2019-10-30 11:34:10
Design of moldable hydrogels for biomedical applications using dynamic covalent boronic esters
B. Marco-Dufort, M.W. Tibbitt*
Macromolecular Engineering Laboratory, Department of Mechanical and Process Engineering, ETH Zürich, Zürich 8092, Switzerland
Conventional polymeric materials based on thermoset plastics or thermoplastics are widely used as biomedical materials. While thermosets and thermoplastics are attractive for a range of applications, their relatively static material properties may limit them. Recent efforts in material design have focused on engineering response and adaptive networks based on dynamic covalent chemistry. The installation of reversible chemical reagents in the network backbone can break and modify the bonds in the network and rearrange the materials on the experimental time scale. We provide a detailed discussion of borate chemistry and provide guidance for adjusting binding based on synthetic modifications. We explain how network topology and connectivity affect the macro nature of the assembled network. In addition, we discuss how these design principles can be used in the basics of borate-based hydrogels and in emerging biomedical applications. The use of borate as a dynamic covalent crosslink will continue to produce materials with emerging dynamic properties, and the design principles presented here will help to make the next generation of borate-based biomaterials.
© 2018 The Authors. Published by Elsevier Ltd. This is an open access article under the CC BY license
1.Introduction
Dynamic covalent chemistry provides an attractive approach to the fabrication of soft materials in which the strength of covalent bonds is combined with the reversibility of non-covalent interactions. The polymer network is formed by physical or chemical crosslinking of functional monomers or polymer precursors. The physically crosslinked network is combined with non-covalent reversible interactions such as hydrogen bonding, hydrophobic interactions or metal-ligand coordination. These usually result in a net effect of shear thinning (viscous flow when shear increases) and self-healing (changes in gel properties after shear arrest) because these bonds can respond to external stimuli including motility. Breaking and reforming. However, the resulting structure is generally unstable to small environmental disturbances and lacks robustness. On the other hand, chemically crosslinked networks are bonded together by permanent covalent bonds. They produce elastic gels with higher mechanical properties than physical networks, but the irreversibility of chemical cross-linking limits their use in applications that require shear thinning and self-healing.
Recent research in polymer network design and engineering has introduced a new class of soft materials based on dynamic covalent chemistry that combines the advantages of physical and chemical cross-linking materials. In this method, the reversible covalent bond is installed in the network backbone and can be broken and modified during the experimental time. Thus, due to the exchange of bonds, these dynamic covalent networks can be rearranged, making stress relaxation and material flow possible. In general, the binding affinities of dynamic covalent chemistry are sensitive to changes in environmental conditions and thus have susceptibility to material properties. Chemicals used in the formation of dynamic covalent networks include transesterification, Diels-Alder (DA) cycloaddition, and borate complexation. These enable the manufacture of processable and recyclable trimers, temperature-induced self-healing networks and injectable hydrogels, respectively. In each case, the ability to break and reform bonds at the molecular scale results in dynamic changes on a macroscopic scale.
In this review, we outline how borate bonds can be used to engineer dynamic covalent networks and gels. After introducing dynamic covalent chemistry and borate linkages, we provide a detailed discussion of how chemistry affects the formation kinetics and thermodynamics of borate formation and fracture. Then, we discuss how the formation and topology of the network affect the macroscopic nature of the dynamic covalent network. In addition, we investigated basic and emerging biomedical applications that use these chemical and physical insights to make materials using borate crosslinkers. Finally, we conclude with a vision for the future in this area. Collectively, this review is intended for chemists, materials scientists, and bioengineers interested in leveraging dynamic covalent boronate linkages in the design of responsive polymer networks and gels.
1.1. Dynamic covalent chemistry
In dynamic covalent chemistry, the chemical reaction pathways that lead to competition for different products are under thermodynamic control. Since they are joined together by covalent bonds, the resulting structure is not only reversible, but also robust. In such systems, the relative stability of the product, DG0, determines their final distribution. Rather than forming the relative energy DEa they require, like a dynamics control system. As a result, slight modifications to the starting material (composition, space or electrons) or changes in the external environment (such as pH, temperature or concentration) can significantly change the thermodynamic equilibrium. Dynamic covalent systems are therefore able to adapt to changing conditions because their molecular composition can be easily assembled and broken down into different product distributions based on changes in chemical equilibrium.
Thus, the dynamic portion can be incorporated into the polymer network to effect rearrangement of the key connections, as shown in FIG. By continuously disconnecting and reforming the bonds, dynamic covalent networks can adapt to physical or chemical cues (such as mechanical loads, pH, or temperature), resulting in highly processable and self-healing materials with frequency-dependent mechanical properties. For example, disulfide cross-linking (Fig. 1a) has long been determined to be reversible. Later, people used light and pH to study the formation and cleavage of disulfide bonds to create a reversible network. Another common strategy for generating dynamic networks involves the DA cycloaddition reaction. Chen et al. A highly crosslinked polymer network was synthesized by the DA reaction of the furan and maleimide moieties. Network rearrangement after heating facilitates reverse transcription of the DA reaction. In another study, Skene and Lehn reversibly exchanged repeating monomers using the acyl hydr function in dynamic polyamides. In each of these reactions, the sequence of bond breaking and formation involves a transition to reduced network connectivity; the bond must be broken before another bond is formed. When a bond cleavage sequence occurs without losing connectivity, as in a reversible exchange reaction, different mechanical properties can also be achieved. Prominently, Montana and others. Forming an irreversible epoxy network, the topology can be rearranged by a transesterification reaction (Fig. 1c) in which the reorganization of the bonds occurs without depolymerization.
It is critical that the kinetics associated with dynamic covalent bond formation is usually slow because the bond must be long enough to produce a stable and robust structure. This typically requires the use of a catalyst, application of heat or drastically changing the pH to balance the thermodynamically stable product over the experimental time frame. As a result, the current focus in the field of dynamic covalent networks is to find materials that are suitable for biomedical applications.
1.2. Borate ester as a dynamic covalent crosslinker
Borate crosslinked networks have become a compelling dynamic material. The reversible condensation reaction of boric acid with cis 1,2- or cis 1,3 diol to form a cyclic ester (Fig. 1b) occurs in an aqueous solution. Under mild conditions, no catalyst is required at ambient temperature. This enables the formation of borate esters under physiological conditions. In addition, a simple proximity group effect can be used to adjust the reactivity of the boronic acid groups to provide materials with adjustable mechanical properties ranging from mechanical static to extremely ductile. Starting from a preliminary study of the use of boric acid in medicine, borate esters have become a common cross-linking motif in biomaterial design. The behavior of these materials is directly dependent on the basic bonding chemistry of the selected borate, so a reasonable design depends on a deep understanding of how to adjust the kinetics and thermodynamics of borate bond formation and fracture.
1.3. Biomedical applications of boric acid
Boric acid has been widely used in medicine due to its low toxicity and biological functionality. One of the earliest and most famous biomedical uses of boric acid is as an enzyme inhibitor. In 2003, the US Food and Drug Administration (FDA) approved bortezomib as the first proteasome inhibitor for human use. The anticancer drug consists of a dipeptide containing boric acid which competitively binds to a hydroxyl group in the active site of the serine protease. Upon binding, a reversible tetrahedral intermediate is formed which mimics the normal transition state and inhibits enzymatic activity.
Another important biomedical use of boric acid is as a synthetic chemical sensor for biomolecules. In 1959, Lorand and Edwards calculated the binding affinities of various polyols to phenylborate ions by measuring the corresponding pH drop after mixing in aqueous solution. They show that the reversible interaction between boric acid and diol changes when external conditions (such as pH and temperature) change. Conversely, the addition of free diol to an aqueous solution containing boric acid will also change the equilibrium, resulting in a relevant change in measurable properties such as pH or solubility. This finding laid the foundation for real-time molecular sensing of many biologically important glycol-containing molecules. Although sensing of other molecules such as ATP or even heavy metal ions has been demonstrated, research has focused on the detection of sugars. A typical device design is to immobilize the boric acid moiety on the substrate. The selective binding of sugar to the device is accompanied by changes in thermodynamic equilibrium which result in changes in the measurable properties of the system. For example, when certain boric acids are combined with sugars, the optical sensor relies on changes in UVeVis absorption, while the fluorescence sensor measures the difference in intensity of the bound and unbound fluorophore-functionalized boric acid. Some recent comments have highlighted the extensive advances in the field of molecular sensing for sugars, especially the boric acid of glucose.
2-(4-Aminophenyl)ethylamineCatalog No.:AA00703L CAS No.:13472-00-9 MDL No.:MFCD00008194 MF:C8H12N2 MW:136.1943 |
2-MethoxybenzaldehydeCatalog No.:AA003HGX CAS No.:135-02-4 MDL No.:MFCD00003308 MF:C8H8O2 MW:136.1479 |
3-QuinolinecarboxaldehydeCatalog No.:AA00125C CAS No.:13669-42-6 MDL No.:MFCD00006768 MF:C10H7NO MW:157.1687 |
1-Fluorocyclopropane-1-carboxylic acidCatalog No.:AA0012QP CAS No.:137081-41-5 MDL No.:MFCD18205954 MF:C4H5FO2 MW:104.0797 |
2-Bromo-5-acetylpyridineCatalog No.:AA001A05 CAS No.:139042-59-4 MDL No.:MFCD04974527 MF:C7H6BrNO MW:200.0326 |
2-Bromo-5-cyanopyridineCatalog No.:AA001B8J CAS No.:139585-70-9 MDL No.:MFCD00234141 MF:C6H3BrN2 MW:183.0054 |
4-Bromo-2,3-difluorophenolCatalog No.:AA001J5S CAS No.:144292-32-0 MDL No.:MFCD08061907 MF:C6H3BrF2O MW:208.9882 |
2-ChloroquinoxalineCatalog No.:AA001K4P CAS No.:1448-87-9 MDL No.:MFCD00043907 MF:C8H5ClN2 MW:164.5917 |
2-Bromo-5-methylphenolCatalog No.:AA003GNE CAS No.:14847-51-9 MDL No.:MFCD11100989 MF:C7H7BrO MW:187.0339 |
3-Methoxyazetidine, HClCatalog No.:AA001L0A CAS No.:148644-09-1 MDL No.:MFCD06804514 MF:C4H10ClNO MW:123.5813 |

4-MethoxyphenolCatalog No.:AA001M5C CAS No.:150-76-5 MDL No.:MFCD00002332 MF:C7H8O2 MW:124.1372 |
2-Methylpyrazole-3-carboxylic acidCatalog No.:AA001RS3 CAS No.:16034-46-1 MDL No.:MFCD00464253 MF:C5H6N2O2 MW:126.1133 |
Tetrabutylammonium bromideCatalog No.:AA001UP1 CAS No.:1643-19-2 MDL No.:MFCD00011633 MF:C16H36BrN MW:322.3677 |
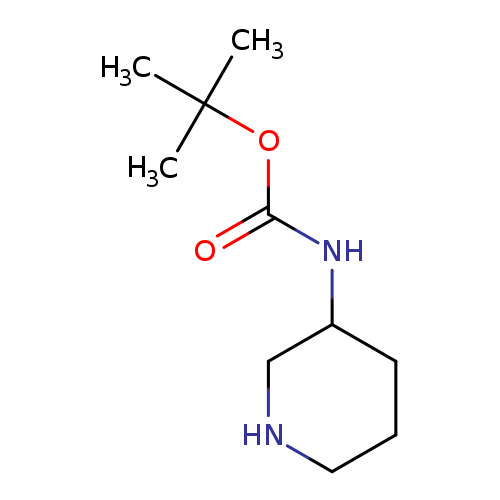
3-Boc-aminopiperidineCatalog No.:AA003JPK CAS No.:172603-05-3 MDL No.:MFCD03839941 MF:C10H20N2O2 MW:200.2780 |
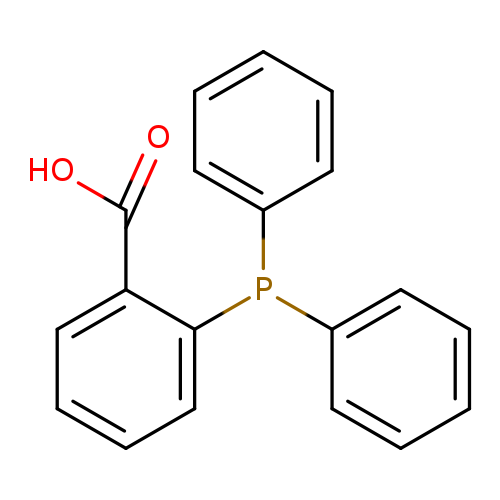
2-(Diphenylphosphino)benzoic acidCatalog No.:AA0037WS CAS No.:17261-28-8 MDL No.:MFCD00674024 MF:C19H15O2P MW:306.2950 |
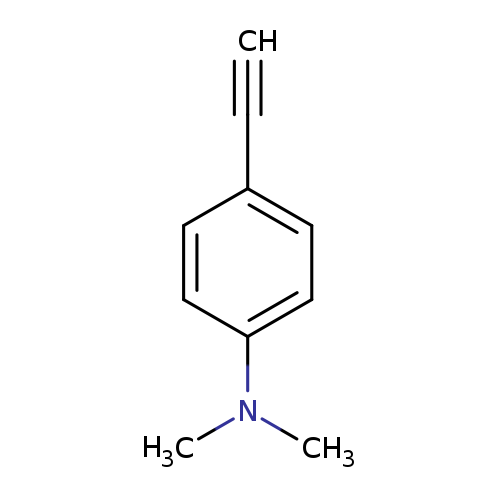
4-Dimethylaminophenyl acetyleneCatalog No.:AA00AOTU CAS No.:17573-94-3 MDL No.:MFCD00168816 MF:C10H11N MW:145.2010 |
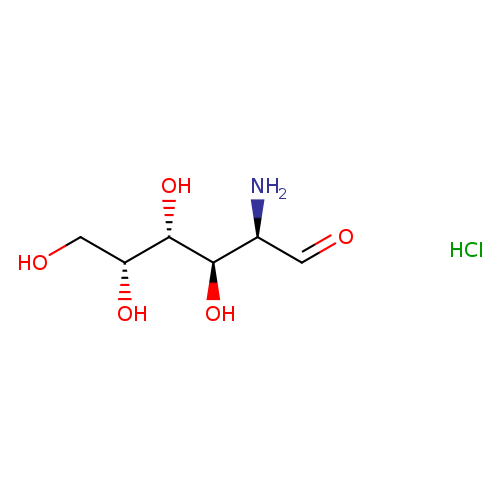
D-Galactosamine, HClCatalog No.:AA00253E CAS No.:1772-03-8 MDL No.:MFCD00135830 MF:C6H14ClNO5 MW:215.6321 |
4,6-Dichloro-2-methylpyrimidineCatalog No.:AA0025N8 CAS No.:1780-26-3 MDL No.:MFCD00090472 MF:C5H4Cl2N2 MW:163.0047 |
N-4-Boc-aminocyclohexanoneCatalog No.:AA0026UR CAS No.:179321-49-4 MDL No.:MFCD00798168 MF:C11H19NO3 MW:213.2735 |
Tris(4-fluorophenyl)phosphineCatalog No.:AA0035P8 CAS No.:18437-78-0 MDL No.:MFCD00013553 MF:C18H12F3P MW:316.2569 |
Methyl 3-fluoro-4-nitrobenzoateCatalog No.:AA003543 CAS No.:185629-31-6 MDL No.:MFCD08444027 MF:C8H6FNO4 MW:199.1359 |
Mono-methyl isophthalateCatalog No.:AA003S2W CAS No.:1877-71-0 MDL No.:MFCD00029972 MF:C9H8O4 MW:180.1574 |
3-Cyanophenylacetic acidCatalog No.:AA002GF3 CAS No.:1878-71-3 MDL No.:MFCD06411079 MF:C9H7NO2 MW:161.1574 |
N-Boc-hexahydro-1H-azepin-4-oneCatalog No.:AA003SR7 CAS No.:188975-88-4 MDL No.:MFCD03788435 MF:C11H19NO3 MW:213.2735 |
5-Bromo-2-chlorobenzaldehydeCatalog No.:AA002DQP CAS No.:189628-37-3 MDL No.:MFCD08445659 MF:C7H4BrClO MW:219.4631 |
1-Methylindole-3-carboxaldehydeCatalog No.:AA002E97 CAS No.:19012-03-4 MDL No.:MFCD00014570 MF:C10H9NO MW:159.1846 |
3-Trifluoromethyl-1H-pyrazoleCatalog No.:AA002DEO CAS No.:20154-03-4 MDL No.:MFCD00115018 MF:C4H3F3N2 MW:136.0752 |
3,4-Dimethoxybenzyl bromideCatalog No.:AA003835 CAS No.:21852-32-4 MDL No.:MFCD09833606 MF:C9H11BrO2 MW:231.0864 |
4,4-DifluorocyclohexanolCatalog No.:AA0033S1 CAS No.:22419-35-8 MDL No.:MFCD10000566 MF:C6H10F2O MW:136.1398 |
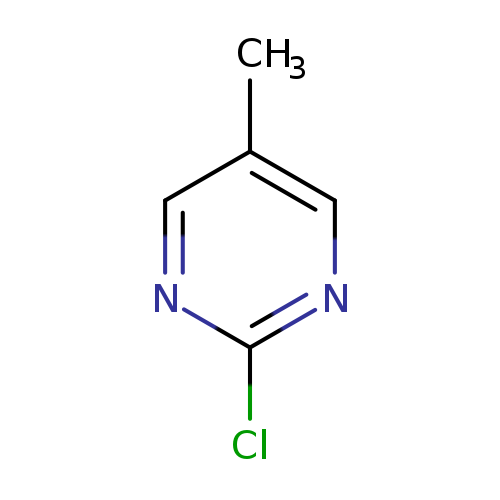
2-Chloro-5-methylpyrimidineCatalog No.:AA003GWH CAS No.:22536-61-4 MDL No.:MFCD09260903 MF:C5H5ClN2 MW:128.5596 |
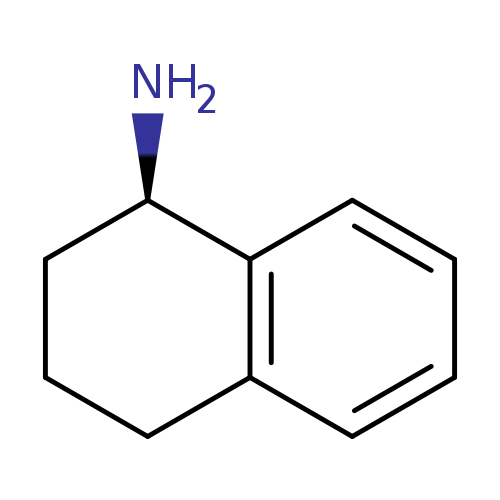
(R)-1,2,3,4-Tetrahydronaphthalen-1-amineCatalog No.:AA003BZE CAS No.:23357-46-2 MDL No.:MFCD00671629 MF:C10H13N MW:147.2169 |
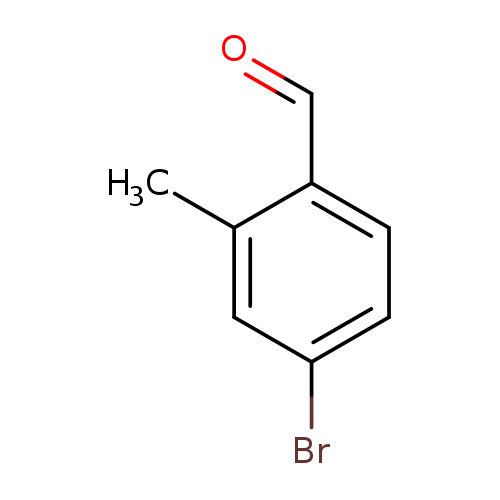
4-Bromo-2-methylbenzaldehydeCatalog No.:AA0033UU CAS No.:24078-12-4 MDL No.:MFCD07787171 MF:C8H7BrO MW:199.0446 |
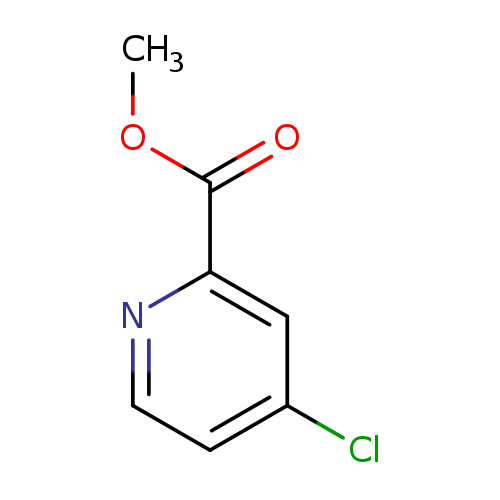
Methyl 4-chloropicolinateCatalog No.:AA002O7T CAS No.:24484-93-3 MDL No.:MFCD04116183 MF:C7H6ClNO2 MW:171.5810 |
Methyl 2,2,2-trichloroacetimidateCatalog No.:AA003RIG CAS No.:2533-69-9 MDL No.:MFCD00001759 MF:C3H4Cl3NO MW:176.4290 |
4-Chloro-3-fluoropyridineCatalog No.:AA0033WP CAS No.:2546-56-7 MDL No.:MFCD03453233 MF:C5H3ClFN MW:131.5354 |
2,5-DifluorobenzaldehydeCatalog No.:AA002T91 CAS No.:2646-90-4 MDL No.:MFCD00010327 MF:C7H4F2O MW:142.1029 |
2-(2-Aminoethyl)pyridineCatalog No.:AA0032RW CAS No.:2706-56-1 MDL No.:MFCD00006367 MF:C7H10N2 MW:122.1677 |
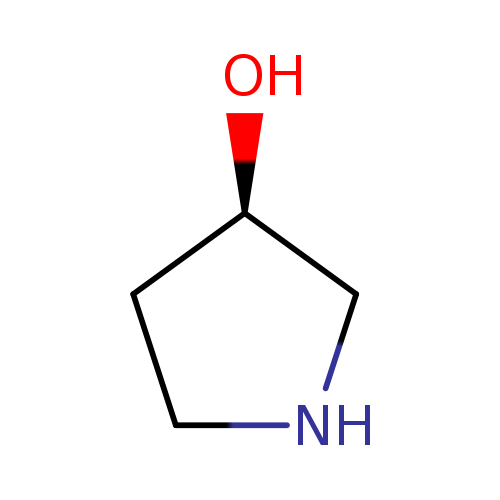
(R)-Pyrrolidin-3-olCatalog No.:AA007LRX CAS No.:2799-21-5 MDL No.:MFCD00145220 MF:C4H9NO MW:87.1204 |
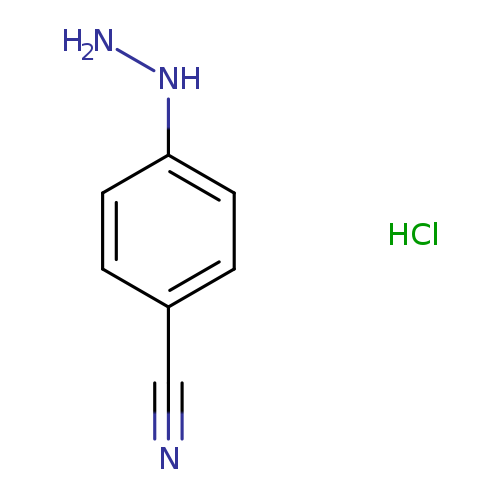
4-Cyanophenylhydrazine, HClCatalog No.:AA002VTU CAS No.:2863-98-1 MDL No.:MFCD00673994 MF:C7H8ClN3 MW:169.6115 |
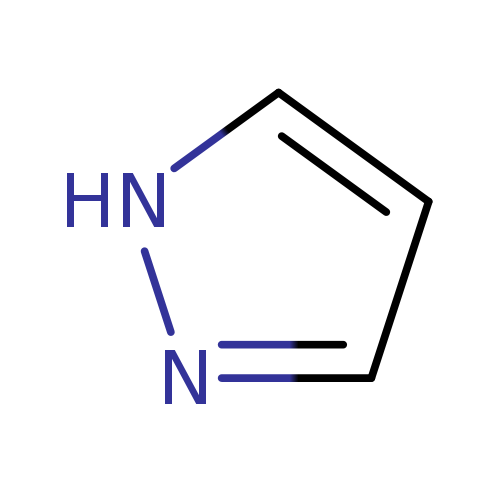
PyrazoleCatalog No.:AA002WGP CAS No.:288-13-1 MDL No.:MFCD00005234 MF:C3H4N2 MW:68.0773 |