2020-03-18 14:54:28
Siti Khadijah Hubadillah, Mohd Hafiz Dzarfan Othman *, Mukhlis A. Rahman, A.F. Ismail, Juhana Jaafar
1.Introduction
‘Pollution — if you don’t kill it, it will kill you’. One of the greatest problems that the world is facing today is water pollution which has caused grave and irreparable damages to the environment and human society. According to a statistical study made by The International Tanker Owners Pollution Federation (ITOPF), there were two prominent oil spill cases recorded of more than 700 tonnes of spillage in 2015 (ITOPF, 2015). Besides, with the emergence of industrial development such as transportation, steel, and petrochemical, a large amount of oily wastewater is continuously produced on a daily basis. Thereby, it shows that oily wastewater is the main pollutant emitted into water and endangers the environment and human health. Conventional oily wastewater treatment methods such as gravity separation, coagulation, flocculation, de-emulsification, and air flotation have been used before the water can be discharged into seas, lakes, or rivers (Zhong et al., 2003; Stack et al., 2005; Bensadok et al., 2007; Binner et al., 2013). However, these methods show limitations in terms of complex operation processes, expensive methods, and low efficiency especially when the oil droplet is in micron size. In addition, water treatment in industrial applications is limited to $500/m2 to $1000/m2 (Koros and Mahajan, 2000). Moreover, the limit for oily wastewater concentration allowable for discharge based on Malaysian environmental quality regulations is only 10 mg/L (Effluent Standard, 2009). The limit has been stated due to oily wastewater may contain hazardous pollutants such as fats, hydrocarbons, and petroleum fractions such as diesel oil, gasoline, and kerosene. Therefore, finding a cost-effective and high-efficiency treatment method for oily wastewater is essential and crucial for the future.
Recently, membrane separation, which is known as worldwide technology, has been proven to be an effective approach to deal with oily wastewater (Das et al., 2017; Yu et al., 2017). This is due to its high oil removal efficiency and cost-effective method. Among the large numbers of studies on oily wastewater treatment using membranes, ceramic membranes has getting an extensive concern as a promising method due to the unique thermal, chemical, and mechanical properties that give significant advantages over polymeric membranes, stainless steel membranes, and conventional filtration techniques (e.g., rotary drum, centrifugation, and decantation) (Li, 2007; Wang et al., 2014; Zheng and Fan, 2012). The remarkable physical and chemical stabilities of ceramic membranes allow them to offer a reproducible performance over a long service life that translates into a lower operating cost (Sondhi et al., 2003; Lei et al., 2017). Lahore and Goodboy are the pioneers for using ceramic membranes for oily wastewater applications (Lahiere and Goodboy, 1993). They used commercial alumina ceramic membrane (Membralox® technology) manufactured by Socie´ te De´ s Ce´ ramique´ s Technique´ s and obtained a 94% of oil rejection with a flux of 12.26 m3/day. Also, the ceramic membrane used in the work possessed a similar performance after a simple cleaning process. The only drawback associated with the ceramic membrane is that it is slightly pricier than its polymeric counterparts. Thus, towards utilizing ceramic membrane in oily wastewater treatment cost-effectively, the preparation of ceramic membrane from low-cost materials especially kaolin clay ( $15/kg) has been extensively studied. In comparison, alumina cost $250/kg (Hubadillah et al., 2016c). According to Mittal et al., kaolin provides low plasticity and high refractory properties to the membrane (Mittal et al., 2011). On top of that, kaolin is hydrophilic and prevents membrane fouling (Mgbemena et al., 2013). Abbasi et al. also investigated the effect of hydrophilicity of kaolin membrane and recorded a fouling percentage of less than 30% after 120 min, with the oil concentration of about 1000 ppm and a good performance of 89% and 94% of chemical oxygen demand (COD) and total organic carbon (TOC) rejection, respectively (Abbasi et al., 2010). Kumar et al. obtained a 99.98% of oil rejection and a flux of 3.16 10—5 m/s at 69 kPa by employing kaolin membrane prepared from mixing kaolin with other inexpensive ceramic materials like ball clay, feldspar, and quartz (Vinoth and Kumar, 2015).
The first kaolin membrane was developed in 1999 by Huang and co-workers for nitrogen separation (Huang et al., 1999). They prepared the kaolin membrane by mixing sodium carboxymethylcellulose (CMC), water, kaolin, and alumina followed by the pressing method to produce a flat sheet kaolin membrane. However, due to the high cost of the pressing method, slip and tape casting fabrication methods have been implemented in the preparation of the flat sheet kaolin membrane (Mohammadi and Pak, 2003; Zyryanov and Karakchiev, 2007; Nandi et al., 2008). The extrusion method has also been implemented in producing flat membranes in the planar module (Liu and Chou, 2000). A few years after that, the extrusion method has been further used in tubular ceramic membrane invention (Zhou et al., 2008; Chaves et al., 2015). In view of usage efficiency, the tubular-shaped membrane possesses a high area to volume ratio compared to those of flat, planar, and disc-shaped membranes. Unfortunately, it is still incomparable with hollow fiber membrane configuration prepared via phase inversion/ sintering technique which possesses more advantages such as high surface area, high flux due to of the presence of finger-like structure, low transmembrane pressures, low-pressure drop across the module, and easy backwashing (Staff, 2011). Up to date, there are more than 100 publications and patents on the ceramic membrane in hollow fiber configuration used for various applications.
Considering such research trends with contemplation on reducing the price of ceramic membrane, this study focused on the preparation and characterization of inexpensive kaolin hollow fiber membrane (KHFM) via phase inversion/sintering technique and the evaluation of separation performance in the treatment of oily wastewater from palm oil mill effluent (POME), car wash, and cafe´. In our previous work, we have successfully prepared the KHFM from 37.5 wt% of kaolin content, 10 mL/min of bore fluid flow rate, and sintering temperature ranging from 1200 to 1500 °C with 100% oil rejection from cafe´ oily wastewater (Hubadillah et al., 2017c). However, the flux obtained was very low (<50 L/m2h). Addressing the limitation of previous work, this paper focused on the KHFM preparation and characterization by exploring various phase inversion/sintering technique parameters. A morphological study was conducted for the KHFM prepared using different content, bore fluid flow rate, and sintering temperature to identify the best KHFM. In order to increase the size of finger-like structures on the KHFM cross-sectional structure, the influence of calcium carbonate (CaCO3) and polyethylene glycol (PEG) as pore agents were also studied. The performance of KHFM on oily wastewater was identified in terms of chemical oxygen demand (COD), turbidity, total dissolved solids (TDS), conductivity, and total organic carbon (TOC).
2.Experimental procedures
2.1.Materials
Kaolin clay (<1 µm) used in this study was purchased from BG Oil Chem Sdn. Bhd., Malaysia. On the basis of weight per- cent, the kaolin clay contains 51.52% of SiO2, 36.9% of Al2O3, 0.96% of Fe2O3, 0.58% of CaO, 0.08% of MgO, and 0.22% of SO3 (Hubadillah et al., 2016c,b,a). Polyethersulfone (PESf) (Radel A-300, Ameco Performance), N-methyl-2-pyrrolidone (NMP) (HPLC grade, Rathbone), and polyethyleneglycol 30- di polyhydroxy stearate (Arlacel P135, CRODA) were used as polymer binder, solvent, and dispersant, respectively. Calcium carbonate (CaCO3, QRec) and polyethylene glycol (PEG) at three different molecular weight (400, 1500, and 30000) were used to study the effect of pore agents. All these materials were used without further purification. Oily wastewater was supplied by Kluang Oil Palm Processing Sdn. Bhd. (2°13010.232 400 N, 103°32043.864800 E), a car wash in Taman Skudai Baru (1°30028.627200 N, 103°38017.743200 E), and Meranti cafe´, Universiti Teknologi Malaysia (UTM), Malaysia (1°33034.97 0400 N, 103°3801.492800 E). The size of oil droplet in all oily wastewater samples were then characterized using particle size analyzer (PSA, Zetasizer) and illustrated in Fig. 1, whereas Table 1 shows the characterization of the collected oily wastewaters.
2.2.Preparation of kaolin suspensions
The KHFM was fabricated based on the phase inversion/sin- tearing technique method. Preceding the fabrication process, ceramic suspensions with different kaolin contents were prepared by mixing kaolin and Arlacel P135 with NMP and then ball milled at 30 rpm for 48 h. After the addition of PESf, the suspensions were further ball milled for another 48 h to achieve uniform dispersion. The prepared suspensions were then degassed in vacuum with gentle stirring for 1 h. The compositions of the suspensions with different kaolin contents used are listed in Table 2. Suspension-A was used throughout the study. For the effect of pore agents, suspension-A was modified by changing the kaolin to pore agent ratio.
2.3.Fabrication of kaolin hollow fiber membranes (KHFM)
For the fabrication of hollow fiber membranes with different kaolin contents and bore fluid flow rates, a tube-in-orifice spinneret with an inner diameter of 0.5 mm and the outer diameter of 1 mm was used. The degassed suspension was transferred to a stainless steel chamber with a spinneret and extruded into the hollow fiber precursors. Tap water was used as both internal and external coagulants. In order to obtain a perfect circular hollow fiber precursors, the internal coagulant (bore fluid) flow rate was varied in the range of 3–20 mL/min. The air gap was kept at 5 cm through the extrusion process. The hollow fiber precursors were then left in tap water at room temperature for at least 24 h for complete solvent exchange. A detailed study on the effect of the extrusion process on the morphology of the ceramic hollow fiber membrane was presented in our previous work (Hubadillah et al., 2016b). The dried hollow fiber precursors were then transferred to a furnace for sintering process by first heating at 600 °C in air at a heating rate of 2 °C/ min for 2 h to remove the polymer binder. Then, the sintering temperature was increased at different target temperatures ranging from 1200 to 1500 °C for 3 h to study the effect of sintering temperature. Finally, the sintered membranes was cooled naturally to room temperature to prevent cracking due to sudden temperature change.
2.4.Characterizations
2.4.1.XRD analysis
X-ray diffraction (XRD, X’pert Pro a1, Philips, Amsterdam, The Netherlands) was conducted in ambient conditions with CuKa radiation (k = 1.5406 A˚) to measure the crystallinity of kaolin powder and KHFM. All samples diffraction patterns were recorded from 10° to 80° 2h with a step size 0.026° and step time of 50 s, operated at 40 kV and 30 mA with a fixed 1/4° anti-scatter slit.
2.4.2.FTIR analysis
Fourier transform infrared spectroscopy (FTIR, Spectrum 100, Perkin Elmer, Waltham, MA) was used to measure the infrared spectrum of absorption for kaolin powder and KHFM. The samples were measured without the addition of KBr in the wavelength range of 4000–600 cm—1 with a resolution of 2 cm—1. The sample compartment was evacuated during acquisition and the contact between the sample and the attenuated total reflectance (ATR) diamond crystal was 2 mm in diameter.
2.4.3.Thermogravimetric analysis
The thermal stability of the various phases in kaolin powder in the transformation into KHFM through the sintering process was studied by thermogravimetric analysis (TGA, TG8120, Rigaku, Japan). The powder was thermally analyzed by placing approximately 25 mg of the powder in an alumina crucible, subjected to a linear heating ramp between 50 and 800 °C at a rate of 10 °C/min and a cooling rate of 50 °C/min.
2.4.4.BET analysis
The specific surface area of kaolin powder was determined by N2 adsorption/desorption isotherms at 196 °C with a Micromeritics instrument (BET, Belsorp Max, BEL Japan Inc., Osaka, Japan). The measurements were performed in the relative pressure range from 0 to 0.99. The classic relative pressure range (P/P0) of 0.05–0.30 was chosen to determine the specific BET surface area. The adsorption/desorption isotherms were used to calculate the pore size distributions with the Barrett–Joyner–Halenda (BJH) method.
2.4.5.Morphological studies
The structures of the cross-sectional areas and surfaces of KHFMs were observed using a scanning electron microscopy (SEM) and field emission scanning electron microscopy(FESEM), respectively, operating at 30 kV at various magnifications. Prior to the SEM and FESEM testings, all KHFMs were sputter-coated with a 0.5 nm gold layer.
2.4.6. Mechanical strength
The mechanical strength of KHFMs was determined by the three-point bending strength method. The three-point bending tests were carried out with an Instron Micro Tester 5848 with a load cell of 2 2 kN (Instron Calibration Laboratory, United Kingdom). Each KHFM with an average length of 25 mm was placed on a span of 5 cm and was loaded at a crosshead speed of 0.25 mm/min until fracture occurred. The step was repeated for three times for each sample.
2.4.7. Porosity and pore size distribution analysis
Mercury intrusion porosimetry (MIP, AutoPore IV, Micromeritics, USA) was used to measure the porosity and pore size distribution of the KHFMs prepared at different sintering temperatures and the addition of pore agents. The KHFMs were broken into pieces and transferred to the 5 cc penetrometer sample holder (Micrometeritics, USA) which was then pressurized from 38.6 to 4.2 × 106 bar for mercury intrusion.
Diethylene glycol monohexyl etherCatalog No.:AA00HC9I CAS No.:112-59-4 MDL No.:MFCD00010703 MF:C10H22O3 MW:190.2799 |
Tetraethylene glycolCatalog No.:AA00388R CAS No.:112-60-7 MDL No.:MFCD00002879 MF:C8H18O5 MW:194.2255 |
Methyl stearateCatalog No.:AA003RZU CAS No.:112-61-8 MDL No.:MFCD00009005 MF:C19H38O2 MW:298.5038 |
Methyl oleate, tech gradeCatalog No.:AA007SNK CAS No.:112-62-9 MDL No.:MFCD00009578 MF:C19H36O2 MW:296.4879 |
Methyl linoleateCatalog No.:AA003RY0 CAS No.:112-63-0 MDL No.:MFCD00009534 MF:C19H34O2 MW:294.4721 |
Myristoyl ChlorideCatalog No.:AA00357C CAS No.:112-64-1 MDL No.:MFCD00000741 MF:C14H27ClO MW:246.8166 |
Palmitoyl chlorideCatalog No.:AA003TGY CAS No.:112-67-4 MDL No.:MFCD00000742 MF:C16H31ClO MW:274.8697 |
1-TridecanolCatalog No.:AA009QSG CAS No.:112-70-9 MDL No.:MFCD00004756 MF:C13H28O MW:200.3608 |
1-BromotetradecaneCatalog No.:AA003E30 CAS No.:112-71-0 MDL No.:MFCD00000228 MF:C14H29Br MW:277.2841 |
1-TetradecanolCatalog No.:AA003EKZ CAS No.:112-72-1 MDL No.:MFCD00004757 MF:C14H30O MW:214.3874 |
1-(Dimethylamino)tetradecaneCatalog No.:AA0032GE CAS No.:112-75-4 MDL No.:MFCD00053736 MF:C16H35N MW:241.4558 |
Stearoyl chlorideCatalog No.:AA0035JI CAS No.:112-76-5 MDL No.:MFCD00000744 MF:C18H35ClO MW:302.9229 |
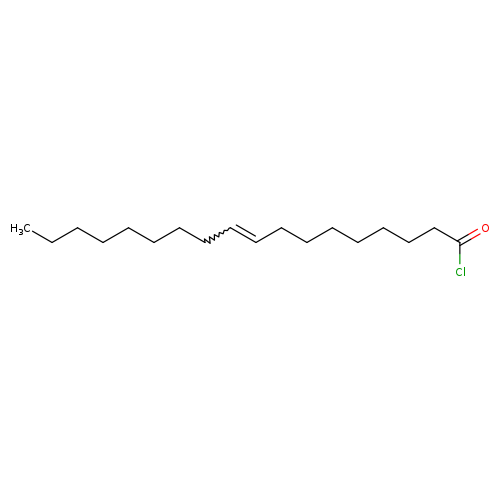
Oleoyl chlorideCatalog No.:AA003TEH CAS No.:112-77-6 MDL No.:MFCD00134332 MF:C18H33ClO MW:300.9070 |
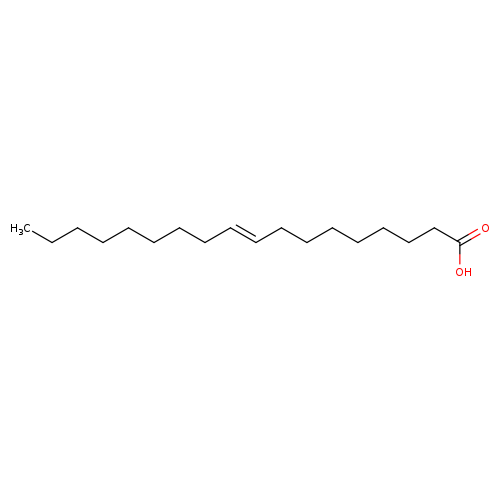
Elaidic acidCatalog No.:AA0078SX CAS No.:112-79-8 MDL No.:MFCD00063954 MF:C18H34O2 MW:282.4614 |
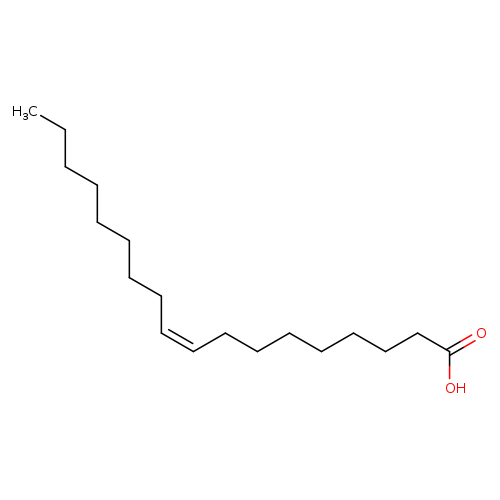
Oleic acid, naturalCatalog No.:AA009QSI CAS No.:112-80-1 MDL No.:MFCD00064242 MF:C18H34O2 MW:282.4614 |
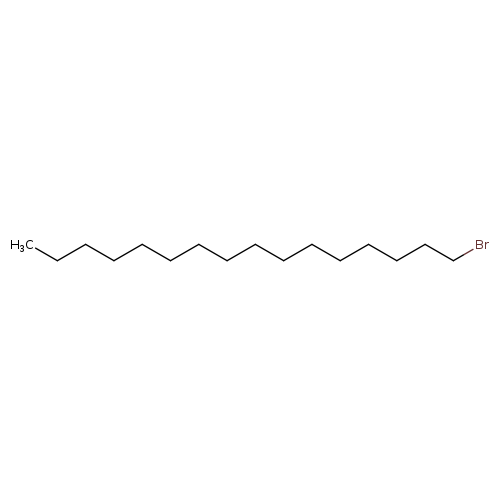
1-BromohexadecaneCatalog No.:AA00388M CAS No.:112-82-3 MDL No.:MFCD00000230 MF:C16H33Br MW:305.3372 |
(Z)-Docos-13-enamideCatalog No.:AA003PVD CAS No.:112-84-5 MDL No.:MFCD00882379 MF:C22H43NO MW:337.5829 |
Behenic acidCatalog No.:AA003PS8 CAS No.:112-85-6 MDL No.:MFCD00002807 MF:C22H44O2 MW:340.5836 |
(Z)-docos-13-enoic acidCatalog No.:AA007S0U CAS No.:112-86-7 MDL No.:MFCD00063188 MF:C22H42O2 MW:338.5677 |
1-OctadeceneCatalog No.:AA0032QZ CAS No.:112-88-9 MDL No.:MFCD00009003 MF:C18H36 MW:252.4784 |
1-BromooctadecaneCatalog No.:AA0032NI CAS No.:112-89-0 MDL No.:MFCD00000231 MF:C18H37Br MW:333.3904 |
Oleylamine, tech gradeCatalog No.:AA0035CQ CAS No.:112-90-3 MDL No.:MFCD00066507 MF:C18H37N MW:267.4931 |
Stearyl alcoholCatalog No.:AA0032QY CAS No.:112-92-5 MDL No.:MFCD00002823 MF:C18H38O MW:270.4937 |
N-EicosaneCatalog No.:AA003PUB CAS No.:112-95-8 MDL No.:MFCD00009344 MF:C20H42 MW:282.5475 |
Isocyanic Acid n-Octadecyl EsterCatalog No.:AA003R0U CAS No.:112-96-9 MDL No.:MFCD00002041 MF:C19H37NO MW:295.5032 |
Sodium hexadecyl sulfateCatalog No.:AA003U9W CAS No.:1120-01-0 MDL No.:MFCD00047766 MF:C16H33NaO4S MW:344.4856 |
Octadecyltrimethylammonium bromideCatalog No.:AA0083NE CAS No.:1120-02-1 MDL No.:MFCD00043171 MF:C21H46BrN MW:392.5006 |
NonanamideCatalog No.:AA0083NA CAS No.:1120-07-6 MDL No.:MFCD00025540 MF:C9H19NO MW:157.2533 |
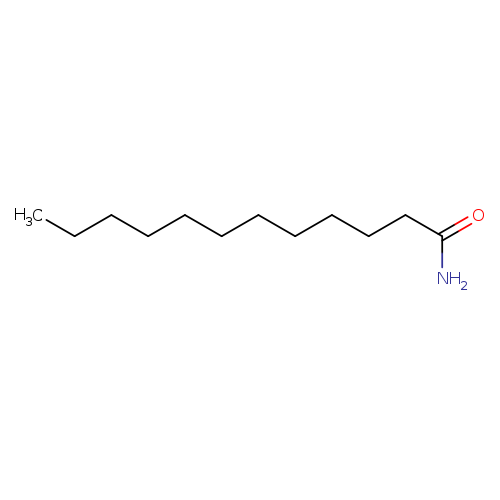
LauramideCatalog No.:AA003PSH CAS No.:1120-16-7 MDL No.:MFCD00025532 MF:C12H25NO MW:199.3330 |
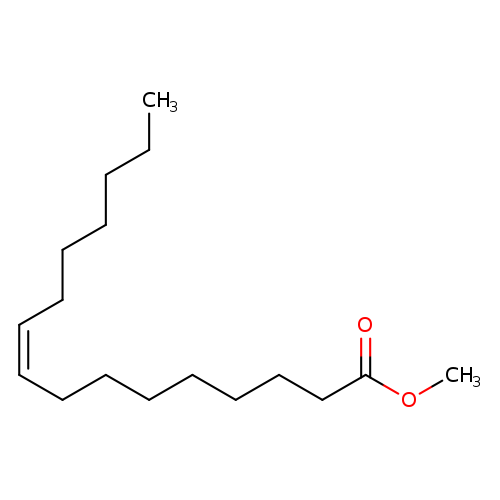
(Z)-Methyl hexadec-9-enoateCatalog No.:AA003RYX CAS No.:1120-25-8 MDL No.:MFCD00042911 MF:C17H32O2 MW:268.4348 |
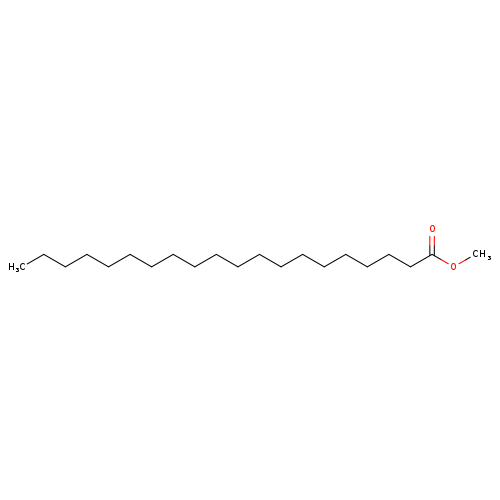
Arachidic acid methyl esterCatalog No.:AA003NUI CAS No.:1120-28-1 MDL No.:MFCD00009014 MF:C21H42O2 MW:326.5570 |
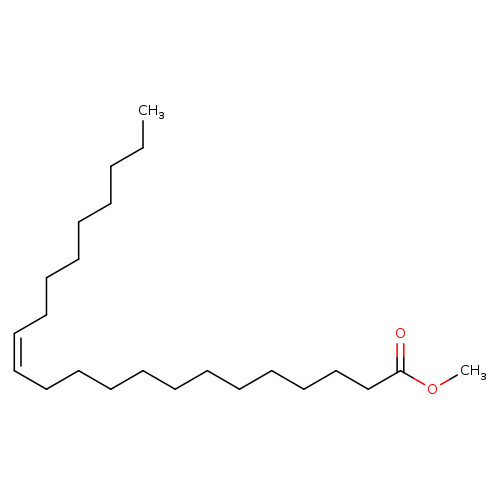
(Z)-Methyl docos-13-enoateCatalog No.:AA003PVF CAS No.:1120-34-9 MDL No.:MFCD00027343 MF:C23H44O2 MW:352.5943 |
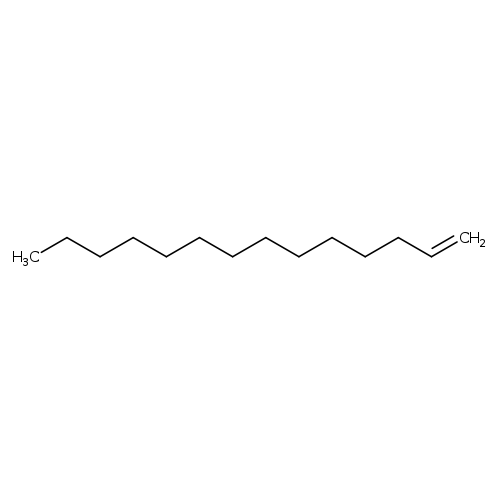
1-TetradeceneCatalog No.:AA003EL0 CAS No.:1120-36-1 MDL No.:MFCD00008981 MF:C14H28 MW:196.3721 |
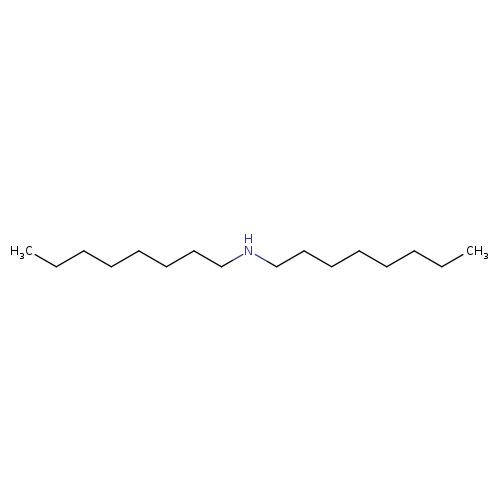
DioctylamineCatalog No.:AA003PLL CAS No.:1120-48-5 MDL No.:MFCD00009557 MF:C16H35N MW:241.4558 |
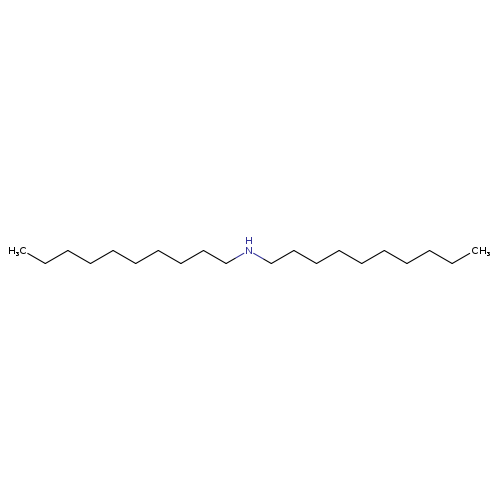
DidecylamineCatalog No.:AA0034MW CAS No.:1120-49-6 MDL No.:MFCD00048396 MF:C20H43N MW:297.5621 |
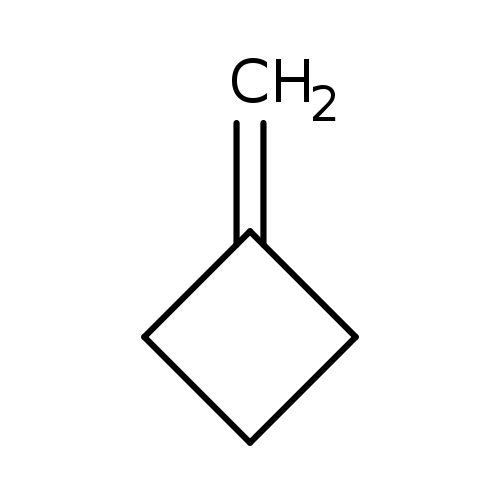
MethylenecyclobutaneCatalog No.:AA003S14 CAS No.:1120-56-5 MDL No.:MFCD00001335 MF:C5H8 MW:68.1170 |
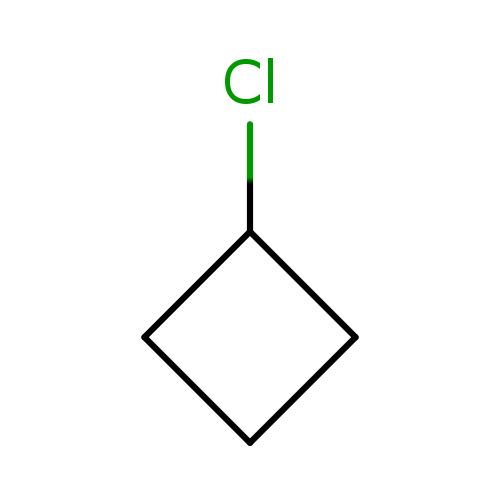
ChlorocyclobutaneCatalog No.:AA00HC9J CAS No.:1120-57-6 MDL No.:MFCD00013736 MF:C4H7Cl MW:90.5514 |
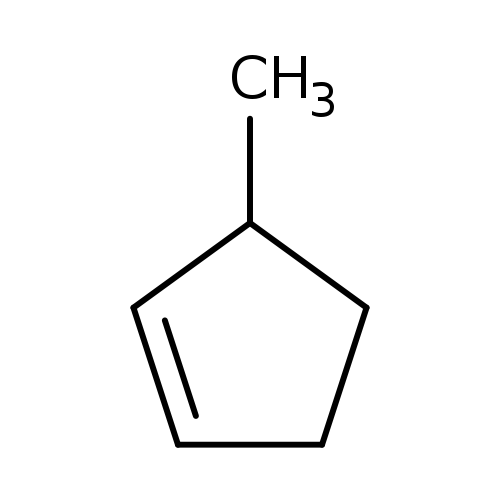
Cyclopentene, 3-methyl-Catalog No.:AA0083JD CAS No.:1120-62-3 MDL No.:MFCD00053260 MF:C6H10 MW:82.1436 |
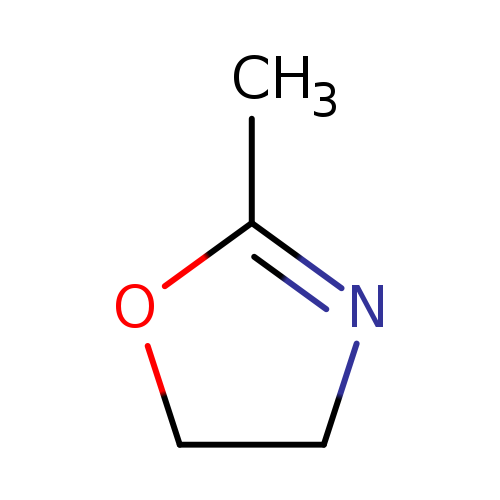
2-Methyl-2-oxazolineCatalog No.:AA003B69 CAS No.:1120-64-5 MDL No.:MFCD00005298 MF:C4H7NO MW:85.1045 |
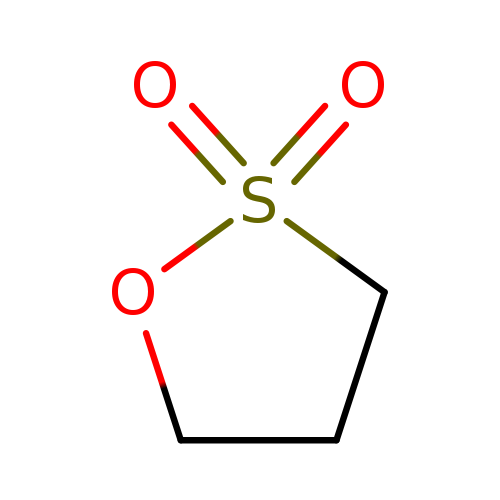
1,3-PropanesultoneCatalog No.:AA0032JB CAS No.:1120-71-4 MDL No.:MFCD00005355 MF:C3H6O3S MW:122.1429 |
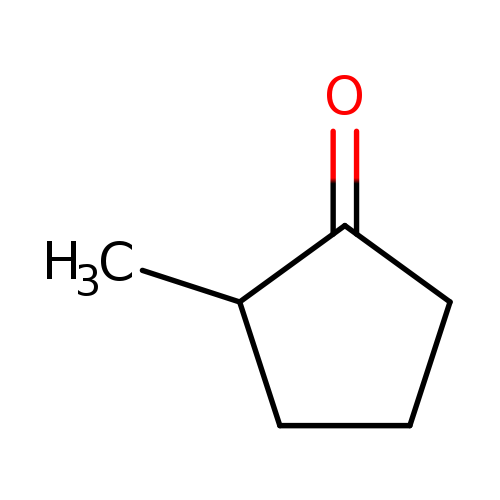
2-MethylcyclopentanoneCatalog No.:AA008RUZ CAS No.:1120-72-5 MDL No.:MFCD00001414 MF:C6H10O MW:98.1430 |
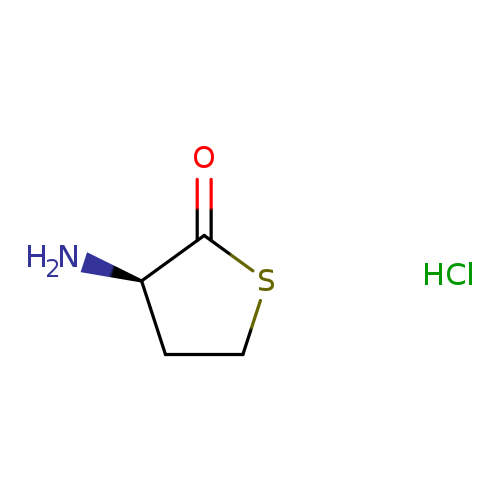
D-HOMOCYSTEINE THIOLACTONE HYDROCHLORIDECatalog No.:AA009NVL CAS No.:1120-77-0 MDL No.:MFCD00077785 MF:C4H8ClNOS MW:153.6304 |
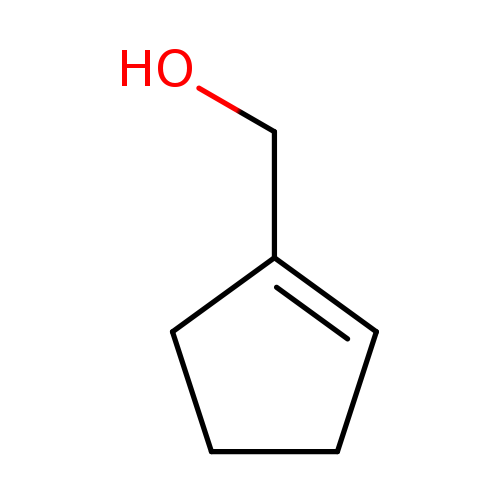
1-Cyclopentene-1-methanolCatalog No.:AA0083J2 CAS No.:1120-80-5 MDL No.:MFCD19646923 MF:C6H10O MW:98.1430 |
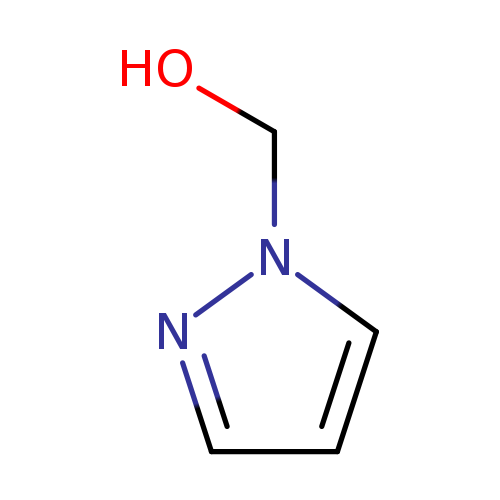
Pyrazol-1-yl-methanolCatalog No.:AA007CEO CAS No.:1120-82-7 MDL No.:MFCD00955584 MF:C4H6N2O MW:98.1032 |
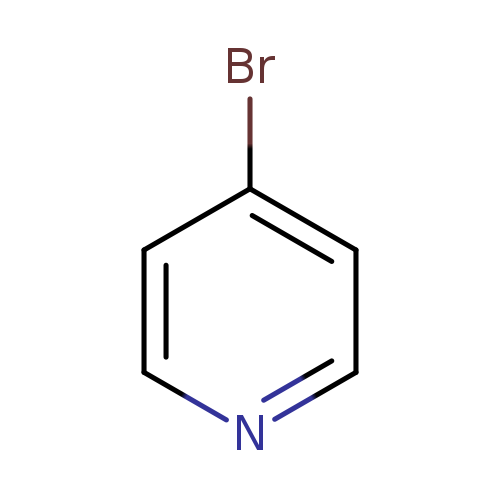
4-BromopyridineCatalog No.:AA003893 CAS No.:1120-87-2 MDL No.:MFCD29043770 MF:C5H4BrN MW:157.9960 |
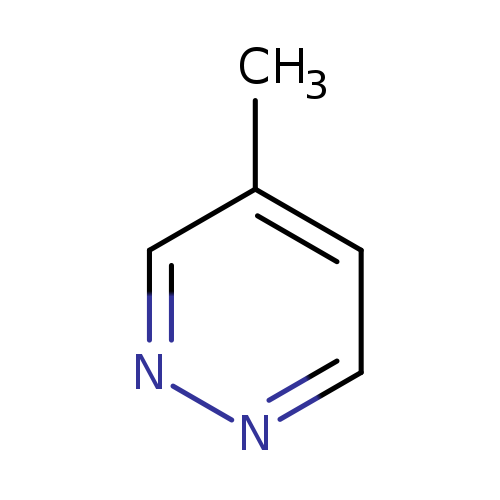
4-MethylpyridazineCatalog No.:AA00HC9K CAS No.:1120-88-3 MDL No.:MFCD00011594 MF:C5H6N2 MW:94.1145 |
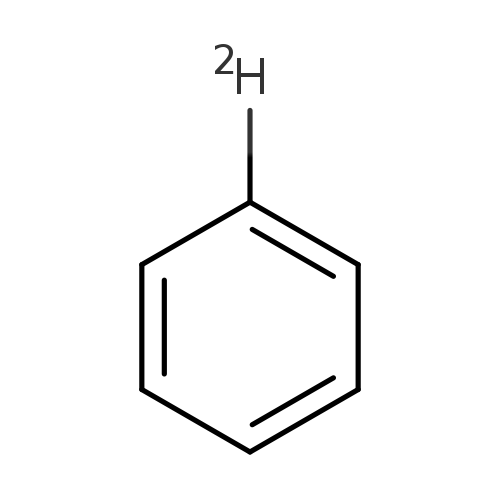
Benzene-dCatalog No.:AA008SNI CAS No.:1120-89-4 MDL No.:MFCD00003011 MF:C6H5D MW:79.1180 |
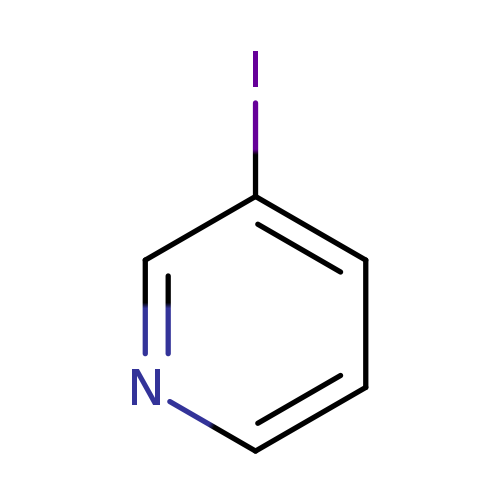
3-IodopyridineCatalog No.:AA0033NQ CAS No.:1120-90-7 MDL No.:MFCD00023553 MF:C5H4IN MW:204.9964 |
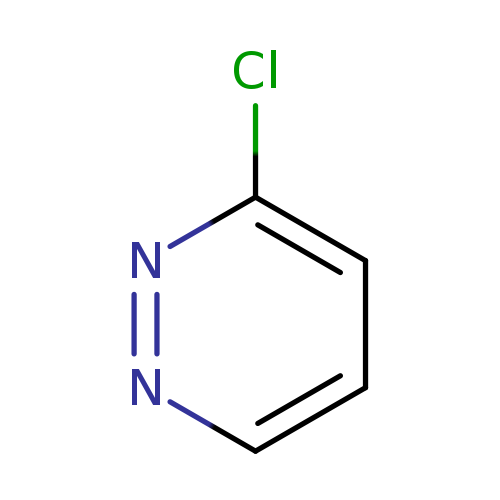
3-ChloropyridazineCatalog No.:AA0033LV CAS No.:1120-95-2 MDL No.:MFCD06801356 MF:C4H3ClN2 MW:114.5330 |
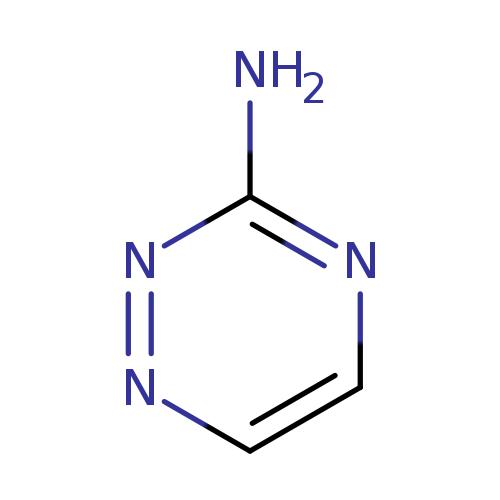
3-Amino-1,2,4-triazineCatalog No.:AA003IQV CAS No.:1120-99-6 MDL No.:MFCD00006459 MF:C3H4N4 MW:96.0907 |
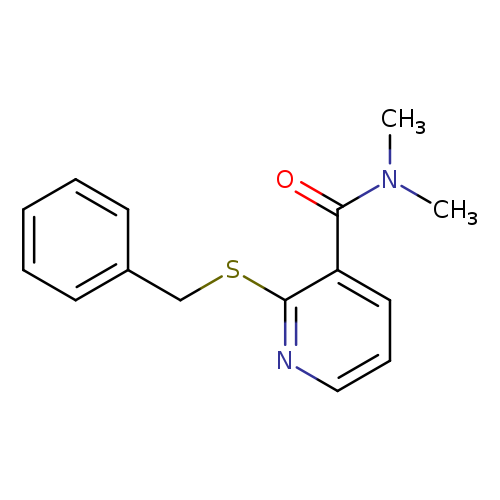
2-(Benzylthio)-N,N-dimethylnicotinamideCatalog No.:AA0092WY CAS No.:112006-57-2 MDL No.:MFCD08316155 MF:C15H16N2OS MW:272.3653 |
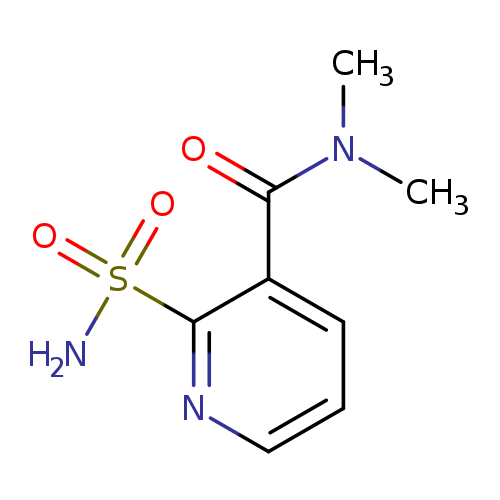
N,N-Dimethylnicotinamide-2-sulfonamideCatalog No.:AA007CVF CAS No.:112006-75-4 MDL No.:MFCD03265507 MF:C8H11N3O3S MW:229.2562 |
1,5-Diphenyl-1h-pyrazole-3-carbaldehydeCatalog No.:AA007UUF CAS No.:112009-28-6 MDL No.:MFCD04115088 MF:C16H12N2O MW:248.2793 |
(R)-1-Chloro-3-phenylpropan-2-olCatalog No.:AA01BAWQ CAS No.:112009-61-7 MDL No.:MFCD07366986 MF:C9H11ClO MW:170.6360 |
5,8-Epoxy-13-cis Retinoic Acid(Mixture of Diastereomers)Catalog No.:AA0083MV CAS No.:112018-12-9 MDL No.:MFCD28898464 MF:C20H28O3 MW:316.4345 |
(R)-Diethyl 2-aminosuccinate hydrochlorideCatalog No.:AA007CV0 CAS No.:112018-26-5 MDL No.:MFCD11225843 MF:C8H16ClNO4 MW:225.6699 |
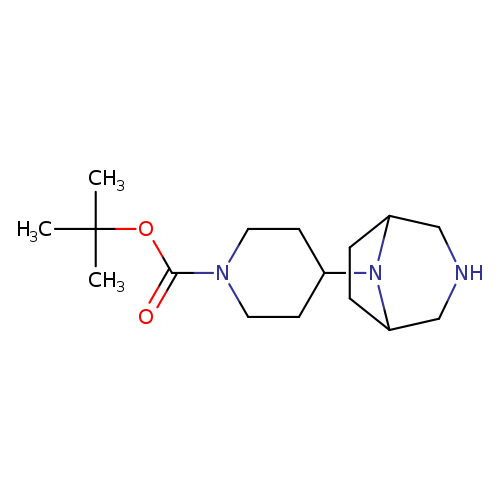
tert-Butyl 4-(3,8-diazabicyclo[3.2.1]octan-8-yl)piperidine-1-carboxylateCatalog No.:AA009231 CAS No.:1120214-86-9 MDL No.:MFCD14279202 MF:C16H29N3O2 MW:295.4204 |
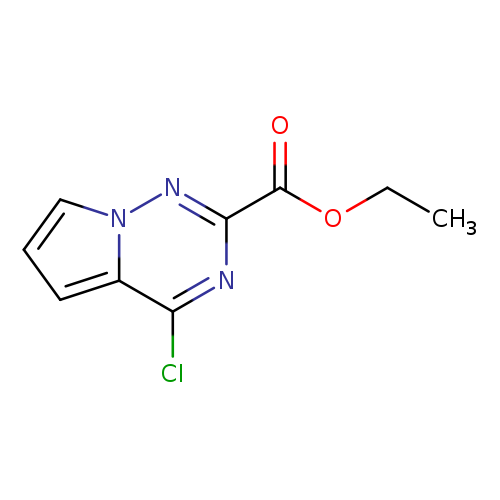
Ethyl 4-chloropyrrolo[1,2-f][1,2,4]triazine-2-carboxylateCatalog No.:AA008TT8 CAS No.:1120214-92-7 MDL No.:MFCD11044886 MF:C9H8ClN3O2 MW:225.6317 |
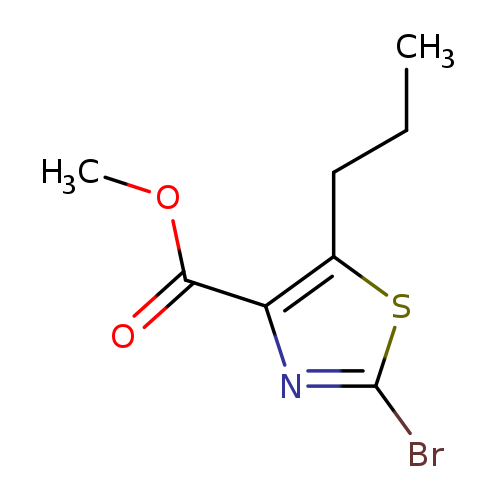
Methyl 2-bromo-5-propylthiazole-4-carboxylateCatalog No.:AA0083MS CAS No.:1120214-96-1 MDL No.:MFCD11706993 MF:C8H10BrNO2S MW:264.1395 |
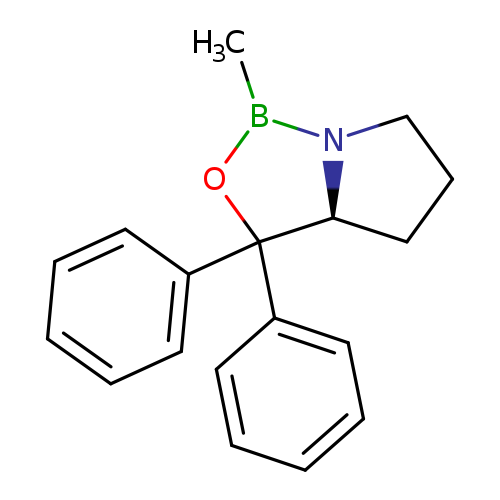
(S)-2-Methyl-CBS-oxazaborolidineCatalog No.:AA00324W CAS No.:112022-81-8 MDL No.:MFCD00078439 MF:C18H20BNO MW:277.1685 |
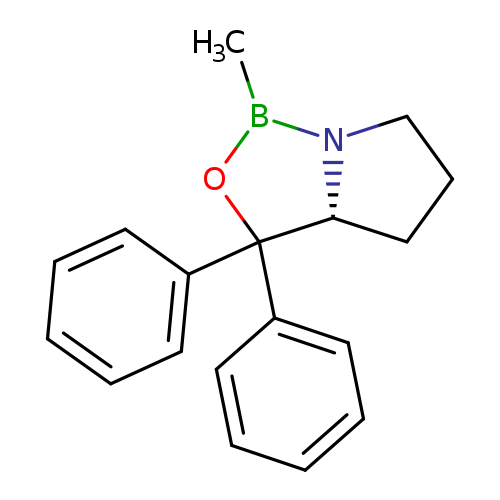
(R)-2-Methyl-CBS-oxazaborolidineCatalog No.:AA00324S CAS No.:112022-83-0 MDL No.:MFCD00078440 MF:C18H20BNO MW:277.1685 |
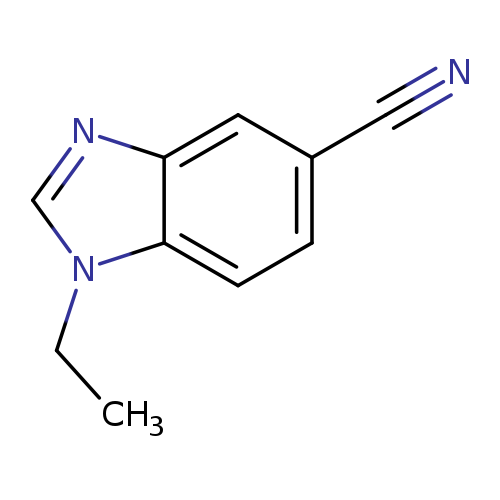
1-Ethyl-1,3-benzodiazole-5-carbonitrileCatalog No.:AA008VIU CAS No.:1120244-47-4 MDL No.:MFCD12827521 MF:C10H9N3 MW:171.1986 |
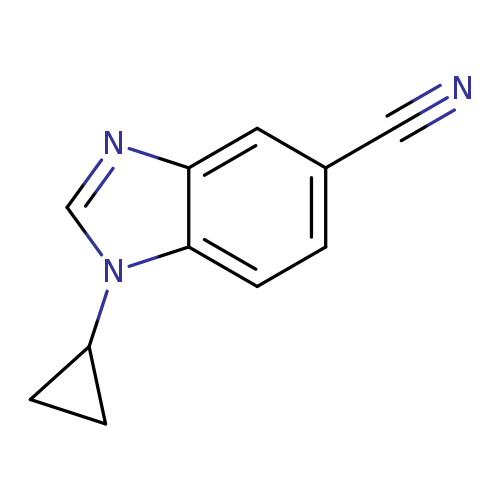
1-Cyclopropyl-1,3-benzodiazole-5-carbonitrileCatalog No.:AA00944C CAS No.:1120244-55-4 MDL No.:MFCD13881256 MF:C11H9N3 MW:183.2093 |
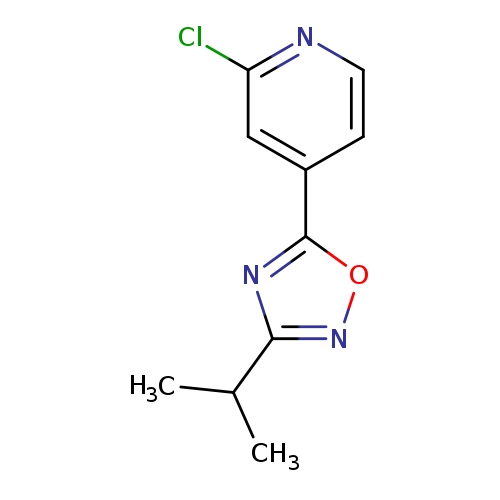
2-chloro-4-[3-(propan-2-yl)-1,2,4-oxadiazol-5-yl]pyridineCatalog No.:AA01AKFZ CAS No.:1120245-52-4 MDL No.:MFCD13881437 MF:C10H10ClN3O MW:223.6589 |