2019-09-24 21:40:09
| AA0035EC | Promotion |
 |
$10/5g, in stock San Diego |
$15/25g, in stock San Diego |
|
| $40/100g, in stock San Diego | |
| $150/500g, in stock San Diego |
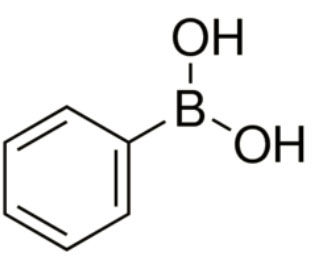
Phenylboronic acid, or benzeneboronic acid is widely utilized in various cross coupling reactions for example Mizoroki-Heck and Suzuki-Miyaura coupling reactions, etc. It serves as a receptor for carbohydrates, antimicrobial agents and enzyme inhibitors in biological reactions. It acts as a reagent in palladium-catalyzed stereoselective Heck-type reaction and as a catalyst in Ni(II) pincer complex and Pd(II) pyridoxal hydrazone metallacycles. It is also employed as a sensor for neutron capture therapy for cancer, transmembrane transport and bioconjugation and labeling of proteins and cell surface.
Literature References
Reagent for protection of diols as their cyclic boronates: J. Am. Chem. Soc., 80, 2443 (1958); Tetrahedron, 25, 477 (1969), which are also useful in GC and GC-MS. This has been utilized to trap cis-diols, in order to prevent over-oxidation during dihydroxylation reactions catalyzed by Osmium(VIII) oxide: Chem. Lett., 1721 (1988); J. Org. Chem., 63, 7322 (1998).
Promotes the ortho-hydroxalkylation of phenols by aldehydes. The cyclic boronate, formed via a [3,3] sigmatropic rearrangement, is the key intermediate: Synthesis, 365 (1979):
With citronellal, hexahydrocannabinoids are formed: J. Chem. Soc., Perkin 1, 605 (1992).
Acts as a template for Diels-Alder reactions, by forming boronate linkages with a hydroxydiene and a hydroxydienophile: Synthesis 1171 (1991); for Nicolaou's application to synthesis of the CD ring system of taxol, see: J. Chem. Soc., Chem. Commun., 1118 (1992); J. Am. Chem. Soc., 117, 634 (1995):
For use in the formation of an oxazaborolidine catalyst for use in enantioselective reductions, see note under (S)-(-)-ɑ,ɑ-Diphenylprolinol.
Forms a stable chiral acyloxyborane (CAB) catalyst with tartaric acid derivatives, which catalyze hetero Diels-Alder reactions, e.g. between aldehydes and 1-Methoxy-3-trimethylsiloxy-1,3-butadiene, to give enantioselectively, dihydro-4-pyrone derivatives: Tetrahedron, 50, 979 (1994).
The most widely-used reaction of arylboronic acids is the Pd-catalyzed Suzuki synthesis of unsymmetrical biaryls: Synth. Commun., 11, 513 (1981):
For illustrative example, see: Org. Synth., 75, 53 (1997).
For a brief feature on uses of this reagent in Organic synthesis, see: Synlett, 2679 (2006). For further information and reviews on boronic acid chemistry, see Appendix 5.
Asoh, T. A.; Takaishi, K.; Kikuchi, A. Adhesion of poly(vinyl alcohol) hydrogels by the electrophoretic manipulation of phenylboronic acid copolymers. J. Mater. Chem. B 2015, 3 (33), 6740-6745.
Tsuchido, Y.; Sakai, Y.; Aimu, K.; Hashimoto, T.; Akiyoshi, K.; Hayashita, T. The design of phenylboronic acid azoprobe-polyamidoamine dendrimer complexes as supramolecular sensors for saccharide recognition in water. New J. Chem. 2015, 39 (4), 2620-2626.