2020-03-16 10:00:46
Minghui Wang, Ao Niua, Zhibo Gonga, Zhengwen Xua, Lili Lia, Bo Lia, Jinxin Wang
Over 85% of strokes are ischemic which occurs when a blood vessel in the neck or brain is blocked. The ischemic penumbra is the area surrounding the ischemic core, neuron cells suffer from moderate ischemia and dysfunction but not death, which is potentially reversible when reperfusion happens. Therefore, neuroprotective agent that promote neuron survive and stabilize penumbra is a promising strategy for ischemic stroke therapy.
Danshen is a widely used Chinese herbal medicine in treating vascular diseases such as hypertension and ischemic stroke.2 Tanshinone II-A is the major lipophilic pharmacological constituent of Danshen. The neuroprotective efficacy of tanshinone II A in stroke has been confirmed in both transient and permanent focal cerebral ischemia mice models.3–4 However, the high lipophilicity and extreme aqueous insolubility of tanshinone IIA limit its further application.
To explore the potential clinical application of tanshinone IIA, our initial thought was to modify tanshinone IIA to improve the water solubility and drug release profile. Based on the tanshinone IIA structure-activity relationship and chemical structure modification position, we chose Przewaquinone A, which is also one of the most important ingredients of Danshen and has neuroprotective effects (Fig. 1). The only difference between Przewaquinone A and tanshinone II A is a methoxy group in position 17. As with tanshinone II-A, the water solubility of przewaquinone A is also unsatisfying.
Polyethylene glycol (PEG), which has been widely used to improve the pharmacokinetic behavior of drugs in prodrug design, is usually applied as an effective way for improving the pharmacokinetics of natural products with biological activities.6–14 Most importantly, PEG has been proven safe and approved by the FDA for systemic human use.15
Firstly, we conjugated przewaquinone A with PEG with different spacers, including L-glycine (L-Gly), L-phenylalanine (L-Phe), L-alanine (L-Ala) and two tripeptides (Gly-Glu-Gly, Gly-Asp-Gly). The preparation of the conjugates was started from the introduction of carboxyl to both terminals of PEG through a method introduced by Bersani et al.16 (Scheme 1). Meanwhile, tanshinone IIA was oxidized to obtain the przewaquinone A (Scheme 2).
We chose amino acids and tripeptides as spacers because of their relative appropriate drug release velocity.17 Furthermore, amino acids could contribute to the cell penetrability of nature compounds because PepT1.18 3a–5d have two przewaquinone A (2) molecules and three different spacers respectively. Compounds 6a–7d involved two different tripeptides to further increase the przewaquinone A ratio in the conjugates (Scheme 3.).
All final conjugates were characterized by FT-IR. For instance, the typical FT-IR spectra of carboxylic-PEG 2000 (1a) and przewaquinone A-Phe-PEG 2000 conjugate (5a) were shown in Fig. 2. In the spectrum of PEG 2000, the broad bands located at 3482 cm−1 and 3126 cm−1 are, respectively, due to the adsorption of OeH stretching vibrations of
hydroxyl ends on carboxylic-PEG. Several new IR signals, like the absorption bands at 1737 cm−1 and 1627 cm−1, attributed to the carboxyl groups of 5a (Fig. 2). 3a and 4b were also identified by MALDI-Tof. The theoretical average weights of 3a and 4b are 2698.8 and 4726.8, which are very close to the MALDI-Tof results.
Water solubility of these prodrugs enhanced remarkably by introducing PEG into przewaquinone A (averagely increased more than 107 times) (Table 1). There was no large difference between conjugates with different PEG molecular weights with the same amino acid linker. Although higher molecular weight of PEG means more hydrogen- bonding acceptors, its lower drug loading capability may lead to the UV value lower, which was applied to calculate solubility.
Drug loading capability shows no big difference for 3a–5d. However, 6a–6d and 7a–7d have significantly higher drug payload. That could be easily explained by the strategy of using amino acid with two carboxy acids group to load more 2. In addition, drug loading ability decreased as the increase of PEG molecular weight in each group, which could be explained by two reasons. Firstly, terminal concentration would decrease if the molecular weight of the PEG increases. That will decrease the conjugate rate of PEG and przewaquinone A. Secondly, basing on the calculation formula, which is przewaquinone A% = (mprzewaquinone A/prodrug) × 100%, larger polymeric molecular weight leading to lower theoretical przewaquinone A ratio was reasonable.
In vitro hydrolysis assay in human plasma of these polymers was carried out to evaluate the drug release behavior of PEGylated przewaquinone A by incubating in human plasma at 37 °C. 3a, 3d, 4a, 4d, 5a, 5d and 7d were selected to compare the influence of molecular weight of PEG and amino acid spacers on drug release properties.
The results were shown in Fig. 4. Firstly, for 3a, 3d, and 4a, 4d, conjugates with higher molecular weight have a slower drug releasing rate, which can also be observed for other polymer conjugates.19 It may because the actual molar of primary drug would decrease if the molecular weight of PEG increases. Hydrolyzation hindrance in these polymers also decreased due to the steric hindrance.17 For example, glycine connected conjugate (3a) has higher releasing rate than phenylalanine connected conjugate (5a). Moreover, types of hydrolytic enzyme in human plasma may also has great influence on the releasing properties of conjugates. That could explain why some spacers with larger hin- drance still had higher releasing rates.
Then, we chose the prodrug 3a and przewaquinone A to examine the in vivo release behavior of our prodrugs. After injection, concentration of przewaquinone A and the 3 was monitored by HPLC. However, only compound 3 was detected in plasma after prodrug 3a administered intravenously (Fig. 5a). This means that the in vivo amide bond of prodrug 3a was much more easily to be broken than ester bond (Fig. 6). Przewaquinone A (2) was detected after 5 mins (Fig. 5b). 3a and 2 reached to their highest concentration in nearly 240 mins and 5 mins respectively, suggesting that 3a was released from prodrug in a sustained manner over a long period of time without a burst, which verified the effectiveness of our prodrug strategy.
To further confirm the effectiveness of PEG conjugates on protecting neuron in vivo, a murine model of focal cerebral ischemia was induced by intra-luminal transient middle cerebral artery occlusion (tMCAO). Compound 3a was administered through caudal vein after reperfusion at doses of 4, 8 and 16 mg/kg daily (QD) for 3 days. Gigaton (15 mg/kg) was selected as the positive control. The neurological deficit was evaluated using Bederson's score.20–21 As a result, the application of compound 3a (16 mg/kg) led to a significant reduction of the observed neurological deficit after 72 h compared to the model group as well as the ignition group (Fig. 8B). Consistent with the improved neurological function, there was a 31.4% reduction in the infarct volume in rats treated with compound 3a (16 mg/kg) compared to the model, and significantly less than the gigaton group (Fig. 8C). Meanwhile, the water content of 3a (16 mg/kg) decreased significantly compared to the model and positive control (Fig. 8D). Overall, the neuroprotective activity of 3a is stronger than that of ignition, indicating 3a has potential for further development as a neuroprotective drug.
In summary, we designed and synthesized a series of novel PEG- amino acid-przewaquinone conjugations. A based on the prodrug strategy. In vivo drug release assay confirmed that 3a could release active przewaquinone A in a sustained manner after intravenous injection, which proved that PEG prodrug might be an effective way to extend the half-life of some potent natural products. Meanwhile, compound 3a was efficacious in a mice tMCAO model, indicating that 3a could be a promising candidate for neuroprotective drugs. These data greatly proved that the PEG conjugation strategy is a quite effective way to improve the pharmacokinetics even the in vivo activity of many natural products. We believe that this meaningful and potential strategy could bring light on resolving the related important problems of numerous valuable natural products on clinical use in the future.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influ- ence the work reported in this paper.
Acknowledgements
This work was supported financially by the Key Research & Development Program in Jiangsu [NO. BE2015683]; the Introduction Program of Leading Scientific and Technological Entrepreneurship in Nanjing [NO. 2013B14007].
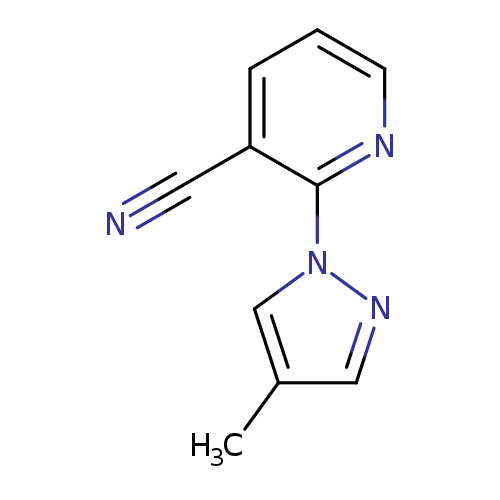
2-(4-Methyl-1H-pyrazol-1-yl)nicotinonitrileCatalog No.:AA007V6L CAS No.:1119391-07-9 MDL No.:MFCD11053985 MF:C10H8N4 MW:184.1973 |
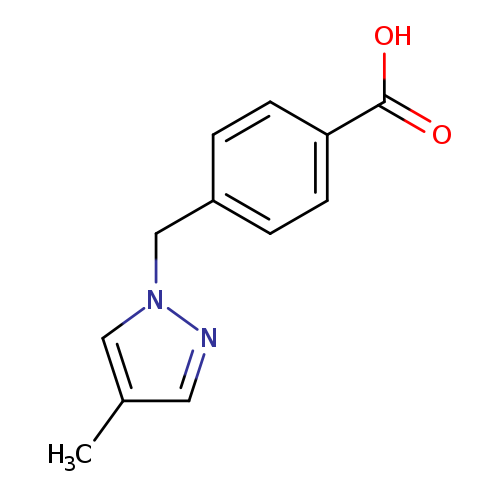
4-[(4-METHYL-1H-PYRAZOL-1-YL)METHYL]BENZOIC ACIDCatalog No.:AA01EK8C CAS No.:1119391-16-0 MDL No.:MFCD12030920 MF:C12H12N2O2 MW:216.2359 |
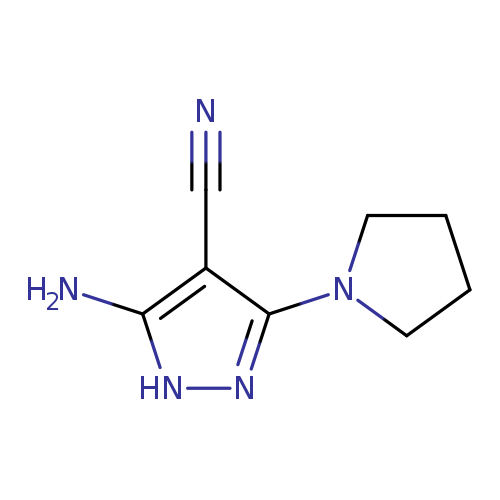
5-Amino-3-(pyrrolidin-1-yl)-1H-pyrazole-4-carbonitrileCatalog No.:AA00HC4G CAS No.:1119391-48-8 MDL No.:MFCD07325241 MF:C8H11N5 MW:177.2064 |
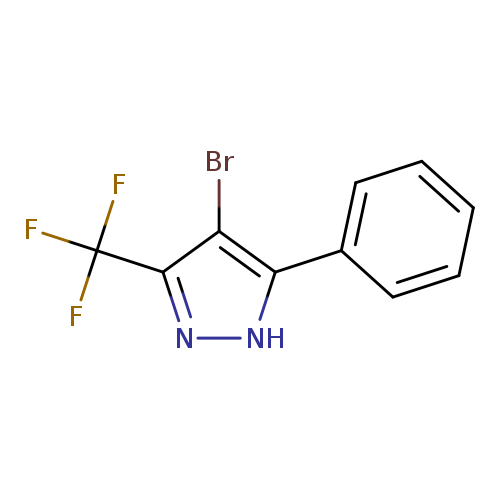
4-bromo-5-phenyl-3-(trifluoromethyl)-1H-pyrazoleCatalog No.:AA00IV6Z CAS No.:1119391-55-7 MDL No.:MFCD00153731 MF:C10H6BrF3N2 MW:291.0672 |
7,7-dimethyl-1-({[4-(pyridin-2-yl)piperazin-1-yl]sulfonyl}methyl)bicyclo[2.2.1]heptan-2-oneCatalog No.:AA00ITEV CAS No.:1119391-83-1 MDL No.:MFCD12910773 MF:C19H27N3O3S MW:377.5010 |
3-[(3,4-dimethoxyphenyl)methyl]-1-[4-(trifluoromethoxy)phenyl]thioureaCatalog No.:AA00IXAA CAS No.:1119391-84-2 MDL No.:MFCD01567616 MF:C17H17F3N2O3S MW:386.3887 |
4-(5-bromo-2-methoxyphenyl)-1H-1,2,3-triazole-5-carbonitrileCatalog No.:AA00IVBN CAS No.:1119391-96-6 MDL No.:MFCD02662324 MF:C10H7BrN4O MW:279.0928 |
4-(4-Bromophenyl)-1h-1,2,3-triazole-5-carbonitrileCatalog No.:AA00IVB4 CAS No.:1119392-12-9 MDL No.:MFCD04154173 MF:C9H5BrN4 MW:249.0668 |
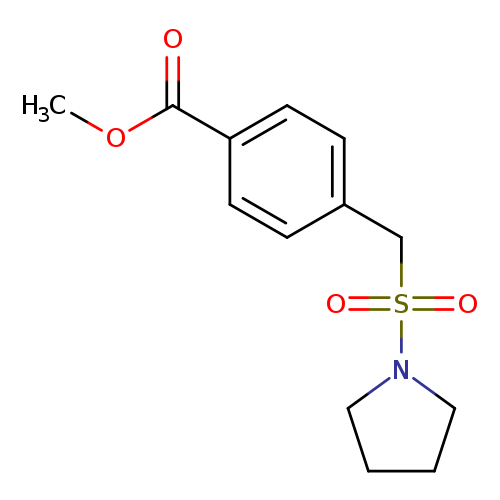
Methyl 4-[(pyrrolidine-1-sulfonyl)methyl]benzoateCatalog No.:AA01EH71 CAS No.:1119407-35-0 MDL No.:MFCD13960330 MF:C13H17NO4S MW:283.3434 |
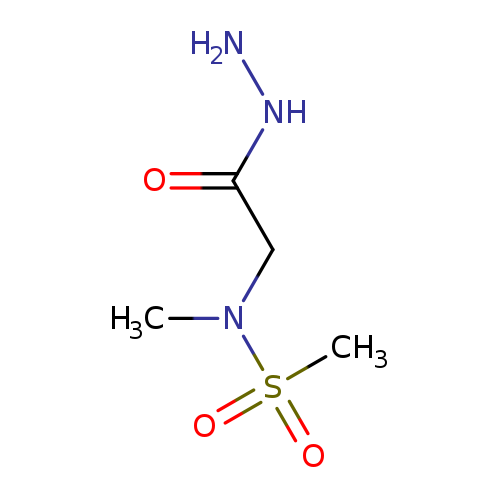
N-(2-Hydrazinyl-2-oxoethyl)-N-methylmethanesulfonamideCatalog No.:AA008VGU CAS No.:1119445-29-2 MDL No.:MFCD12827522 MF:C4H11N3O3S MW:181.2134 |
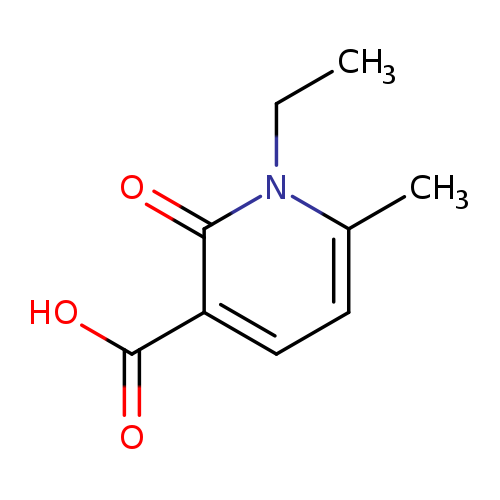
1-Ethyl-6-methyl-2-oxo-1,2-dihydro-3-pyridinecarboxylic acidCatalog No.:AA0083XB CAS No.:1119446-11-5 MDL No.:MFCD12198442 MF:C9H11NO3 MW:181.1885 |
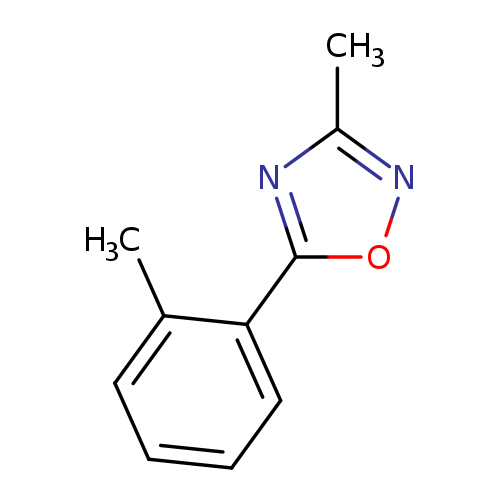
3-Methyl-5-(2-methylphenyl)-1,2,4-oxadiazoleCatalog No.:AA00HC4L CAS No.:1119448-03-1 MDL No.:MFCD13961845 MF:C10H10N2O MW:174.1992 |
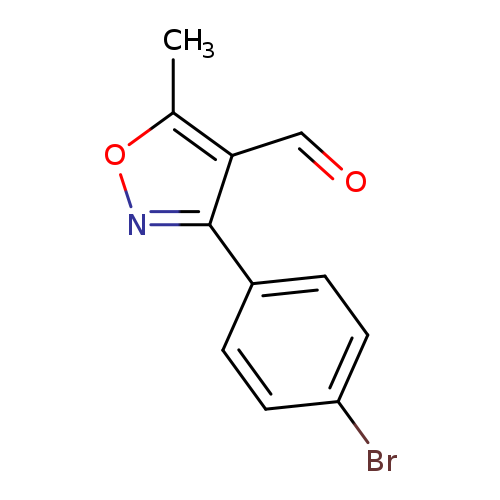
3-(4-Bromophenyl)-5-methylisoxazole-4-carboxaldehydeCatalog No.:AA003I0V CAS No.:1119449-35-2 MDL No.:MFCD11656166 MF:C11H8BrNO2 MW:266.0907 |
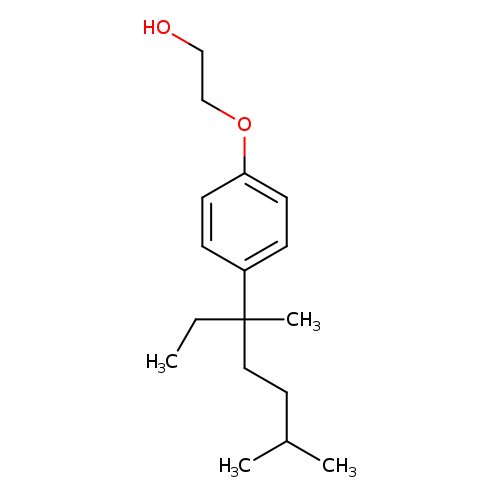
4-(3',6'-Dimethyl-3'-heptyl)phenol MonoethoxylateCatalog No.:AA007V6H CAS No.:1119449-37-4 MDL No.:MFCD11869787 MF:C17H28O2 MW:264.4030 |
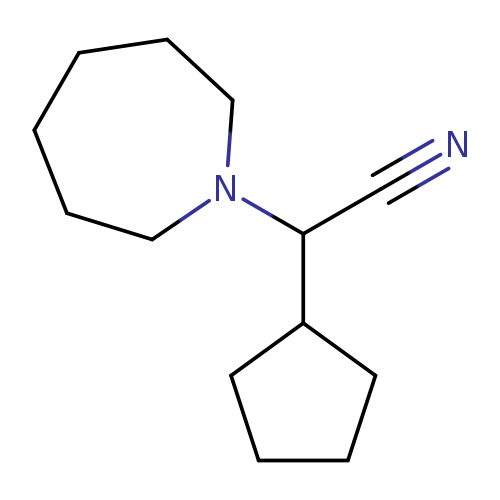
Azepan-1-yl(cyclopentyl)acetonitrileCatalog No.:AA008VI4 CAS No.:1119449-72-7 MDL No.:MFCD12026893 MF:C13H22N2 MW:206.3272 |
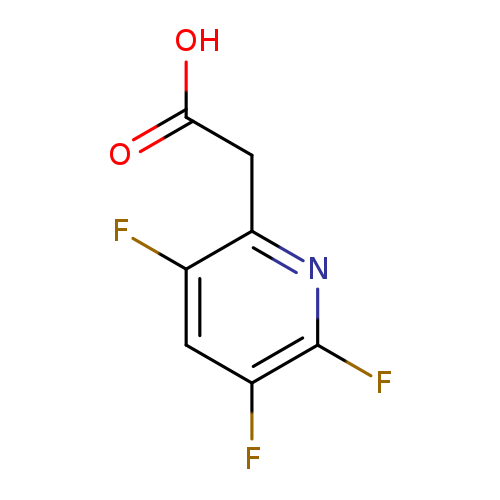
(3,5,6-Trifluoropyridin-2-yl)acetic acidCatalog No.:AA008VKG CAS No.:1119450-11-1 MDL No.:MFCD03001145 MF:C7H4F3NO2 MW:191.1074 |
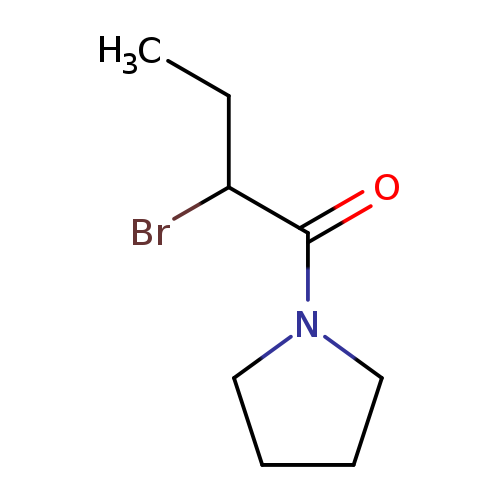
1-(2-Bromobutanoyl)pyrrolidineCatalog No.:AA008VHN CAS No.:1119450-19-9 MDL No.:MFCD03030369 MF:C8H14BrNO MW:220.1069 |
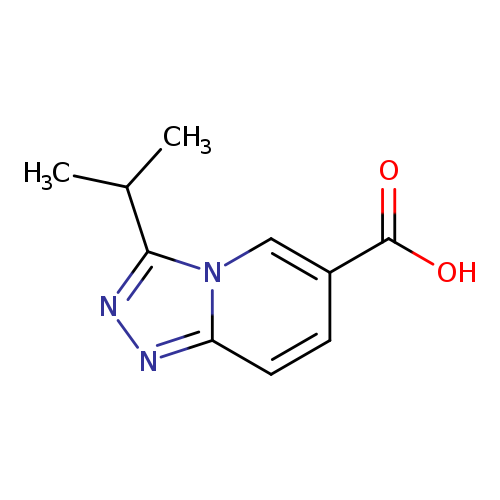
3-Isopropyl[1,2,4]triazolo[4,3-a]pyridine-6-carboxylic acidCatalog No.:AA00HC6W CAS No.:1119450-90-6 MDL No.:MFCD07376865 MF:C10H11N3O2 MW:205.2132 |
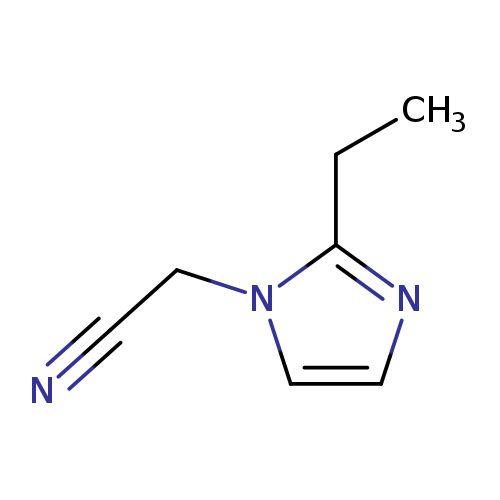
2-(2-Ethyl-1H-imidazol-1-yl)acetonitrileCatalog No.:AA008VBG CAS No.:1119451-03-4 MDL No.:MFCD12026943 MF:C7H9N3 MW:135.1665 |
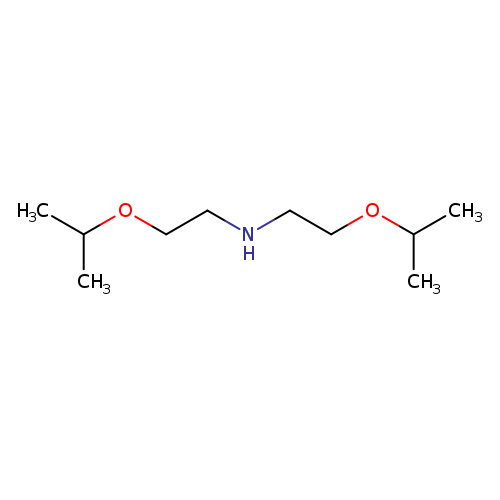
Bis(2-isopropoxyethyl)amineCatalog No.:AA00HC6Z CAS No.:1119451-05-6 MDL No.:MFCD06797066 MF:C10H23NO2 MW:189.2951 |
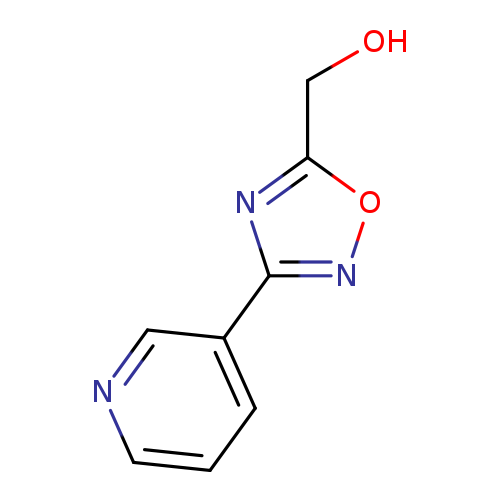
(3-Pyridin-3-yl-1,2,4-oxadiazol-5-yl)methanolCatalog No.:AA008VDE CAS No.:1119451-10-3 MDL No.:MFCD12027101 MF:C8H7N3O2 MW:177.1601 |
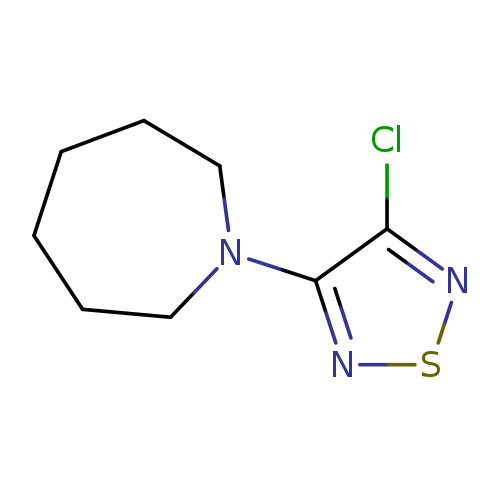
1-(4-Chloro-1,2,5-thiadiazol-3-yl)azepaneCatalog No.:AA008VIM CAS No.:1119451-34-1 MDL No.:MFCD12027294 MF:C8H12ClN3S MW:217.7190 |
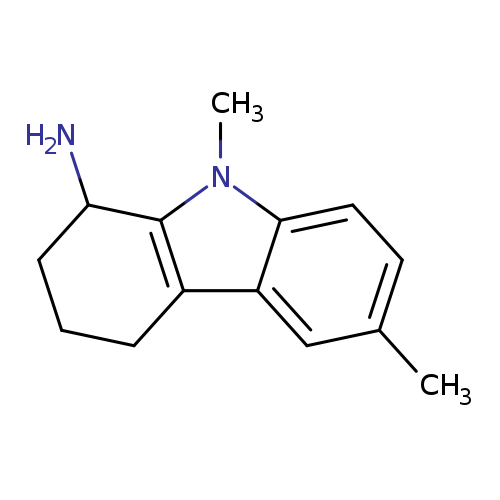
6,9-Dimethyl-2,3,4,9-tetrahydro-1h-carbazol-1-amineCatalog No.:AA008V9W CAS No.:1119451-37-4 MDL No.:MFCD12027302 MF:C14H18N2 MW:214.3061 |
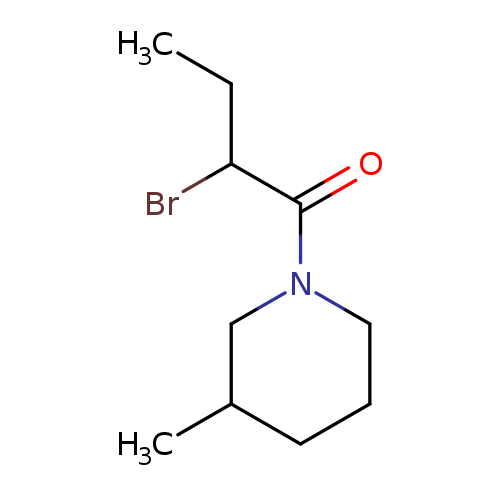
1-(2-Bromobutanoyl)-3-methylpiperidineCatalog No.:AA008V4C CAS No.:1119451-43-2 MDL No.:MFCD12027316 MF:C10H18BrNO MW:248.1600 |
4,6-Dimethyl-5-morpholin-4-ylpyrimidin-2-amineCatalog No.:AA008VFV CAS No.:1119451-57-8 MDL No.:MFCD12027400 MF:C10H16N4O MW:208.2602 |
5-Ethyl-4-methylisoxazole-3-carboxylic acidCatalog No.:AA0090EH CAS No.:1119452-16-2 MDL No.:MFCD12026904 MF:C7H9NO3 MW:155.1513 |
5-Methyl-4-[(4-methylpiperidin-1-yl)methyl]isoxazole-3-carboxylic acid HClCatalog No.:AA0090GC CAS No.:1119452-18-4 MDL No.:MFCD12026931 MF:C12H18N2O3 MW:238.2829 |
2-(5-Thioxo-4,5-dihydro-1h-1,2,4-triazol-3-yl)benzoic acidCatalog No.:AA008V8Z CAS No.:1119452-21-9 MDL No.:MFCD12027006 MF:C9H7N3O2S MW:221.2358 |
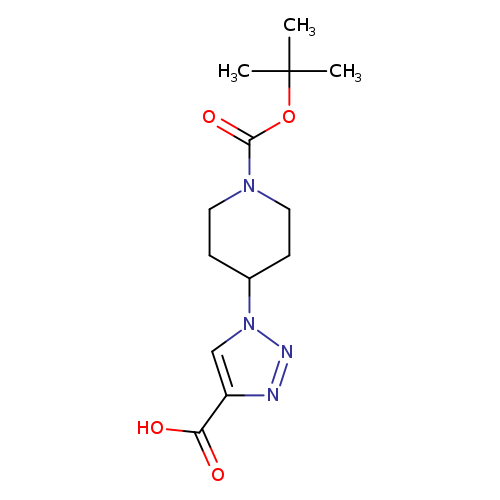
1-[1-(tert-Butoxycarbonyl)piperidin-4-yl]-1h-1,2,3-triazole-4-carboxylic acidCatalog No.:AA0090PU CAS No.:1119452-31-1 MDL No.:MFCD12027299 MF:C13H20N4O4 MW:296.3223 |
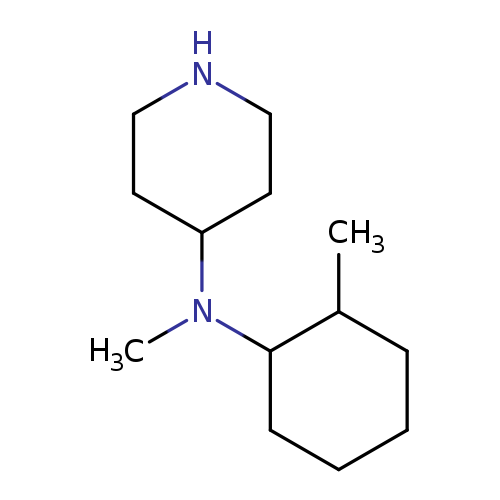
N-Methyl-n-(2-methylcyclohexyl)piperidin-4-amineCatalog No.:AA008V2Z CAS No.:1119452-90-2 MDL No.:MFCD12027204 MF:C13H26N2 MW:210.3589 |
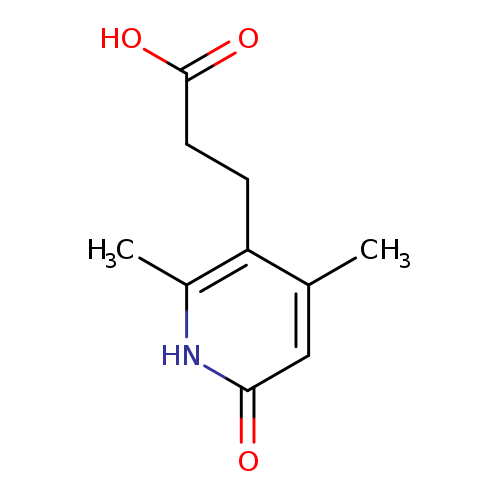
3-(2,4-Dimethyl-6-oxo-1,6-dihydropyridin-3-yl)propanoic acidCatalog No.:AA008V7I CAS No.:1119453-13-2 MDL No.:MFCD12027398 MF:C10H13NO3 MW:195.2151 |
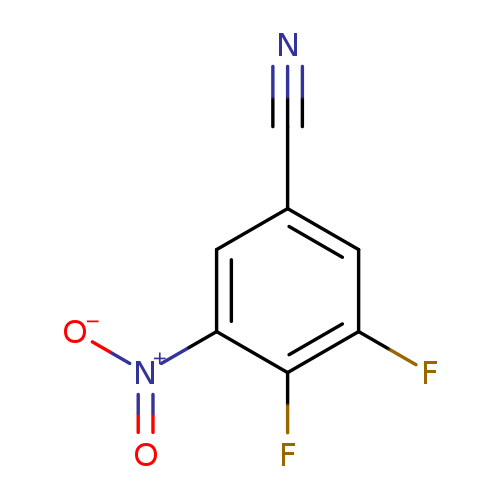
3,4-Difluoro-5-nitrobenzonitrileCatalog No.:AA008UIN CAS No.:1119454-07-7 MDL No.:MFCD11110377 MF:C7H2F2N2O2 MW:184.0998 |
Methyl 5-chloro-2-(chlorosulfonyl)benzoateCatalog No.:AA01A280 CAS No.:1119454-19-1 MDL No.:MFCD22380314 MF:C8H6Cl2O4S MW:269.1018 |
4-(2,4-Dimethoxyphenyl)phenolCatalog No.:AA00HC96 CAS No.:1119454-89-5 MDL No.:MFCD18312918 MF:C14H14O3 MW:230.2592 |
2,3-Difluoro-5-nitrophenolCatalog No.:AA008XMR CAS No.:1119455-04-7 MDL No.:MFCD12026384 MF:C6H3F2NO3 MW:175.0897 |
1-tert-butyl-5-ethyl-1H-pyrazole-4-carboxylic acidCatalog No.:AA019UXH CAS No.:1119459-44-7 MDL No.:MFCD11858063 MF:C10H16N2O2 MW:196.2462 |
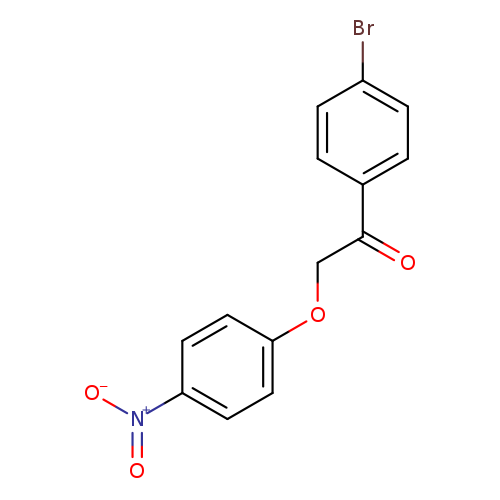
1-(4-Bromophenyl)-2-(4-nitrophenoxy)ethanoneCatalog No.:AA007V6E CAS No.:111946-84-0 MDL No.:MFCD00428549 MF:C14H10BrNO4 MW:336.1375 |
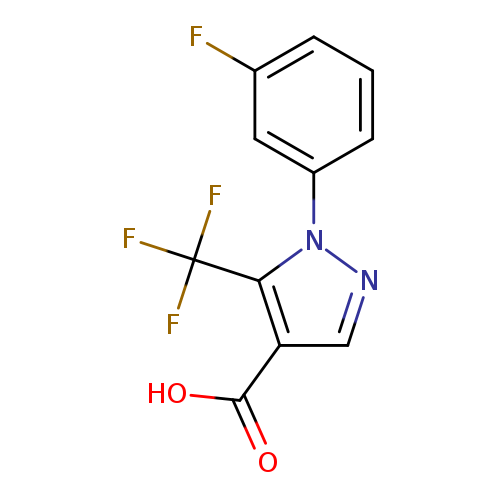
1-(3-Fluorophenyl)-5-(trifluoromethyl)-1H-pyrazole-4-carboxylic acidCatalog No.:AA01C0IK CAS No.:1119489-35-8 MDL No.:MFCD16418002 MF:C11H6F4N2O2 MW:274.1712 |
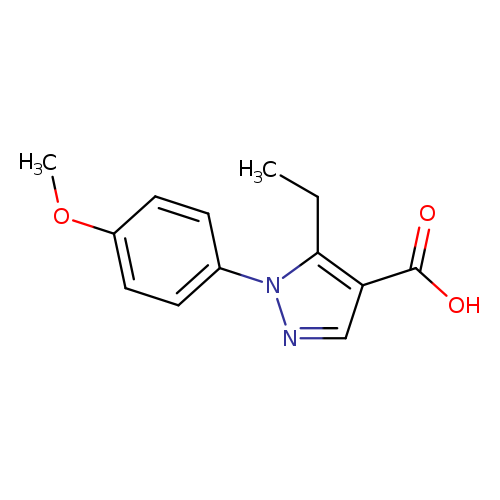
5-Ethyl-1-(4-methoxyphenyl)-1h-pyrazole-4-carboxylic acidCatalog No.:AA01ACUM CAS No.:1119490-22-0 MDL No.:MFCD14462876 MF:C13H14N2O3 MW:246.2619 |
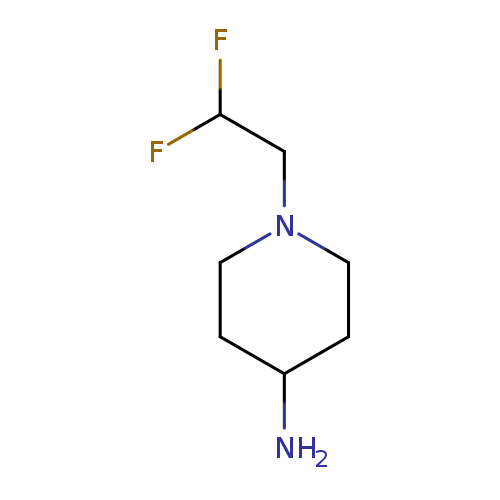
1-(2,2-Difluoro-ethyl)-piperidin-4-ylamineCatalog No.:AA0092O5 CAS No.:1119499-74-9 MDL No.:MFCD10699009 MF:C7H14F2N2 MW:164.1963 |
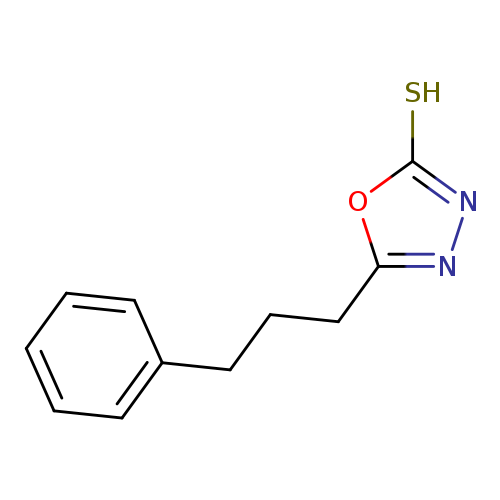
5-(3-phenylpropyl)-1,3,4-oxadiazole-2-thiolCatalog No.:AA01AAAI CAS No.:1119505-40-6 MDL No.:MFCD10695393 MF:C11H12N2OS MW:220.2908 |
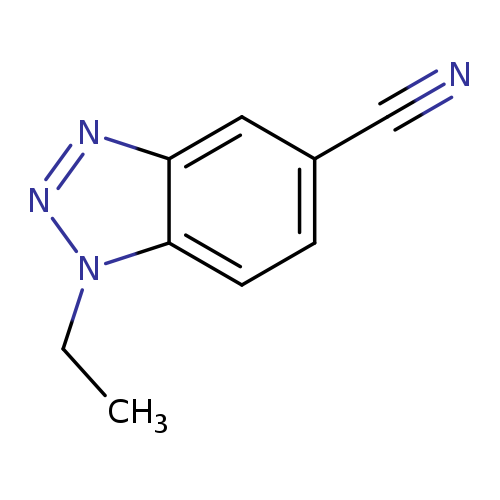
1-Ethyl-1,2,3-benzotriazole-5-carbonitrileCatalog No.:AA007UYZ CAS No.:1119505-52-0 MDL No.:MFCD13881240 MF:C9H8N4 MW:172.1866 |
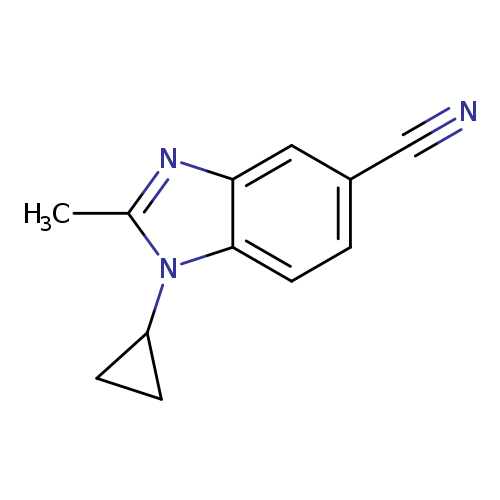
1-Cyclopropyl-2-methyl-1,3-benzodiazole-5-carbonitrileCatalog No.:AA009448 CAS No.:1119505-56-4 MDL No.:MFCD13881257 MF:C12H11N3 MW:197.2358 |
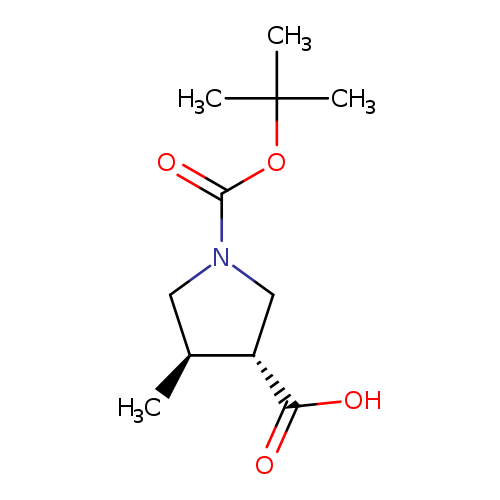
(3R,4R)-1-(tert-Butoxycarbonyl)-4-methylpyrrolidine-3-carboxylic acidCatalog No.:AA0083Q9 CAS No.:1119512-35-4 MDL No.:MFCD15071830 MF:C11H19NO4 MW:229.2729 |
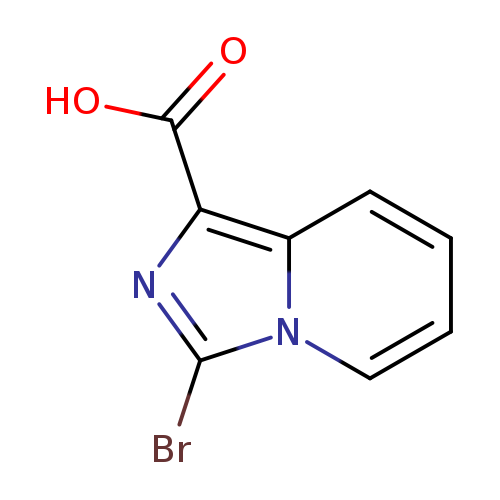
3-Bromoimidazo[1,5-a]pyridine-1-carboxylic acidCatalog No.:AA0094JV CAS No.:1119512-48-9 MDL No.:MFCD11846504 MF:C8H5BrN2O2 MW:241.0415 |

GLP-1(7-36) AcetateCatalog No.:AA01EOKC CAS No.:1119517-19-9 MDL No.:MFCD18074453 MF: MW: |
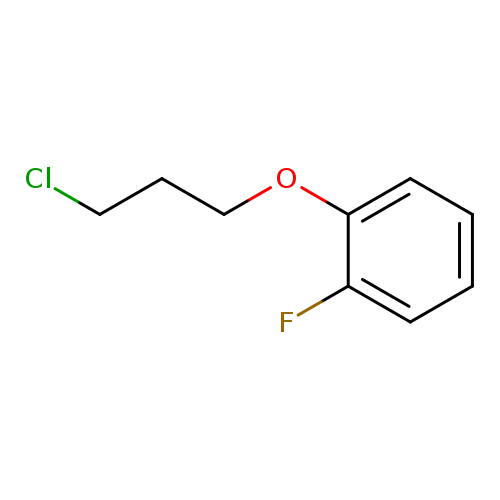
1-(3-chloropropoxy)-2-fluorobenzeneCatalog No.:AA01DUWN CAS No.:111952-54-6 MDL No.:MFCD09734161 MF:C9H10ClFO MW:188.6265 |
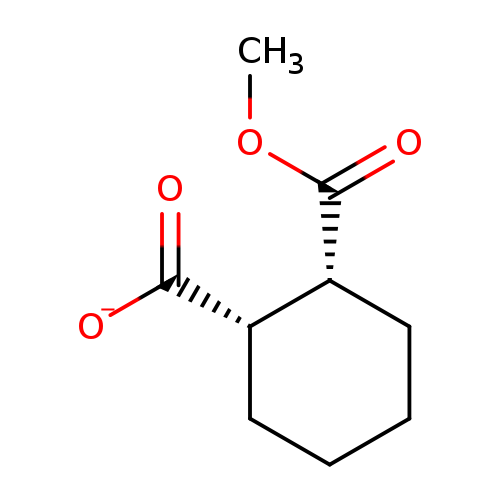
Cis-2-carbomethoxycyclohexane-1-carboxylic acidCatalog No.:AA002UNN CAS No.:111955-05-6 MDL No.:MFCD01311242 MF:C9H13O4- MW:185.1971 |
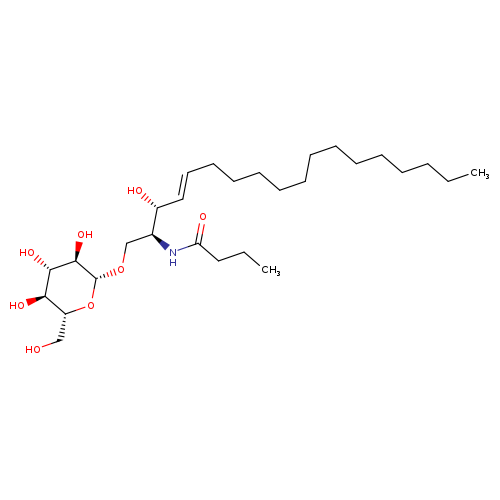
β-D-Glucosyl C4-CeramideCatalog No.:AA008W46 CAS No.:111956-45-7 MDL No.:MFCD27977950 MF:C28H53NO8 MW:531.7223 |
(9H-Carbazol-9-ylmethyl)dimethylamineCatalog No.:AA01EQOK CAS No.:111960-22-6 MDL No.:MFCD00022221 MF:C15H16N2 MW:224.3009 |
9-(Pyrrolidin-1-ylmethyl)-9H-carbazoleCatalog No.:AA00J06I CAS No.:111960-23-7 MDL No.:MFCD00958842 MF:C17H18N2 MW:250.3382 |
2-Hydroxy-n-[2-(pyridin-4-yl)ethyl]propanamideCatalog No.:AA01B87U CAS No.:111960-94-2 MDL No.:MFCD24568996 MF:C10H14N2O2 MW:194.2304 |
[(4-nitrophenyl)methyl](propan-2-yl)amine hydrochlorideCatalog No.:AA019WJO CAS No.:111961-43-4 MDL No.:MFCD00083671 MF:C10H15ClN2O2 MW:230.6913 |
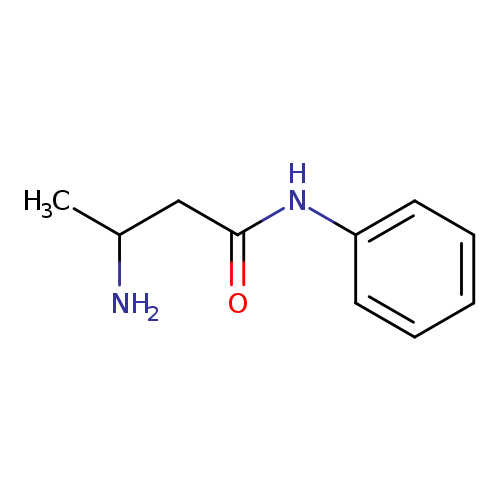
3-AMINO-N-PHENYLBUTANAMIDECatalog No.:AA01DYCM CAS No.:111961-65-0 MDL No.:MFCD12160005 MF:C10H14N2O MW:178.2310 |
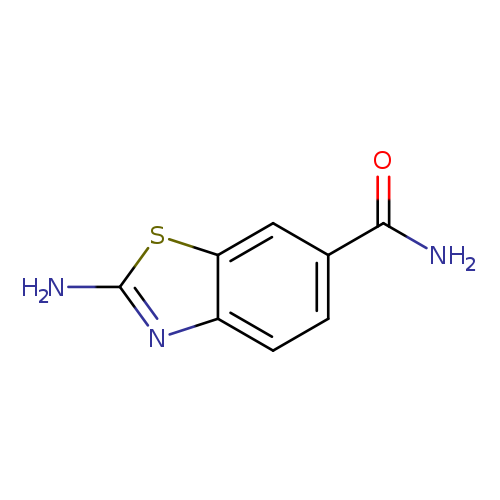
2-Aminobenzothiazole-6-carboxamideCatalog No.:AA008V3Z CAS No.:111962-90-4 MDL No.:MFCD01466259 MF:C8H7N3OS MW:193.2257 |
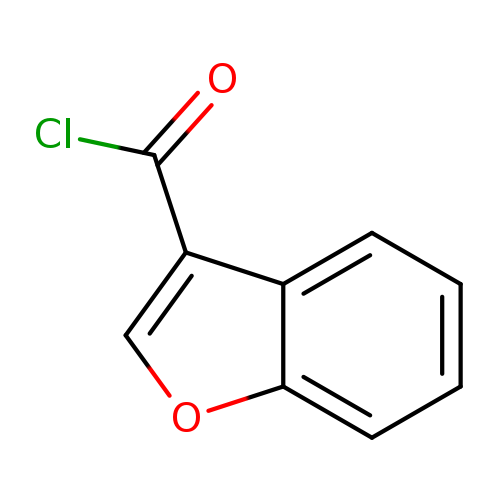
Benzofuran-3-carbonyl chlorideCatalog No.:AA0083PU CAS No.:111964-21-7 MDL No.:MFCD11841066 MF:C9H5ClO2 MW:180.5878 |
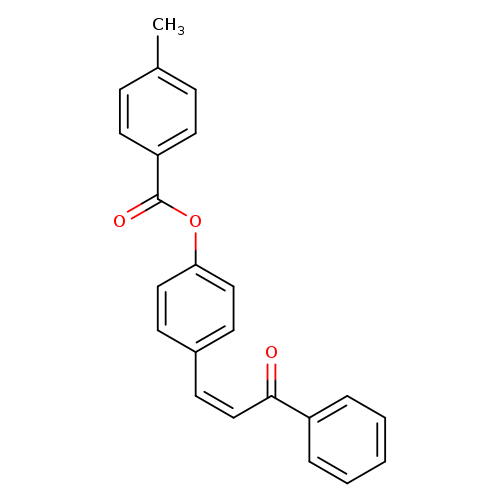
4-[(1Z)-3-oxo-3-phenylprop-1-en-1-yl]phenyl 4-methylbenzoateCatalog No.:AA00ISQQ CAS No.:111965-04-9 MDL No.:MFCD00169176 MF:C23H18O3 MW:342.3872 |
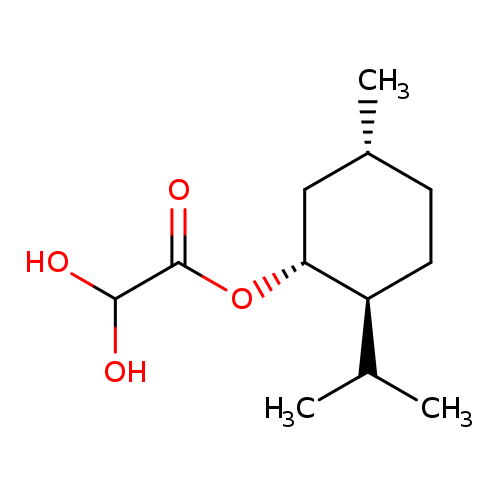
L-Menthyl glyoxylate hydrateCatalog No.:AA003BEN CAS No.:111969-64-3 MDL No.:MFCD04037548 MF:C12H22O4 MW:230.3007 |
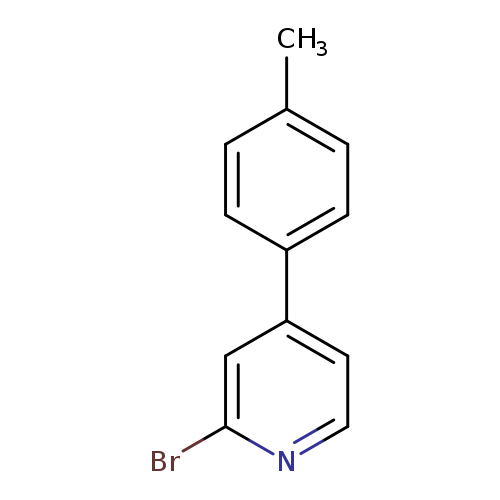
2-bromo-4-(4-methylphenyl)pyridineCatalog No.:AA01BC84 CAS No.:111971-30-3 MDL No.:MFCD11869542 MF:C12H10BrN MW:248.1185 |
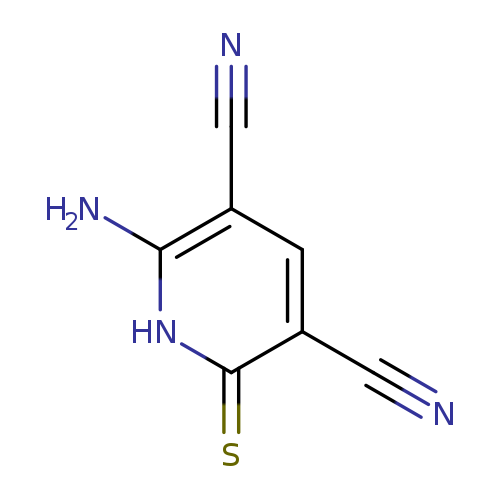
3,5-Pyridinedicarbonitrile, 6-amino-1,2-dihydro-2-thioxo-Catalog No.:AA007UY6 CAS No.:111971-56-3 MDL No.:MFCD06382875 MF:C7H4N4S MW:176.1985 |