2019-11-13 15:02:41
Santigopal Mondal,† Santhivardhana Reddy Yetra,† Subrata Mukherjee,† and Akkattu T. Biju*,‡
Organic Chemistry Division, CSIR-National Chemical Laboratory, Dr. Homi Bhabha Road, Pune 411008, India
Department of Organic Chemistry, Indian Institute of Science, Bangalore 560012, India
1. INTRODUCTION
Since the seminal, independent, isolation of free carbenes by the Bertrand1 and Arduengo2 groups, N-heterocyclic carbene(NHC)-based organocatalysis has emerged as an effective and sophisticated synthetic strategies for the rapid construction of medicinally and biologically important molecules from simple starting materials.3 In general, NHCs are useful for the umpolung of aldehydes. The addition of carbenes to aldehydes generate the nucleophilic enaminol intermediate known as the “Breslow intermediates” (Figure 1).4 The two important transformations proceeding via the umpolung strategy are
benzoin condensation5 and Stetter reaction.6 Moreover, NHCs are also employed in the conjugate umpolung of α,β-unsaturated aldehydes, and these reactions proceed via the generation of homoenolate equivalents.7 In addition, the reaction of NHC with α-functionalized aldehydes such as α-chloroaldehydes, α-epoxyaldehydes or ketenes could result in the generation of NHC-bound enolates.8 A wide variety of carbocycles, heterocycles and acyclic molecules can be accessed using the NHCumpolung concept, and the use of chiral NHCs in the process results in the synthesis of enantiomerically enriched compounds.
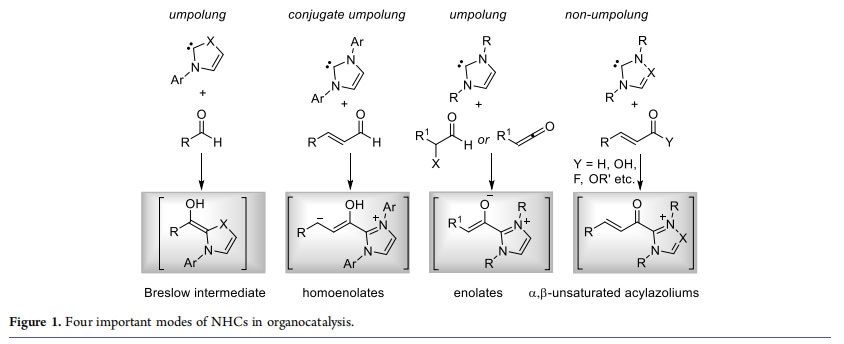
In addition to the application of NHCs in umpolung strategies, carbenes are also useful as catalysts in non-umpolung transformations.9 An important mode of reactivity in this domain proceeds through the α,β-unsaturated acylazoliums. In many cases, α,β-unsaturated acylazolium intermediate acts as a bis-electrophile allowing the conjugate addition of various bisnucleophiles followed by 1,2-addition to form a wide variety of carbocycles and heterocycles. Important methods for the generation of α,β-unsaturated acylazoliums include (i) the reaction of α,β-unsaturated aldehydes with NHCs in the presence of stoichiometric oxidants, (ii) the reaction of ynals with NHCs in the absence of external oxidants, (iii) the reaction of 2-bromoenals and rarely 3-bromoenals with NHCs, (iv) the reaction of NHCs with α,β unsaturated esters, thioester, or acyl fluorides and more recently, and (v) the reaction of NHCs with α,β-unsaturated acids and amides (Figure 2). The purpose of this Account is to summarize the recent developments in our laboratory on the NHC-catalyzed generation of α,β-unsaturated acylazolium intermediates and their subsequent reactions with bis-nucleophiles thereby shedding light on the power of this NHC-bound intermediate in organocatalysis. To put things in proper perspective, adequate description of related work carried out by others is also included in this Account.

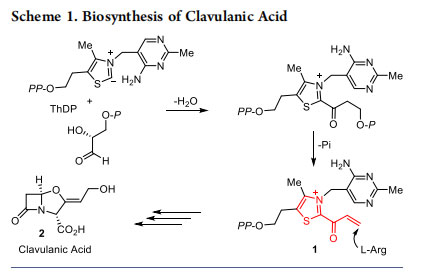
Analogous to the NHC-catalyzed umpolung of aldehydes, the chemistry of α,β-unsaturated acylazoliums also has a biological origin. α,β-Unsaturated acylazoliums were found to be the key intermediates in the biosynthesis of clavulanic acid (2). In 2007, Merski and Townsend successfully demonstrated that the conjugate addition of L-arginine to the α,β-unsaturated acylazolium 1 derived from thiamine diphosphate (ThDP, vitamin B1) is the key step in the biosynthesis of the potent β- lactamase inhibitor clavulanic acid (2) (Scheme 1).10 The strategies for the enantioselective construction of carbocycles and heterocycles proceeding via the NHC-catalyzed generation of α,β-unsaturated acylazolium intermediates, developed in our laboratory are presented in the following sections.
2. [3 + 3] ANNULATION OF α,β-UNSATURATED ACYLAZOLIUMS WITH 1,3-BISNUCLEOPHILES
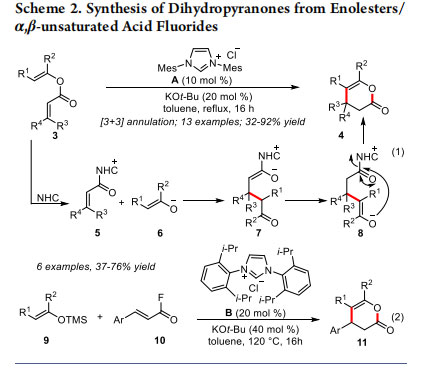
In 2009, Lupton and co-workers demonstrated that NHCs can catalyze the intramolecular cyclization reactions of α,β-unsaturated enol esters 3 leading to the synthesis of dihydropyranones 4. The reaction proceeds via the intermediacy of the α,β-unsaturated acylazolium 5 (Scheme 2, eq 1).11 The reaction is initiated by the 1,2-addition of NHC generated from the imidazolium salt A to the enol ester leading to the α,β-unsaturated acylazolium 5 and the enolate 6. Michael addition of the enolate 6 to the azolium 5 generates the NHC-bound enolate 7, which undergoes a proton transfer to form the azolium 8. The intramolecular acylation then affords the dihydropyranone 4. A variety of enol esters with β,β-disubstitutions underwent smooth annulation reactions to afford the dihydropyranone products in good yields (up to 92%). Moreover, the Lupton group demonstrated that NHCs can catalyze the [3 + 3] annulation reaction of TMS enol ethers 9 with α,β-unsaturated acid fluorides 10 leading to the synthesis of dihydropyranones 11 in 37−76% yields (eq 2).
After Zeitler successfully demonstrated that α,β-unsaturated acylazoliums can be reliably generated from ynals,12 coupling of ynals with enolic C-nucleophiles such as kojic acids 12 proceeding via the enantioselective Claisen rearrangement was disclosed by Bode and co-workers (Scheme 3).13 The expected dihydropyranone product was unstable under the reaction conditions. In the presence of methanol, the ring-opened product 13 was formed in good yields (78−98%) and excellent enantioselectivities (up to >99%). In addition to kojic acid, pyruvic esters and β-naphthol afforded the corresponding functionalized dihydropyranone under identical conditions.
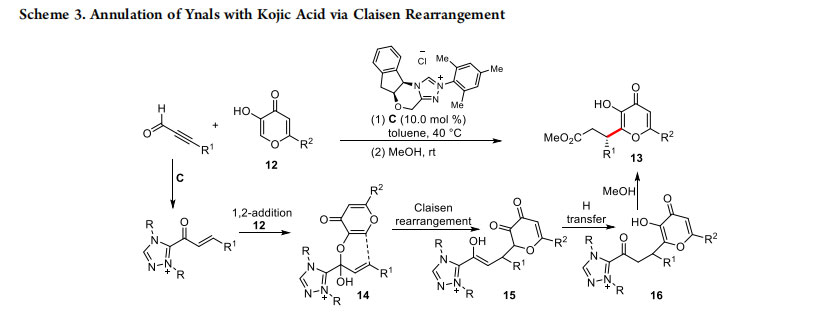
Mechanistically, the reaction proceeds via the addition of NHC to the ynals generating the key α,β-unsaturated acylazolium intermediate. The 1,2-addition of kojic acid 12 to the intermediate acylazolium generates the hemiacetal 14, which undergoes a Claisen rearrangement to give the enol intermediate15. Rapid proton transfer to the intermediate 16 followed by methanolysis afforded the observed product 13.
In 2010, De Sarkar and Studer disclosed an elegant conjugate addition of soft carbon nucleophiles such as 1,3-dicarbonyl compounds 17 to catalytically generated α,β-unsaturated acylazoliums generated from enals 18 under NHC-catalysis in the presence of the bisquinone oxidant 20 (Scheme 4, eq 3).14

The reaction afforded functionalized dihydropyranones 19 in good to excellent yields (51−92%) using NHC generated from the triazolium salt D. A wide variety of α,β-unsaturated aldehydes were well tolerated under the optimized reaction conditions and nucleophiles such as β-diketones and β-ketoesters underwent smooth annulation reaction to afford the desired products. In the same year, Xiao and co-workers disclosed an efficient and atom-economic NHC-catalyzed annulation reaction of ynals with 1,3-diketones or 1,3-keto esters for the synthesis of dihydropyranones (eq 4).15 In the presence of NHC generated from the imidazolium salt A and KOt-Bu, the desired dihydropyranones were obtained in moderate to good yields (41−74%). Subsequently, Xiao and co-workers developed the enantioselective version of this [3 + 3] annulation reaction using NHC generated from the chiral triazolium salt ent-C under base-free conditions.16 In addition, an enantioselective version of the oxidative NHC-catalyzed dihydropyranone synthesis was developed by the You group.17
Furthermore, the generation of α,β-unsaturated acylazoliums from 2-bromoenals followed by their interception with 1,3-dicarbonyl compounds was demonstrated by the Ye18 and Yao groups.19
Inspired by these studies, we envisioned a unified strategy for the enantioselective synthesis of dihydropyranones and dihydropyridinones by the NHC-catalyzed formal [3 + 3] annulation of 2-bromoenals with readily available 1,3-dicarbonyl compounds or primary vinylogous amides. Treatment of 2-bromoenal 21 with 1,3-dicarbonyl compound 22 in the presence of carbene generated from the chiral triazolium salt C using DABCO as the base and LiOAc as the additive furnished the dihydropyranones 23 in excellent yields (up to 96%) and enantioselectivities (up to 99%) (Table 1).20 For good reactivity and selectivity, the combination of two bases was found to be important. A broad range of 2-bromoenals as well as 1,3-dicarbonyl compounds were well tolerated under the optimized reaction condition.
Notably, the reaction proceeds under mild conditions and relatively low catalyst loading (5 mol %). Employing primary vinylogous amides 24 as the bisnucleophile resulted in the enantioselective synthesis of dihydropyridinones 25 (Table 2).20 The protection of the vinylogous amide nitrogen was not required for this annulation and the amide-bond forming side reaction was not observed under the present
conditions.

Mechanistically, the reaction proceeds via the generation of free carbene from C followed by its addition to 2-bromoenal 21 and subsequent proton transfer. The resulting Breslow intermediate 26,
4 then undergoes debromination to afford the key α,β-unsaturated acylazolium intermediate 27 (Scheme 5). Nucleophilic addition of 22 or 24 to intermediate 27 generates the NHC-bound enol intermediate 28, which undergoes proton transfer and a subsequent intramolecular acylation resulting in the formation of the dihydropyranones 23 or dihydropyridinones 25 via the azolium 29. To get insight into the mode of enantioinduction, DFT studies were carried out. These studies indicated that the addition of acetyl acetone from below the plane containing the triazolium moiety (leading to the intermediate 28a) is energetically more favored than the approach from above the plane of the triazolium (leading to the intermediate 28b). This is because the addition of nucleophile from below the plane is stabilized by possible intramolecular hydrogen bonding between the carbonyl oxygen and enol hydrogen, which is absent when the nucleophile approaches from above the plane of the azolium moiety.

Motivated by the success of this reaction, we have envisioned the interception of the chiral α,β-unsaturated acylazolium intermediates with various cyclic and acyclic bisnucleophiles. Employing enolizable aldehydes 30 as the coupling partner for 2-bromoenals 21 resulted in a chemoselective NHC-catalyzed enantioselective cross-coupling for the synthesis of 4,5-disubstituted dihydropyranones 31 (Table 3).21 It is noteworthy that the enantioselective formation of dihydropyranones took place in favor of eight possible byproducts (two γ-butyrolactones, four benzoin products, and two Stetter products). The use
of NHC generated from the chiral triazolium salt C using Na2CO3 as the base afforded the desired products in good yields (41−96%) and excellent enantioselectivities (87−99%) under mild reaction conditions and relatively low catalyst loading.
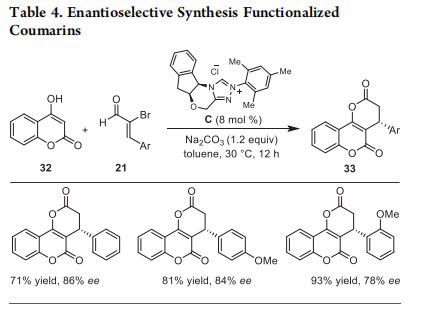
Various electron-donating and -withdrawing substituents on β- aryl ring of 2-bromoenals as well as aliphatic 2-bromoenals are well tolerated under the optimized reaction conditions. Additionally, a series of enolizable aldehydes also underwent smooth annulation reaction to form the desired product. Moreover, the heterocyclic C−H acids such as 4-hydroxy coumarins (32) were successfully employed as the 1,3-bisnucleophilic component for the interception of α,β- unsaturated acylazolium, generated from 2-bromoenals and NHCs, for the synthesis of biologically important coumarinfused dihydropyranones 33. 22 The NHC generated from imidazolium salt A was efficient for this transformation, resulting in various oxygen heterocycles, and the target products 33 were obtained in 37−95% yield. The use of N-methyl quinolinones as the bisnucleophile afforded quinolinone-fused dihydropyranones in high yields. The enantioselective version of this reaction using NHC generated from the chiral triazolium salt C furnished the coumarin-fused dihydropyranones in up to 86% ee (Table 4). Competition experiments revealed that the presence of electron-withdrawing groups on β-aryl ring of 21 enhances the reaction rate.


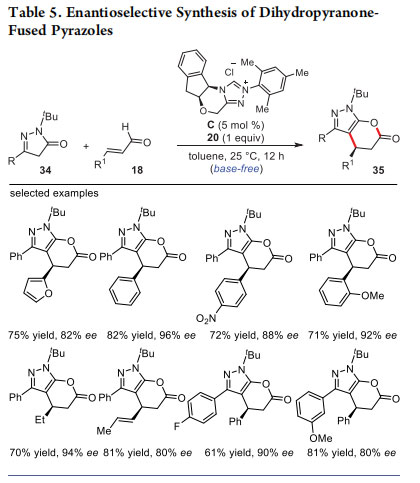
Subsequently, the use of pyrazolones as bisnucleophiles in oxidative NHC-catalysis was investigated. The formal [3 + 3] annulation of pyrazolones 34 with α,β-unsaturated acylazoliums generated from enals 18 under oxidative conditions (using bisquinone 20) afforded the dihydropyranone-fused pyrazoles 35 in good yields (up to 82%) and ee values (up to 96%) (Table 5).23 The reaction proceeds under mild, operationally simple and base-free conditions. Interestingly, in the absence of the NHC catalyst, simple Knoevenagel condensation of enals with 34 occurred. A wide variety of functional groups on the enal as well as on the pyrazolone moiety were well tolerated under the reaction conditions. Moreover, the β,β-disubstituted enal underwent smooth annulation reaction to afford the desired product. Considering the importance of functionalized pyrazoles in the pharmaceutical and agricultural industries, the NHC-catalyzed enantioselective routes to these compounds under metal-free conditions are attractive.
3. [3 + 2] ANNULATION OF α,β-UNSATURATED ACYLAZOLIUMS WITH 1,2-BISNUCLEOPHILES
In view of the interesting results obtained with the interception of α,β-unsaturated acylazoliums with 1,3-bisnucleophiles for the synthesis of six-membered heterocycles, we then focused our attention on 1,2-bisnucleophiles for the synthesis of fivemembered rings. Treatment of 3-hydroxy oxindoles 36 with catalytically generated α,β-unsaturated acylazoliums under oxidative conditions resulted in the enantioselective synthesis of spiro γ-butyrolactones 37 in good yields and enantioselectivity with moderate diastereoselectivities (Table 6).24 The conjugated addition of the C-nucleophile to α,β unsaturated acylazolium followed by intramolecular acylation afforded the desired spirocyclic product in the presence of carbene generated from chiral triazolium salt C and DBU under oxidative conditions. The choice of base was crucial to avoid the undesired ester formation and several functional groups were tolerated under the optimized reaction conditions. Our mechanistic investigation revealed the possibility of the formation of isatin from 36 in presence oxidant and a subsequent [3 + 2] annulation with the catalytically generated homoenolate intermediates from enal and NHCs (Scheme 6).25
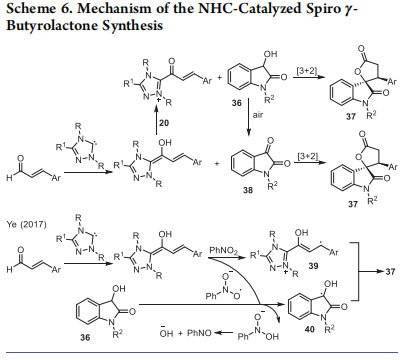
Considering the oxidation of dioxindoles to the corresponding isatins in air and in the absence of external oxidants, it is reasonable to assume the possibility of a [3 + 2] annulation of NHC-homoenolate generated from enals with the in situ generated isatins 38 (Scheme 6). A closely related NHCcatalyzed oxidative [3 + 2] annulation of dioxindoles with enals for the synthesis of spiro γ-butyrolactones 37 was uncovered by Ye and co-workers.26 Interestingly, the use of nitrobenzene as a single electron oxidant enables the generation of radical cation 39 (single electron oxidation of the homoenolate) and the enol radical 40 from dioxindole. The coupling of the two radicals is the key for the formation of 37 (Scheme 6). Similar strategies for the [3 + 2] annulation of α,β-unsaturated acylazolium leading to five-membered nitrogen heterocycles were developed by Lu and Du, Huang, Ye, and Chi groups.27
4. REACTION OF α,β-UNSATURATED ACYLAZOLIUMS WITH α-SUBSTITUTED 1,3-DICARBONYLS
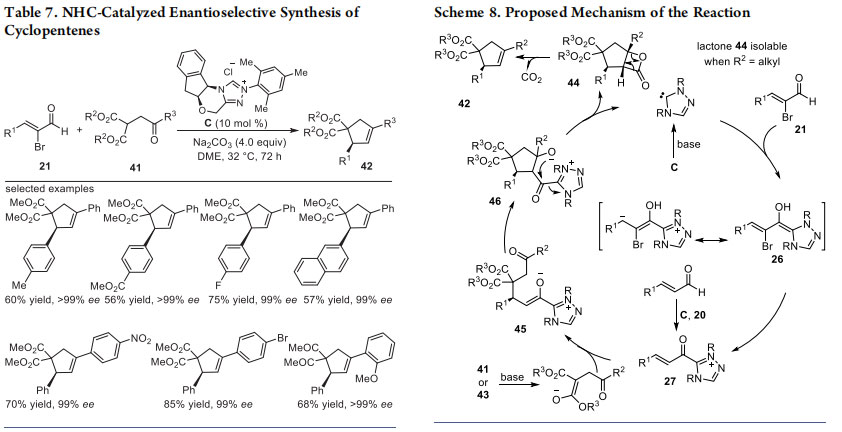
Next, we focused our attention on the use of α-substituted 1,3- dicarbonyls as the coupling partner for α,β-unsaturated acylazoliums. The NHC-catalyzed reaction of α,β-unsaturated acylazoliums generated from 2-bromoenals with malonic ester 41 bearing a γ-aroyl group resulted in the enantioselective synthesis of functionalized cyclopentenes 42 (Table 7).28 This cascade reaction follows a Michael intramolecular aldol-β-lactonization-decarboxylation sequence to deliver the products in good yields and excellent ee values. The use of NHC generated from C in the presence of Na2CO3 in DME was found
to be optimal for this reaction. A variety of substitution pattern on the β-aryl ring of 2-bromoenal as well as substitution on the γ-aroyl moiety were tolerated well under the optimized reaction conditions to afford the desired product in moderate to good yields (40−85% yield) and high enantioselectivities (up to>99%). Moreover, the reaction resulted in the diastereoselective and enantioselective synthesis of cyclopentane-fused β-lactones when performed with malonate derivatives bearing an alkyl group at the γ-position.
The interception of chiral α,β-unsaturated acylazolium with malonates 43 having alkyl group at the γ-position was disclosed independently by Studer and co-workers. The enantioselective synthesis of highly substituted β-lactones 44 were achieved through oxidative carbene catalysis using NHC generated from chiral triazolium salt C and oxidant 20 with LiCl as a cooperative Lewis acid (Scheme 7).29a Moreover, a closely related interception of α,β-unsaturated acylazoliums with donor−acceptor cyclopropanes for the selective synthesis of cyclopentane-fused β-lactones was demonstrated by Lupton and coworkers.

A tentative mechanism for this NHC-catalyzed cyclopentene/highly substituted β-lactone synthesis is described in Scheme 8. The key chiral α,β-unsaturated acylazolium intermediate 27 was generated from 21 and NHC generated from C. Alternatively, 21 can also be formed from enal and C using the oxidant 20. Conjugate addition of enolate generated from 41/43 onto intermediate 27 resulted in the NHC-bound enolate intermediate 45 formation. The intermediate 45 can undergo a highly selective intramolecular aldol reaction leading to the formation of the cyclopentane intermediate 46. β-Lactonization
of intermediate 46 followed by the release of free NHC afforded the cyclopentane-fused β-lactone 44. In the case of substrate 41, a rapid decarboxylation of 44 resulted in the formation of the cyclopentene 42. The driving force for the rapid decarboxylation of 44 (when R2 = aryl) might probably due to the enhanced stability of 42 via formation of the styrenic double bond.
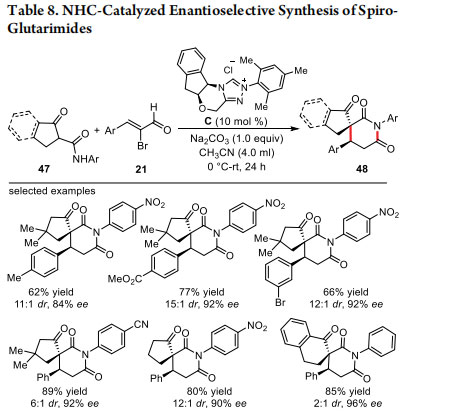
Recently, we have employed α-substituted cyclic β-ketoamides 47 as the coupling partner for α,β-unsaturated acylazoliums. The formal [3 + 3] annulation of 2-bromoenals 21 with 47 furnished diastereoselective and enantioselective route to spiro-glutarimide derivatives 48 (Table 8).30 Again, the carbene generated from C in the presence of Na2CO3 in CH3CN provided the product bearing two contiguous stereocenters including one all-carbon quaternary spirocenter. A broad range of β-aryl-α-bromoenals and cyclic β-ketoamides were well tolerated under the optimized reaction conditions and delivered the desired products in high yields (up to 95%), enantiose lectivities (up to 96%) and diastereoselectivities (up to 17:1).
Mechanistically, this [3 + 3] annulation proceeds via the Michael addition-proton transfer-intramolecular amidation sequence to deliver the product. Notably, the aliphatic 2-bromoenal afforded the spirocyclic product in poor yield and selectivity. However, good yield and selectivity was observed while performing the reactions with β-alkyl enal under oxidative conditions.
5. MICHAEL−MICHAEL-LACTONIZATION CASCADE INVOLVING α,β-UNSATURATED ACYLAZOLIUMS
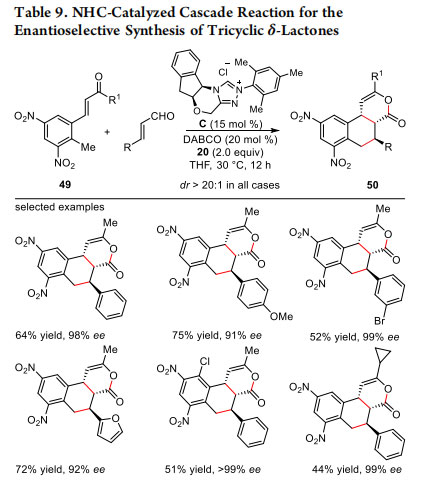
NHCs are known to catalyze several cascade or domino reactions.31 Cascade reaction involving α,β-unsaturated acylazoliums are usually initiated by Michael addition and the final catalyst regeneration takes place via an acylation reaction. Highly efficient and enantioselective cascade reactions via α,β- unsaturated acylazoliums initiated by Michael addition for the synthesis of functionalized δ-lactones was demonstrated by the groups of Hui,32 Studer,33 Ye,34 Chi,35 and Xu.36 We have recently employed dinitrotoluene derivatives 49 bearing two nitro groups to trap the catalytically generated α,β-unsaturated
acylazolium under oxidative conditions in a cascade reaction following the Michael/Michael/lactonization sequence. In the presence of NHC generated from C using DABCO, the functionalization of benzylic C(sp3)-H bond of 49 resulted in the synthesis of tricyclic δ-lactone 50 as a single diastereomer with three-contiguous stereocenters. The generality of this mild, atom-economic and highly stereoselective cascade reaction was demonstrated by performing the reaction with a broad range of aromatic and heteroaromatic enals (Table 9).37 Moreover, the synthetic utility of this methodology was further extended by converting the tricyclic δ-lactones to synthetically useful compounds while preserving the enantiopurity.
Mechanistically, the reaction proceeds via the generation of chiral α,β-unsaturated acylazolium intermediate 27, generated by the nucleophilic attack of NHC generated from C onto the enal followed by the oxidation of the generated Breslow intermediate 26 using the oxidant 20 (Scheme 9). The conjugated addition of the anion generated from 49 from the bottom face of the azolium 27 under basic conditions could result in the NHC-bound enolate intermediate 51 formation, which could undergo a second intramolecular 1,4-addition to the vinyl ketone moiety to provide the enolate intermediate 52. Intramolecular acylation of 52 afforded the desired δ-lactone 50 concomitant with the NHC catalyst regeneration.

Cascade reactions proceeding via α,β-unsaturated acylazoliums, initiated by aza-Michael addition enabled the enantioselective synthesis of nitrogen heterocycles as reported by the groups of Hui32 and Chi.38 We have recently used this concept for the N−H functionalization of indoles leading to the highly selective synthesis of pyrroloquinolines. While using indole derivative 53 (bearing a COCF3 group at the 3-position) as the nucleophilic component, the reaction proceeds via an azaMichael/Michael/lactonization sequence to afford the tetracyclic products 54 in good yields, diastereoselectivities, and enantioselectivities (Table 10).39 The carbene generated from the triazolium salt C using DABCO under oxidative conditions using 20 in DMSO was optimal for high reactivity and selectivity, and the reaction tolerates a broad range of functional groups. It is noteworthy that the reaction features a simultaneous improvement in reactivity/selectivity employing polar aprotic solvents.
6. TANDEM DIENOLATE-ENOLATE ADDITION TO α,β-UNSATURATED ACYLAZOLIUMS
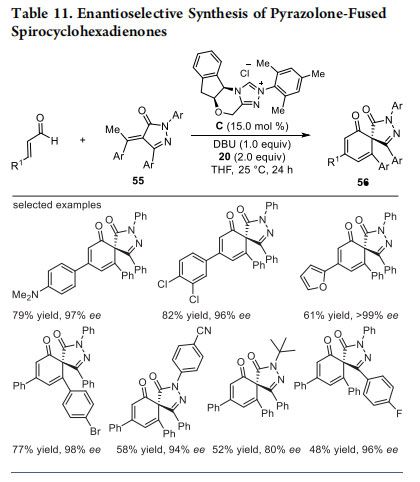
Inspired by the addition of enolates from acyclic/cyclic 1,3- dicarbonyls to α,β-unsaturated acylazoliums, we envisioned the extension of this concept to dienolates. The addition of dienolates to α,β-unsaturated acylazoliums in a formal [3 + 3] pathway for the construction of all-carbon six-membered rings has been demonstrated by Lupton,40 Wang,41 and Ye groups.42 However, the simultaneous interception of α,β-unsaturated acylazoliums using dienolate/enolate generated from the single precursor was unknown. We have used α-arylidene pyrazolinones 55 as the precursor for the tandem generation of dienolate/enolate, which reacts with α,β-unsaturated acylazoliums under oxidative conditions in a [3 + 3] fashion for the synthesis of pyrazolone-fused spirocyclohexadieneones 56 (Table 11).43 This cascade reaction proceeds in a vinylogous Michael addition/spiroannulation/dehydrogenation sequence. In the presence of NHC generated from C using DBU, and excess oxidant 20, the products bearing an all-carbon quaternary stereocenter are formed in moderate to good yields and excellent ee values. Under the optimized reaction conditions, a variety of substituents on the β-aryl ring of enals and α-arylidene pyrazolinones were well tolerated.

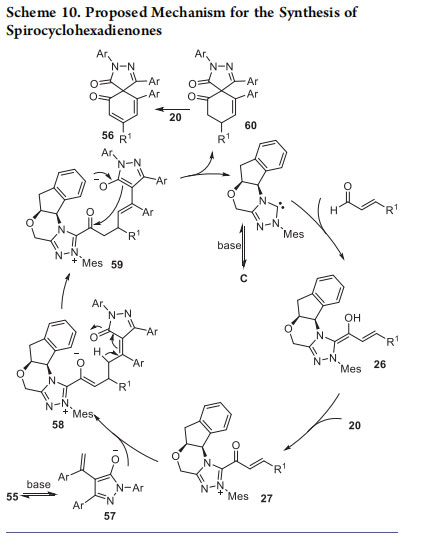
The mechanism of this formal [3 + 3] annulation reaction leading to spirocyclic frameworks is shown in Scheme 10. The reaction proceeds via the generation of chiral α,β-unsaturated acylazolium intermediate 27 from enals and C using 20 via the Breslow intermediate 26, and the dienolate 57 generated from the pyrazolinone 55. Reaction of these intermediates generated the NHC-bound enolate 58, which under basic conditions afforded the second dienolate intermediate 59. Intramolecular acylation of 59 resulted in the formation of 60, which underwent oxidation to provide the spirocyclohexadienone.
7. CONCLUSIONS
This Account has summarized the rich and fascinating chemistry of NHC-catalyzed generation of α,β-unsaturated acylazoliums followed by their interception with various nucleophiles. This is one of the important non-umpolung modes of NHC reactivity. As explained, these key intermediates can be generated from enals (under oxidative conditions), 2-bromoenals, ynals and various acid derivatives. We have demonstrated the interception of α,β-unsaturated acylazoliums with various cyclic and acyclic 1,3-bisnucleophiles for the enantioselective synthesis of dihydropyranones and dihydropyridinones in a formal [3 + 3] pathway. When a malonic ester derivative having a γ-benzoyl group was used as the coupling partner for α,β-unsaturated acylazoliums, functionalized cyclopentenes were obtained via a
cascade process involving Michael-intramolecular aldol-β- lactonization-decarboxylation sequence. Moreover, the enantioselective synthesis of spiro-glutarimides has been achieved by using cyclic β-ketoamides, and the reaction proceeds in a Michael addition-intramolecular amidation pathway. Additionally, the α,β-unsaturated acylazoliums have been employed for cascade processes for the enantioselective synthesis of tricyclic δ-lactones and pyrroloquinolines by using dinitrotoluene derivatives and suitably substituted N−H indoles respectively, and these reactions proceed via the (aza) Michael/Michael/ lactonization sequence. Furthermore, the enantioselective synthesis of pyrazolone-fused spirocyclohexadienones has been developed by using α-arylidene pyrazolinones as the bisnucleophile for the tandem generation of dienolate/enolate, this formal [3 + 3] annulation proceeds via the vinylogous Michael/spiroannulation/dehydrogenation sequence to afford spirocyclic compounds with an all-carbon quaternary stereocenter. Although tremendous development has occurred in this area, some aspects of the chemistry of these reactive intermediates are not well understood yet. For instance, the addition of oxygen/nitrogen nucleophiles in a 1,4-pathway is not well explored in this realm of NHC-catalysis. Further advances in this area will provide new reactions involving this key
electrophilic intermediate. It is reasonable to believe that NHCcatalysis using α,β-unsaturated acylazoliums will continue to flourish and result in more developments in the years to come.
AUTHOR INFORMATION
Corresponding Author
*E-mail: [email protected].
ORCID
Akkattu T. Biju: 0000-0002-0645-8261
Notes
The authors declare no competing financial interest.
Biographies
Santigopal Mondal was born in 1990 in South 24 Parganas, (WB), India. He completed his M.Sc. from IIT Delhi in 2013. He has recently completed his Ph.D. from the research group of Prof. A. T. Biju at the
CSIR-NCL, Pune working on enantioselective NHC-catalyzed transformations. Presently, he is a postdoctoral fellow at the University of Pennsylvania with Professor Jeffrey D. Winkler. Santhivardhana Reddy Yetra was born in 1988 in Guntur, (AP), India. He completed his M.Sc. from Andhra University in 2010. In 2016, he completed his Ph.D. from the research group of Prof. A. T. Biju at the CSIR-NCL, Pune working on asymmetric catalysis using NHCs. Currently, he is a postdoctoral fellow with Professor Lutz Ackermann at the University of Göttingen, in Germany.
Subrata Mukherjee was born in 1991 in Burdwan, (WB), India. He completed his B.Sc. (Hons) in Chemistry at Calcutta University in 2011, and his M.Sc. at the IIT-Madras in 2014. Currently, he is a Ph.D. student in the research group of Prof. A. T. Biju. His research focuses on asymmetric catalysis by using NHCs and related chemistry.
Akkattu T. Biju received his Ph.D. under the guidance of Dr. Vijay Nair at the CSIR-NIIST, Trivandrum, India. Subsequently, he was a postdoctoral fellow with Prof. Tien-Yau Luh at the National Taiwan University, Taipei and an Alexander von Humboldt fellow with Prof.
Frank Glorius at the Westfalische Wilhelms-Universita ̈ t Mu ̈ ̈nster, Germany. In June 2011, he began his independent research career at the CSIR-NCL, Pune. Since June 2017, he has been an Associate Professor in the Department of Organic Chemistry, Indian Institute of Science, Bangalore. His research focuses on the development of transitionmetal-free reactions using NHC organocatalysis and aryne chemistry, and their applications in organic synthesis.
■ ACKNOWLEDGMENTS
We are grateful to the generous financial support provided by Board of Research in Nuclear Sciences (BRNS), Government of India (Grant No. 37(2)/14/49/2014-BRNS/), and the Indian Institute of Science, Bangalore (start-up grant for A.T.B.) for our work on NHC-catalysis. We thank Dr. Anup Bhunia, Dr. Atanu Patra, and Mr. Arghya Ghosh for experimental and intellectual contributions. Sa.M. and Su.M. thank UGC for the fellowship and S.R.Y. thanks CSIR-New Delhi for senior research fellowship.
2-(6-Hydroxy-2,3-dihydrobenzofuran-3-yl)acetic acidCatalog No.:AA0000KK CAS No.:1000414-37-8 MDL No.:MFCD13184245 MF:C10H10O4 MW:194.1840 |
(S)-Methyl 2-(6-hydroxy-2,3-dihydrobenzofuran-3-yl)acetateCatalog No.:AA0000KJ CAS No.:1000414-38-9 MDL No.:MFCD20488619 MF:C11H12O4 MW:208.2106 |
1-[(tert-Butyl)oxycarbonyl]-4-(4-methoxyphenyl)pyrrolidine-3-carboxylic acidCatalog No.:AA0000KI CAS No.:1000415-75-7 MDL No.:MFCD02089476 MF:C17H22NO5- MW:320.3603 |
[(1-isobutylpiperidin-3-yl)methyl]amineCatalog No.:AA00JDIH CAS No.:1000416-60-3 MDL No.:MFCD10003323 MF:C10H22N2 MW:170.2951 |
4-Aminopicolinic acidCatalog No.:AA0000KU CAS No.:100047-36-7 MDL No.:MFCD23703519 MF:C6H6N2O2 MW:138.1240 |
3H-Pyrazolo[3,4-d]pyrimidin-3-one, 1,2-dihydro-6-(methylthio)-Catalog No.:AA0092R9 CAS No.:100047-42-5 MDL No.:MFCD09754087 MF:C6H6N4OS MW:182.2030 |
5-Methyl-1h-pyrrole-3-carboxylic acidCatalog No.:AA0000KP CAS No.:100047-52-7 MDL No.:MFCD09991919 MF:C6H7NO2 MW:125.1253 |
Methyl 5-methylisoxazole-4-carboxylateCatalog No.:AA0000KN CAS No.:100047-54-9 MDL No.:MFCD09756501 MF:C6H7NO3 MW:141.1247 |
5-Bromo-3-methylpyrazin-2-olCatalog No.:AA0000LT CAS No.:100047-56-1 MDL No.:MFCD22548397 MF:C5H5BrN2O MW:189.0100 |
4,5-Dimethyl-isoxazole-3-carboxylic acidCatalog No.:AA0000LR CAS No.:100047-61-8 MDL No.:MFCD05667138 MF:C6H7NO3 MW:141.1247 |
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carboxylic acidCatalog No.:AA0000LQ CAS No.:100047-66-3 MDL No.:MFCD03982044 MF:C6H6N2O3 MW:154.1234 |
2-(Bromomethyl)-2-butylhexanoic acidCatalog No.:AA0000LO CAS No.:100048-86-0 MDL No.:MFCD18206921 MF:C11H21BrO2 MW:265.1872 |
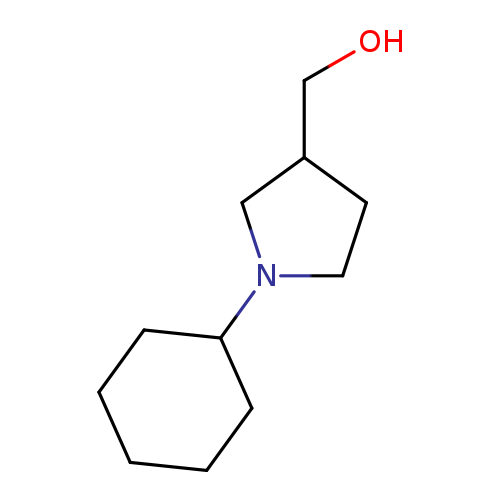
(1-Cyclohexylpyrrolidin-3-yl)methanolCatalog No.:AA009RNN CAS No.:100049-71-6 MDL No.:MFCD10003885 MF:C11H21NO MW:183.2905 |
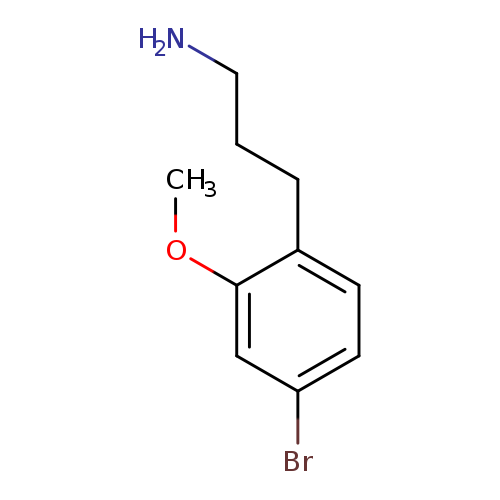
3-(4-Bromo-2-methoxyphenyl)propan-1-amineCatalog No.:AA01A6JR CAS No.:1000504-26-6 MDL No.:MFCD09913736 MF:C10H14BrNO MW:244.1283 |
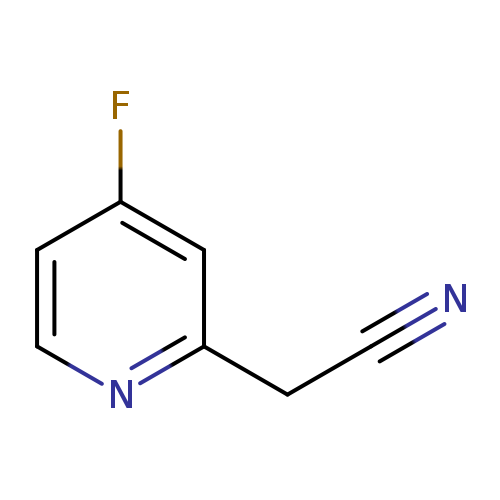
2-(4-Fluoropyridin-2-yl)acetonitrileCatalog No.:AA0000LF CAS No.:1000504-35-7 MDL No.:MFCD09924401 MF:C7H5FN2 MW:136.1264 |
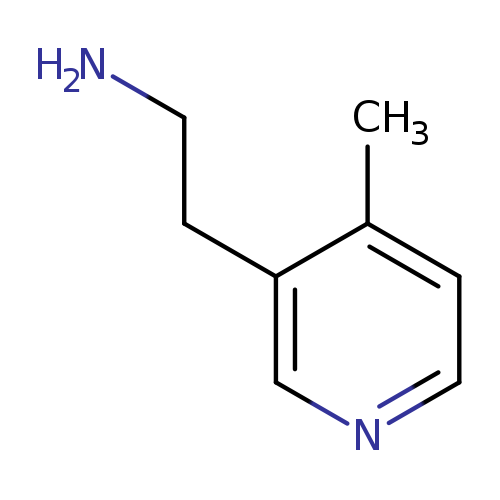
2-(4-methylpyridin-3-yl)ethan-1-amineCatalog No.:AA01A0GV CAS No.:1000504-51-7 MDL No.:MFCD09924452 MF:C8H12N2 MW:136.1943 |
3-(2-methyl-pyridin-4-yl)-propylamineCatalog No.:AA01DUT0 CAS No.:1000507-72-1 MDL No.:MFCD09914784 MF:C9H14N2 MW:150.2209 |
N-[(1E)-2-{[(2E)-2-(Hydroxyimino)cyclopentyl]methyl}-cyclopentylidene]hydroxylamineCatalog No.:AA01F3GW CAS No.:100051-43-2 MDL No.:MFCD00956640 MF:C11H18N2O2 MW:210.2728 |
3-(6-methylpyridin-2-yl)propan-1-amineCatalog No.:AA01BA1E CAS No.:1000510-50-8 MDL No.:MFCD09912911 MF:C9H14N2 MW:150.2209 |
2-[2-(dimethylamino)phenyl]acetonitrileCatalog No.:AA01EJOO CAS No.:1000512-16-2 MDL No.:MFCD09923695 MF:C10H12N2 MW:160.2157 |
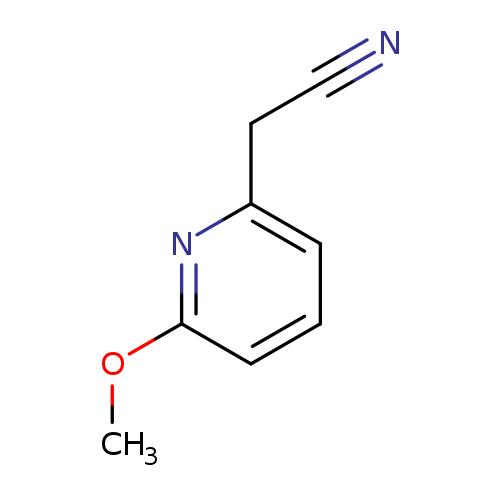
2-(6-Methoxypyridin-2-yl)acetonitrileCatalog No.:AA0000L8 CAS No.:1000512-48-0 MDL No.:MFCD09923742 MF:C8H8N2O MW:148.1619 |
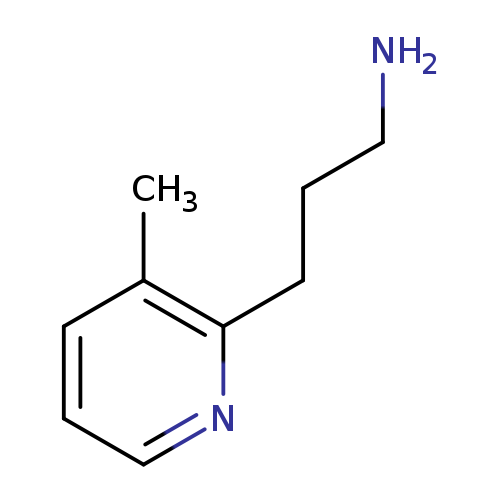
3-(3-Methylpyridin-2-yl)propan-1-amineCatalog No.:AA01AUGT CAS No.:1000512-96-8 MDL No.:MFCD09913294 MF:C9H14N2 MW:150.2209 |
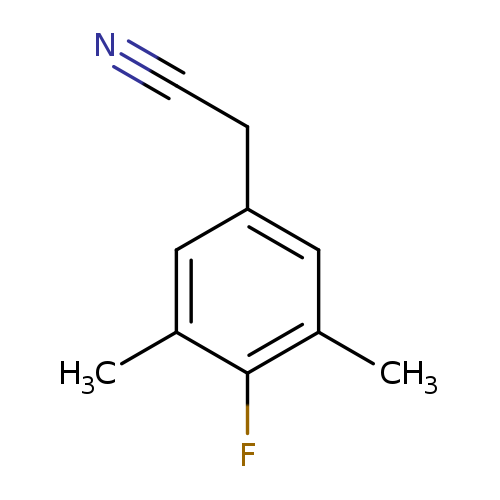
4-Fluoro-3,5-dimethylphenylacetonitrileCatalog No.:AA00H91Y CAS No.:1000513-61-0 MDL No.:MFCD09832374 MF:C10H10FN MW:163.1915 |
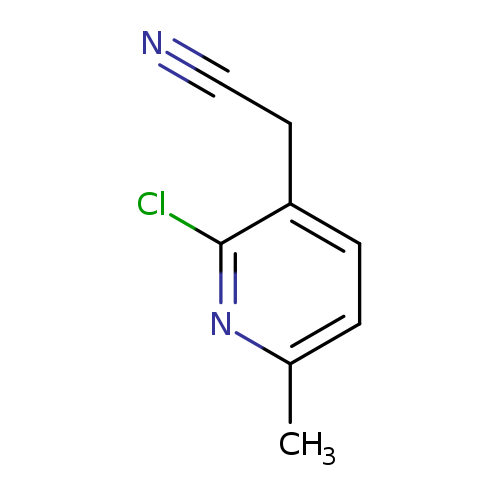
2-(2-Chloro-6-methylpyridin-3-yl)acetonitrileCatalog No.:AA01BRF7 CAS No.:1000514-91-9 MDL No.:MFCD09924116 MF:C8H7ClN2 MW:166.6076 |
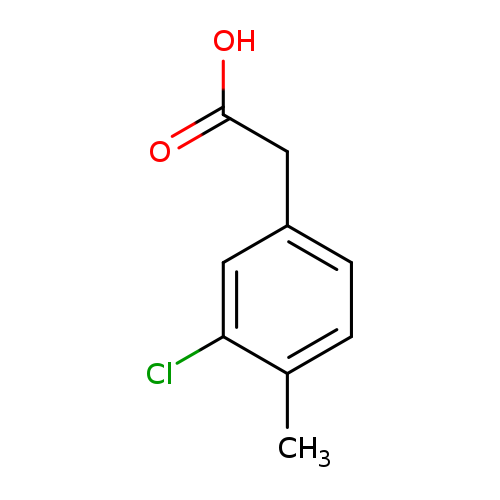
2-(3-chloro-4-methylphenyl)acetic acidCatalog No.:AA01C78Z CAS No.:1000515-63-8 MDL No.:MFCD09924904 MF:C9H9ClO2 MW:184.6196 |
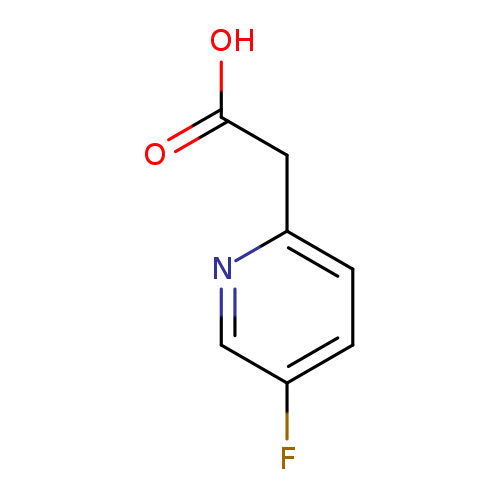
2-(5-Fluoropyridin-2-yl)acetic acidCatalog No.:AA0000MD CAS No.:1000515-83-2 MDL No.:MFCD09924935 MF:C7H6FNO2 MW:155.1264 |
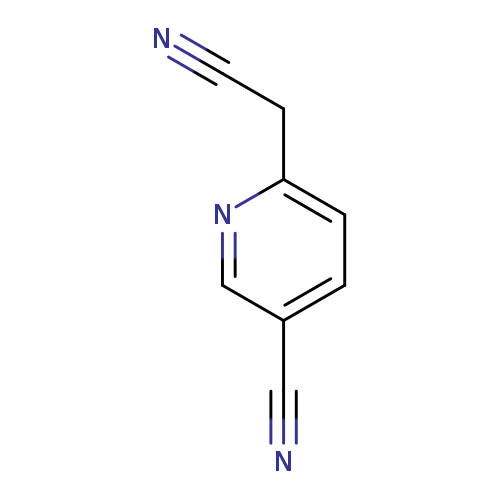
6-(Cyanomethyl)nicotinonitrileCatalog No.:AA00ISA8 CAS No.:1000516-33-5 MDL No.:MFCD09924285 MF:C8H5N3 MW:143.1454 |
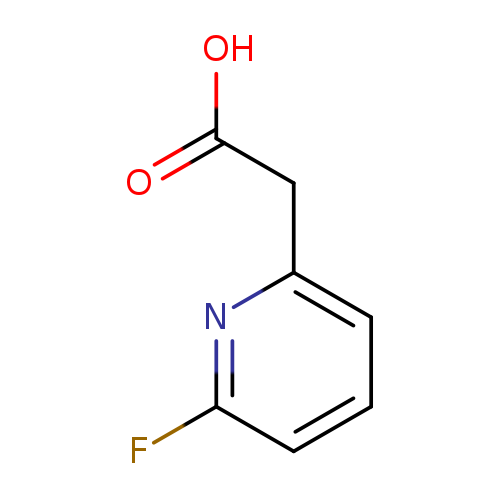
2-(6-Fluoropyridin-2-yl)acetic acidCatalog No.:AA0000MA CAS No.:1000517-25-8 MDL No.:MFCD09925118 MF:C7H6FNO2 MW:155.1264 |
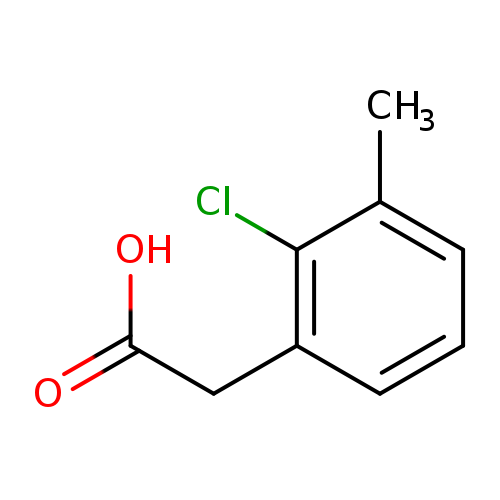
2-(2-chloro-3-methylphenyl)acetic acidCatalog No.:AA01BDNZ CAS No.:1000518-49-9 MDL No.:MFCD09925311 MF:C9H9ClO2 MW:184.6196 |
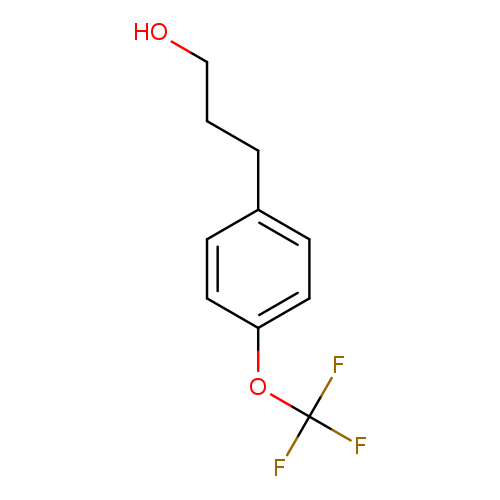
3-(4-(Trifluoromethoxy)phenyl)propan-1-olCatalog No.:AA0000M7 CAS No.:1000519-40-3 MDL No.:MFCD09927393 MF:C10H11F3O2 MW:220.1883 |
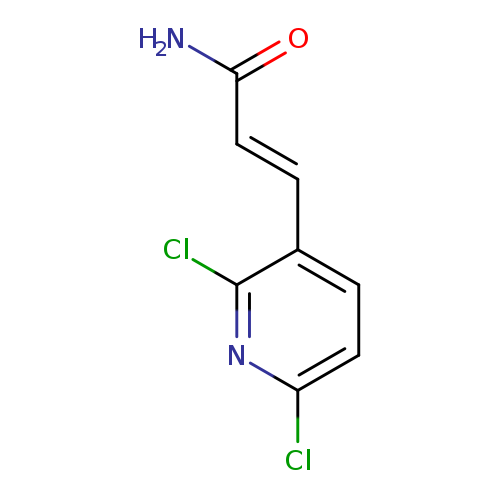
(2E)-3-(2,6-Dichloropyridin-3-yl)prop-2-enamideCatalog No.:AA01EH9U CAS No.:1000519-96-9 MDL No.:MFCD09911658 MF:C8H6Cl2N2O MW:217.0520 |
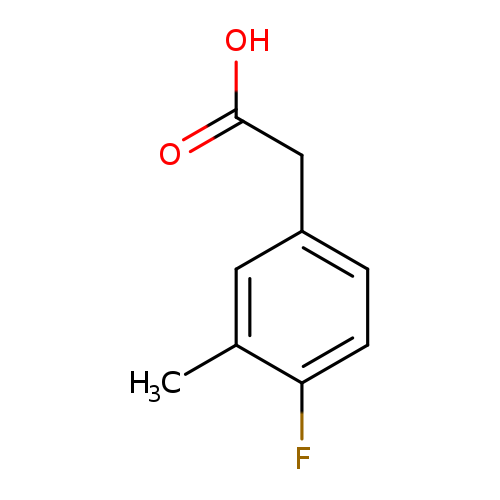
4-Fluoro-3-methylphenylacetic acidCatalog No.:AA0000M6 CAS No.:1000520-92-2 MDL No.:MFCD09832241 MF:C9H9FO2 MW:168.1650 |
3-(3-methylpyridin-4-yl)propan-1-amineCatalog No.:AA01DX26 CAS No.:1000521-04-9 MDL No.:MFCD09913295 MF:C9H14N2 MW:150.2209 |
3-(6-methoxypyridin-3-yl)propan-1-amineCatalog No.:AA01EI4W CAS No.:1000521-37-8 MDL No.:MFCD09913331 MF:C9H14N2O MW:166.2203 |
2-Pyridineethanol,5-fluoro-Catalog No.:AA00IL1A CAS No.:1000521-75-4 MDL No.:MFCD09926281 MF:C7H8FNO MW:141.1429 |
2-(3-Chloro-5-(trifluoromethyl)pyridin-2-yl)acetic acidCatalog No.:AA0000M3 CAS No.:1000522-34-8 MDL No.:MFCD09925056 MF:C8H5ClF3NO2 MW:239.5790 |
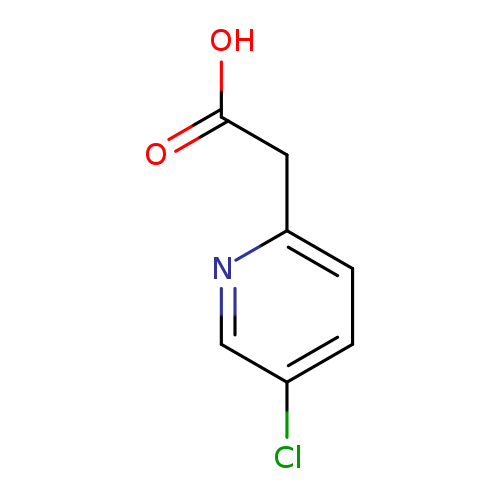
2-(5-Chloropyridin-2-yl)acetic acidCatalog No.:AA0000M2 CAS No.:1000522-43-9 MDL No.:MFCD09925072 MF:C7H6ClNO2 MW:171.5810 |
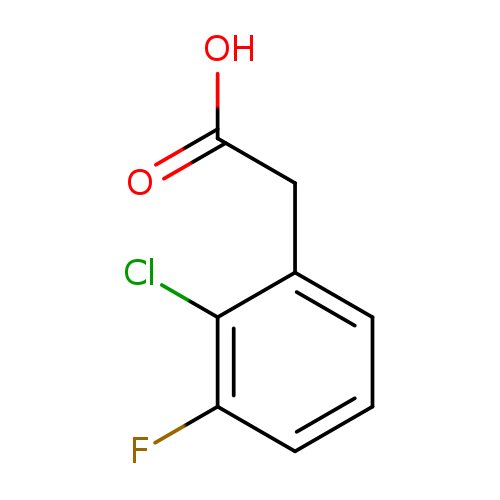
2-(2-Chloro-3-fluorophenyl)acetic acidCatalog No.:AA0000M1 CAS No.:1000523-07-8 MDL No.:MFCD09925136 MF:C8H6ClFO2 MW:188.5834 |
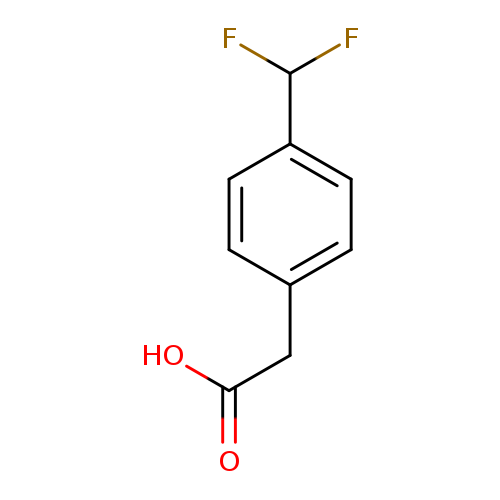
2-[4-(difluoromethyl)phenyl]acetic acidCatalog No.:AA01A224 CAS No.:1000524-74-2 MDL No.:MFCD09925293 MF:C9H8F2O2 MW:186.1554 |
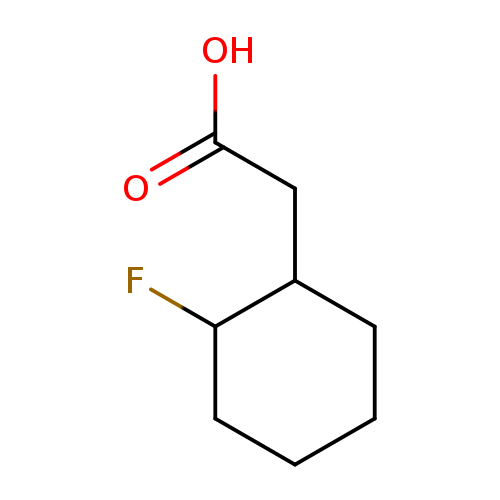
2-(2-fluorocyclohexyl)acetic acidCatalog No.:AA01B8W1 CAS No.:1000525-30-3 MDL No.:MFCD09925345 MF:C8H13FO2 MW:160.1860 |
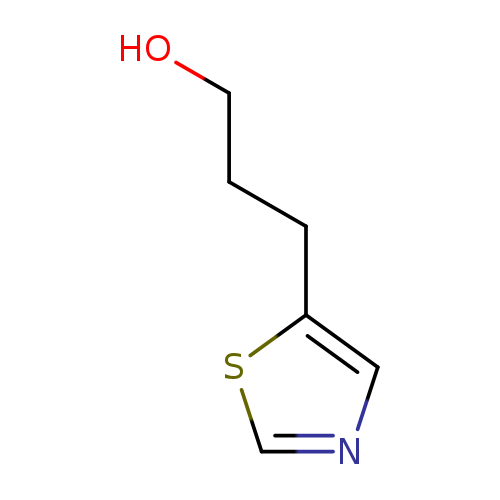
3-(1,3-thiazol-5-yl)propan-1-olCatalog No.:AA01C5I5 CAS No.:1000527-93-4 MDL No.:MFCD09927118 MF:C6H9NOS MW:143.2068 |
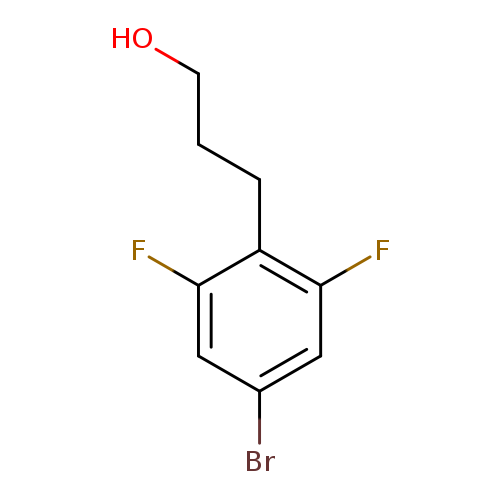
3-(4-bromo-2,6-difluorophenyl)propan-1-olCatalog No.:AA01FRLM CAS No.:1000528-24-4 MDL No.:MFCD09927147 MF:C9H9BrF2O MW:251.0680 |
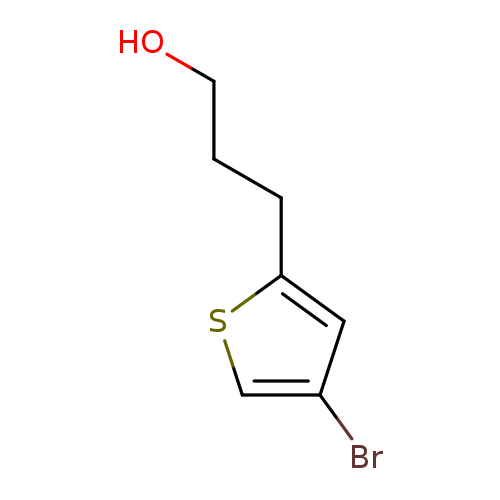
3-(4-Bromothiophen-2-yl)propan-1-olCatalog No.:AA01BBN9 CAS No.:1000529-80-5 MDL No.:MFCD09927273 MF:C7H9BrOS MW:221.1148 |
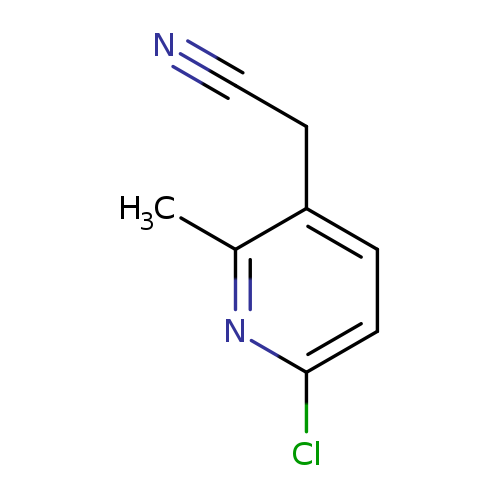
2-(6-chloro-2-methylpyridin-3-yl)acetonitrileCatalog No.:AA01A1ZG CAS No.:1000529-85-0 MDL No.:MFCD09924248 MF:C8H7ClN2 MW:166.6076 |
2-(3-bromothiophen-2-yl)ethan-1-amineCatalog No.:AA01BTPV CAS No.:1000532-83-1 MDL No.:MFCD08448951 MF:C6H8BrNS MW:206.1034 |
tert-Butyl 6-(aminomethyl)pyridine-3-carboxylateCatalog No.:AA01DQHD CAS No.:1000535-97-6 MDL No.:MFCD09927475 MF:C11H16N2O2 MW:208.2569 |
2-(4-(Trifluoromethyl)pyridin-2-yl)acetonitrileCatalog No.:AA0000MW CAS No.:1000536-10-6 MDL No.:MFCD09923755 MF:C8H5F3N2 MW:186.1339 |
2-(3-tert-butylphenyl)ethan-1-amineCatalog No.:AA01A5KE CAS No.:1000538-58-8 MDL No.:MFCD09924666 MF:C12H19N MW:177.2860 |
Benzenamine, N-(1,2-dimethylpropyl)-Catalog No.:AA0000N9 CAS No.:100054-14-6 MDL No.:MFCD11138705 MF:C11H17N MW:163.2594 |
N-(2,3-Dimethoxybenzyl)ethanamineCatalog No.:AA0000N5 CAS No.:100054-84-0 MDL No.:MFCD07405845 MF:C11H17NO2 MW:195.2582 |
1H-Indazole-5-acetonitrileCatalog No.:AA0091F0 CAS No.:1000543-14-5 MDL No.:MFCD09924142 MF:C9H7N3 MW:157.1720 |
2-(4-Methyl-3-(trifluoromethyl)phenyl)acetic acidCatalog No.:AA0000MR CAS No.:1000544-72-8 MDL No.:MFCD09832293 MF:C10H9F3O2 MW:218.1725 |
2-(Isoquinolin-6-yl)acetic acidCatalog No.:AA0000MP CAS No.:1000545-64-1 MDL No.:MFCD09925161 MF:C11H9NO2 MW:187.1947 |
2-[2-(3-Bromophenyl)ethyl]-2,3-dihydro-1h-isoindole-1,3-dioneCatalog No.:AA00IOED CAS No.:1000546-99-5 MDL No.:MFCD09923439 MF:C16H12BrNO2 MW:330.1760 |
2-(3-(Difluoromethyl)phenyl)acetic acidCatalog No.:AA0000MN CAS No.:1000547-15-8 MDL No.:MFCD09925304 MF:C9H8F2O2 MW:186.1554 |
4-Fluoro-3-methylphenylacetonitrileCatalog No.:AA009OS1 CAS No.:1000548-41-3 MDL No.:MFCD09832240 MF:C9H8FN MW:149.1649 |
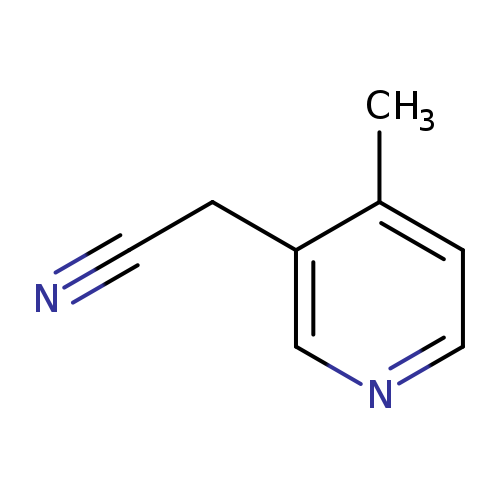
2-(4-methylpyridin-3-yl)acetonitrileCatalog No.:AA01ACKN CAS No.:1000548-83-3 MDL No.:MFCD09923740 MF:C8H8N2 MW:132.1625 |
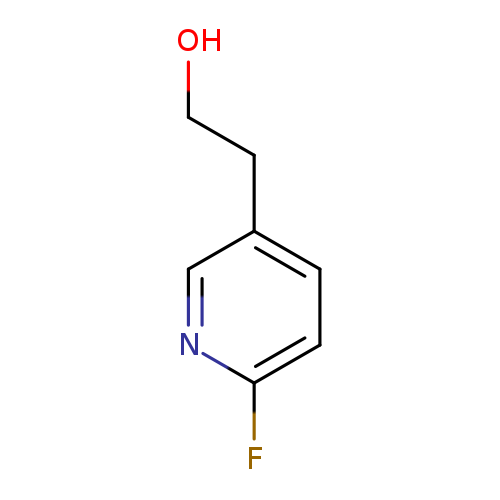
2-(6-Fluoropyridin-3-yl)ethan-1-olCatalog No.:AA01DUTB CAS No.:1000549-35-8 MDL No.:MFCD09926311 MF:C7H8FNO MW:141.1429 |
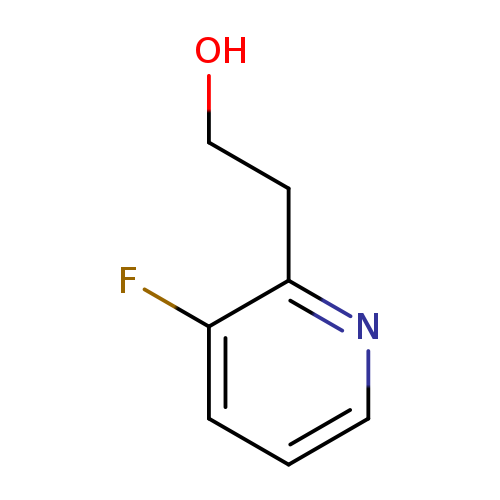
2-(3-Fluoropyridin-2-yl)ethan-1-olCatalog No.:AA0000NR CAS No.:1000549-37-0 MDL No.:MFCD09926580 MF:C7H8FNO MW:141.1429 |
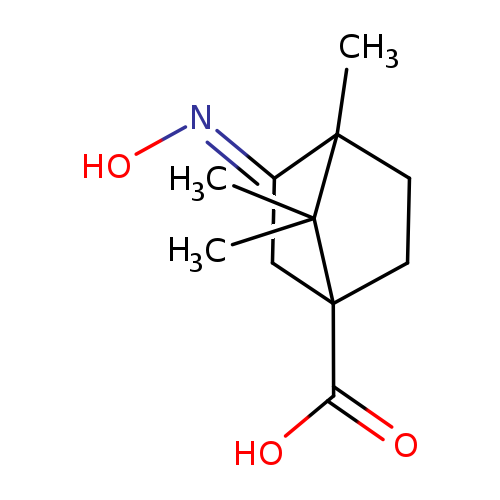
(3E)-3-(HYDROXYIMINO)-4,7,7-TRIMETHYLBICYCLO[2.2.1]HEPTANE-1-CARBOXYLIC ACIDCatalog No.:AA008X8P CAS No.:100055-50-3 MDL No.:MFCD03659904 MF:C11H17NO3 MW:211.2576 |
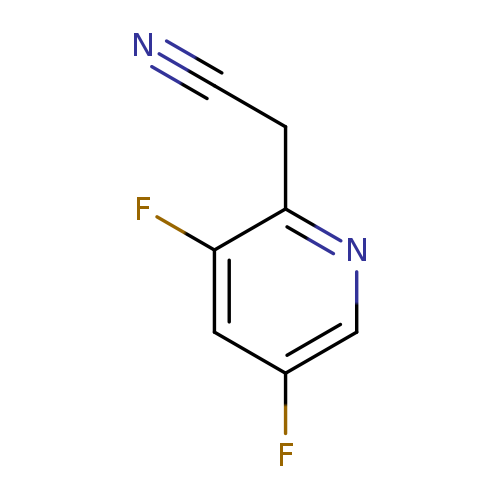
2-(3,5-difluoropyridin-2-yl)acetonitrileCatalog No.:AA01AKSI CAS No.:1000557-40-3 MDL No.:MFCD09924171 MF:C7H4F2N2 MW:154.1169 |
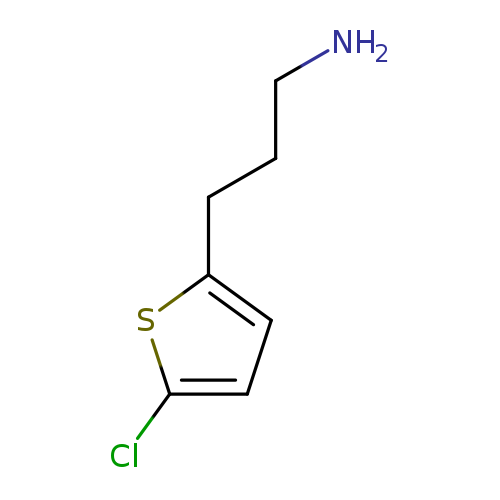
3-(5-chlorothiophen-2-yl)propan-1-amineCatalog No.:AA01B14R CAS No.:1000564-79-3 MDL No.:MFCD09913007 MF:C7H10ClNS MW:175.6790 |
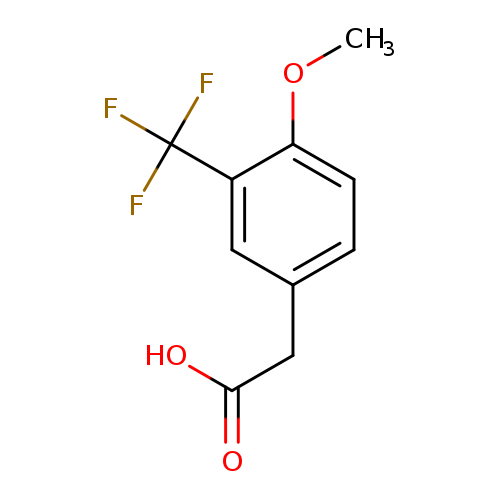
4-Methoxy-3-(trifluoromethyl)phenylacetic acidCatalog No.:AA0000ND CAS No.:1000566-45-9 MDL No.:MFCD09832302 MF:C10H9F3O3 MW:234.1719 |
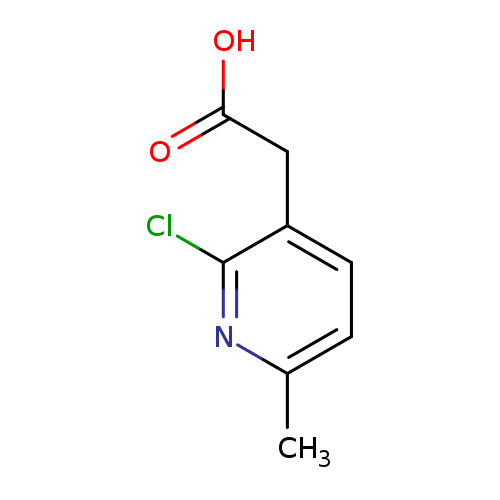
2-(2-chloro-6-methylpyridin-3-yl)acetic acidCatalog No.:AA01BB7G CAS No.:1000567-19-0 MDL No.:MFCD09925188 MF:C8H8ClNO2 MW:185.6076 |
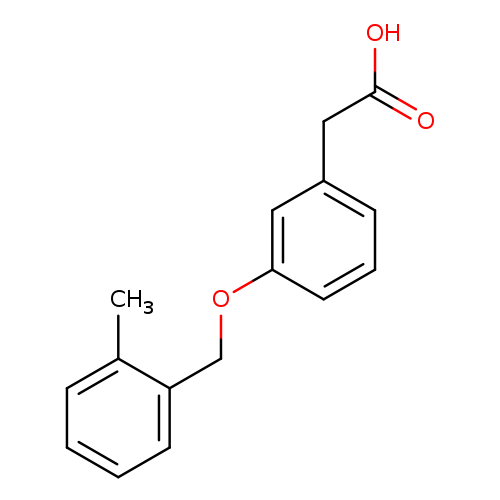
2-(3-[(2-Methylphenyl)methoxy]phenyl)acetic acidCatalog No.:AA01AB2P CAS No.:1000567-84-9 MDL No.:MFCD09925286 MF:C16H16O3 MW:256.2964 |
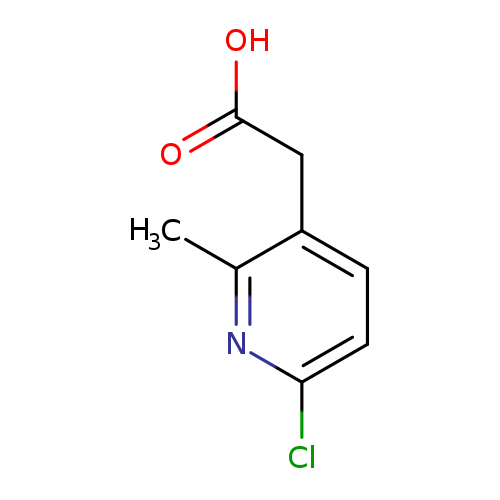
2-(6-chloro-2-methylpyridin-3-yl)acetic acidCatalog No.:AA01A1ME CAS No.:1000568-04-6 MDL No.:MFCD09925308 MF:C8H8ClNO2 MW:185.6076 |
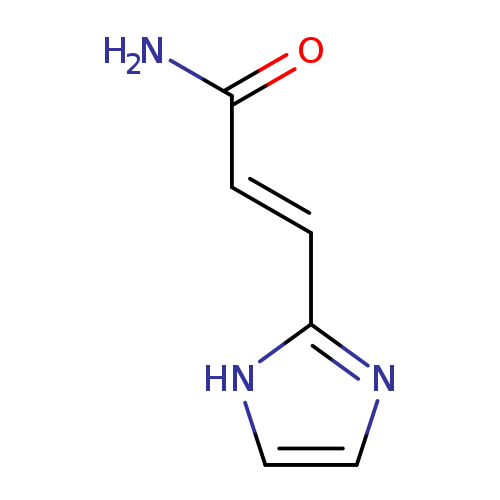
(2E)-3-(1H-imidazol-2-yl)prop-2-enamideCatalog No.:AA01EM2A CAS No.:1000568-69-3 MDL No.:MFCD09910639 MF:C6H7N3O MW:137.1393 |
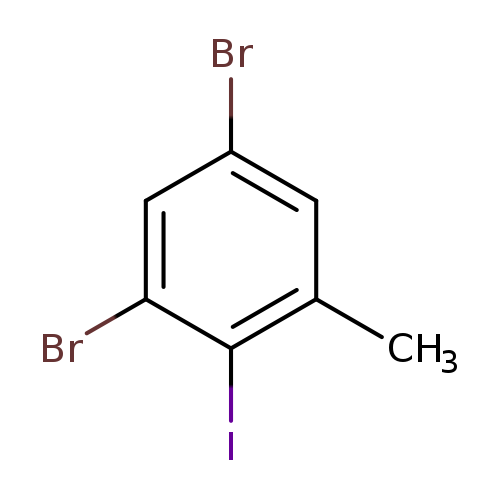
3,5-Dibromo-2-iodotolueneCatalog No.:AA01FFEO CAS No.:1000571-43-6 MDL No.:MFCD09800828 MF:C7H5Br2I MW:375.8271 |

2-Amino-5-bromo-3-(trifluoromethyl)benzonitrileCatalog No.:AA01F9M7 CAS No.:1000571-53-8 MDL No.:MFCD09800831 MF:C8H4BrF3N2 MW:265.0300 |
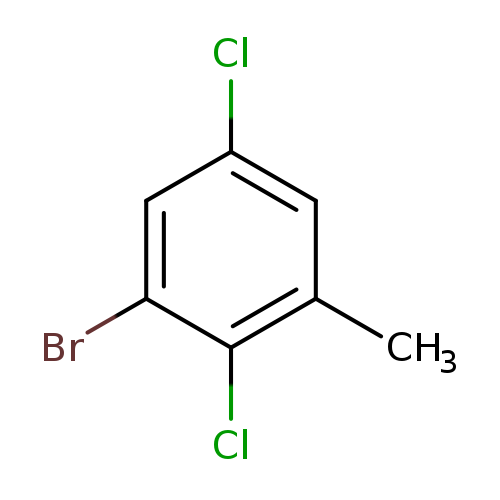
3-Bromo-2,5-dichlorotolueneCatalog No.:AA01FF9H CAS No.:1000571-63-0 MDL No.:MFCD09800834 MF:C7H5BrCl2 MW:239.9246 |
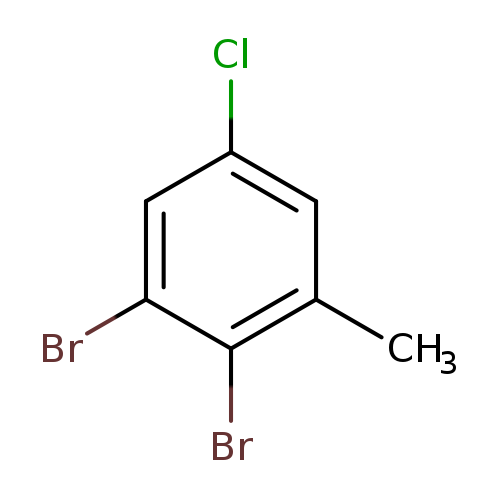
5-Chloro-2,3-dibromotolueneCatalog No.:AA01EQFW CAS No.:1000571-68-5 MDL No.:MFCD09800835 MF:C7H5Br2Cl MW:284.3756 |
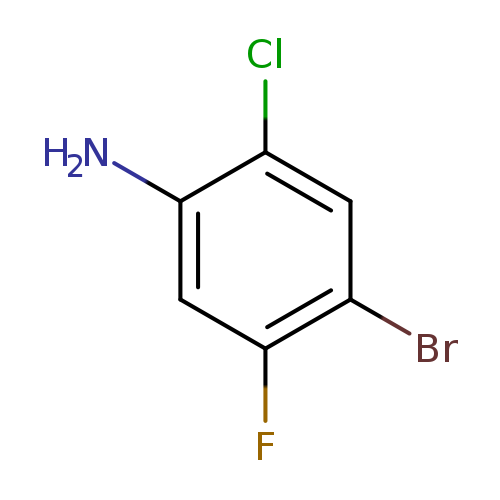
4-Bromo-2-chloro-5-fluoroanilineCatalog No.:AA008XKL CAS No.:1000572-63-3 MDL No.:MFCD09878155 MF:C6H4BrClFN MW:224.4581 |
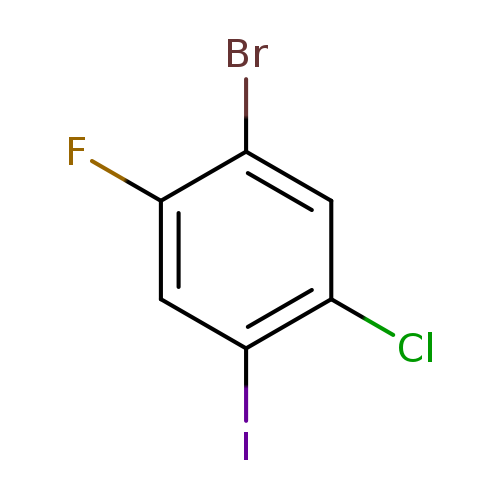
1-Bromo-5-chloro-2-fluoro-4-iodobenzeneCatalog No.:AA01FD7G CAS No.:1000572-73-5 MDL No.:MFCD09878157 MF:C6H2BrClFI MW:335.3400 |
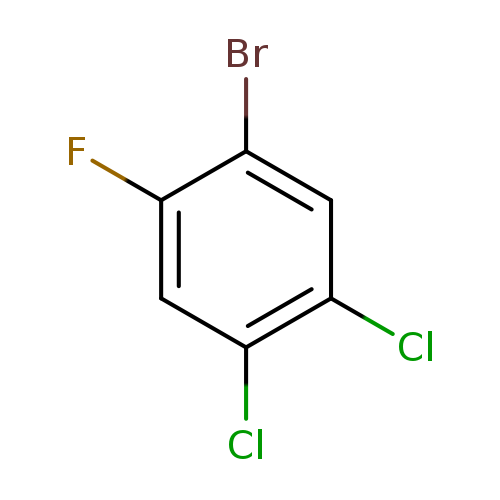
1-Bromo-4,5-dichloro-2-fluorobenzeneCatalog No.:AA0000OK CAS No.:1000572-78-0 MDL No.:MFCD09878158 MF:C6H2BrCl2F MW:243.8885 |
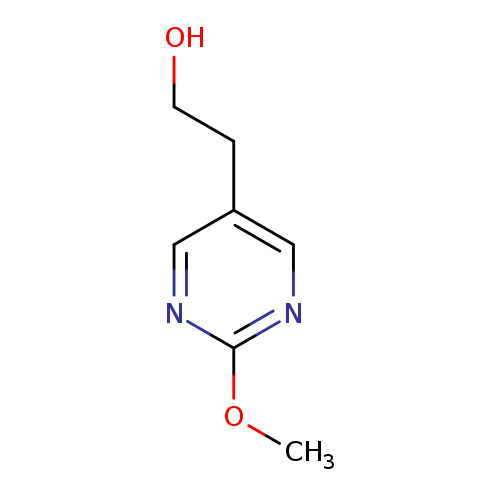
2-(2-methoxypyrimidin-5-yl)ethan-1-olCatalog No.:AA01BRQG CAS No.:1000572-80-4 MDL No.:MFCD09926333 MF:C7H10N2O2 MW:154.1665 |
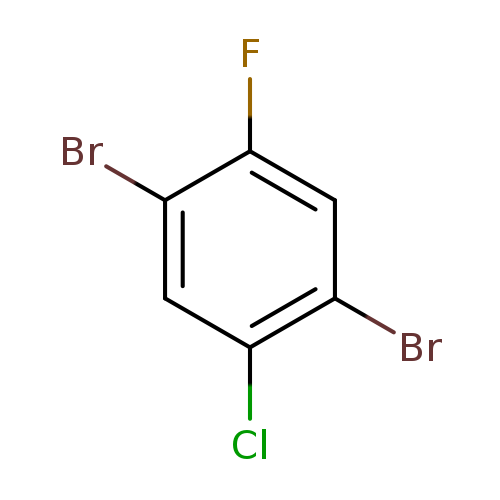
1,4-Dibromo-2-chloro-5-fluorobenzeneCatalog No.:AA008XK5 CAS No.:1000572-83-7 MDL No.:MFCD09878159 MF:C6H2Br2ClF MW:288.3395 |
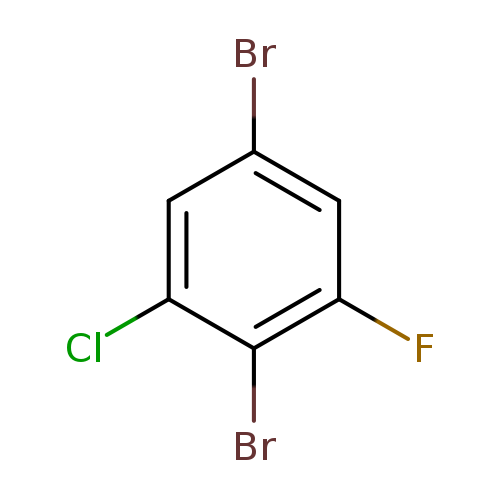
2,5-dibromo-1-chloro-3-fluorobenzeneCatalog No.:AA0095CK CAS No.:1000572-88-2 MDL No.:MFCD09878162 MF:C6H2Br2ClF MW:288.3395 |
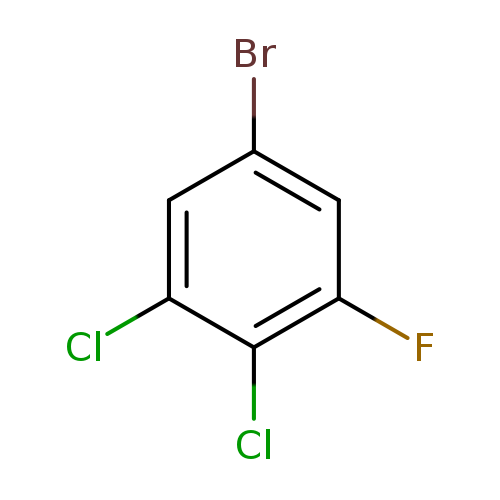
1-Bromo-3,4-dichloro-5-fluorobenzeneCatalog No.:AA0000OJ CAS No.:1000572-93-9 MDL No.:MFCD09878164 MF:C6H2BrCl2F MW:243.8885 |
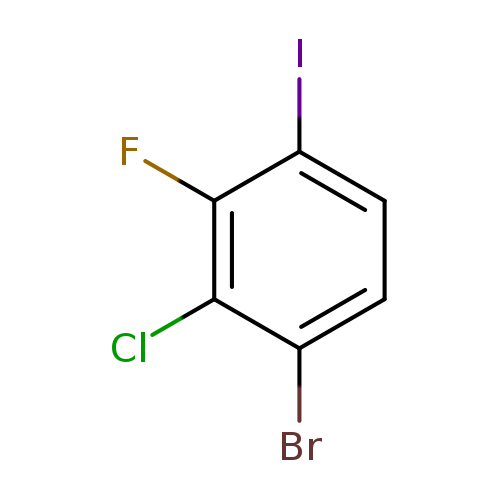
1-Bromo-2-chloro-3-fluoro-4-iodobenzeneCatalog No.:AA0000OI CAS No.:1000573-03-4 MDL No.:MFCD09878167 MF:C6H2BrClFI MW:335.3400 |
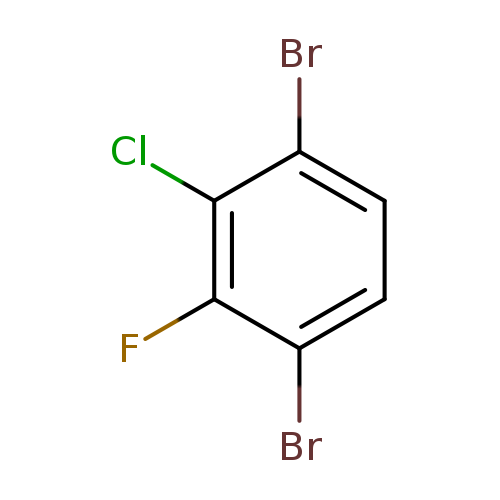
1-Chloro-3,6-dibromo-2-fluorobenzeneCatalog No.:AA01EQG3 CAS No.:1000573-09-0 MDL No.:MFCD09878168 MF:C6H2Br2ClF MW:288.3395 |
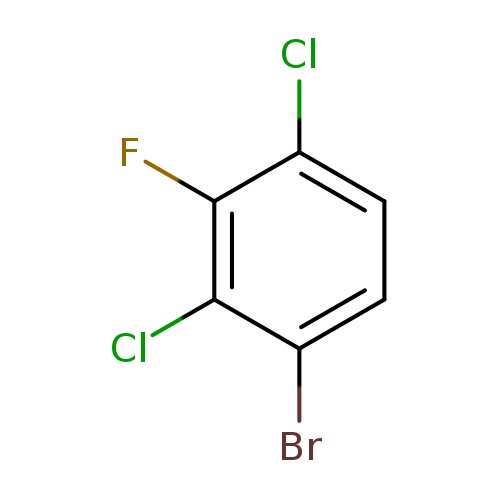
1-Bromo-2,4-dichloro-3-fluorobenzeneCatalog No.:AA0000OH CAS No.:1000573-15-8 MDL No.:MFCD09878169 MF:C6H2BrCl2F MW:243.8885 |
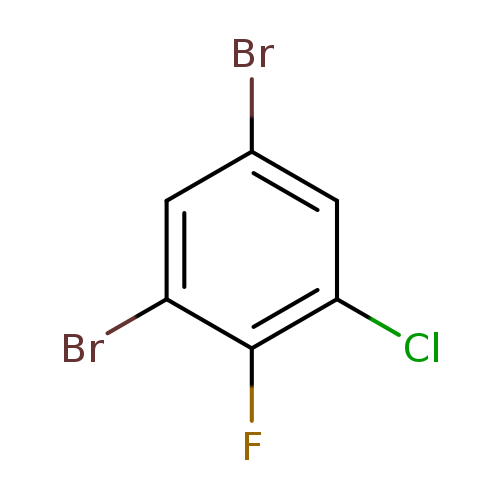
1-Chloro-3,5-dibromo-2-fluorobenzeneCatalog No.:AA00H926 CAS No.:1000573-27-2 MDL No.:MFCD09878171 MF:C6H2Br2ClF MW:288.3395 |
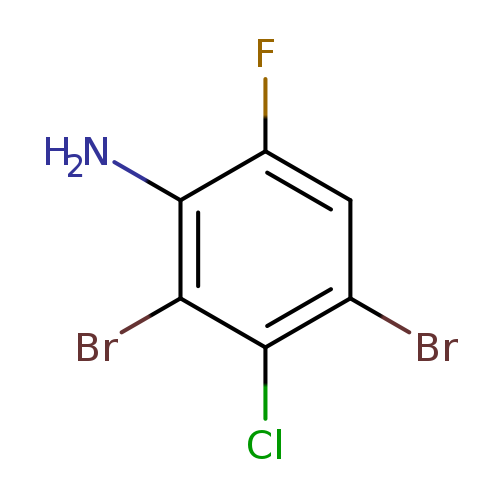
2,4-Dibromo-3-chloro-6-fluoroanilineCatalog No.:AA01F4HK CAS No.:1000573-39-6 MDL No.:MFCD09878174 MF:C6H3Br2ClFN MW:303.3541 |
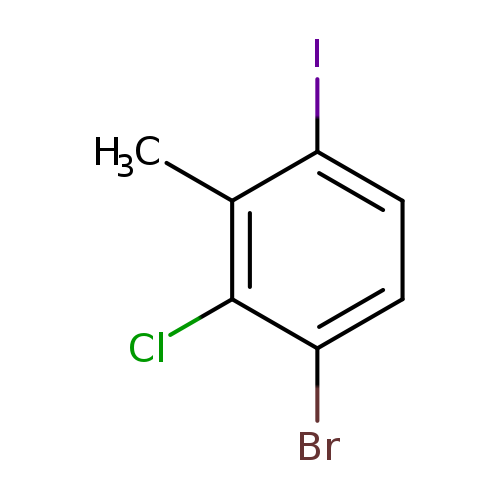
1-Bromo-2-chloro-4-iodo-3-methylbenzeneCatalog No.:AA0000OG CAS No.:1000573-57-8 MDL No.:MFCD09878177 MF:C7H5BrClI MW:331.3761 |
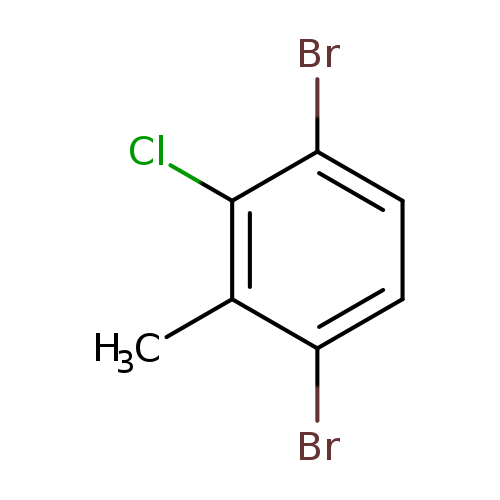
3,6-Dibromo-2-chlorotolueneCatalog No.:AA01EQG9 CAS No.:1000573-62-5 MDL No.:MFCD09878178 MF:C7H5Br2Cl MW:284.3756 |
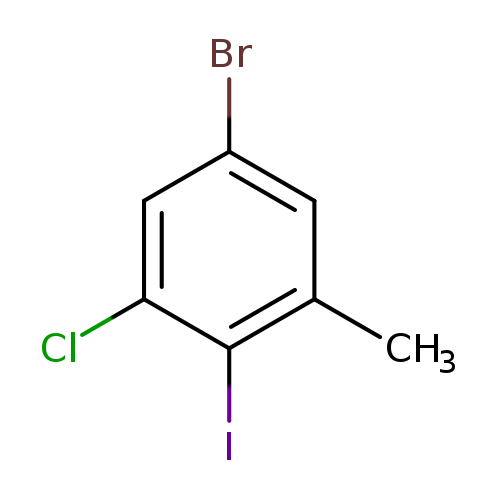
5-Bromo-1-chloro-2-iodo-3-methylbenzeneCatalog No.:AA0000OF CAS No.:1000573-87-4 MDL No.:MFCD09878185 MF:C7H5BrClI MW:331.3761 |

4-Bromo-3-chloro-2-nitroanilineCatalog No.:AA0000OE CAS No.:1000573-99-8 MDL No.:MFCD09878187 MF:C6H4BrClN2O2 MW:251.4652 |
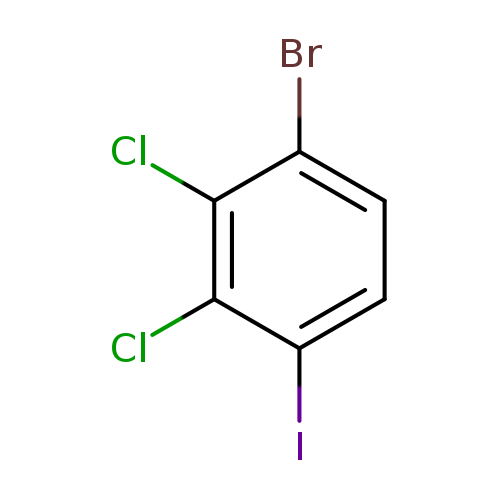
1-Bromo-2,3-dichloro-4-iodobenzeneCatalog No.:AA0093OA CAS No.:1000574-05-9 MDL No.:MFCD09878189 MF:C6H2BrCl2I MW:351.7946 |
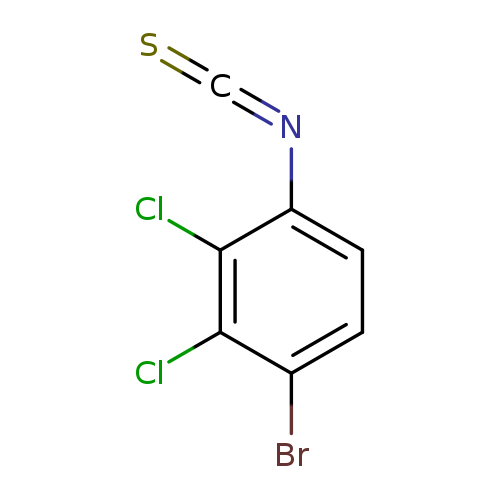
4-Bromo-2,3-dichlorophenyl isothiocyanateCatalog No.:AA01FBCY CAS No.:1000574-11-7 MDL No.:MFCD09878190 MF:C7H2BrCl2NS MW:282.9725 |
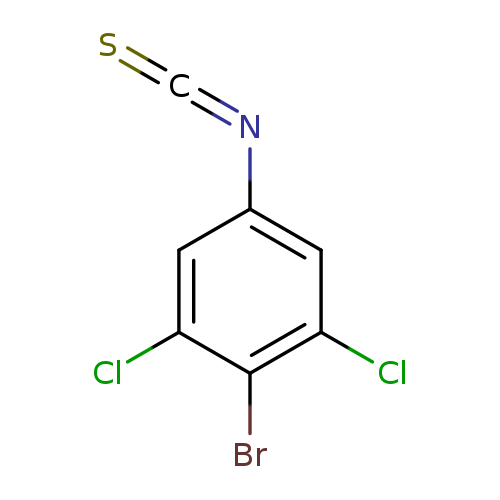
4-Bromo-3,5-dichlorophenylisothiocyanateCatalog No.:AA01F3JT CAS No.:1000574-23-1 MDL No.:MFCD09878194 MF:C7H2BrCl2NS MW:282.9725 |
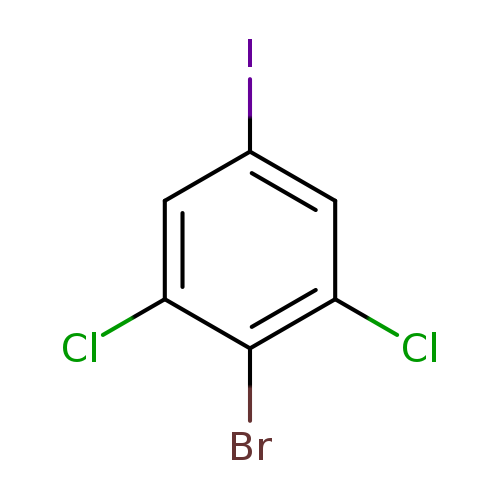
4-Bromo-3,5-dichloroiodobenzeneCatalog No.:AA008XKM CAS No.:1000574-29-7 MDL No.:MFCD09878196 MF:C6H2BrCl2I MW:351.7945 |
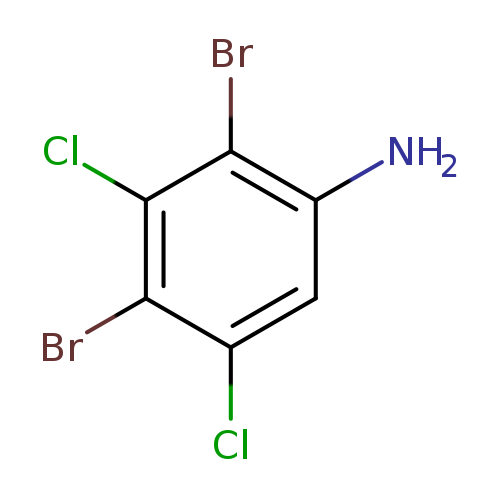
2,4-Dibromo-3,5-dichloroanilineCatalog No.:AA01FEPB CAS No.:1000574-35-5 MDL No.:MFCD09878197 MF:C6H3Br2Cl2N MW:319.8087 |
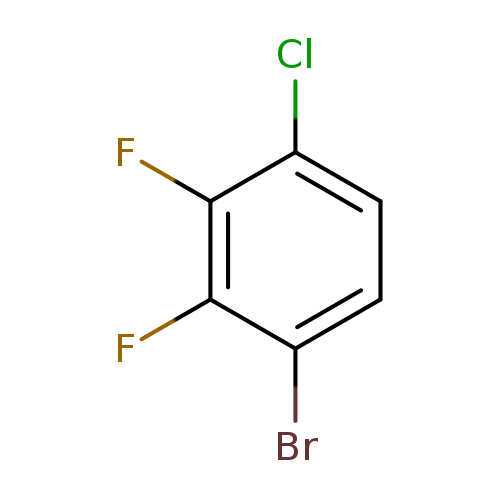
4-Chloro-2,3-difluorobromobenzeneCatalog No.:AA00H928 CAS No.:1000574-47-9 MDL No.:MFCD09878201 MF:C6H2BrClF2 MW:227.4339 |
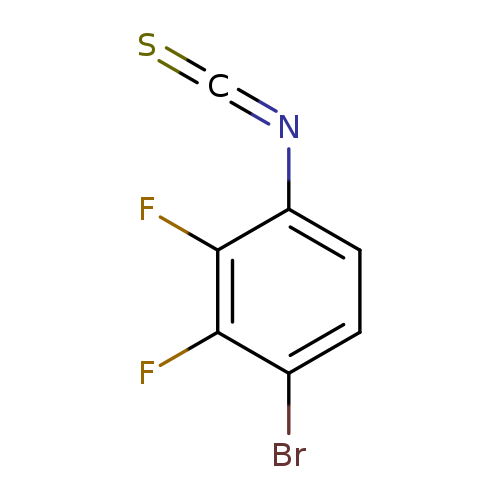
4-Bromo-2,3-difluorophenyl isothiocyanateCatalog No.:AA01FBCZ CAS No.:1000574-53-7 MDL No.:MFCD09878202 MF:C7H2BrF2NS MW:250.0633 |
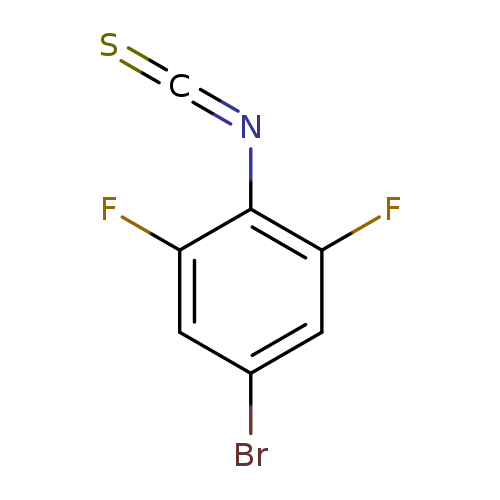
4-Bromo-2,6-difluorophenyl isothiocyanateCatalog No.:AA01FBD0 CAS No.:1000574-64-0 MDL No.:MFCD00123917 MF:C7H2BrF2NS MW:250.0633 |
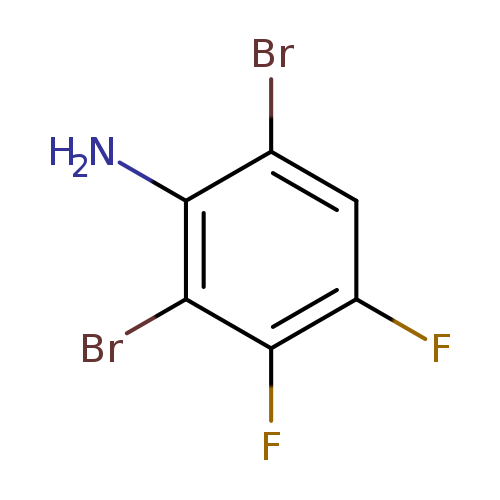
2,6-Dibromo-3,4-difluoroanilineCatalog No.:AA01FEPD CAS No.:1000574-69-5 MDL No.:MFCD09878204 MF:C6H3Br2F2N MW:286.8995 |
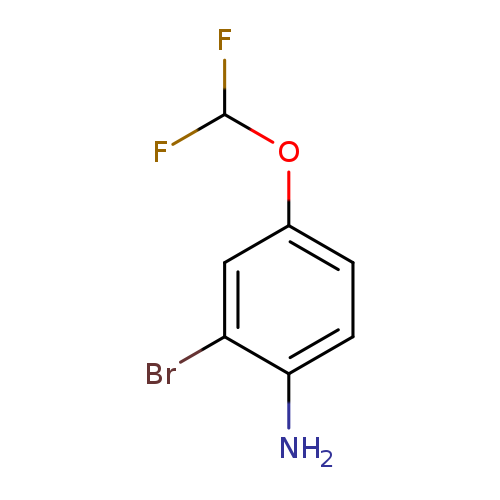
2-Bromo-4-(difluoromethoxy)anilineCatalog No.:AA01F3YH CAS No.:1000574-79-7 MDL No.:MFCD09878206 MF:C7H6BrF2NO MW:238.0294 |
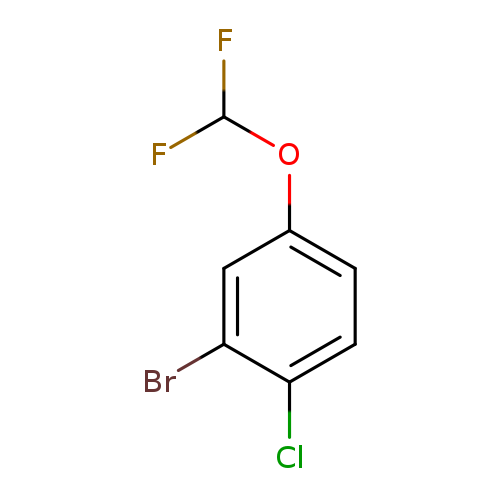
2-Bromo-1-chloro-4-(difluoromethoxy)benzeneCatalog No.:AA008XK8 CAS No.:1000574-90-2 MDL No.:MFCD09878208 MF:C7H4BrClF2O MW:257.4599 |
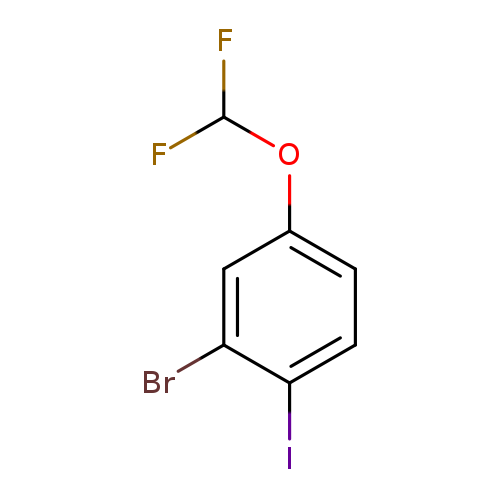
2-Bromo-4-(difluoromethoxy)-1-iodobenzeneCatalog No.:AA01FD7H CAS No.:1000575-02-9 MDL No.:MFCD09878210 MF:C7H4BrF2IO MW:348.9113 |
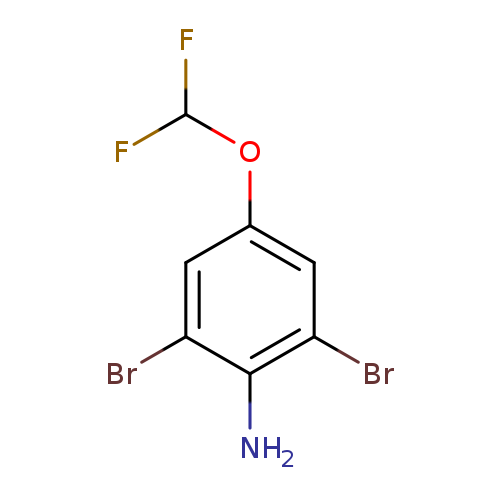
2,6-Dibromo-4-(difluoromethoxy)anilineCatalog No.:AA01FCF3 CAS No.:1000575-08-5 MDL No.:MFCD12547870 MF:C7H5Br2F2NO MW:316.9255 |
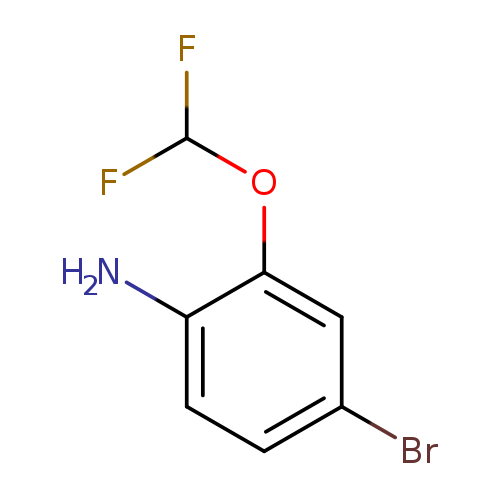
4-Bromo-2-(difluoromethoxy)anilineCatalog No.:AA01CA3G CAS No.:1000575-14-3 MDL No.:MFCD09878213 MF:C7H6BrF2NO MW:238.0294 |
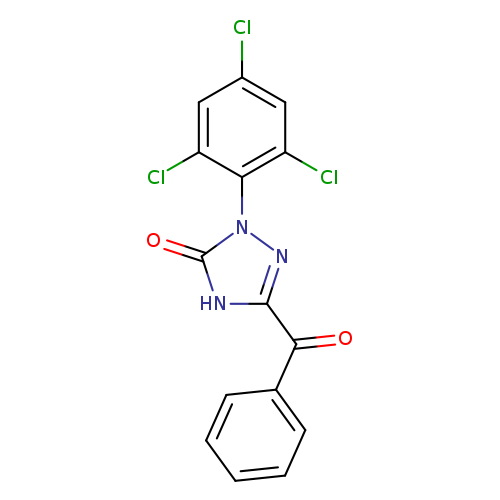
5-Benzoyl-2-(2,4,6-trichlorophenyl)-1,2-dihydro-3H-1,2,4-triazol-3-oneCatalog No.:AA01F4TV CAS No.:1000575-15-4 MDL No.:MFCD09954987 MF:C15H8Cl3N3O2 MW:368.6019 |
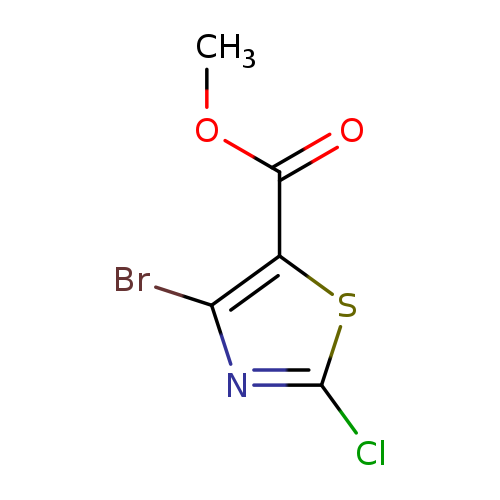
Methyl 4-Bromo-2-chlorothiazole-5-carboxylateCatalog No.:AA01FA4I CAS No.:1000575-16-5 MDL No.:MFCD09878034 MF:C5H3BrClNO2S MW:256.5048 |
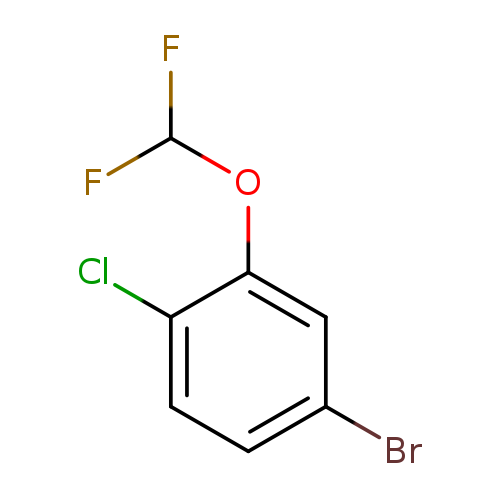
4-Bromo-1-chloro-2-(difluoromethoxy)benzeneCatalog No.:AA01DLKF CAS No.:1000575-20-1 MDL No.:MFCD09878214 MF:C7H4BrClF2O MW:257.4599 |
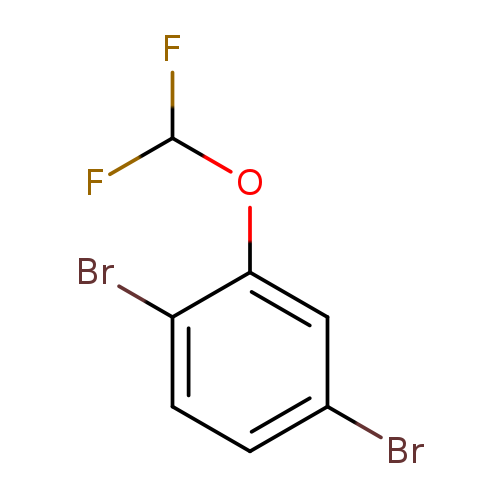
1,4-Dibromo-2-(difluoromethoxy)benzeneCatalog No.:AA01CAA9 CAS No.:1000575-26-7 MDL No.:MFCD09878215 MF:C7H4Br2F2O MW:301.9109 |
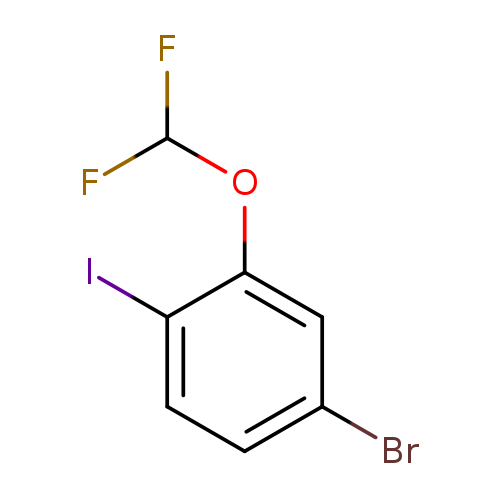
4-Bromo-2-(difluoromethoxy)-1-iodobenzeneCatalog No.:AA01FCF4 CAS No.:1000575-32-5 MDL No.:MFCD09878216 MF:C7H4BrF2IO MW:348.9113 |
4-Bromo-2-(difluoromethoxy)phenylisothiocyanateCatalog No.:AA01F2QJ CAS No.:1000575-37-0 MDL No.:MFCD09878217 MF:C8H4BrF2NOS MW:280.0893 |
4-Fluoro-3-methoxyphenylisothiocyanateCatalog No.:AA01F3YK CAS No.:1000575-99-4 MDL No.:MFCD09800755 MF:C8H6FNOS MW:183.2027 |