2020-03-17 13:00:39
ZIF(Zn & Co)@wool fabric composites
Two ZIFs containing different metal centers (Zn & Co) were directly grown within the wool fabric matrix to prepare two different composites namely; ZIF-8@wool fabric and ZIF-67@wool fabric, respectively. Composites of ZIF@wool fabrics were synthesized by mixing the metal salts and organic ligand of 2-methyl imidazole in the presence of wool fabrics which exhibit various functional groups for potential interactions with metal ions (Zn & Co) and 2-methyl imidazole. As displayed in Fig. 1, the negative groups of wool fabrics (OH, SH and NH) act as active sites for binding with metal ions (Zn & Co) via electrostatic/coordination bond and with 2-methyl imidazole via hydrogen bonding [6,15,16,45,46]. Owing to its several binding sites, wool fabric can serve as a mixed linker and participate in the interaction with metal ions (Zn & Co) instead of 2-methyl imidazole [6,15,16,45,46]. Metal ions can bind with NH of imidazole from aside and with the functional groups of wool from the other side. The prepared composites were characterized by using X-ray diffraction (XRD), infrared (FTIR) and scanning electron microscope (SEM).
The diffraction of X-ray was applied for ZIF powder, wool fabrics and ZIF@wool fabric composites, and the diffraction data were presented in Fig. 2. For ZIF powder, four diffraction patterns have appeared at 2h = 7.4°, 10.4°, 12.8°, and 18.1°. These diffraction peaks are characteristic for ZIF (8 & 67) and matched with the previously published data [49,50]. Two broad diffraction peaks were recorded for wool fabric at 2h = 9.2° and 20.2° [15]. In the case of composites (ZIF@wool fabric), all diffraction peaks of ZIF were notably recorded which confirmed the formation of ZIF within the matrix of wool fabric.
The function groups, as well as the interaction between ZIF and wool, were further emphasized by using FTIR spectra. The FTIR absorbance spectra of wool fabric, ZIF and ZIF@wool fabric composites were presented in Fig. 3. For untreated wool, absorption peaks of NH stretching vibration and NH deformation appeared at 3276 and 1450 cm—1, respectively. Absorption peaks at 1639, 1533 and 1228 cm—1 were assigned to amide I, II and III, respectively. While absorption peaks at 2958 cm—1 and 1170 cm—1 were corresponding to the aliphatic CAH asymmetric stretching and the aliphatic CAC stretching, respectively [15]. For ZIF ZIFs, the appeared absorption bands at 418, 759–1457, 2908, 2985 and 3679 were associated with Zn-N stretch, entire vibration of imidazole ring, aliphatic CAH, aromatic CAH and bending NAH, respectively.
In case of ZIF@wool fabric composites, besides the absorption peaks of wool, peaks of M+2–N stretch and entire vibration of imi- dazole ring were obviously observed at 418, 759–1457. These data are supported the XRD data and further proved the formation of ZIFs inside the wool building units.
The morphological features and surface structures for ZIF@wool fabric composites were investigated by electron microscope and the micrographs were shown in Fig. 4. It was observed that deposits were diffused on the whole surface of wool fabrics. EDX analyses displayed separated signals for Zn (Fig. 4a) and Co (Fig. 4b) elements besides signals of C, O and S elements for wool structure. These indicate that the deposits onto fabrics were both of ZIF-8 and ZIF-67 which consequently confirmed the immediate formation of ZIFs within wool fabric structure. From micrographs, the wool fibrils were densely enveloped with ZIFs and the shape of ZIF was dependent on metal type. Particles like shape were observed for ZIF-8 (Fig. 4a) and ZIF-67 with hexagonal crystalline structure (Fig. 4b) was clearly seen onto wool fabrics. The morphological structure of ZIF (8 & 67) showed on the surface of wool was similar to that reported in the literature for their corresponding ZIF [49,50], which is considered as another evidence for successful formation of ZIFs within fabrics.
The amounts of metals (Zn & Co) and ZIFs onto the wool fabrics were measured and collected in Table 1. The estimated amounts of ZIFs in the composites were 128.8 and 118.3 mg/g for ZIF-8 and ZIF- 67, respectively. Considering the chemical formula of ZIFs (C8H10N4Zn for ZIF-8 and C8H10N4Co for ZIF-67), metal contents (Zn and Co) were calculated based on ZIF contents to be 33.0 mg/ g for Zn and 31.5 mg/g for Co. However, ZIF-8@wool fabric composite showed slightly higher contents (ZIF/M+2) compared to that of ZIF-67@wool fabric composite, the differences could be neglected due to its small value by including the standard deviation values.
Colorimetric parameters (L*, a*, b*, K/S and Abs) for wool fabrics and composites were summarized in Table 1. The data displayed that, the color of wool fabrics was not changed by incorporation of ZIF-8 attributing to the white color of this type of ZIF. Meanwhile, the direct insertion of ZIF-67 was accompanied by the change in color from creamy-white to reddish-blue (violet color). The violet was the typical color of ZIF-67 as seen for the powder form of ZIF and this color confirmed the formation of ZIF inside the building units of wool fabrics.
Removal of 2-naphthol from water
The prepared ZIF-8@wool fabric and ZIF-67@wool fabric composites were applied in the removal of 2-naphthol as a pharmaceutical intermediate from water. The untreated wool fabric was used as a reference to compare the efficiency of composites. The adsorption of 2-naphthol onto wool fabric and ZIF@wool fabric composites was firstly confirmed by measuring the infrared spectra as presented in Fig. 5. The IR spectrum of 2-naphthol showed various important absorption peaks at 3245, 3050, 1633–1577, 1467, 1371, 742 and 516 cm—1 which are referred to the OAH stretching, aromatic CAH stretching, aromatic C@C, CAH in-plane bending, CAO stretching, CAC in-plane bending, OAH out-of-plane deformation, and CAC in-plane deformation, respectively, as assigned in literature [24]. After adsorption of 2-naphthol, all the aforementioned absorption peaks for OAH, CAH, C@C, and CAC of 2-naphthol molecule were clearly recorded, confirming the adsorption of 2-naphthol onto wool fabric and ZIF@wool fabric composites.
Kinetics of 2-naphthol adsorption
The effect of contact time onto the removal amounts of 2- naphthol (Qt, mg/g) from water was studied up to 60 hrs and the results were presented in Fig. 6. For all experiments, the adsorption of 2-naphthol was very fast in the first 10 hrs, followed by forming a plateau shape until reached the equilibrium after 48 hrs. Similar profiles were observed for wool and ZIF@wool composites, but, the composites exhibited considerably higher adsorption contents than wool fabric. The higher affinity of composites is referred to the several binding sites including binding sites of wool and ZIF. After 24 hrs, the adsorbed amounts of 2-naphthol onto ZIF@wool composites were (338.3–356.0 mg/g; 84.6–89.0%) double of that on the wool (172 mg/g; 43.0%). In comparison with ZIF-67@wool fabric composite, the relatively higher adsorption capacity was observed for ZIF-8@wool fabric composites (356 mg/g) which may be related to the higher ZIF content. This further explained the assumption of increment in adsorption capacity by increasing the binding sites within composites.
Kinetics for adsorption of 2-naphthol onto wool fabric and ZIF@wool fabric composites were investigated by applying pseudo-first and pseudo-second-order models. Parameters of kinetics including, adsorption capacities at equilibrium (QE), rate constants (K1 & K2) and correlation coefficients (R2) are all collected in Table 2. For further confirmation, the suitability of the kinetic model, Chi-square data (v2) as a statistical test [55], were exported from the fitting figure and the values were introduced in Table 2. From the data in Table 2, adsorption of 2-naphthol onto wool and composites was fully fitted to the pseudo-first-order model rather than pseudo-second-order model, however similar (R2 = 0.99) was recorded for both models. This fitting is confirmed by two things; chi-square values and calculated adsorption capacities at equilibrium. Chi-square values are quite smaller in the case of the pseudo-first-order model compared to that of the pseudo-second-order model. Values of the calculated we are much closer to that of the experimental QE for pseudo-first-order model.
For pseudo-first-order, the rate constant (K1) was increased by a factor of 2 after incorporation of ZIF within wool fabrics, and it was enlarged from 47.5 10—3 (mg/g h) for wool fabric to 94.7 10—3 (mg/g h) for ZIF-8@wool fabric composite. This reflects the acceleration of 2-naphthol adsorption by the insertion of ZIF. The relevant assumption of pseudo-first-order kinetic is that the main - rate-determining step of the 2-naphthol adsorption onto wool fabric and composites is physisorption [56]. Such postulation describes that the chemical interaction happens between two main reactants (2-naphthol and composites), however, the adsorption rate is originally depending on only the concentration of composites. In the adsorption process, the formation of actual bonds such as covalent or ionic between reactants is characterized for chemisorption, while the physisorption adsorption included weak interactions (e.g. hydrophobic bonding, van der Waals interactions ion-dipole, dipole-dipole interactions, hydrogen bonding, and coordination bonding) [5,57,58]. Consequently, the adsorption of 2- naphthol is basically dependent on the active sites in composites which is more relevant to ZIF content and hence increasing the ZIF contents within wool fabrics could be resulted in much higher 2-naphthol uptake. The lower rate constant (79.5 10—3 mg/g h) for ZIF-67@wool composite than that for ZIF-8@wool composite is attributed to the lower ZIF content and subsequently, is further supported such hypothesis.
Isotherm
The uptake values (QE, mg/g) of 2-naphthol onto wool and composites were plotted with the initial concentration of 2-naphthol (mg/L) in Fig. 7. For the three adsorbent materials (wool fabric, ZIF-8@wool fabric, and ZIF-67@wool fabric), plateau shape profiles were observed. The adsorption values of 2-naphthol were gradually grown with its initial concentration up to the optimal concentration (1 g/L) and further increment in the initial concentration over this limit was not accompanied by a notable change in the adsorption values. The adsorption capacities were followed the order of ZIF-8@wool fabric > ZIF-67@wool fabric >> wool fabric. This explained the fundamental role of ZIF in the adsorption of 2- naphthol onto wool fabrics.
The linear fitting of 2-naphthol adsorption onto wool and composites were applied for both isotherm models; Langmuir and Freundlich, and the fitting graphs were presented in supplementary data (Fig. S1). Parameters of isotherm (max, L, F, n, and R2) and Chi-square (v2) values for both models were calculated and recorded in Table 3. The presented data confirmed that the 2-naphthol adsorption was well adapted to the Langmuir model via realizing the best linearization (R2 = 0.99) and smaller v2 values. The calculated maximum uptake capacities were significantly increased from 197.1 mg/g for wool fabric to 371.2–391.1 mg/g for ZIF@wool fabric composites and the ZIF-8 exhibited much higher capacity compared to that of ZIF-76. By incorporation of 12.9% ZIF, the uptake capacity of wool fabric against 2-naphthol was enlarged by 50%. Such isotherm of Langmuir is characterized by the formation of monolayer from adsorbate (2-naphthol) on the homogeneous surface of adsorbents (wool & ZIF@wool composites) [59]. Molecules of 2-naphthol are supposed to be adsorbed onto wool or composites through their active binding sites and each active site occupied with only one molecule. Therefore, the adsorption of 2-naphthol is governed by the availability of active sites onto wool/composites and forming one adsorption layer.
The maximum capacities for removal of 1- and 2-naphthol from water by using different adsorbent materials were summarized in Table 4 for comparing with that reported in the current study (338.4–356.0 mg/g). Very low adsorption capacities (0.12–33.7 mg/g) were observed for carbon nanotube-based composite, bamboo hydrochars and anion-cation organopalygroskite [35,37,40]. The application of montmorillonite modified with the surfactant, cross-linked poly(styrene-co-divinyl benzene) resin and sawdust results in acceptable adsorption of 2-naphthol (193.8 – 272.2) [23,34,36]. Modified diatomite, graphene oxide/Fe3O4@- polyaniline and b-cyclodextrin/graphene oxide nanosheets were used in removing 1-naphthol with lower capacity [60,61]. Accord- ing to our knowledge, the highest recorded adsorption capacities in literature was 331.6–339.0 mg/g for 1-naphthol by using sulfonated graphene nanosheets and ZIF-67 [33,38], which are much lower than that obtained in the current work. Taking into account the aforementioned data, using of the so-prepared ZIF@wool fabric composite exhibited the best removal results of 2-naphthol from water. Moreover, the prepared composite is considered as an easily applicable compared to the other mentioned adsorbents.
3.5.Regeneration of ZIF@wool fabric composites
The regeneration of composites towards the adsorption of 2- naphthol was studied in order to check the reusing efficacy of composites in the removal of 2-naphthol. The regeneration of composites was performed through repetitive cycles of adsorption/ desorption up to 4 cycles. The adsorption capacities of 2- naphthol onto the regenerated ZIF@wool composites were shown in Fig. 8a. The regenerated composites showed good efficiency in 2-naphthol adsorption and the adsorption capacities were marginally affected by the regeneration process. After 4 regenerated cycles, the adsorption capacity of the composites (311.2–mg/g) was reduced by only 8–9%. XRD and FTIR absorbance spectra were measured for the regenerated composites as shown in Fig. 8b and c. These data declared that; ZIF still exists inside composites after 4 regeneration cycles and hence the composites show good stability against repetitive wash. From XRD patterns, the amount of ZIF onto composites was reduced by the regeneration process due to its leaching out into the environment during the different processes (adsorption & desorption). However, diminishing in ZIF contents is explained the lowering of adsorption capacities for the regenerated composites, the adsorption efficiency of ZIF@- wool composites was substantial-good after the regeneration process. As a conclusion, the data suggested that; further enlargement in the adsorption of 2-naphthol and higher regeneration cycles could be both obtained by the incorporation of higher contents from ZIF within wool fabrics.
Adsorption chemistry
The adsorption mechanism of 2-naphthol onto ZIF@wool fabric composites were suggested based on the data of kinetics and isotherm, as presented in Fig. 9, in consideration of the chemical structure and the nature of reactants. 2-naphthol has an aromatic structure and composites have several active sites. The physisorption nature of adsorption reflects that the adsorption of 2-naphthol onto composites is dominantly carried out through weak interactions represented in van der Waals interactions, hydrophobic bonding, ion-dipole interactions, dipole-dipole interactions, hydrogen bonding and coordination bonding [5,57,58]. The weak interactions are formed between the functional groups of the whole composites (OH, NH, SH) and OH in the 2-naphthol. Moreover, interaction between the two aromatic rings (phenyl in 2-naphthol and imidazolium ring in composite) could be expressed as p - p interaction [38,62]. The considerable enlargement in
2-naphthol adsorption by incorporation of ZIF within wool fabrics is corresponding to the several functional groups of ZIF characterized for composites which subsequently increases the availability of interaction formation between composites and 2-naphthol. Additionally, the physical deposition or trapping of 2-naphthol molecules onto pores and/or intermolecular spaces of composites can’t be ignored.
4.Conclusion
Removal of 2-naphthol as a pharmaceutical intermediate from water by using the prepared ZIF (Zn & Co)@wool fabric composites, was systematically studied. The preparation process of ZIF@wool composites was confirmed by XRD, FTIR, SEM, and EDX. Material contents (ZIF/M+2), as well as colorimetric data, were measured for the as-prepared composites. The application of ZIF@wool composites was performed for removal of 2-naphthol. Adsorption of 2- naphthol onto the prepared composites well complied with pseudo-first-order kinetic model and Langmuir isotherm profile. The data showed that adsorption of 2-naphthol is physisorption and carried out through weak interaction with the functional groups of ZIF@wool composites. The adsorption reaction rate was accelerated by a factor of two, due to the incorporation of ZIF within wool fabrics. The adsorption capacities were enlarged from 172.0 mg/g for wool to 338.3 – 356.0 mg/g for composites within 48 hrs contact time. The regeneration and reusing study reflected the good constancy of composites as the adsorption capacity was lessen by 8–9% only after regeneration for 4 cycles.
As a conclusion, data presented in the current study showed significant findings in the removal of 2-naphthol from water using applicable adsorptive composite based on ZIF and wool fabric. Comparable with conventional techniques for removal of 2-naphthol, the current work exhibited valuable advantages represented inapplicability, simplicity, cost-effectiveness, and regeneration of composites. Additionally, controlling in the removal of 2-naphthol and/or numbers of recycling process is governed by the contents of ZIFs in the composites. Further work can be studied for the removal of other organic pollutants using such composites with different metal-organic frameworks or fabrics.
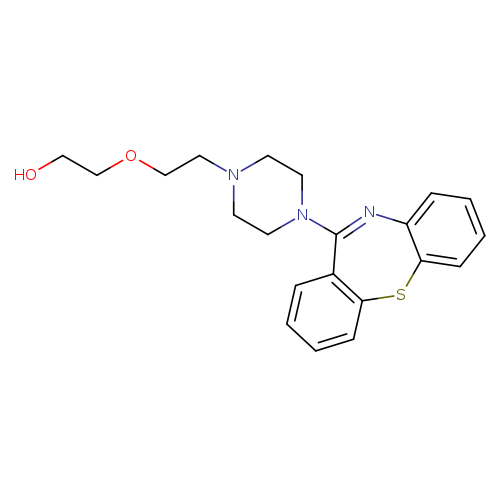
2-[2-(4-{2-thia-9-azatricyclo[9.4.0.0^{3,8}]pentadeca-1(11),3,5,7,9,12,14-heptaen-10-yl}piperazin-1-yl)ethoxy]ethan-1-olCatalog No.:AA003EN8 CAS No.:111974-69-7 MDL No.:MFCD00866699 MF:C21H25N3O2S MW:383.5071 |
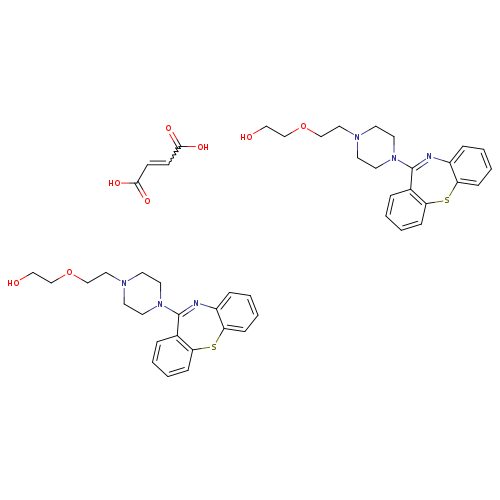
(2E)-but-2-enedioic acid; bis(2-[2-(4-{2-thia-9-azatricyclo[9.4.0.0^{3,8}]pentadeca-1(15),3,5,7,9,11,13-heptaen-10-yl}piperazin-1-yl)ethoxy]ethan-1-ol)Catalog No.:AA0038WL CAS No.:111974-72-2 MDL No.:MFCD03423782 MF:C46H54N6O8S2 MW:883.0864 |
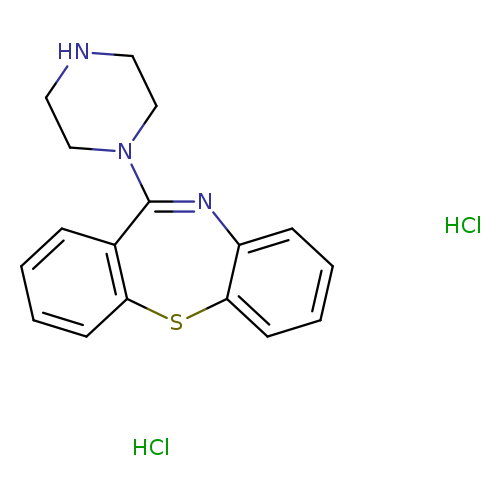
11-Piperazinodibenzo[b,f][1,4]thiazepine DiHClCatalog No.:AA003AWB CAS No.:111974-74-4 MDL No.:MFCD08703302 MF:C17H19Cl2N3S MW:368.3239 |
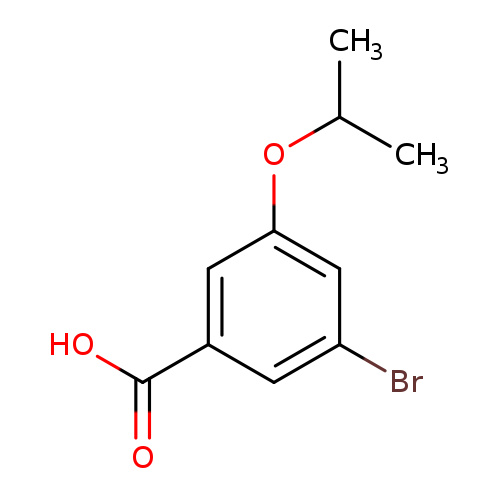
3-Bromo-5-isopropoxybenzoic acidCatalog No.:AA008SRU CAS No.:1119779-04-2 MDL No.:MFCD16618944 MF:C10H11BrO3 MW:259.0965 |
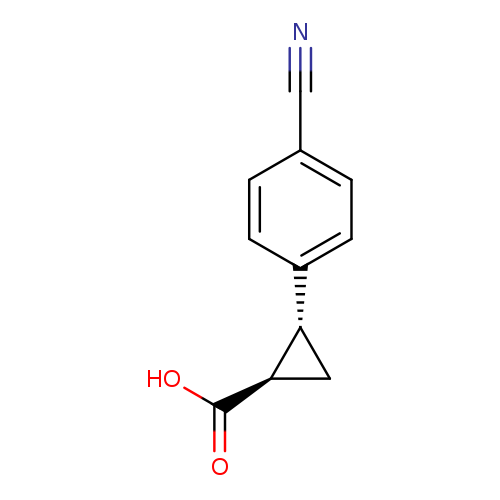
rac-(1R,2R)-2-(4-cyanophenyl)cyclopropane-1-carboxylic acid, transCatalog No.:AA01A2Q7 CAS No.:1119807-15-6 MDL No.:MFCD30704374 MF:C11H9NO2 MW:187.1947 |
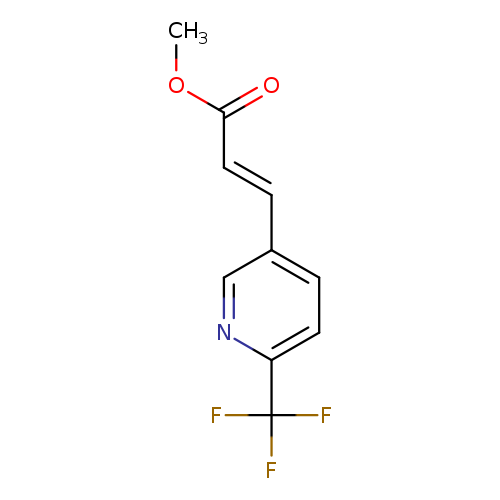
methyl (2E)-3-[6-(trifluoromethyl)pyridin-3-yl]prop-2-enoateCatalog No.:AA00HC9B CAS No.:1119807-17-8 MDL No.:MFCD27578513 MF:C10H8F3NO2 MW:231.1712 |
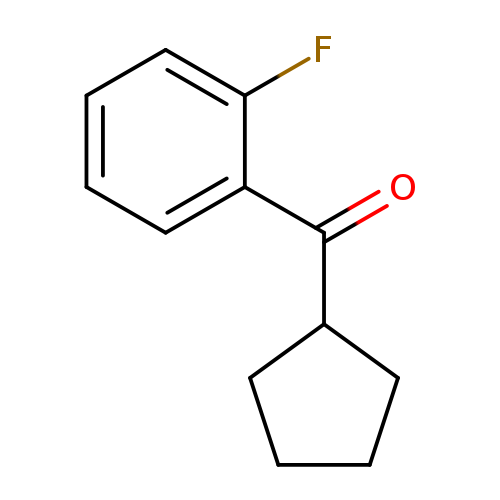
Cyclopentyl(2-fluorophenyl)methanoneCatalog No.:AA003653 CAS No.:111982-45-7 MDL No.:MFCD01861125 MF:C12H13FO MW:192.2294 |
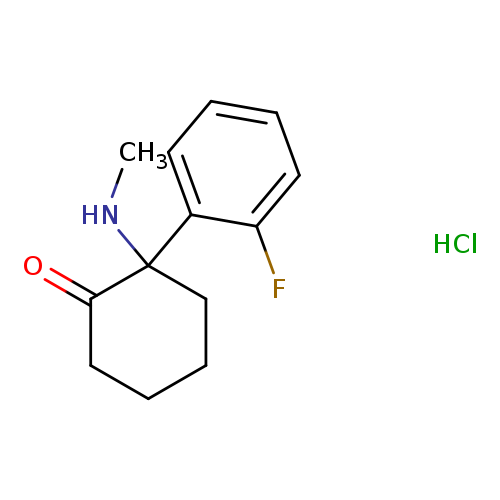
2-(2-fluorophenyl)-2-(methylamino)-cyclohexanone,monohydrochlorideCatalog No.:AA01EPM5 CAS No.:111982-49-1 MDL No.: MF:C13H17ClFNO MW:257.7316 |
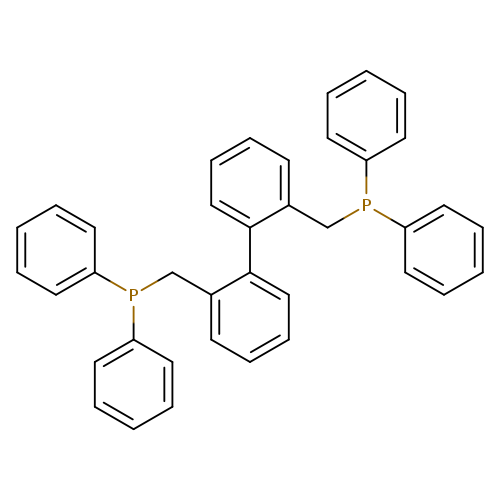
BISBICatalog No.:AA01D8Z2 CAS No.:111982-81-1 MDL No.:MFCD28139680 MF:C38H32P2 MW:550.6082 |
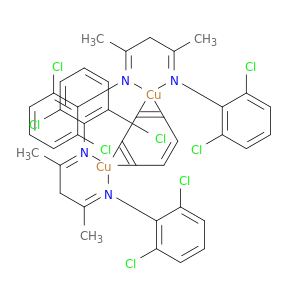
μ-Benzenebis[N,N'-(1,3-dimethyl-1,3-propanediylidene)bis(2,6-dichlorobenzenaminato)] dicopper(I), benzene adductCatalog No.:AA01FQVM CAS No.:1119821-62-3 MDL No.:MFCD12911904 MF:C40H34Cl8Cu2N4 MW:981.4408 |

Silane,[(1-methylethylidene)bis(4,1-phenyleneoxy)]bis[(1,1-dimethylethyl)dimethyl-Catalog No.:AA01EIC1 CAS No.:111983-38-1 MDL No.: MF: MW: |
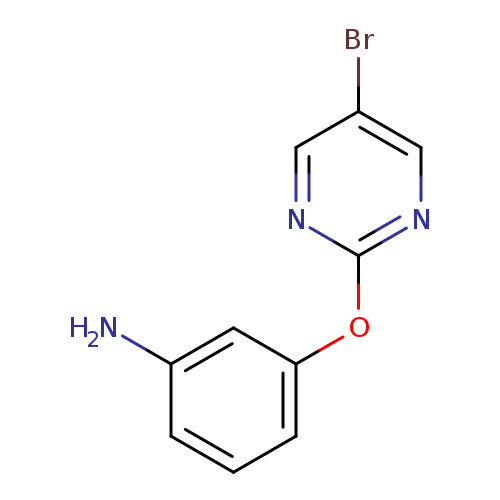
3-[(5-Bromopyrimidin-2-yl)oxy]anilineCatalog No.:AA008RZG CAS No.:111986-67-5 MDL No.:MFCD03646355 MF:C10H8BrN3O MW:266.0940 |

3-phenyl-1-(pyridin-2-yl)-4,5-dihydro-1H-pyrazol-5-oneCatalog No.:AA01E8YU CAS No.:111987-96-3 MDL No.:MFCD07809165 MF: MW: |
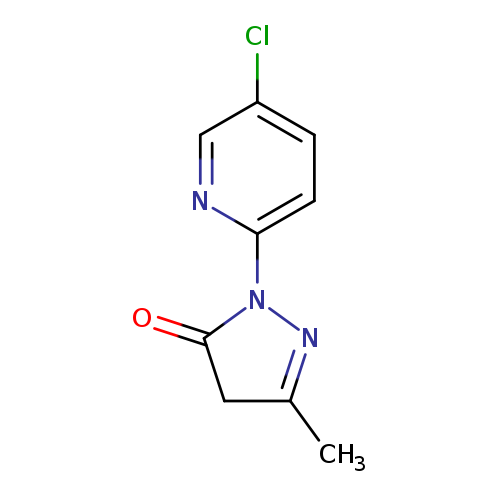
1-(5-chloropyridin-2-yl)-3-methyl-4,5-dihydro-1H-pyrazol-5-oneCatalog No.:AA01BS3D CAS No.:111988-00-2 MDL No.:MFCD20226245 MF:C9H8ClN3O MW:209.6323 |
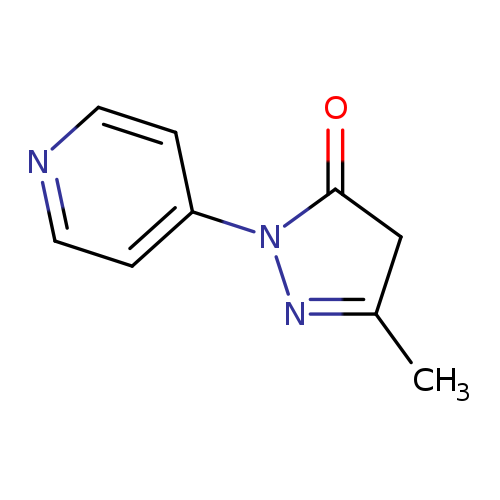
3-methyl-1-(pyridin-4-yl)-4,5-dihydro-1H-pyrazol-5-oneCatalog No.:AA01A5PP CAS No.:111988-01-3 MDL No.:MFCD12134544 MF:C9H9N3O MW:175.1873 |
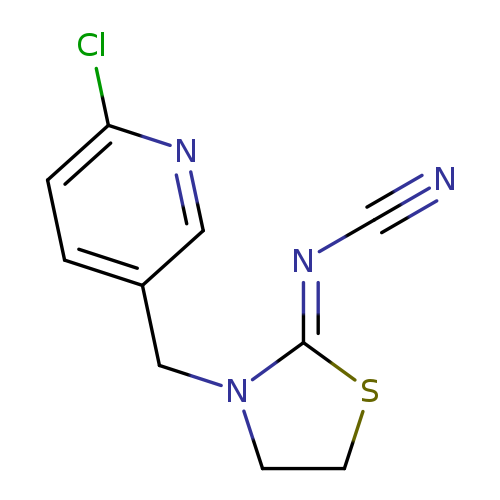
ThiaclopridCatalog No.:AA00HC9C CAS No.:111988-49-9 MDL No.:MFCD02101042 MF:C10H9ClN4S MW:252.7233 |
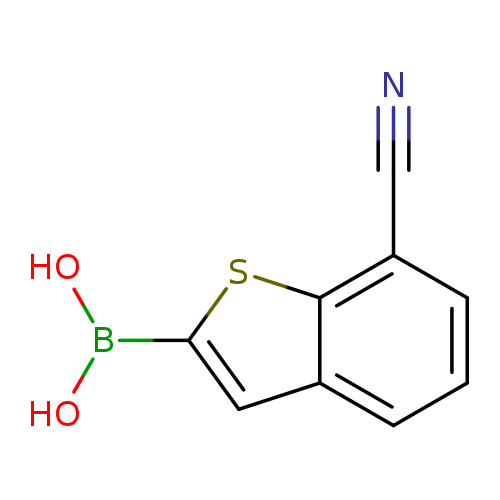
7-Cyanobenzo[b]thiophen-2-ylboronic acidCatalog No.:AA008TSS CAS No.:1119899-37-4 MDL No.:MFCD16877453 MF:C9H6BNO2S MW:203.0254 |
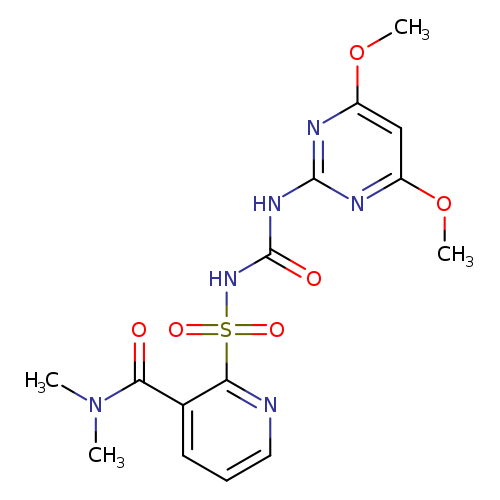
2-({[(4,6-dimethoxypyrimidin-2-yl)carbamoyl]amino}sulfonyl)-N,N-dimethylpyridine-3-carboxamideCatalog No.:AA0083NS CAS No.:111991-09-4 MDL No.:MFCD00203303 MF:C15H18N6O6S MW:410.4050 |
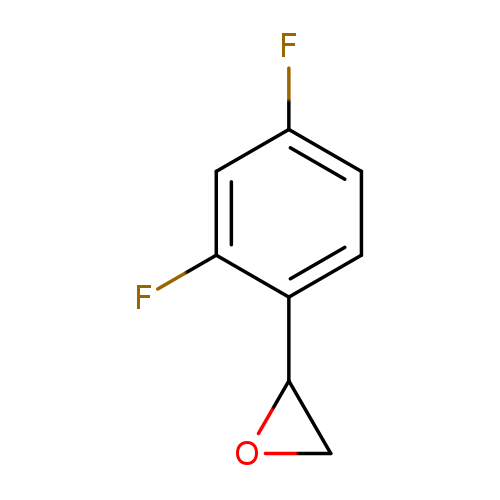
2-(2,4-difluorophenyl)oxiraneCatalog No.:AA019UXZ CAS No.:111991-12-9 MDL No.:MFCD09741026 MF:C8H6F2O MW:156.1294 |
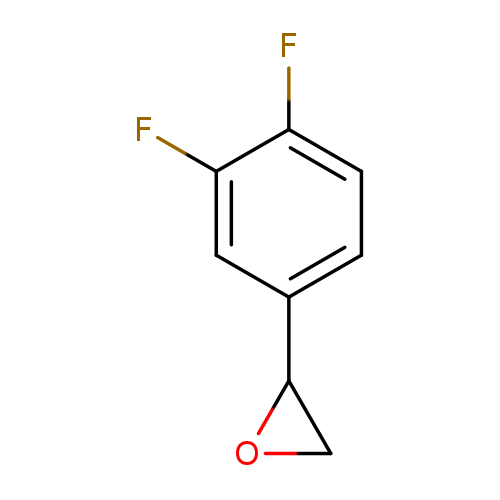
2-(3,4-Difluorophenyl)oxiraneCatalog No.:AA003EQ3 CAS No.:111991-13-0 MDL No.:MFCD09728790 MF:C8H6F2O MW:156.1294 |
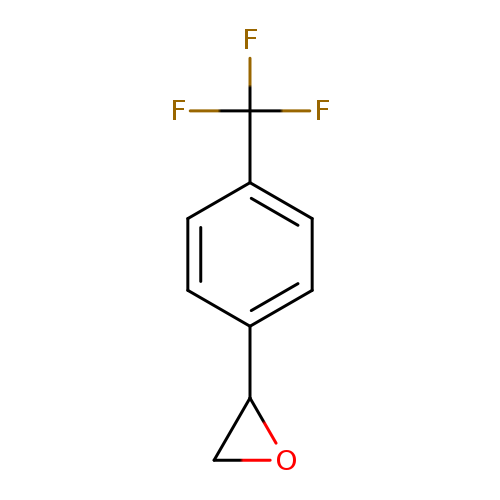
Oxirane, [4-(trifluoromethyl)phenyl]-Catalog No.:AA007CVL CAS No.:111991-14-1 MDL No.:MFCD09950047 MF:C9H7F3O MW:188.1465 |
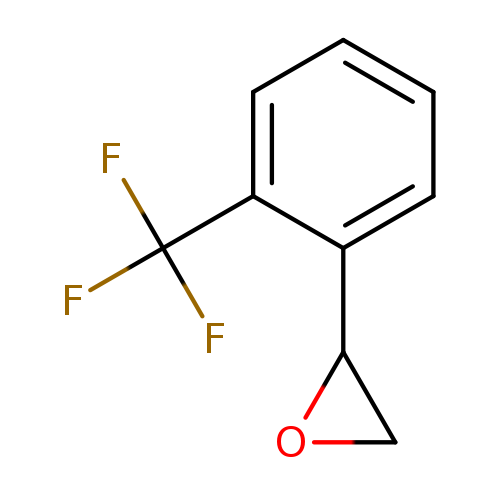
2-[2-(trifluoromethyl)phenyl]oxiraneCatalog No.:AA009U3T CAS No.:111991-15-2 MDL No.:MFCD09734108 MF:C9H7F3O MW:188.1465 |
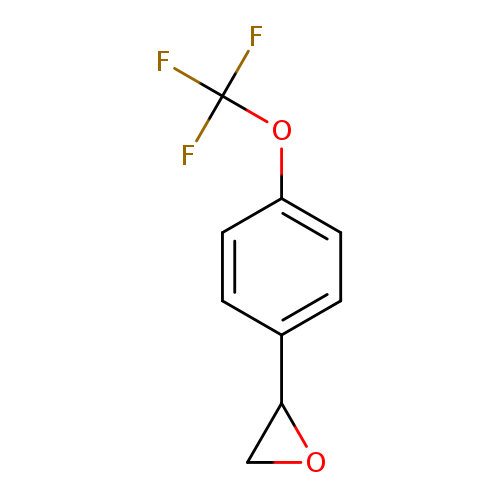
2-[4-(Trifluoromethoxy)phenyl]oxiraneCatalog No.:AA0083NQ CAS No.:111991-17-4 MDL No.:MFCD09932938 MF:C9H7F3O2 MW:204.1459 |
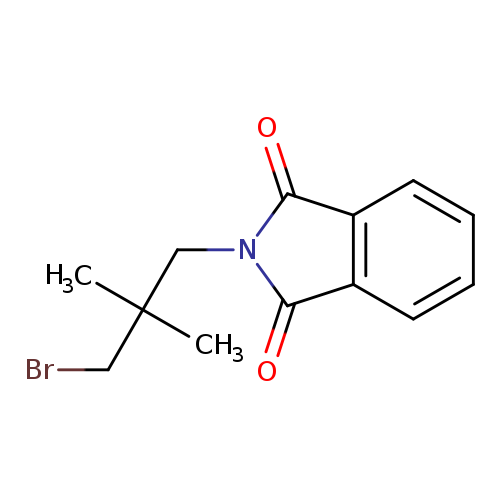
2-(3-Bromo-2,2-dimethylpropyl)isoindoline-1,3-dioneCatalog No.:AA0090IW CAS No.:111992-61-1 MDL No.:MFCD12028466 MF:C13H14BrNO2 MW:296.1598 |
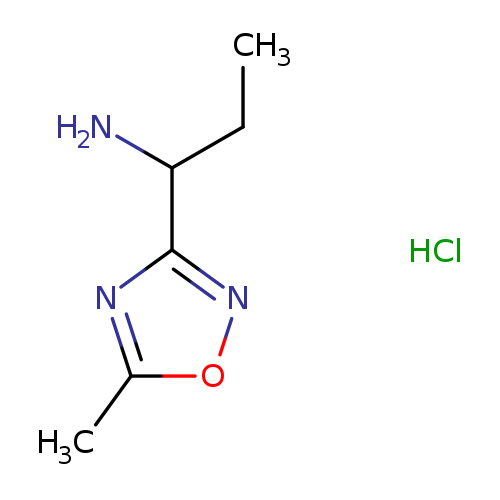
1-(5-methyl-1,2,4-oxadiazol-3-yl)propan-1-amine hydrochlorideCatalog No.:AA019TFP CAS No.:111997-68-3 MDL No.:MFCD21602643 MF:C6H12ClN3O MW:177.6320 |
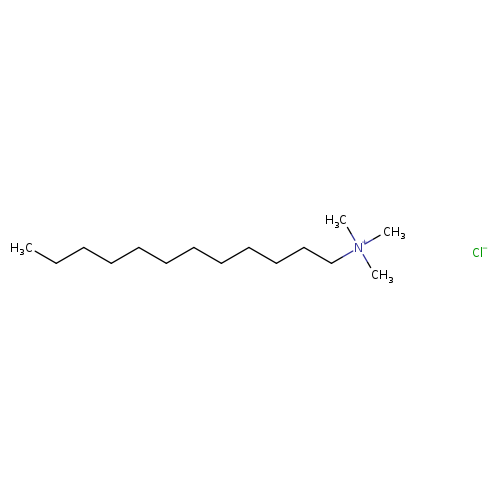
Dodecyltrimethylammonium chlorideCatalog No.:AA0038WK CAS No.:112-00-5 MDL No.:MFCD00041974 MF:C15H34ClN MW:263.8902 |
Hexadecyltrimethylammonium chlorideCatalog No.:AA0034X1 CAS No.:112-02-7 MDL No.:MFCD00011773 MF:C19H42ClN MW:319.9965 |
Trimethylstearylammonium chlorideCatalog No.:AA00HC9G CAS No.:112-03-8 MDL No.:MFCD00050188 MF:C21H46ClN MW:348.0496 |
OctadecyltrichlorosilaneCatalog No.:AA003894 CAS No.:112-04-9 MDL No.:MFCD00000484 MF:C18H37Cl3Si MW:387.9309 |
Nonanoic acidCatalog No.:AA0083JQ CAS No.:112-05-0 MDL No.:MFCD00004433 MF:C9H18O2 MW:158.2380 |
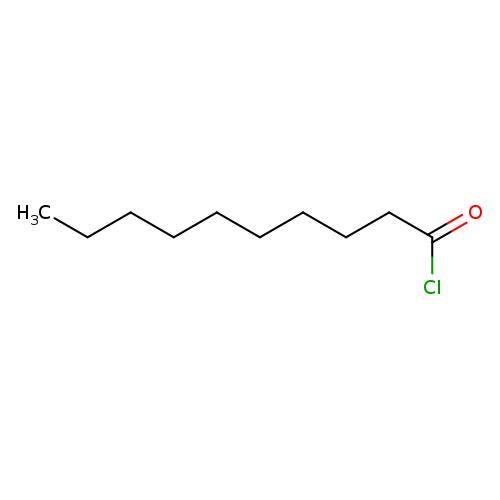
Decanoyl chlorideCatalog No.:AA008QZU CAS No.:112-13-0 MDL No.:MFCD00000771 MF:C10H19ClO MW:190.7103 |
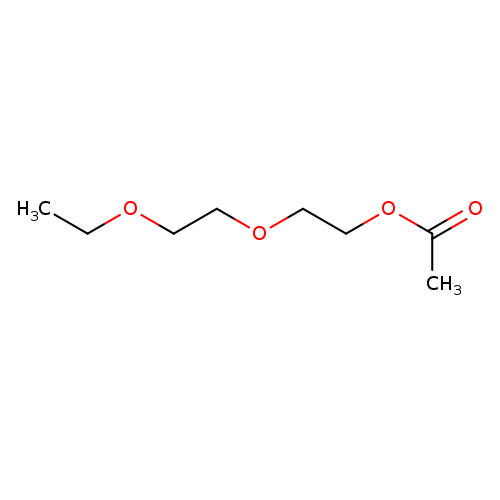
2-(2-Ethoxyethoxy)ethyl acetateCatalog No.:AA003PFA CAS No.:112-15-2 MDL No.:MFCD00041928 MF:C8H16O4 MW:176.2102 |
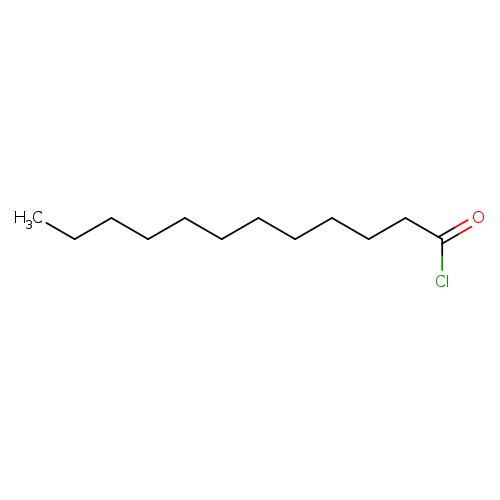
Lauroyl chlorideCatalog No.:AA003PSQ CAS No.:112-16-3 MDL No.:MFCD00000740 MF:C12H23ClO MW:218.7634 |
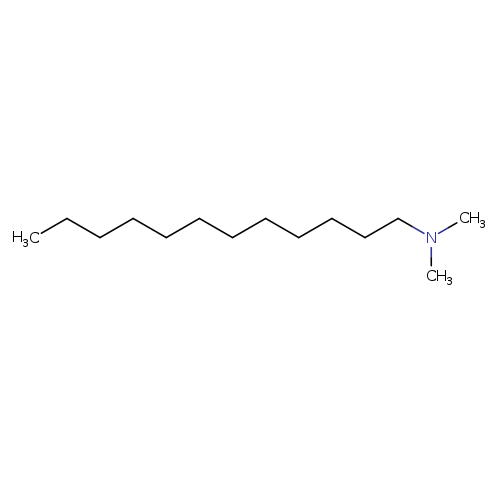
N,N-DimethyldodecylamineCatalog No.:AA00358N CAS No.:112-18-5 MDL No.:MFCD00008970 MF:C14H31N MW:213.4026 |
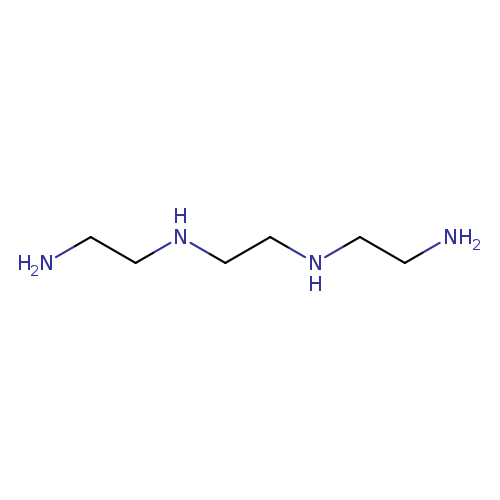
TriethylenetetramineCatalog No.:AA00HC9H CAS No.:112-24-3 MDL No.:MFCD00149562 MF:C6H18N4 MW:146.2339 |
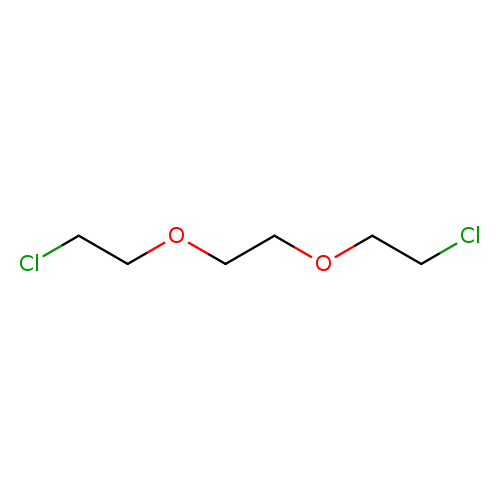
1,2-Bis(2-chloroethoxy)ethaneCatalog No.:AA003AX1 CAS No.:112-26-5 MDL No.:MFCD00000976 MF:C6H12Cl2O2 MW:187.0643 |
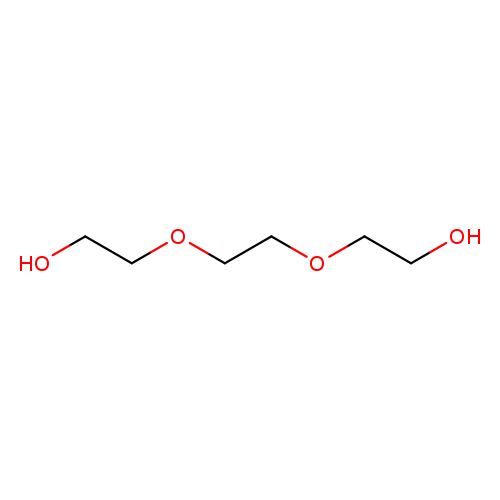
Triethylene glycolCatalog No.:AA009NX8 CAS No.:112-27-6 MDL No.:MFCD00002880 MF:C6H14O4 MW:150.1730 |
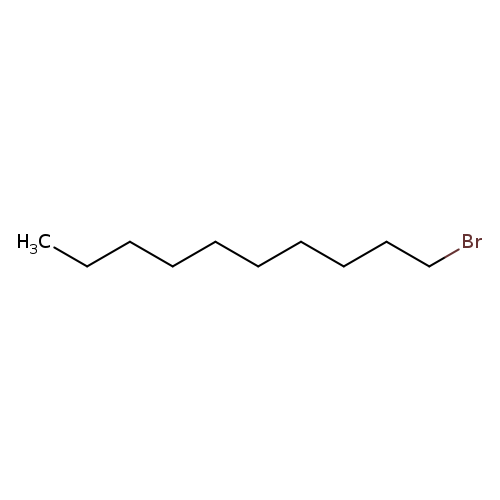
1-BromodecaneCatalog No.:AA0034M3 CAS No.:112-29-8 MDL No.:MFCD00000221 MF:C10H21Br MW:221.1777 |
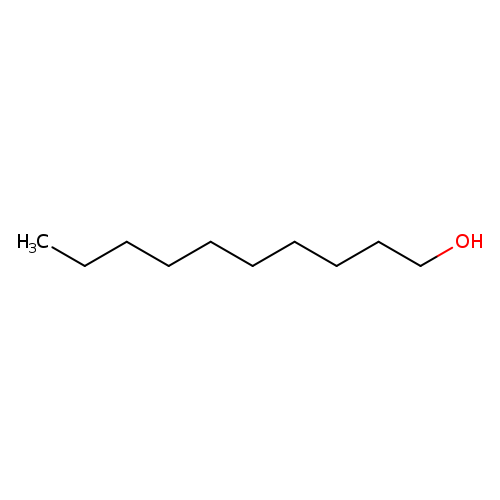
1-DecanolCatalog No.:AA0034M2 CAS No.:112-30-1 MDL No.:MFCD00004747 MF:C10H22O MW:158.2811 |
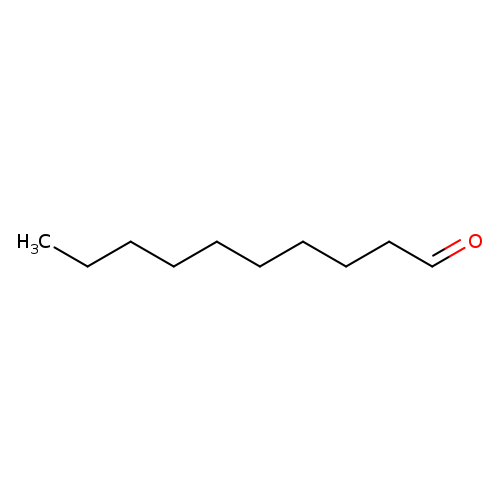
DecanalCatalog No.:AA003P6K CAS No.:112-31-2 MDL No.:MFCD00007031 MF:C10H20O MW:156.2652 |
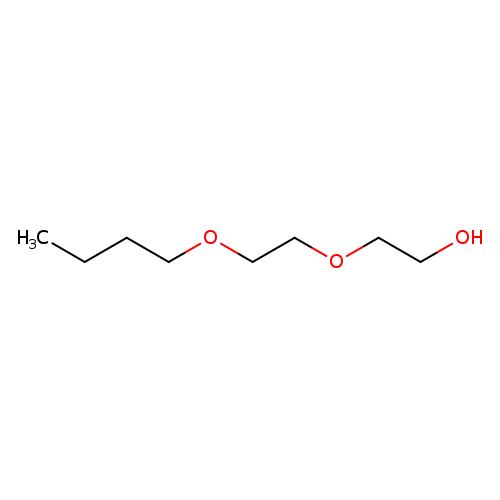
2-(2-Butoxyethoxy)ethanolCatalog No.:AA0034HZ CAS No.:112-34-5 MDL No.:MFCD00002881 MF:C8H18O3 MW:162.2267 |
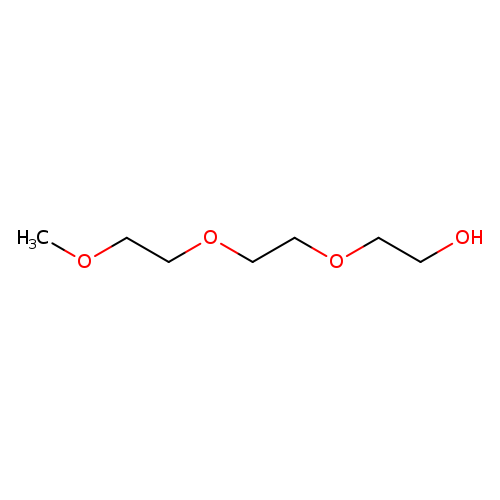
Triethylene glycol monomethyl etherCatalog No.:AA0082P1 CAS No.:112-35-6 MDL No.:MFCD00002875 MF:C7H16O4 MW:164.1995 |
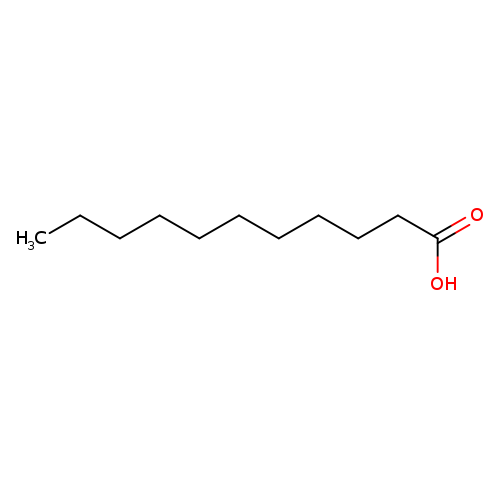
Undecanoic acidCatalog No.:AA003QRO CAS No.:112-37-8 MDL No.:MFCD00002730 MF:C11H22O2 MW:186.2912 |
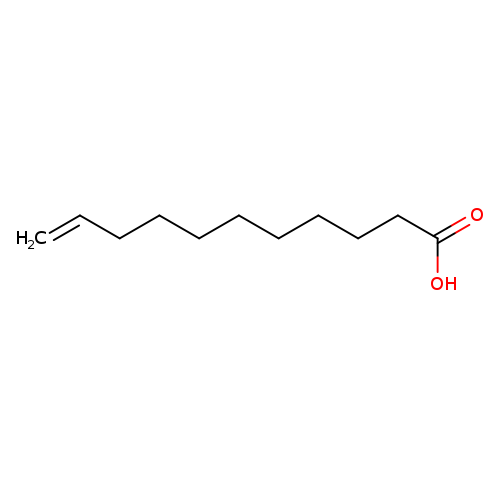
Undec-10-enoic acidCatalog No.:AA003V75 CAS No.:112-38-9 MDL No.:MFCD00004442 MF:C11H20O2 MW:184.2753 |
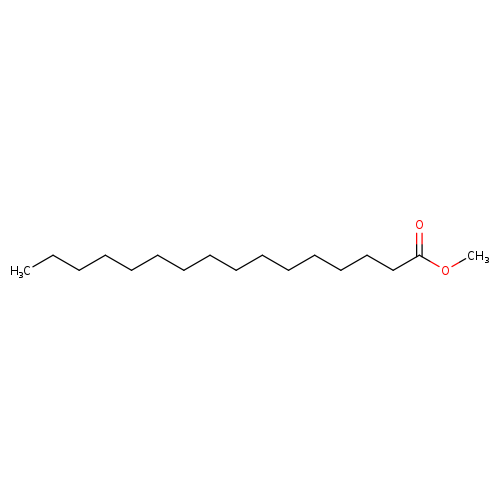
Methyl palmitateCatalog No.:AA003RYW CAS No.:112-39-0 MDL No.:MFCD00008994 MF:C17H34O2 MW:270.4507 |
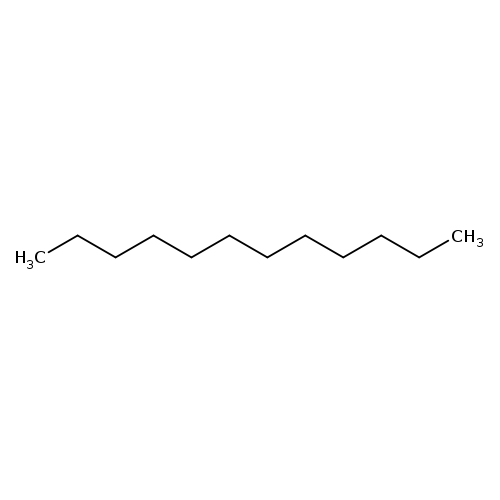
DodecaneCatalog No.:AA003PSI CAS No.:112-40-3 MDL No.:MFCD00008969 MF:C12H26 MW:170.3348 |
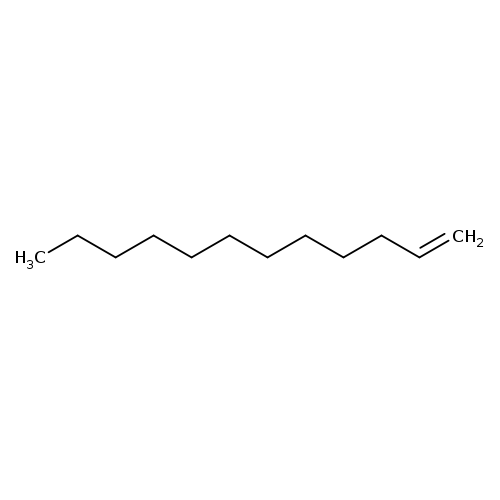
1-DodeceneCatalog No.:AA003E77 CAS No.:112-41-4 MDL No.:MFCD00008961 MF:C12H24 MW:168.3190 |
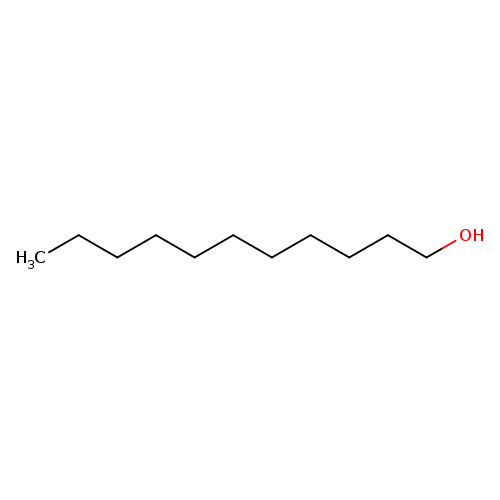
1-UndecanolCatalog No.:AA0032RE CAS No.:112-42-5 MDL No.:MFCD00004751 MF:C11H24O MW:172.3077 |
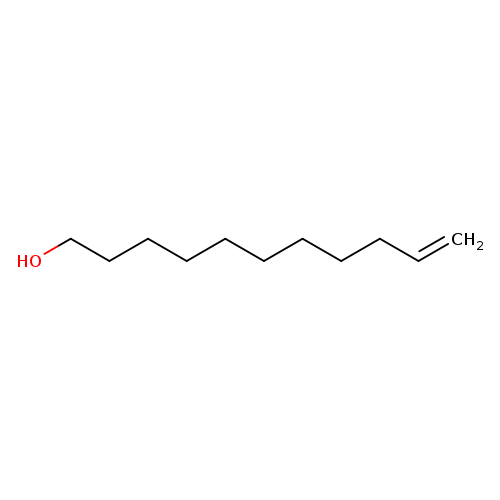
10-Undecen-1-olCatalog No.:AA003DRO CAS No.:112-43-6 MDL No.:MFCD00004750 MF:C11H22O MW:170.2918 |
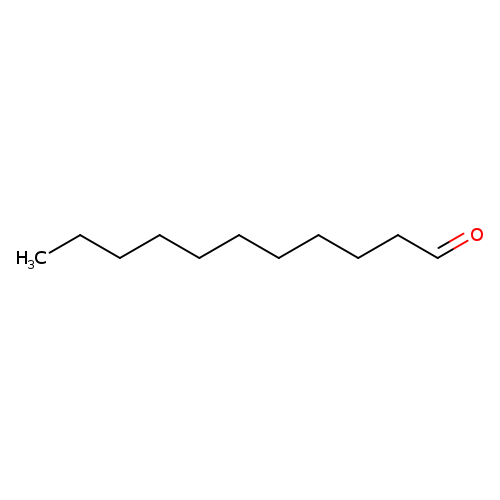
UndecanalCatalog No.:AA003V70 CAS No.:112-44-7 MDL No.:MFCD00007016 MF:C11H22O MW:170.2918 |
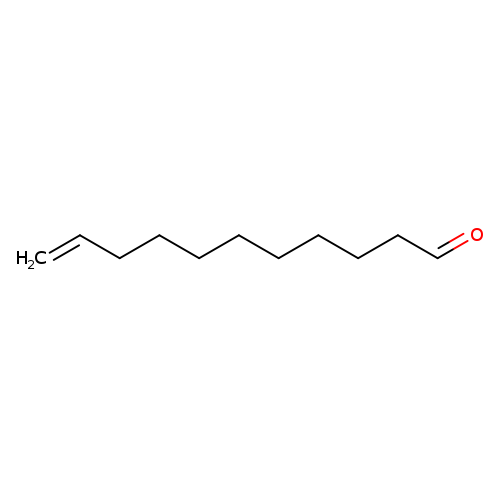
10-UndecenalCatalog No.:AA003DRP CAS No.:112-45-8 MDL No.:MFCD00007032 MF:C11H20O MW:168.2759 |
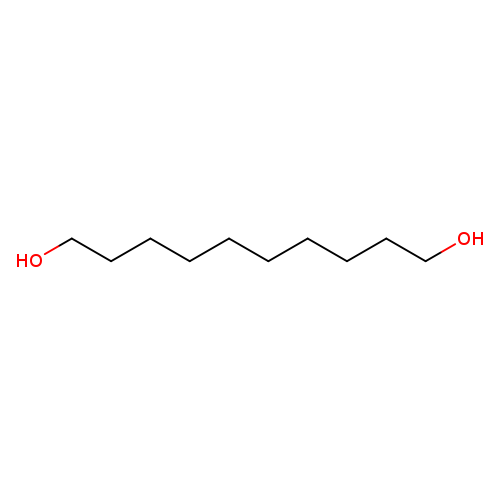
1,10-DecanediolCatalog No.:AA0032GQ CAS No.:112-47-0 MDL No.:MFCD00004749 MF:C10H22O2 MW:174.2805 |
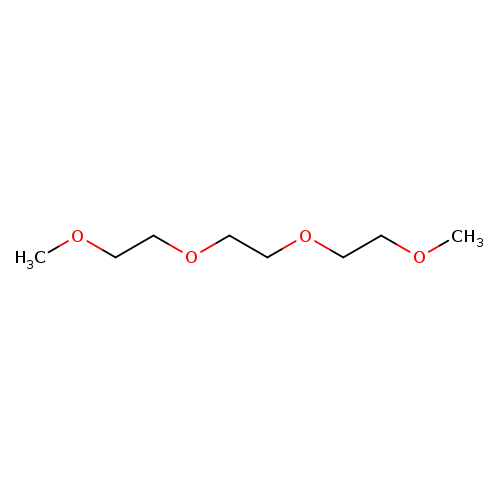
2,5,8,11-TetraoxadodecaneCatalog No.:AA003UZ4 CAS No.:112-49-2 MDL No.:MFCD00008504 MF:C8H18O4 MW:178.2261 |
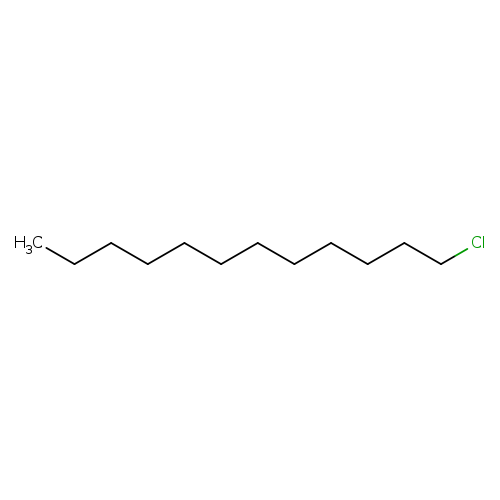
LaurylchlorideCatalog No.:AA00350G CAS No.:112-52-7 MDL No.:MFCD00013688 MF:C12H25Cl MW:204.7799 |
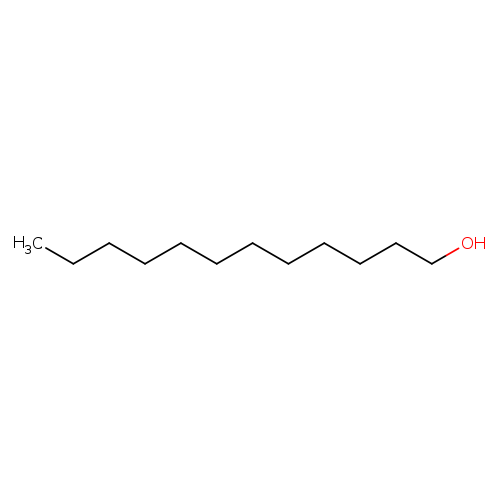
1-DodecanolCatalog No.:AA0032OD CAS No.:112-53-8 MDL No.:MFCD00004753 MF:C12H26O MW:186.3342 |
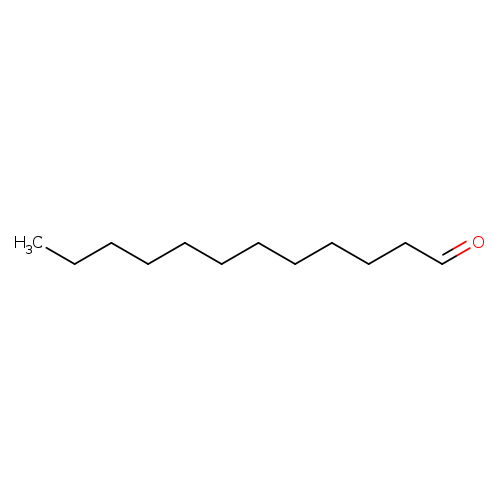
DodecanalCatalog No.:AA003PSV CAS No.:112-54-9 MDL No.:MFCD00007017 MF:C12H24O MW:184.3184 |
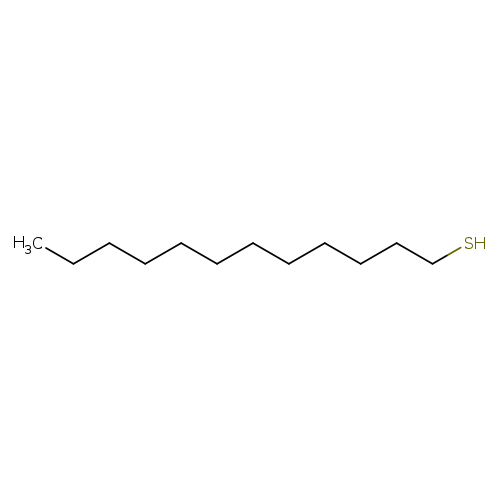
1-DodecanethiolCatalog No.:AA003E76 CAS No.:112-55-0 MDL No.:MFCD00004885 MF:C12H26S MW:202.3998 |
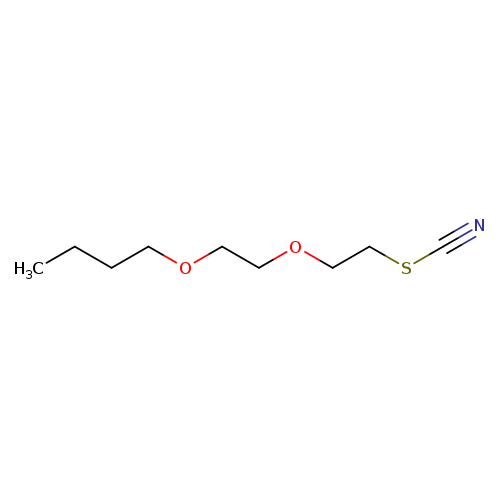
Thiocyanic acid,2-(2-butoxyethoxy)ethyl esterCatalog No.:AA007SV2 CAS No.:112-56-1 MDL No.:MFCD00085536 MF:C9H17NO2S MW:203.3018 |