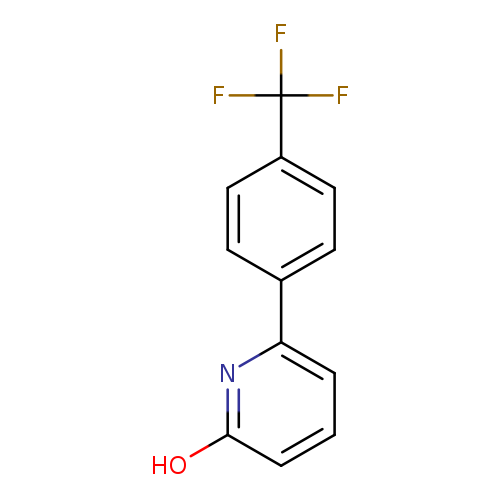
2-Hydroxy-6-(4-trifluoromethylphenyl)pyridineCatalog No.:AA007BF4 CAS No.:1111110-54-3 MDL No.:MFCD11876114 MF:C12H8F3NO MW:239.1932 |
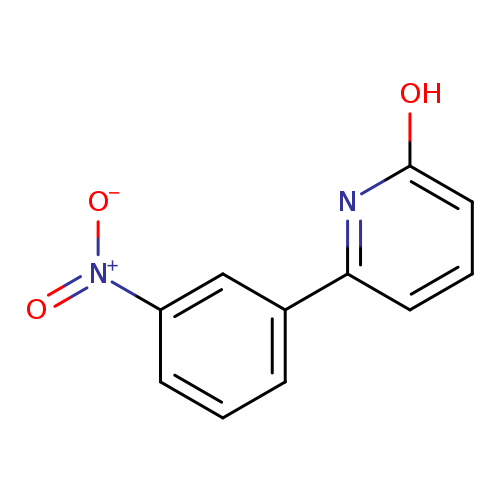
2-Hydroxy-6-(3-nitrophenyl)pyridineCatalog No.:AA007TQJ CAS No.:1111110-56-5 MDL No.:MFCD11876117 MF:C11H8N2O3 MW:216.1928 |
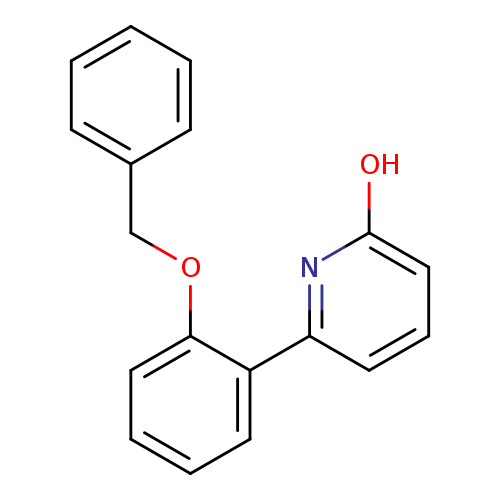
6-(2-Benzyloxyphenyl)-2-hydroxypyridineCatalog No.:AA00HBWH CAS No.:1111111-20-6 MDL No.:MFCD11876203 MF:C18H15NO2 MW:277.3172 |
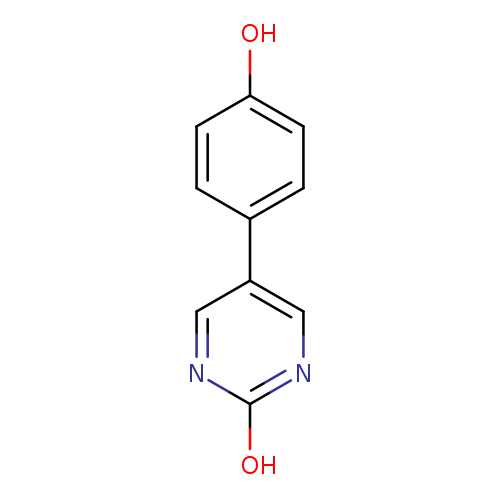
5-(4-Hydroxyphenyl)-2-hydroxypyrimidineCatalog No.:AA00HBXO CAS No.:1111113-77-9 MDL No.:MFCD11876977 MF:C10H8N2O2 MW:188.1827 |
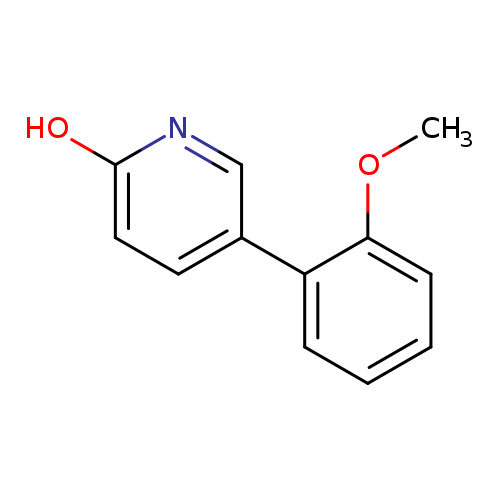
2-Hydroxy-5-(2-methoxyphenyl)pyridineCatalog No.:AA00HBYX CAS No.:1111115-94-6 MDL No.:MFCD11876649 MF:C12H11NO2 MW:201.2212 |
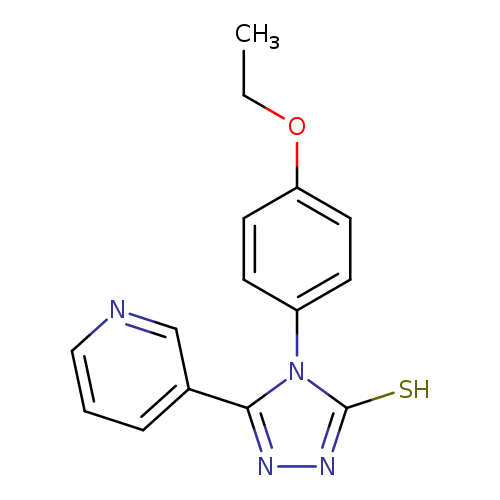
4-(4-Ethoxyphenyl)-5-(pyridin-3-yl)-4H-1,2,4-triazole-3-thiolCatalog No.:AA008U14 CAS No.:111114-78-4 MDL No.:MFCD03953997 MF:C15H14N4OS MW:298.3629 |
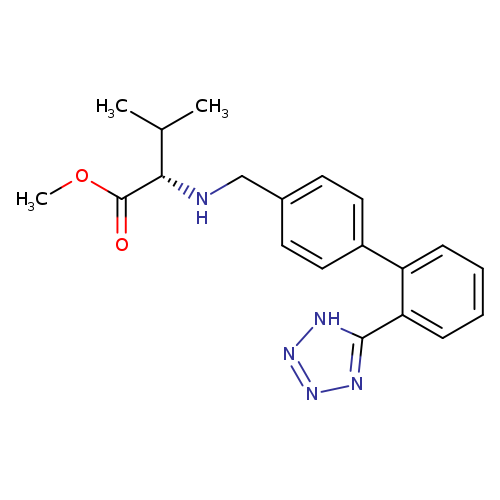
N-[[2'-(2H-Tetrazol-5-yl)[1,1'-biphenyl]-4-yl]methyl]-L-valine methyl esterCatalog No.:AA01EATE CAS No.:1111177-24-2 MDL No.:MFCD29045634 MF:C20H23N5O2 MW:365.4289 |
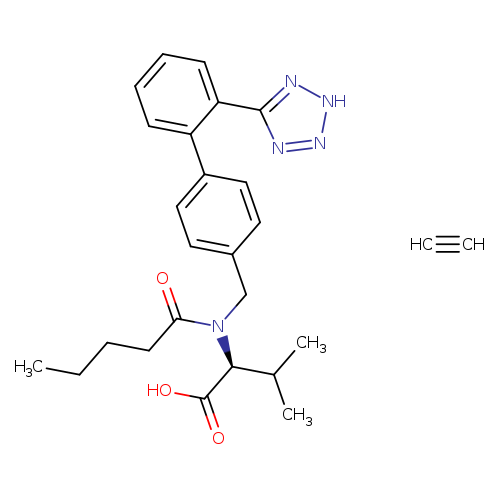
ethyl N-((2'-(1H-tetrazol-5-yl)-[1,1'-biphenyl]-4-yl)Methyl)-N-pentanoyl-L-valinateCatalog No.:AA00954M CAS No.:1111177-30-0 MDL No.:MFCD29045635 MF:C26H31N5O3 MW:461.5560 |
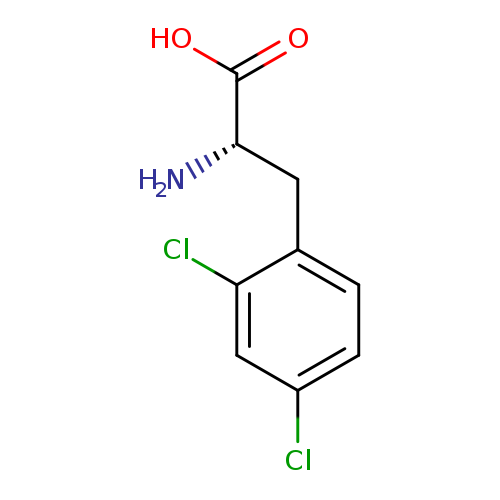
2,4-Dichloro-L-phenylalanineCatalog No.:AA003R44 CAS No.:111119-36-9 MDL No.:MFCD01860882 MF:C9H9Cl2NO2 MW:234.0793 |
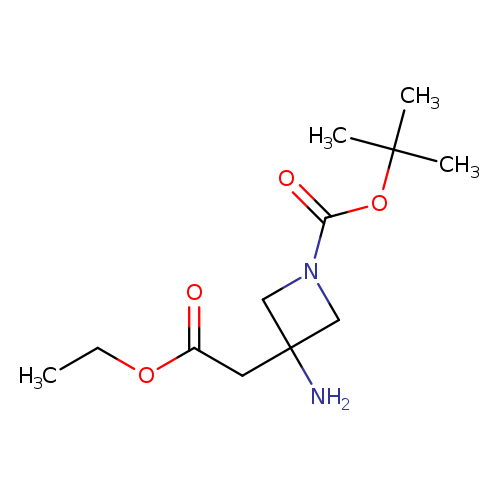
tert-Butyl 3-amino-3-(2-ethoxy-2-oxoethyl)azetidine-1-carboxylateCatalog No.:AA0099CZ CAS No.:1111202-76-6 MDL No.:MFCD18375085 MF:C12H22N2O4 MW:258.3141 |
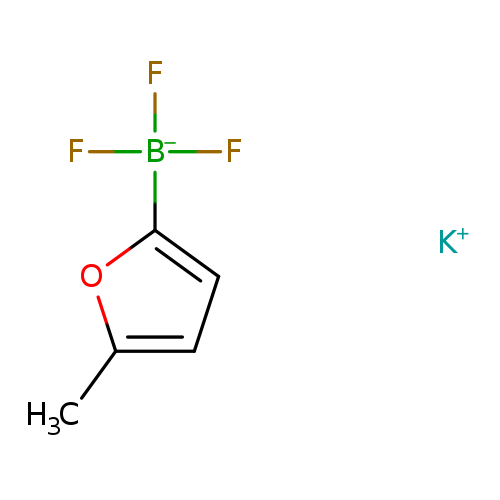
Potassium 5-methylfuran-2-trifluoroborateCatalog No.:AA0082TI CAS No.:1111213-54-7 MDL No.:MFCD09992923 MF:C5H5BF3KO MW:187.9971 |
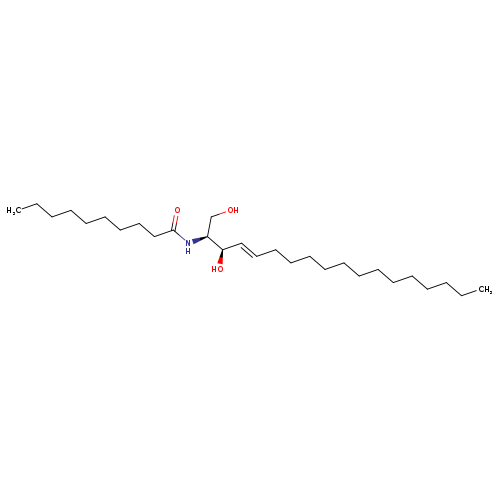
N-DECANOYL-D-ERYTHRO-SPHINGOSINECatalog No.:AA008RV5 CAS No.:111122-57-7 MDL No.:MFCD02259134 MF:C28H55NO3 MW:453.7412 |
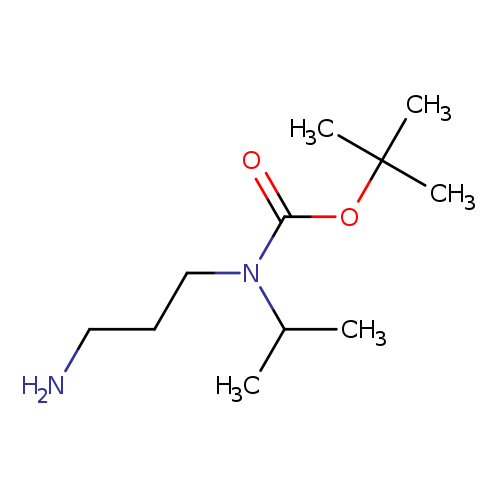
tert-Butyl (3-aminopropyl)(isopropyl)carbamateCatalog No.:AA0099D0 CAS No.:1111236-12-4 MDL No.:MFCD11102239 MF:C11H24N2O2 MW:216.3205 |
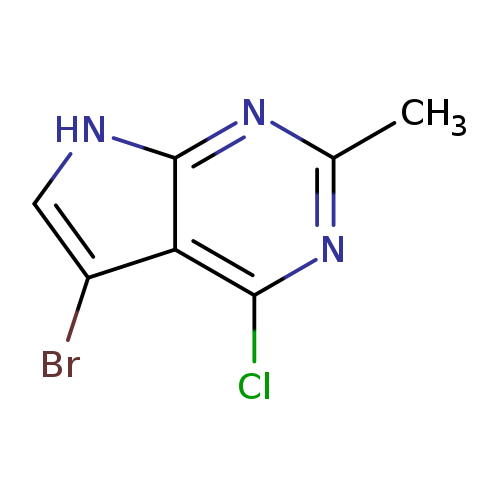
5-Bromo-4-chloro-2-methyl-7h-pyrrolo[2,3-d]pyrimidineCatalog No.:AA007BEZ CAS No.:1111237-76-3 MDL No.:MFCD16249868 MF:C7H5BrClN3 MW:246.4917 |
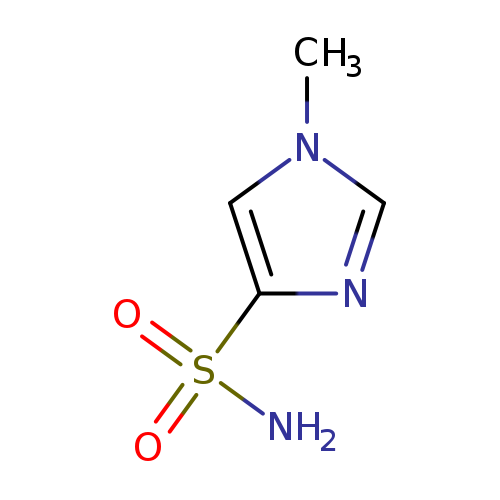
1-Methylimidazole-4-sulfonamideCatalog No.:AA007BEM CAS No.:111124-90-4 MDL No.:MFCD02091382 MF:C4H7N3O2S MW:161.1823 |
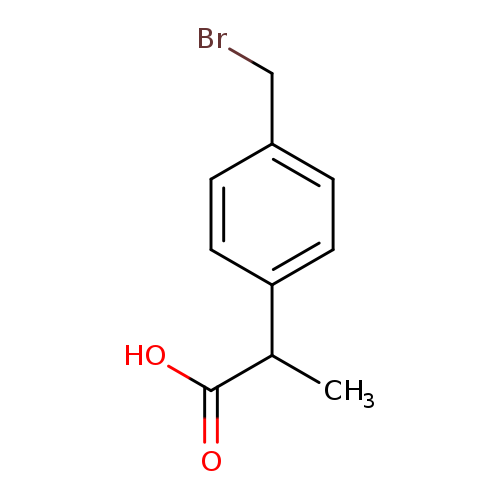
2-(4-(Bromomethyl)phenyl)propanoic acidCatalog No.:AA003ESB CAS No.:111128-12-2 MDL No.:MFCD02093445 MF:C10H11BrO2 MW:243.0971 |
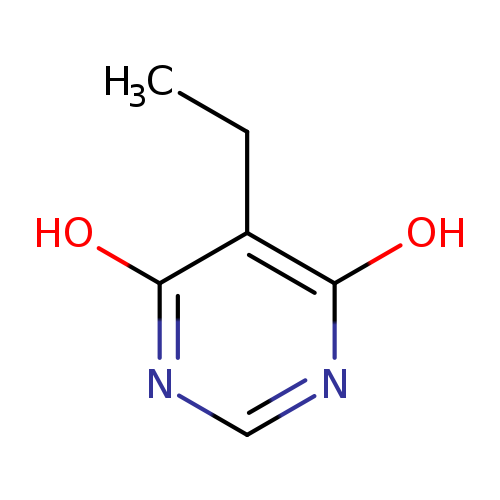
5-Ethyl-6-hydroxypyrimidin-4(3h)-oneCatalog No.:AA009O0X CAS No.:111129-64-7 MDL No.:MFCD00192047 MF:C6H8N2O2 MW:140.1399 |
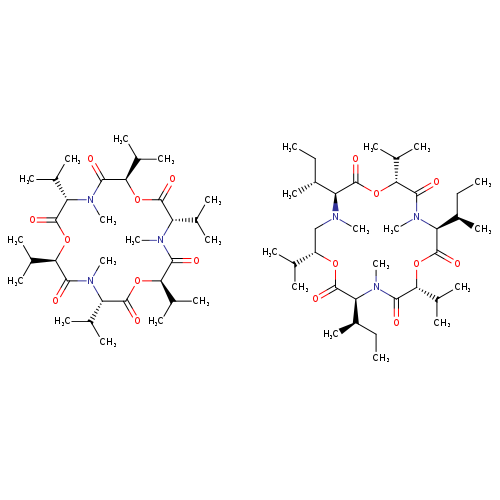
ENNIATIN FROM MICROBIAL SOURCECatalog No.:AA0094B4 CAS No.:11113-62-5 MDL No.:MFCD00214209 MF:C69H122N6O17 MW:1307.7370 |
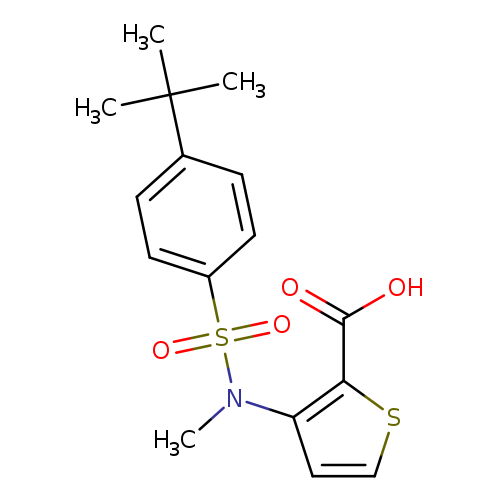
3-(N-methyl4-tert-butylbenzenesulfonamido)thiophene-2-carboxylic acidCatalog No.:AA00IMNS CAS No.:1111300-43-6 MDL No.:MFCD19104880 MF:C16H19NO4S2 MW:353.4564 |
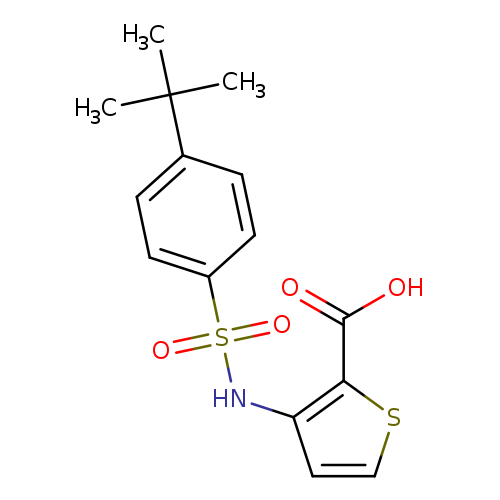
3-(4-tert-butylbenzenesulfonamido)thiophene-2-carboxylic acidCatalog No.:AA00J0JR CAS No.:1111300-51-6 MDL No.:MFCD19105036 MF:C15H17NO4S2 MW:339.4298 |
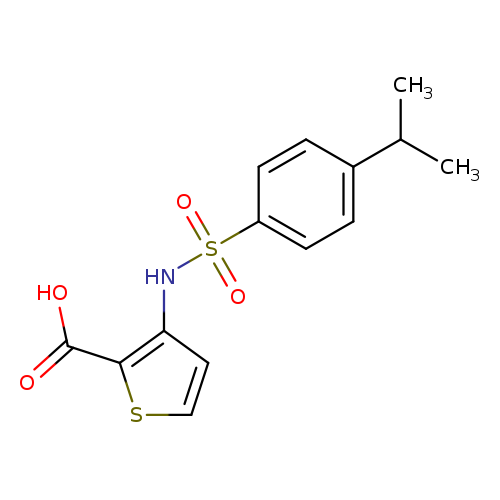
3-[4-(propan-2-yl)benzenesulfonamido]thiophene-2-carboxylic acidCatalog No.:AA00J0HT CAS No.:1111301-77-9 MDL No.:MFCD13696882 MF:C14H15NO4S2 MW:325.4032 |
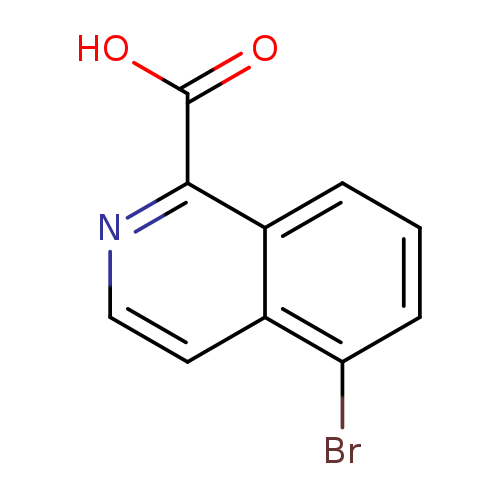
5-Bromoisoquinoline-1-carboxylic acidCatalog No.:AA00923Q CAS No.:1111311-65-9 MDL No.:MFCD17215758 MF:C10H6BrNO2 MW:252.0641 |
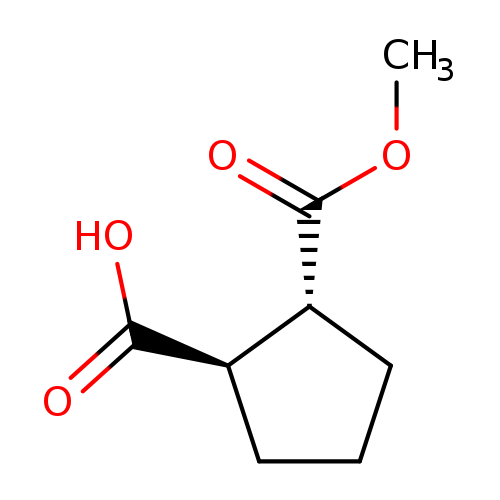
trans-2-(Methoxycarbonyl)cyclopentanecarboxylic acidCatalog No.:AA008WSW CAS No.:111138-44-4 MDL No.:MFCD01311174 MF:C8H12O4 MW:172.1785 |
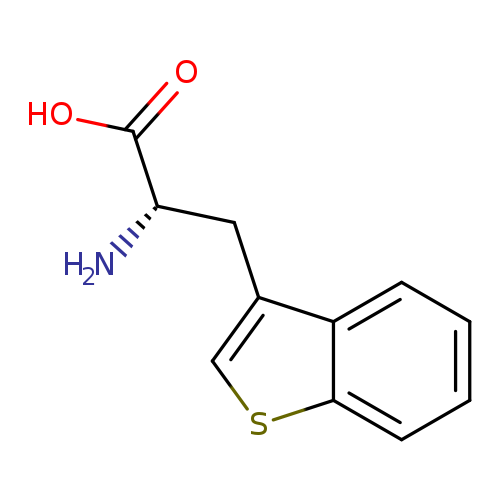
D-3-BenzothienylalanineCatalog No.:AA0037W9 CAS No.:111139-55-0 MDL No.:MFCD00079683 MF:C11H11NO2S MW:221.2755 |
k-CarrageenanCatalog No.:AA00HC16 CAS No.:11114-20-8 MDL No.:MFCD00151514 MF:C24H36O25S2-- MW:788.6576 |
3-(2-Fluorophenoxy)propanenitrileCatalog No.:AA0082TB CAS No.:111140-91-1 MDL No.:MFCD01569390 MF:C9H8FNO MW:165.1643 |
3-(4-Carbamoylphenoxy)propionic acidCatalog No.:AA008UFL CAS No.:111140-92-2 MDL No.:MFCD09948835 MF:C10H11NO4 MW:209.1986 |
8-Fluoro-4-chromanoneCatalog No.:AA003NFK CAS No.:111141-00-5 MDL No.:MFCD09744011 MF:C9H7FO2 MW:166.1491 |
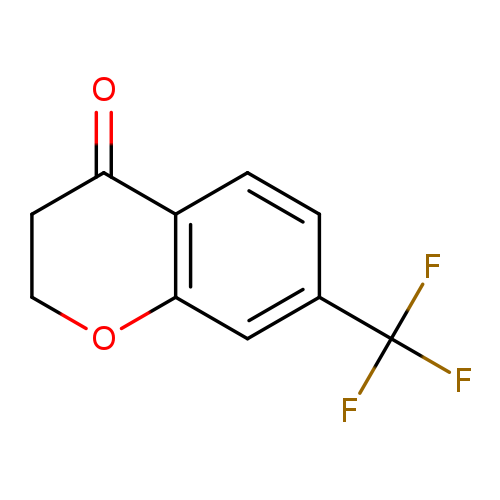
7-(Trifluoromethyl)chroman-4-oneCatalog No.:AA007BEF CAS No.:111141-02-7 MDL No.:MFCD09998165 MF:C10H7F3O2 MW:216.1566 |
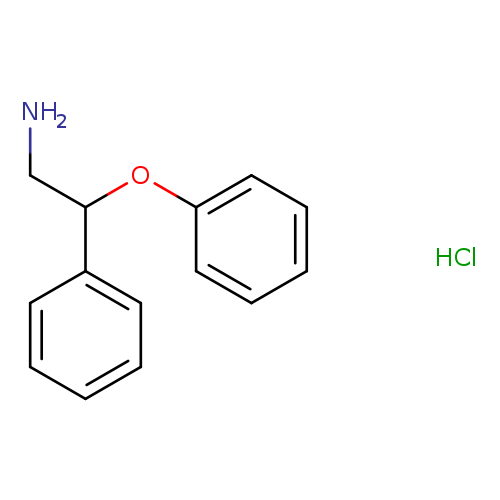
(2-amino-1-phenylethoxy)benzene hydrochlorideCatalog No.:AA01BFCB CAS No.:111143-04-5 MDL No.:MFCD02103429 MF:C14H16ClNO MW:249.7359 |
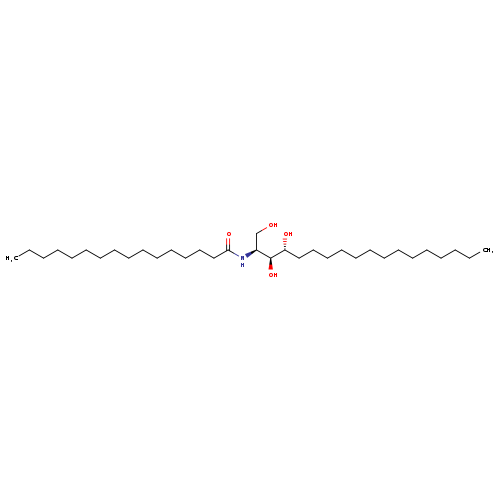
N-[(1S,2S,3R)-2,3-dihydroxy-1-(hydroxymethyl)heptadecyl]-hexadecanamideCatalog No.:AA01EPQZ CAS No.:111149-09-8 MDL No.:MFCD08703057 MF:C34H69NO4 MW:555.9160 |
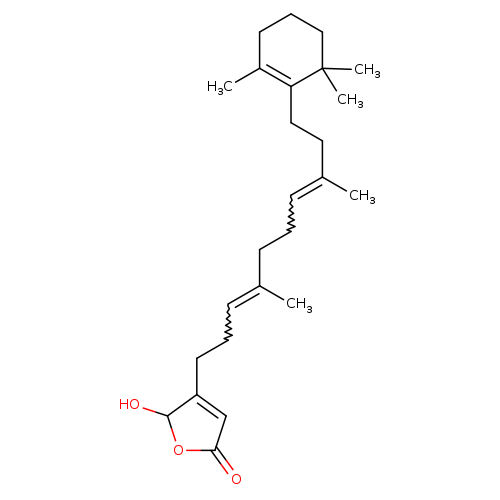
LuffariellolideCatalog No.:AA01E600 CAS No.:111149-87-2 MDL No.:MFCD00211056 MF:C25H38O3 MW:386.5674 |
EnduracidinCatalog No.:AA000X9U CAS No.:11115-82-5 MDL No.: MF:C107H140Cl2N26O32 MW:2373.3175 |
NF 110Catalog No.:AA007BED CAS No.:111150-22-2 MDL No.:MFCD09038569 MF:C41H28N6Na4O17S4 MW:1096.9101 |
4-(3-Methylphenyl)piperidineCatalog No.:AA008R3Q CAS No.:111153-83-4 MDL No.:MFCD03839929 MF:C12H17N MW:175.2701 |
TakinibCatalog No.:AA01DUWJ CAS No.:1111556-37-6 MDL No.:MFCD11822633 MF:C18H18N4O2 MW:322.3611 |
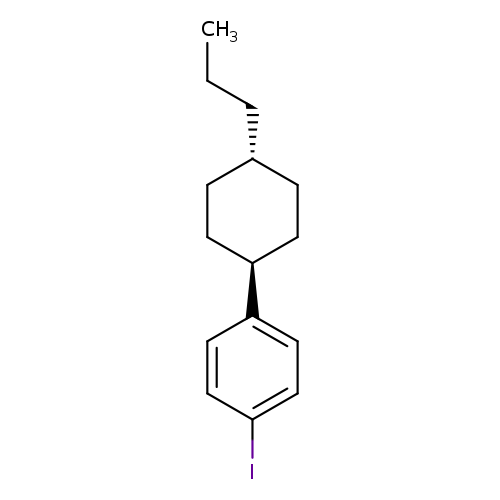
1-Iodo-4-(trans-4-n-propylcyclohexyl)benzeneCatalog No.:AA008SGU CAS No.:111158-11-3 MDL No.:MFCD06658188 MF:C15H21I MW:328.2317 |
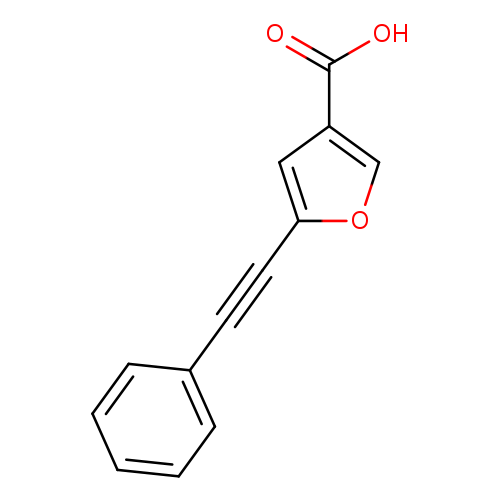
5-(2-Phenylethynyl)furan-3-carboxylic acidCatalog No.:AA019U5Q CAS No.:1111580-55-2 MDL No.:MFCD11839727 MF:C13H8O3 MW:212.2008 |
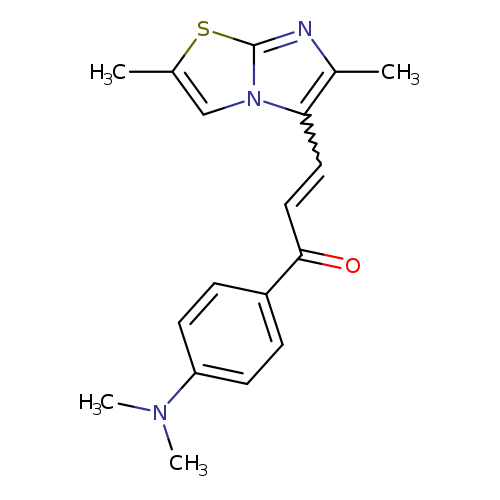
1-[4-(dimethylamino)phenyl]-3-{2,6-dimethylimidazo[2,1-b][1,3]thiazol-5-yl}prop-2-en-1-oneCatalog No.:AA01C3L6 CAS No.:1111591-52-6 MDL No.:MFCD11780684 MF:C18H19N3OS MW:325.4280 |
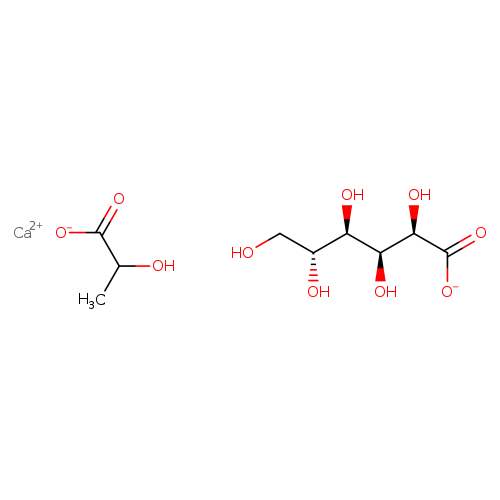
Calcium lactate gluconateCatalog No.:AA008YD6 CAS No.:11116-97-5 MDL No.:MFCD01320836 MF:C9H16CaO10 MW:324.2953 |