2020-03-10 12:15:20
Ankur Harish Shah, Ivan Djordjevic, Terry W.J. Steele
School of Materials Science and Engineering, Nanyang Technological University, Singapore
1.Introduction
Suturing and stapling techniques have been a clinical standard for hundreds of years, despite their well-known drawbacks. Suturing is skill-dependent and relatively slow in an application (Durkaya et al., 2005). Replacement with biocompatible liquid glue would enable rapid interventions by minimally trained first responders. Numerous hurdles need to be overcome to produce appropriate nontoxic glue formulations, as the design parameters need to incorporate a simplified approach that is safe for general use. An ideal tissue adhesive should be liquid on the application, crosslink into mechanically compliant films instantly, and form tissue covalent bonds even in the presence of interfacial water layers. (Bhagat and Becker, 2017). Currently, no commercial tissue adhesives meet these requirements, but a crosslinking method based on carbenes seeks to address the current deficiencies.
Carbenes are short-lived intermediates that insert into molecule in proximity (including water/solvent molecules) non-selectively forming covalent bonds via molecular X–H insertion (Brunner et al., 1980). The carbene precursor diazirine has been grafted onto dendrimers such as poly amidoamine (PAMAM) that resulted in water-soluble PAMAM-g- diazirine (PDz) adhesives. Dendrimers are a key design choice—they avoid polymer entanglement, allowing high solute formulations at low viscosities (Ping et al., 2015a; O'Rorke et al., 2017; Nanda et al., 2018; Singh et al., 2018). Due to rapid dissociation of UVA-activated diazirine to carbene, PDz adhesive has rapid curing (< 1 min), tunable elasticity and adhere to wet substrates towards tissue bonding applications (Mogal et al., 2014a; Feng et al., 2016). Aryl-diazirine is conjugated (grafted) onto 5th generation dendrimer (G5-PAMAM, 28 kDa) to achieve liquid-to-elastomer light-activated transition in aqueous medium. Despite these attractive features, PDz adhesives still have limitations to overcome. Upon removal of water, neat PDz is a viscous liquid, with viscosities (> 50 Pa s) (Shah et al., 2019) that restricts syringe based application, surface wetting, and adhesion strength (< 10 kPa) (Feng et al., 2016). Presence of water (O–H insertion) competes with crosslinks between dendrimer molecules and proteins on tissue surfaces. Leading to weak adhesion strength (Nanda et al., 2018). This effect has been partially mediated by replacing water with liquid oligomers/polymers such as low molecular weight polyethylene glycol (PEG Mw = 400 Da; abbreviated to PEG400 further in text) (Shah et al., 2019). PAMAM dendrimers are known to be miscible with liquid PEG400 forming homogeneous polymer blends (Ristić and Mijović, 2009; Natali and Mijovic, 2009). Motivated by those findings, we produced binary blends of PDz and liquid PEG400 that improve the mechanical modulus by a factor of 5 (Shah et al., 2019) over the originally conceived aqueous PDz formulations (Nanda et al., 2018).
Binary PDz/PEG400 formulations have previously demonstrated shear moduli above 100 kPa with 70% PDz content, but such high solute concentration (PDz) limits the adhesive performance to 5 kPa (Shah et al., 2019). The target modulus of 10–100 kPa is selected based on variation of elastic moduli of soft tissues in the range of 10–400 kPa (Saraf et al., 2007). The target adhesion strength of 10 kPa is selected based on the adhesion strength of commercial/clinically-approved fi- brin based adhesives (Lang et al., 2014). To achieve a higher level of wet adhesion with a lower solute content, the PDz/PEG400 binary adhesive is reformulated with tertiary high molecular weight PEGs (2 kDa, 6 kDa and 10 kDa) in order to increase the probability of intermolecular dendrimer bridging, and therefore increase the cohesive strength while minimizing dynamic viscosity of pure PDz. This strategy exploits the observation that carbene preferentially inserts within H–O- bonds versus H–C- bonds (Blencowe et al., 2008; Moss, 2006). There- fore, it is hypothesized that the loading of original PDz/PEG400 with long, linear molecular chains of PEG (Mn = 2 kDa, 6 kDa and 10 kDa) would increase the modulus and adhesion strength. Tertiary blend (TB) adhesive formulations are obtained by mixing high molecular weight PEG polymers with binary PDz/PEG400 (TB-control) adhesive. The percentage weight of macromolecules (% w/w) in TB formulations and their respective nomenclature are identified in Table 1.
The concentration of PDz is kept constant in all investigated formulations in order to assess the influence of long-chain PEGs on TB mechanical and adhesive performance. Mechanical properties of TBs are investigated by real-time photorheometry and lap shear adhesion on wet tissue-mimicking (hydrated collagen) substrates. Furthermore, swelling and overall percentage of leachates from UVA-crosslinked TB in aqueous medium (saline buffer) are also presented.
2.Materials and methods
2.1.Materials
G5-PAMAM (Mn = 28.8 kDa) dendrimer is purchased from Dendritech, USA. 3-[4-(bromomethyl) phenyl]-3-(trifluoromethyl)-dia- zine (referred to as “diazirine”) is purchased from TCI, Japan. Methanol and polyethylene glycols (PEG, Mn = 400 Da, 2 kDa, 6 kDa and 10 kDa) are purchased from Sigma Aldrich and used as received. Dermabond™ tissue adhesive is purchased from Ethicon. Collagen films are purchased from Nippi films, Singapore.
2.2.Preparation of adhesive polymer blends
Diazirine is grafted onto G5-PAMAM dendrimer (128 surface amine functional groups) using previously published method (Nanda et al., 2018). Briefly, 1000 mg of PAMAM and 181.75 mg of diazirine are stirred in 200 mL of methanol for 12 h to achieve 15% conjugation of PDz. The percentage conjugation is calculated as ratio between primary amine and diazirine. PDz solution in methanol (50%) is mixed with pure (liquid) PEG400. This mixture is further blended with 5% PEG solutions (2 kDa, 6 kDa or 10 kDa) in varying weight ratios as listed in Table 1. The solvent (methanol) is removed in a vacuum oven at 37 °C for 96 h. Binary blend of PDz and PEG400 in the 30:70 wt ratio is used as a control (TB-control) in all measurements.
2.3.Dynamic mechanical properties and curing kinetics
The material properties (storage, G′ and loss moduli, G”) of the TBs upon UVA irradiation is assessed in real-time by photorheometry (Fig. 1a, Physica MCR 501, Anton Paar USA). The rheometer is connected to 365 nm UVA source (Omnicure S1000, SG) and samples are irradiated with constant power of 200 mW cm−2. The experimental parameters are fixed at 1% strain at 1 Hz with 100 μm gap thickness (23 °C). The test sample is sheared with a 10 mm stainless steel parallel plate. The dynamic viscosity of the TBs is calculated at 60 s by dividing G" with angular frequency (G"/ω). Gelation point is defined as time/UV dose required for G” = G’ (or tan δ = 1).
2.4.Scanning electron microscopy (SEM) imaging
The surface morphology of photocured TBs is assessed by photocuring 20 mg of blends between two polyethylene terephthalate (PET) films (2 × 2 cm) with an effective dose of 20 J cm−2 (PET film trans-
mission loss ∼ 30%). The cohesively-fractured surface is gold coated
for 30 s and recorded with SEM (Joel 6630), with an accelerating voltage set at 1 kV and working distance of 11 mm.
2.5.Swelling and mass loss studies
Swelling ratio (%) of crosslinked TB is evaluated by pipetting 7.5 μL (5–10 mg) of uncured adhesive between two 1 cm2 PET films subse- quently photocured with an effective UVA dose of 20 J cm−2 (at 200 mW cm−2 for 100 s). The PET film sandwich-structure is stripped, and the cohesively fractured surface of crosslinked TB is immersed in
phosphate buffered saline (PBS; pH 7.4) at 37 °C for 5 and 15 min. The cohesively fractured surface allows maximum surface area for rapid water swelling. Before recording the mass of the hydrated sample, ex- cess moisture is removed by a lint-free paper. The percentage swelling is calculated by weighing swelled sample (Mw) relative to the mass of the dry crosslinked adhesive (M0): % Swelling = [(Mw - M0)/M0]*100. Similar experimental design was used to indirectly determine the amount of leachates by measuring the residual weight after sample immersion into PBS for 60 min. The residual weight was calculated as follows: % Res. W. = [(M0 – Mr)/M0]*100 where Mr represents sample weight after emersion in PBS.
2.6.Lap shear adhesion strength
The adhesion strength of the photocured matrix is based on ASTM standard F2255-05. Each substrate (collagen + glass and PET + glass) were prepared as follows: collagen film and PET film (2 × 2 cm2; thickness 0.02 mm) are each adhered onto a glass slide with double-sided tape. The collagen substrates were incubated in water at 37 °C prior to tests. 10 mg of uncured TB is smeared onto PET surface and clipped onto the hydrated collagen surface forming a “sandwich structure as: glass/PET/TB/collagen/glass. The sandwich is held to- gether by paperclip and photocured through glass/PET transparent
substarte with an effective dose of 20 J cm−2 (irradiation power of 200 mW cm−2 for 100 s). Substrate temperature does not deviate more than 2 °C (data not shown). Shear adhesion strength is obtained using tensile tester (Chatillon Force Measurement Products, USA). The lap sandwich is subjected to a tensile strain rate of 3 mm min−1 with 50 N loading cell. Lap shear adhesion strength is calculated by dividing the
failure load with the total adhesion area (4 cm−2). For positive control, binary adhesive (TB-control; Table 1) is cured with an effective dose of 20 J cm−2 and Dermabond™ (2 min cure time) against hydrated col- lagen film.
2.7.Statistical analysis
The experiments were carried out in triplicate, including photo- rheometry, adhesion, swelling and mass loss studies. Statistical analysis is performed on the data sets using one-way ANOVA (OriginPro soft- ware) and is significant at *p < 0.05. A strong correlation (Pearson's r) is recorded: positive for r > 0.7, negative or inverse for r > −0.7.
3.Results and discussion
3.1.Effective modulus can be tuned up to 250 kPa with 30% PDz content in TB adhesives
Addition of liquid PEG400 reduces the viscosity of the neat PDz (15% grafted with diazirine) by three orders of magnitude; from 55,000 mPa s to 75 mPa s (Shah et al., 2019) which increases up to 27,000 mPa s upon reinforcement with high Mn PEGs. As shown in Fig. 2b, TB-2K(1), TB-6K(1) and TB-10K(1) have similar viscosity (165–225 mPa s) with no influence of the PEG's chain length. Further- more, low-concentration PEG TBs meet Cilurzo et al.'s criteria for syr- ingibility with a 21G needle (≤250 mPa s) (Cilurzo et al., 2011). The viscosity of the high-concentration PEG TBs: TB-2K(10), TB-6K(10) and TB-10K(10), increases as a function of PEG's chain length with a strong positive correlation (Pearson's r: 0.995). TBs with high viscosity (up to 10,000 mPa s) can still be applied by spatula or syringed with tapered tips (Fischer and Fischer, 1995) prior to crosslinking. Part of the mo- tivation in the current formulation design is the broad applicability and simple methodology—such as that required for first responders. In this regard, the bioadhesive would be stored at ambient temperature and activated at ambient temperature—thus, all measurements are taken at ambient temperature which ranges from 20 to 25 °C.
Crosslinking kinetics upon exposure to UVA light is observed from photorheology profiles shown in Fig. 1c and d. There is an immediate increase in G′ and G” moduli due to carbene-induced intermolecular crosslinking. Carbenes covalently crosslink via X–H insertion with molecules in their proximate environment. In the case of TBs, carbenes insert mainly into surface-exposed primary amines from PAMAM den- drimers, –OH end-groups (PEG) and –CH2- groups from either one of the polymers in the blends. Introduction of high Mn PEGs has limited influence on the gelation time compared to (TB-control) as shown in Fig. 1e. Gelation time does not show dependency on increased Mn PEG (from 2 kDa to 10 kDa) at fixed concentration (1% or 10%). TBs achieve 99% of G′ with an UVA dose of 10 J cm−2 with G′ between 35 –250 kPa upon crosslinking saturation (Fig. 1f). Note that the G’ value for TB-10K (10), (248 ± 14 kPa) correlate to neat, solvent- and PEG-free PDz (245 ± 25 kPa) and are a magnitude higher than TB-control (40 ± 2 kPa), both of which were cured with a UVA dose of 60 J cm−2 (Shah et al., 2019).
The results show that the G′ values of TBs can be tuned by PEG chain length and the reinforcement ratio (1% or 10% w/w ratio; Table 1) to match the specific shear modulus of human soft tissues such as stomach (8–45 kPa), ventricle heart wall (60–148 kPa) and liver (37–340 kPa) (Saraf et al., 2007). Vakalopoulos et al. have measured the shear modulus of cured commercial adhesives and sealants with similar conditions as the photorheometry employed herein (1 Hz, 1% strain) and it was found that G′ of cured Dermabond™ (cyanoacrylate) is 40 MPa, Bio-glueTM (two component, BSA/glutaraldehyde) is 25 MPa, Evicel™ (fibrin) is 2 MPa and Tissucol™ (fibrin) 0.6 MPa (Vakalopoulos et al., 2015), which are up to 4 orders of magnitude higher than G′ values of soft tissues (Valtorta and Mazza, 2005; Nicolle and Palierne, 2010). The TB formulations exhibit the range of G’ similar to soft tissues or at least at the same order of magnitude (Umale et al., 2013).
Apart from mechanical moduli the adhesive film deformation pro- file should also mimic the tissues stress-strain J-curve. In particular, the film-forming polymer adhesive should show strain-hardening beha- viour under applied stress. Matching tissue elasticity as well as de- formation profile can prevent stress concentration at the tissue/ad- hesive interface and stop the interfacial adhesion failure. This feature is of particular interest when the adhesive is applied on soft tissues under perpetual dynamic stresses, such as blood vessels. Elastic moduli of blood vessels are strain-dependent, ranging from 100 kPa at 40 mmHg to 1 MPa at 220 mmHg (Peterson et al., 1960; Bergel, 1961; Murdock et al., 2019). As seen in Fig. 1g, all the photocured TBs exhibit strain hardening response to stress with linear elastic Hookean-like behaviour and do not fail under physiologically-relevant mechanical load (150 mmHg = 20 kPa). Photocured TBs show initiation of the strain- hardening behaviour at 10–30% shear strain range, with failure stress directly influenced by the photocured TB modulus (G').
To show that photocured TBs have strain-dependent G', the shear stress-strain profiles up to 10% strain and between 10-30% strain are linearly fitted to obtain averaged G' values. Linear fitting is employed as a gross estimation of the G' and all the adhesive films to have tan δ < 0.1 and therefore considered primarily elastic. As demon- strated in Fig. 1h, the G' values at 1–10% strain are only influenced by the concentration of high Mn PEG and not by the PEG chain size (Mn). At 10–30% shear strain, the recorded G' reaches the values of 12 kPa, 27 kPa and 53 kPa for TB-2K(10), TB-6K(10) and TB-10K(10) respec- tively.
3.2.Interconnected porous morphology of PEG 6K and 10K-reinforced TBs
Evolution of molecular nitrogen due to diazirine UVA-activation to carbene leads to foaming within polymer matrix, which results in the formation of well-defined porous structures (Fig. 2a–h). Neat PDz (Fig. 2g) has the highest number of pores per surface with the pore diameter of ∼50 μm. When mixed with PEG400 (TB-control; Fig. 3h) UVA-activation of diazirine results in largest pore size (> 300 μm) when compared to other polymer blends. Photocured TBs (Fig. 2a–f) display well-defined porous structures with fairly uniform pore sizes. For high-concentration PEG TBs: TB-2K(10), TB-6K(10) and TB-10K (10), the size of the pores reduces as the molecular weight of the PEG increases (Fig. 2b,d,f). This is most likely a result of a synergetic effect between the initial viscosity and nitrogen gas solubility, which is de- pendent on the molecular weight of PEG (Wiesmet et al., 2000). Visc- osity provides resistance to the initial expansion of the pore once the nitrogen gas nucleates. As gas diffuses at a faster rate through PEG400 (in comparison to higher Mn PEGs) the gas evolution results in smaller, interconnected pores (Fig. 2d,f). The balance of pore size and porosity is critical for tissue regeneration where control of pore size leads to op- timal tissue formation within the porous scaffold network (Loh and Choong, 2013; Wu et al., 2018).
3.3.TB at 10% PEG reinforcement have less swelling than TB-control formulation
Photocured TBs show similar swelling behaviour as the binary TB- control. As seen in Fig. 3a, low concentration load (1%) of high Mn PEGs shows a significant increase in percentage swelling with time, measured at 5 min and 15 min points: TB-2K(1), (from 160 ± 6 to 210 ± 30%) and TB-6K(1), (from 140 ± 6 to 230 ± 15%). Immediate leaching is observed, even after 15 min. Water absorption for TB-10K(1), decreased from 250 ± 35 (5 min) to 190 ± 20% (15 min). The swelling of high-concentration PEG TBs (Fig. 3b) is not statistically different between 5 and 15 min. Upon incubation in PBS (pH = 7.4) for 1h, lower amount of unreacted polymer leached out from the TBs when compared to TB-control (i.e. higher % residual weight; Fig. 3c). The relative concentration of –OH groups reduces as the amount of high Mn increases in the TB, which directly affects the site of carbene covalent insertion into PEG chains. As seen in Fig. 3c, 35–45% of crosslinked TB's remained adhered onto PET sheets. Compared to TB-control, four TBs, namely TB-2K(1), TB-6K(10), TB-10K(1) and TB-10K(10) resulted in higher interfacial crosslinking (with PET substrate) as indicated by higher mass retention.
PAMAM dendrimer and PEG polymers in general, swell in hydrated environment. Introduction of high Mn PEGs with fixed PDz content is anticipated to induce higher swelling rate (Yuan et al., 2017) compared to binary TB-control. However, this was not observed as the swelling ratios were statistically homogenous. Of the six formulations, only TB- 10K(10) showed statistically higher swelling. This observation is in accordance with our previously published results where the swelling ratio was independent of PEG400 content up to 50% w/w concentration in binary mixture with PDz (Shah et al., 2019). Uniform swelling would have limited effect on nutrient transport (Park et al., 2009) making the blend an attractive carrier to load hydrophobic drugs and proteins. Overcoming the cationic charges of the dendrimer with PEG chains would further allow safer cell encapsulation within polymer matrix. The improvement in mass retention can be attributed to carbene in- sertion into aliphatic domain of the PEG backbone. Un-crosslinked PEG400 is expected to leach out from the polymer blend while high Mn PEGs are expected to retain in the photocured matrix as a result of higher crosslinking density.
3.4.High Mn PEG reinforcement increases lap shear adhesion strength
The liquid TB adhesives are irradiated with a net 365 nm UVA dose of 20 J cm−2. This dose is selected for two reasons; 1) 99% of storage modulus is achieved by 20 J cm−2 (as evident from photorheometry results; Fig. 1 a,c,d) and 2), the maximum exposure dose of 20 J cm−2 is the upper limit to prevent erythematic response on skin as defined by
the guidelines issued by International Commission on Non-Ionizing Radiation Protection for UVR exposure (Wan et al., 2012; Protection and I.C.o.N.-I.R., 2004). Lap shear stress/strain profiles are shown in Fig. 4a. The adhesion strength is determined from the failure point and the values are compared in Fig. 4b.
The lap shear adhesion strength of the photocured TBs (25–45 kPa) is statistically higher than binary TB-control (17 ± 2 kPa; Fig. 4b). Photocured TB-2K(1) and TB-10K(1) observe shear strength values of 45 ± 3 kPa and 35 ± 1 kPa, respectively, and are statistically higher when compared to 2-min cured Dermabond™ glue (23 ± 8 kPa; Fig. 4b). Adhesion achieved by TBs is superior to GRF/glutaraldehyde (24.5 kPa) (Shojiro et al., 1999) and fibrin glue (14 kPa), however, it should be noted that the values will differ across substrates (Lauto et al., 2008). The improvement in the adhesive performance does not follow the improvement in the mechanical properties of the photocured TBs. For TB-2-10(1) formulations the dynamic viscosity and the G’ are si- milar but show superior adhesive performance compared to TB-2- 10(10) series. This is attributed to improved carbene-induced cross-linking with the –CH2 groups of PEG chains. In a PEG molecule, the mean monomer length is approximated to 0.278 nm (Oesterhelt et al., 1999), and the counter length of PEG400 is 3.1 nm (Ma et al., 2014) which is less than the diameter of G5-PAMAM dendrimer (∼5 nm) (Maiti et al., 2004). Therefore, crosslinking is possible either between dendrimer molecules (self-crosslinking) or between dendrimer and PEG400 (polymer composite crosslinking).
Reinforcing TB-control with longer PEG chains should increase the probability of achieving higher crosslinking density as the reinforcing PEG has longer counter length compared to PDz diameter. Based on the monomer length, the counter length of PEG 2 kDa, 6 kDa and 10 kDa can be approximated as 12.7 nm, 38.1 nm, and 63.1 nm respectively (Ma et al., 2014). With higher chain length, multiple PDz dendrimers can insert onto a single PEG chain, creating a more interconnected matrix. Liquid PEG400 imparts plasticity to the crosslinked matrix, but a partial replacement with PEG 2K–10K increases the displacement and total work needed before failure (as seen with the 1% compositions). With increasing PEG size, crosslinked TBs fail at lower shear strain, but the long polymer chains provide entanglement and nodes for energy dissipation, which may account for TBs ability to handle higher shear stresses before failure. Some of the limitations within the manuscript are the unresolved toxicity of the primary components. However, we have previously demonstrated that subcutaneous implants of PDz without PEG display a moderate immunological response that can be mitigated by amine-capping (Nanda et al., 2018; Gao et al., 2018). Others have demonstrated that neat polycationic PAMAM dendrimers do not migrate from the injection site, and therefore pose little risk outside the immediate implant area (Niki et al., 2015). Other limita- tions include the wet substrates that formulation is restricted to. The miscibility of PEG prevents immersion or underwater applications. It should be noted that these formulations are not limited to photo- activation, but may be voltage activated as well with the proper grafting of functional groups (Singh et al., 2018; Ping et al., 2015b; Gan et al., 2018). Through a combination of bioadhesion and PEG-ac- celerated drug delivery, the formulations may surpass previous at- tempts of short term drug delivery with polyesters (Cheng et al., 2015; Steele et al., 2013; Mogal et al., 2014b).
4.Conclusion
Liquid, polymer adhesive blends were produced from diazirine- grafted dendrimers and PEG macromolecules of different sizes and concentrations. These bioadhesive formulations crosslink rapidly with low doses of UVA light, thus forming an elastic film. The mechanical characteristics of crosslinked blends mimic that of soft tissues thus making them a potential candidate to reinforce or replace sutures and staples. Dynamic modulus and adhesion strength are dependent on the molecular weight of PEG additive and can be tuned by both UVA light intensity and relative concentration of PEG component. This liquid-to- solid polymer composite may be exploited for release of therapeutics, mending tissues, or combination thereof in future surgical interventions.
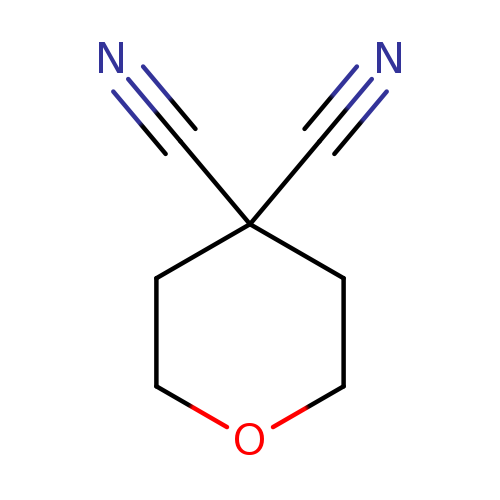
Dihydro-2H-pyran-4,4(3H)-dicarbonitrileCatalog No.:AA008WBR CAS No.:111511-90-1 MDL No.:MFCD19313175 MF:C7H8N2O MW:136.1512 |
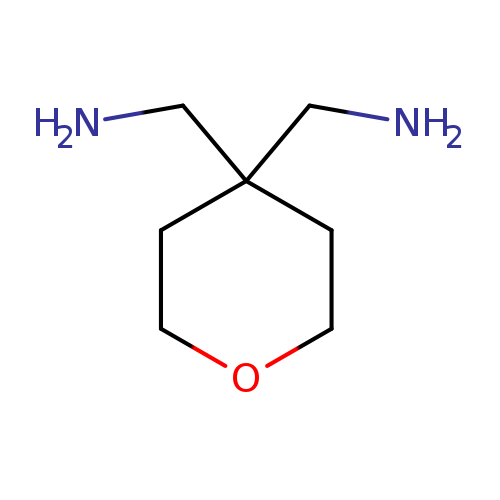
[4-(Aminomethyl)oxan-4-yl]methanamine dihydrochlorideCatalog No.:AA009U5Q CAS No.:111511-91-2 MDL No.:MFCD10699277 MF:C7H16N2O MW:144.2147 |
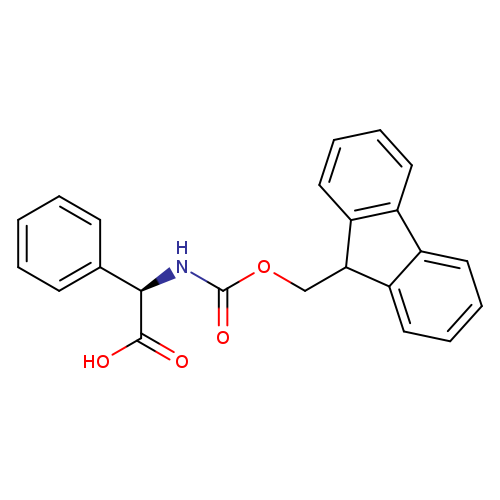
Fmoc-d-phg-ohCatalog No.:AA00HC39 CAS No.:111524-95-9 MDL No.:MFCD00190891 MF:C23H19NO4 MW:373.4013 |
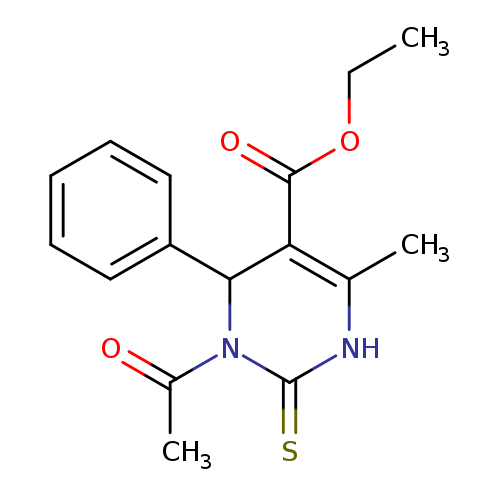
ethyl 3-acetyl-6-methyl-4-phenyl-2-sulfanylidene-1,2,3,4-tetrahydropyrimidine-5-carboxylateCatalog No.:AA00IPSS CAS No.:111535-64-9 MDL No.:MFCD00649136 MF:C16H18N2O3S MW:318.3907 |
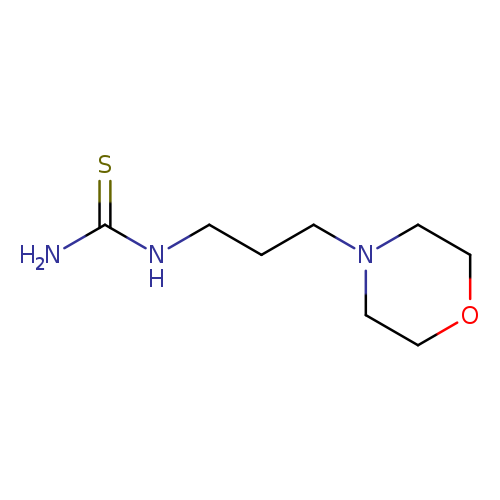
1-(3-Morpholinopropyl)thioureaCatalog No.:AA008253 CAS No.:111538-46-6 MDL No.:MFCD00041378 MF:C8H17N3OS MW:203.3051 |
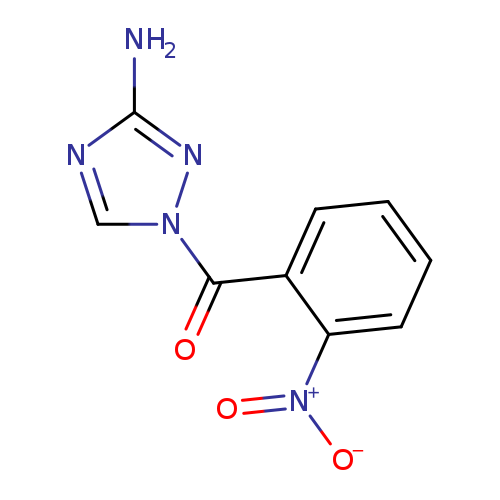
1-(2-nitrobenzoyl)-1H-1,2,4-triazol-3-amineCatalog No.:AA00IX4D CAS No.:111544-25-3 MDL No.:MFCD00170342 MF:C9H7N5O3 MW:233.1836 |
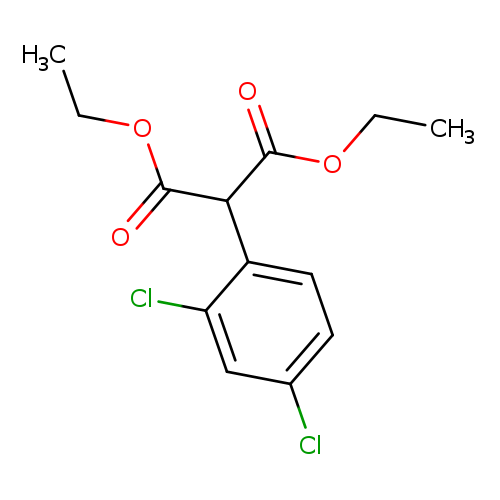
Diethyl 2-(2,4-dichlorophenyl)malonateCatalog No.:AA003PCP CAS No.:111544-93-5 MDL No.:MFCD00831103 MF:C13H14Cl2O4 MW:305.1539 |
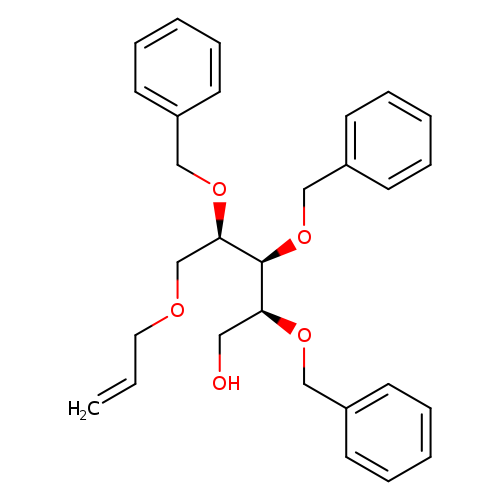
5-O-Allyl-2,3,4-tri-o-benzyl-d-ribitolCatalog No.:AA007AAN CAS No.:111549-97-4 MDL No.:MFCD09750685 MF:C29H34O5 MW:462.5773 |
Benzaldehyde,4,4'-[1,2-ethanediylbis(oxy-2,1-ethanediyloxy)]bis-Catalog No.:AA00824W CAS No.:111550-48-2 MDL No.:MFCD00970744 MF:C20H22O6 MW:358.3851 |
1-[(2R)-piperidin-2-yl]ethan-1-one hydrochlorideCatalog No.:AA01ANCZ CAS No.:111556-21-9 MDL No.:MFCD22682504 MF:C7H14ClNO MW:163.6452 |
2-(1H-Pyrazol-5-yl)anilineCatalog No.:AA003OIM CAS No.:111562-32-4 MDL No.:MFCD09028476 MF:C9H9N3 MW:159.1879 |
4-(2-triphenylenyl)-dibenzothiopheneCatalog No.:AA003AQE CAS No.:1115639-86-5 MDL No.:MFCD32184652 MF:C30H18S MW:410.5289 |
4,4,5,5-Tetramethyl-2-(3-(triphenylen-2-yl)phenyl)-1,3,2-dioxaborolaneCatalog No.:AA008Z86 CAS No.:1115639-92-3 MDL No.:MFCD28130383 MF:C30H27BO2 MW:430.3452 |
3-(Dimethoxymethyl)-1h-pyrazoleCatalog No.:AA008SE8 CAS No.:111573-59-2 MDL No.:MFCD00085025 MF:C6H10N2O2 MW:142.1558 |
2-methyl-2,3-dihydro-1-benzofuran-5-olCatalog No.:AA01DYCK CAS No.:111580-01-9 MDL No.:MFCD24714760 MF:C9H10O2 MW:150.1745 |
Silane,[[(1a,3b,5E,7E,22E)-9,10-secoergosta-5,7,10(19),22-tetraene-1,3-diyl]bis(oxy)]bis[(1,1-dimethylethyl)dimethyl-Catalog No.:AA00IM0W CAS No.:111594-58-2 MDL No.:MFCD11977660 MF:C40H72O2Si2 MW:641.1695 |
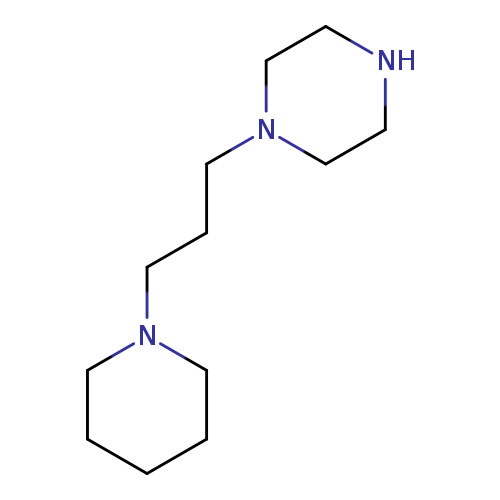
1-(3-Piperidinopropyl)piperazineCatalog No.:AA007SLR CAS No.:111594-93-5 MDL No.:MFCD01320896 MF:C12H25N3 MW:211.3470 |
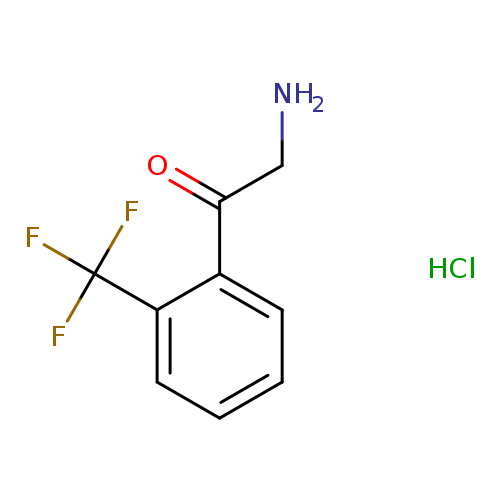
2-amino-1-(2-(trifluoromethyl)phenyl)ethanone hydrochlorideCatalog No.:AA0096A5 CAS No.:111595-55-2 MDL No.:MFCD18382543 MF:C9H9ClF3NO MW:239.6221 |
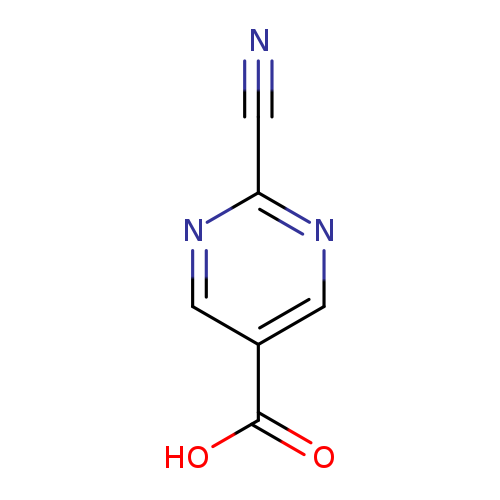
2-Cyanopyrimidine-5-carboxylic acidCatalog No.:AA0095SW CAS No.:1115962-72-5 MDL No.:MFCD12755887 MF:C6H3N3O2 MW:149.1069 |
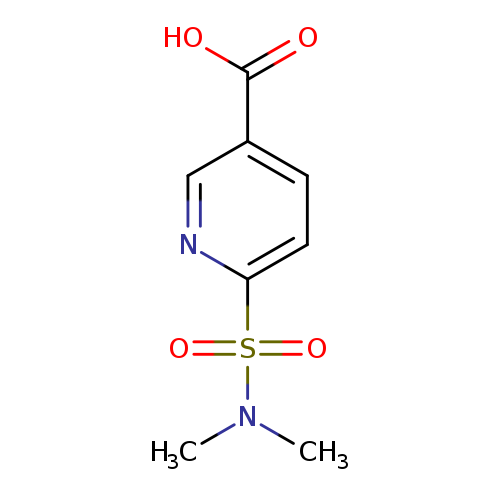
6-(N,N-Dimethylsulfamoyl)nicotinic acidCatalog No.:AA01ACRX CAS No.:1115962-75-8 MDL No.:MFCD18902446 MF:C8H10N2O4S MW:230.2410 |
N-NITROSODIETHANOLAMINECatalog No.:AA003T65 CAS No.:1116-54-7 MDL No.:MFCD00015522 MF:C4H10N2O3 MW:134.1338 |
TrioctylamineCatalog No.:AA0038W1 CAS No.:1116-76-3 MDL No.:MFCD00009560 MF:C24H51N MW:353.6684 |
4,4-Diethoxy-n,n-dimethyl-1-butanamineCatalog No.:AA00HC3B CAS No.:1116-77-4 MDL No.:MFCD00671479 MF:C10H23NO2 MW:189.2951 |
tert-Butyl cyanoacetateCatalog No.:AA0035KC CAS No.:1116-98-9 MDL No.:MFCD00001938 MF:C7H11NO2 MW:141.1677 |
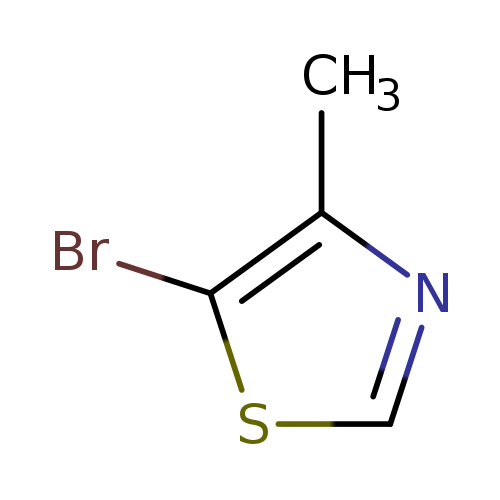
5-Bromo-4-methylthiazoleCatalog No.:AA00345D CAS No.:111600-83-0 MDL No.:MFCD12026322 MF:C4H4BrNS MW:178.0503 |
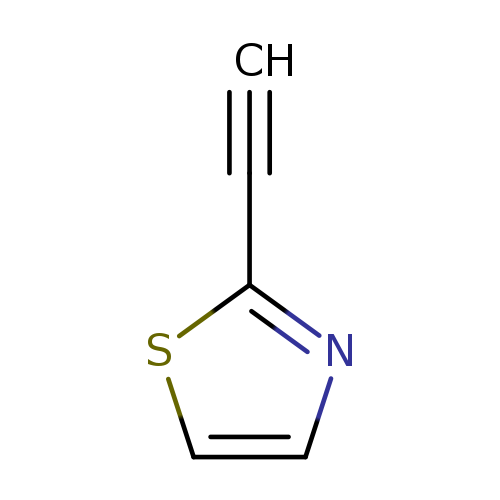
2-Ethynyl-1,3-thiazoleCatalog No.:AA00824K CAS No.:111600-85-2 MDL No.:MFCD13189814 MF:C5H3NS MW:109.1490 |
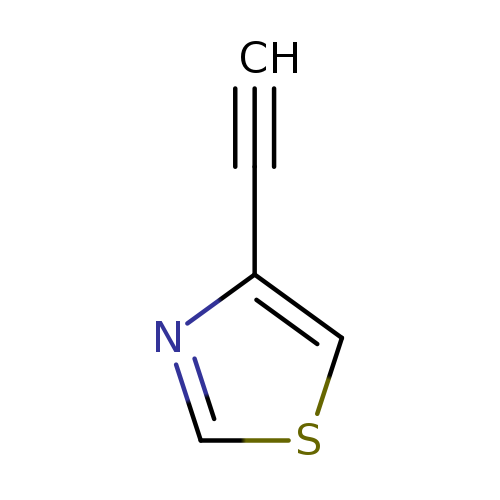
Thiazole, 4-ethynyl-Catalog No.:AA007AAC CAS No.:111600-89-6 MDL No.:MFCD18207415 MF:C5H3NS MW:109.1490 |
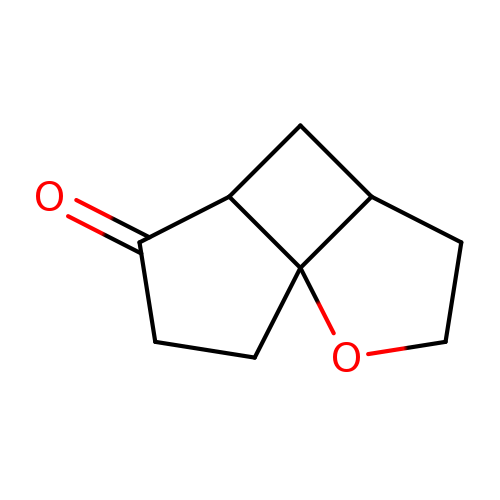
2-oxatricyclo[5.3.0.0,1,5]decan-8-oneCatalog No.:AA01C987 CAS No.:1116036-49-7 MDL No.:MFCD24734657 MF:C9H12O2 MW:152.1904 |
5-Methyl-2-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzonitrileCatalog No.:AA00HC3I CAS No.:1116093-68-5 MDL No.:MFCD10698508 MF:C14H18BNO2 MW:243.1092 |
3-methoxy-2-(tetramethyl-1,3,2-dioxaborolan-2-yl)benzonitrileCatalog No.:AA00PIN1 CAS No.:1116096-84-4 MDL No.:MFCD22494884 MF:C14H18BNO3 MW:259.1086 |
5-methoxy-2-(tetramethyl-1,3,2-dioxaborolan-2-yl)benzonitrileCatalog No.:AA01BG1V CAS No.:1116097-04-1 MDL No.:MFCD22494890 MF:C14H18BNO3 MW:259.1086 |
3,5-Difluoro-4-iodopyridineCatalog No.:AA008X9N CAS No.:1116099-07-0 MDL No.:MFCD13185558 MF:C5H2F2IN MW:240.9774 |
3-oxo-3,4-dihydro-2H-pyrido[3,2-b][1,4]oxazine-7-sulfonyl chlorideCatalog No.:AA0091MH CAS No.:1116135-67-1 MDL No.:MFCD19200313 MF:C7H5ClN2O4S MW:248.6436 |
1H-Pyrrolo[3,2-b]pyridine 4-oxideCatalog No.:AA003EC6 CAS No.:1116136-36-7 MDL No.:MFCD12827548 MF:C7H6N2O MW:134.1353 |
5-Chloro-3-nitro-1h-pyrrolo[3,2-b]pyridineCatalog No.:AA0099C3 CAS No.:1116136-63-0 MDL No.:MFCD16876018 MF:C7H4ClN3O2 MW:197.5786 |
1-(4-(3-Methoxy-4-nitrophenyl)piperazin-1-yl)ethanoneCatalog No.:AA008ZR6 CAS No.:1116229-11-8 MDL No.:MFCD26400580 MF:C13H17N3O4 MW:279.2918 |
7-amino-6-methoxy-1,2,3,4-tetrahydroquinolin-2-oneCatalog No.:AA01AI6G CAS No.:1116232-42-8 MDL No.:MFCD12173108 MF:C10H12N2O2 MW:192.2145 |
Gsk1838705aCatalog No.:AA003A74 CAS No.:1116235-97-2 MDL No.:MFCD19443644 MF:C27H29FN8O3 MW:532.5694 |
Ethyl 1-(Phenylsulfonyl)piperidine-4-carboxylateCatalog No.:AA008Y2D CAS No.:111627-26-0 MDL No.:MFCD00591722 MF:C14H19NO4S MW:297.3700 |
2-Piperazin-1-yl-benzooxazoleCatalog No.:AA008RSO CAS No.:111628-39-8 MDL No.:MFCD04035522 MF:C11H13N3O MW:203.2404 |
2-(CHLOROACETYL)-6,7-DIMETHOXY-1,2,3,4-TETRAHYDROISOQUINOLINECatalog No.:AA008U0L CAS No.:111631-72-2 MDL No.:MFCD00264807 MF:C13H16ClNO3 MW:269.7240 |
1-(5-(Hydroxymethyl)pyrimidin-2-yl)piperidin-4-olCatalog No.:AA008UPI CAS No.:1116339-72-0 MDL No.:MFCD09991528 MF:C10H15N3O2 MW:209.2450 |
S-METHYL-S-(4-PYRIDINYL) SULFOXIMINECatalog No.:AA01B70B CAS No.:1116339-84-4 MDL No.:MFCD11707249 MF:C6H8N2OS MW:156.2055 |
rac-(1R,3S)-3-amino-2,3-dihydro-1H-indene-1-carboxylic acid hydrochloride, cisCatalog No.:AA01ELEY CAS No.:111634-94-7 MDL No.:MFCD09910168 MF:C10H12ClNO2 MW:213.6608 |
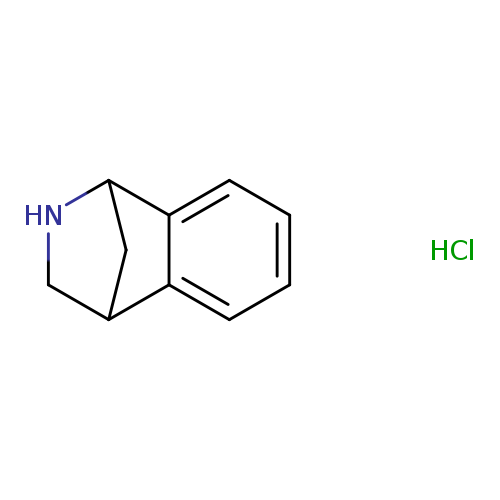
9-azatricyclo[6.2.1.0,2,7]undeca-2,4,6-triene hydrochlorideCatalog No.:AA01C249 CAS No.:111635-04-2 MDL No.:MFCD20680629 MF:C10H12ClN MW:181.6620 |
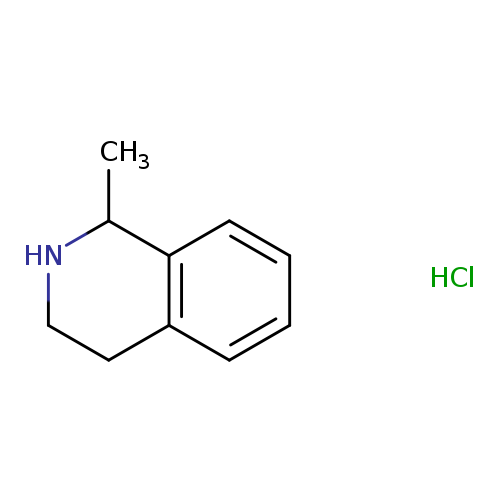
1-Methyl-1,2,3,4-tetrahydroisoquinoline, HClCatalog No.:AA008YYU CAS No.:111635-08-6 MDL No.:MFCD17977125 MF:C10H14ClN MW:183.6779 |
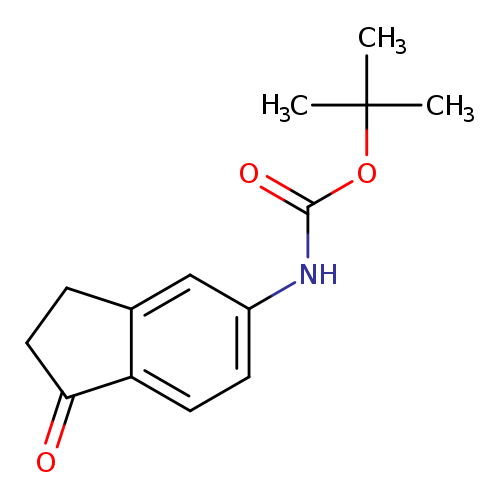
tert-butyl N-(1-oxo-2,3-dihydro-1H-inden-5-yl)carbamateCatalog No.:AA01AKA6 CAS No.:1116358-96-3 MDL No.:MFCD22056444 MF:C14H17NO3 MW:247.2897 |
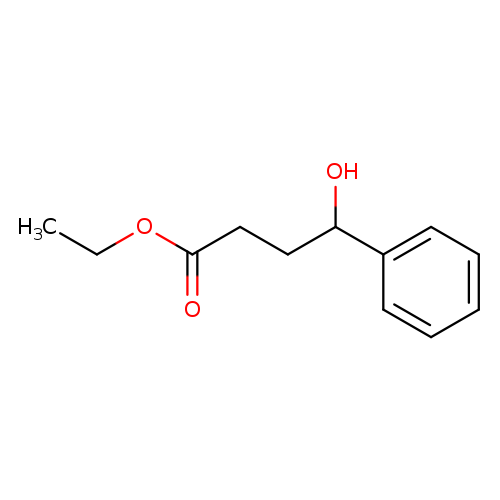
ethyl 4-hydroxy-4-phenylbutanoateCatalog No.:AA01B5BP CAS No.:111636-13-6 MDL No.:MFCD16042046 MF:C12H16O3 MW:208.2536 |
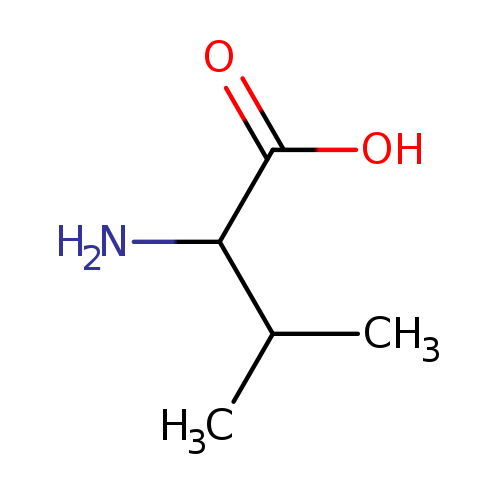
D-Valine-d8Catalog No.:AA01FO0P CAS No.:1116448-82-8 MDL No.: MF:C5H11NO2 MW:117.1463 |
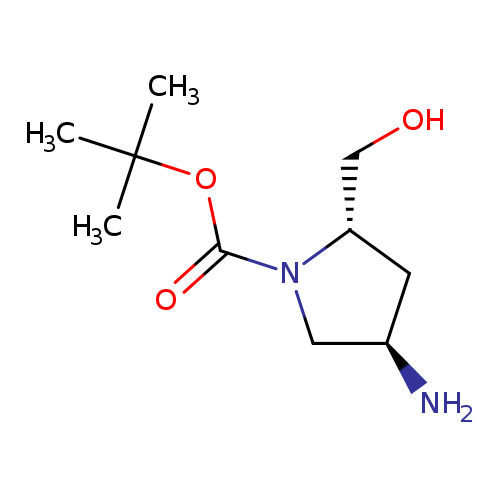
(2S,4R)-tert-Butyl 4-amino-2-(hydroxymethyl)pyrrolidine-1-carboxylateCatalog No.:AA008Y4N CAS No.:1116454-27-3 MDL No.:MFCD11101172 MF:C10H20N2O3 MW:216.2774 |
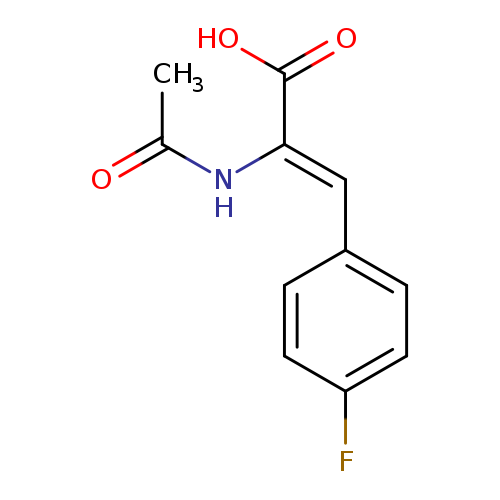
p-Fluoro-α-acetamidocinnamic AcidCatalog No.:AA0079BH CAS No.:111649-72-0 MDL No.:MFCD02683331 MF:C11H10FNO3 MW:223.2004 |
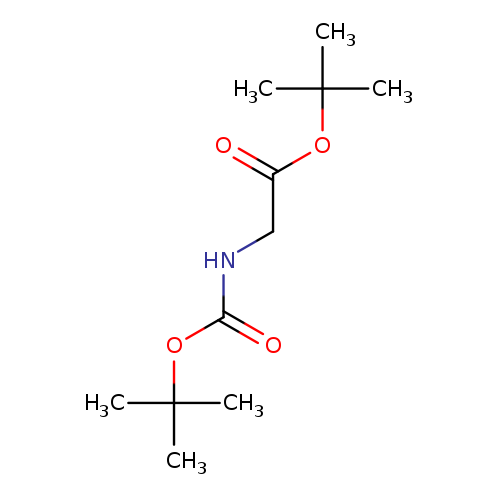
Boc-gly-otbuCatalog No.:AA0079B8 CAS No.:111652-20-1 MDL No.:MFCD00191866 MF:C11H21NO4 MW:231.2887 |
4-(Bromomethyl)-n,n-dimethylaniline hydrobromideCatalog No.:AA019WIC CAS No.:1116572-69-0 MDL No.:MFCD13195837 MF:C9H13Br2N MW:295.0142 |
N-(2,2,2-trifluoroethyl)cyclobutanamine hydrochlorideCatalog No.:AA019Y9B CAS No.:1116589-04-8 MDL No.:MFCD22056387 MF:C6H11ClF3N MW:189.6064 |