2019-11-15 15:18:18
Hong-Gang Cheng, Shuqing Chen, Ruiming Chen, and Qianghui Zhou
1. Introduction
The selective, modular, and efficient assembly of molecular complexity represents one of the most challenging yet fascinating directions in modern synthetic organic chemistry.[1] Therein, the transition-metal-catalyzed C@H functionalization of arenes is among the most attractive strategies.[2] As it can be used to construct carbon–carbon or carbonheteroatom bonds in a direct and selective manner, various game-changing disconnection strategies for organic synthesis have thus been provided. Over decades, this field has attracted much attention from both academic and industrial laboratories.
In this context, Pd/norbornene (NBE) cooperative catalysis, namely the Catellani reaction, is one of the most promising approaches. It utilizes the synergistic interplay of palladium and NBE catalysis to facilitate sequential ortho C@H functionalization and ipso termination of aryl halides. Since the pioneering work by the groups of Catellani[3] and Lautens,[4] this chemistry has attracted considerable attention from the organic synthesis community. Over more than twenty years of development, it has become a powerful strategy for the expeditious synthesis of highly substituted arenes, which are difficult to access through traditional cross-coupling reactions.
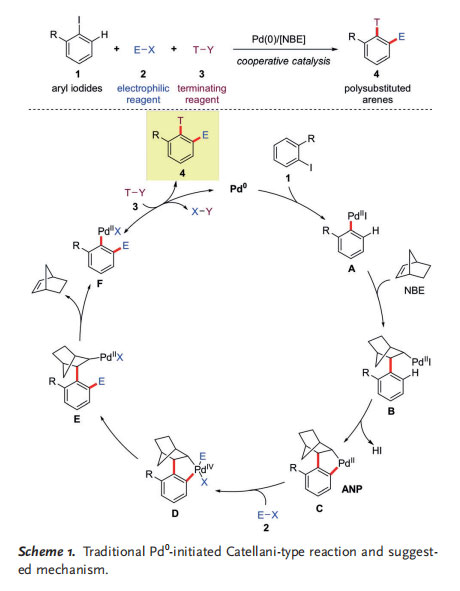
As shown in Scheme 1, in traditional Catellani-type reactions, aryl halides (mainly aryl iodides) are used as the substrates, and a Pd 0 catalyst is required to generate the arylpalladium(II) species A, which can undergo carbopalladation with NBE to form intermediate B. A subsequent ortho C@H activation leads to the formation of the key aryl norbornylpalladacycle C (ANP). Then, oxidative addition of an electrophile 2 (E-X) to C generates PdIV species D, which delivers norbornylpalladium(II) species E upon reductive elimination. If the R group is a hydrogen atom, a second ortho C@H activation will occur, following the same procedure.
Otherwise, because of increased steric interactions between the palladium center and the two ortho substituents, in addition to the lack of a b-hydrogen atom syn to palladium in intermediate E, a retro-carbopalladation to regenerate NBE will take place to provide arylpalladium(II) species F, which undergoes a traditional cross-coupling reaction with the terminating reagent 3 (T-Y) to afford the polysubstituted arene 4 and regenerate the Pd0 species catalyst.[6]
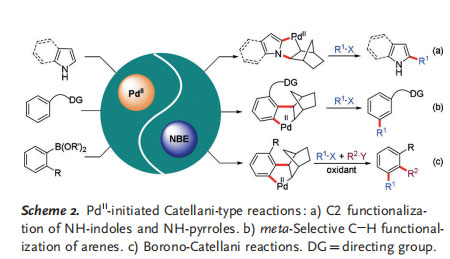
According to the above proposed mechanism, it can be surmised that this kind of reaction may also be initiated by a Pd II catalyst to form the common arylpalladium(II) species from suitable starting materials that are different from aryl halides. Indeed, owing to the efforts of Bach, Yu, and others, a suite of elegant PdII-initiated Catellani-type reactions have been developed in the past few years (Scheme 2). Different from the conventional Catellani reaction, this PdII/NBE cooperative catalysis proceeds with completely different substrates and a different reaction mechanism, and thus the reaction conditions are significantly different or even orthogonal. This emerging concept of PdII/NBE cooperative catalysis has tremendously advanced Catellani-type reactions, therefore opening a new avenue for future developments in this field. Although there have been several elegant reviews and accounts published for Pd/NBE cooperative catalysis,[5] one focusing on these emerging PdII-initiated Catellani-type reactions are still lacking yet highly desired. In this context, we have comprehensively summarized the recent advances and breakthroughs in this direction in this Minireview, hoping to inspire future studies and promote new developments in this field. This Minireview was divided into Sections according to the type of substrate interacting with the PdII catalyst, including NH-indoles and NH-pyrroles, arenes with a directing group, as well as aryl boronic acids and their derivatives.

2. C2 Functionalization of NH-Indoles and NHPyrroles
Indoles and pyrroles are two important classes of heterocycles. Although considerable efforts have been made in the direct C@H functionalization of indoles and pyrroles, the regioselective C2 functionalization of these heterocycles remains a challenge. Recently,adirect C2 functionalization (mainly alkylation and arylation) of NH-indoles and NH-pyrroles was realized by PdII/NBE cooperative catalysis. The Bach group initiated this research and made major contributions.[5j]
In this Section, we have summarized the C2 functionalization of NH-indoles and NH-pyrroles by PdII/NBE cooperative catalysis. The contents discussed have been catalogued according to the functionalization reagents employed.
2.1. C2 Alkylation of NH-Indoles
Studies in this direction were initiated by Bach and coworkers in 2011, who developed reactions between NHindoles and primary alkyl halides (bromides or iodides) that selectively generate C2-alkylated indoles under cooperative PdII/NBE catalysis (Scheme 3).[7, 8] By making use of this facile transformation, an array of structurally diverse 2-alkylindoles were synthesized in moderate to excellent yields.
For 3-substituted NH-indoles, a modified procedure was required, involving more reactive alkyl iodide reagents, a more polar solvent, and air atmosphere (Scheme 3). For electron-deficient NH-indoles, a weaker base such as KHCO3 or K2HPO4 was required so as to prevent direct N-alkylation of the indoles. Lastly, it was found that the addition of water dramatically accelerated this process. Mechanistic studies showed that 3-substituted NH-indoles were suitable substrates whereas N-substituted indoles exhibited no reactivity at all, indicating the pivotal role of the free NH moiety (Scheme 4 a). Moreover, through careful reaction design, key complex 12 and trapping product 13 were isolated and characterized (Scheme 4 a). Based on these results, a reaction mechanism was put forward (Scheme 4 b). Initially, the free N@H bond of indole 5 is activated by PdII, which is followed by NBE insertion to form complex 15.

Cyclopalladation of 15 generates the five-membered palladacycle 16. Then, oxidative addition of alkyl bromide to 16 gives the PdIV complex 17, which undergoes reductive elimination to deliver norbornylpalladium(II) intermediate 18. Lastly, NBE expulsion from 18 followed by protolysis of the resulting intermediate 19 affords the final product, 2-alkylindole 7, and regenerates the PdII catalyst.[8]
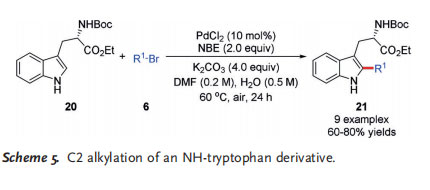
A number of C2-alkylated tryptophan derivatives 21 were prepared in good yields in a similar process from the N-Bocprotected ethyl ester of (S)-tryptophan (20). Significantly, the reaction proceeded without any loss of the enantiomeric purity inherited from the chiral tryptophan substrate(Scheme 5).[9] It should be noted that this transformation requires air to proceed to prevent reduction of PdII to Pd0 , just as for the previously mentioned C3-substituted indole substrates.
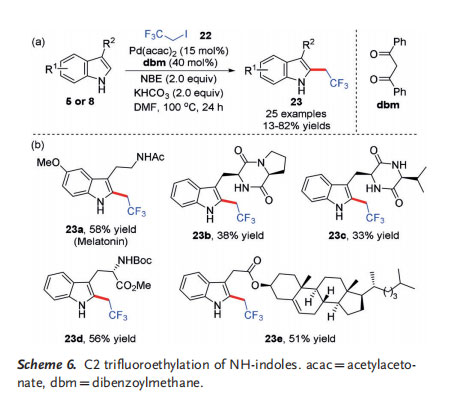
Recently, Liu[10] and co-workers utilized this strategy for the selective C2 trifluoroethylation of indoles with commercially available trifluoroethyl iodide as the alkylating reagent(Scheme 6 a). The reaction displays a wide functional group tolerance, and can even be utilized for the late-stage trifluoroethylation of complex indole derivatives (Scheme 6 b). Preliminary mechanistic studies show that the unique anionic ligand dibenzoylmethane (dbm) plays a critical role in governing the efficiency of this transformation by accelerating the oxidative addition step of the unreactive trifluoroethyl iodide to the ANP intermediate 16. In addition, DFT calculations suggested that the N@H activation of the indole substrate is involved in the rate-determining step.

Remarkably, the synthetic utility of this selective C2 alkylation strategy of indoles was demonstrated by its application in the efficient synthesis of several complex indole alkaloids, such as (:) aspidospermidine (Scheme 7 a),[8a] (:)-goniomitine (Scheme 7 b),[8a] (+)-kopsihainanine A (Scheme 7 c),[11] (@)-aspidophylline A (Scheme 7 d),[12] and (+)-strictamine (Scheme 7 e).[13]

2.2. C2 Arylation of NH-Indoles
Based on the success of the selective C2 alkylation of NHindoles through PdII/NBE cooperative catalysis, the Bach group demonstrated that this chemistry could be extended to C2 arylation by selecting iodobenzene as the coupling partner. However, only one such example was presented in their report in 2011 (Scheme 8 a).[7] Recently, the groups of Xue and Jiang intensively explored this topic, and successfully synthesized a variety of C2-arylated NH-indoles in moderate to excellent yields (Scheme 8 b).[14] It was found that a combination of electron-rich indoles and electron-poor aryl iodides
usually led to good results, probably because the corresponding ortho C@H activation and oxidative addition steps are facilitate.

2.3. C2 Alkylation of NH-Pyrroles
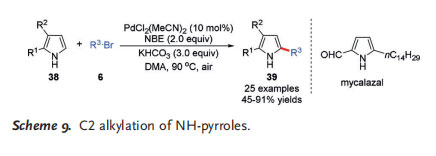
Aside from indole substrates, Bach and co-workers applied the PdII/NBE cooperative catalysis chemistry to NH-pyrroles for selective C2 alkylation, which used to be a very challenging task. As pyrroles are more electron-rich and less acidic than indoles, initial attempts with such pyrroles, for example, 2-phenylpyrrole, were unsuccessful. However, electron-deficient pyrroles are suitable substrates for this transformation, delivering the corresponding alkylated pyrroles 39 in moderate to excellent yields.[15] Utilizing this reaction as the key step, a short synthesis of the lipophilic pyrrole natural product mycalazal was realized (Scheme 9).
3. meta-Selective C@H Functionalization of Arenes
Transition-metal-catalyzed site-selective C@H functionalization has continuously been a highly impactful process in synthetic chemistry.[16] Whereas ortho-selective C@H functionalization reactions of arenes have been well developed, meta-selective C@H functionalization remains a challenge. Recently, several elegant strategies have been developed to address this issue,[16g,i, 17–19] for example, steric-hindrancesensitive borylation,[17] ruthenium-catalyzed meta-selective C@H functionalization,[18] and the use of a U-shaped template.[19] A separate approach using PdII/NBE cooperative catalysis was developed by the groups of Yu, Dong, Zhao, Shi, Ferreira, and others,[20–37] who drew inspiration from the Catellani reaction. As shown in Scheme 10, by taking advantage of the directed ortho palladation and CatellaniQs NBE-mediated insertion/deinsertion, functionalization at the meta position of the arene substrate can be readily achieved.
It should be pointed out that in these meta-selective C@H functionalization processes, stoichiometric silver salts are usually needed, which may act as a base for the C@H activation steps, as an oxidant to prevent the formation of Pd0 species, as well as a halogen anion scavenger to promote the formation of PdIV species. Further studies illustrated the use of this strategy in generating derivatives of phenylacetic acid, b-arylethylamines, benzyl amines, anilines, and phenols.

In this Section, we have summarized the meta-selective C@H functionalization of arenes by PdII/NBE cooperative catalysis. The contents are ordered according to the type of functionalization, including alkylation, arylation, chlorination, amination, and alkynylation. Enantioselective meta C@H functionalization co-catalyzed by a chiral NBE-type mediator and PdII will be discussed separately.
3.1. meta-Selective C@H Alkylation
Inspired by the unique Pd0-initiated Catellani-type reactions of aryl iodides and in line with their continuous interest in directed C@H functionalization, Yu and co-workers developed an elegant approach for the meta C@H alkylation of phenylacetic amides by PdII/NBE cooperative catalysis in 2015 (Scheme 11 a).[20] This transformation provided the first proof of concept that meta C@H alkylation can be achieved through directed ortho C@H activation followed by involvement of NBE as a mediator. The use of a newly developed pyridine-based ligand 49 proved crucial for relaying the palladium catalyst to the meta position by NBE after the initial ortho-C@H activation. Nevertheless, this process suffered from a narrow substrate scope as the alkylating reagent was limited to those without b-hydrogen atoms, for example, iodomethane, ethyl iodoacetate, and benzyl halides. To overcome the aforementioned limitation, the same group introduced a more reactive mediator, 2-carbomethoxynorbornene (52; Yu mediator), in combination with the quinoline-type ligand 51 to successfully develop a more general and efficient meta C@H alkylation process with a broader scope of alkylating reagents (13 examples; Scheme 11b).[21]
Later on, they realized the meta C@H alkylation of benzylsulfonamide (sulfonamide as an ortho-directing group) by using Yu mediator 52 and simple isoquinoline as the ligand, generating the desired meta-alkylated products 54 in 45–72% yield (Scheme 11c).[22] Very recently, Ding and co-workers reported the direct meta alkylation of nosyl-protected methyl esters of phenylalanine derivatives in moderate to high yields(Scheme 11d).[23] In this process, another NBE derivative, diisopropyl bicyclo[2.2.1]hept-5-ene-2,3-dicarboxylate (56), was introduced together with simple pyridine as the optimal combination of mediator and ligand. Significantly, no racemization occurred in this transformation. It should be pointed out that in the meta-selective C@H alkylation processes described above, hindered secondary
alkyl iodides and bromides were all demonstrated to be unsuitable alkylating reagents.
3.2. meta-Selective C@H Arylation
Biaryl motifs are ubiquitous in bioactive natural products and pharmaceuticals.[24] Consequently, extensive efforts have been devoted to the development of efficient methods to assemble biaryls in a rapid fashion. Following the success of meta C@H alkylation reactions of arenes by PdII/NBE cooperative catalysis, the groups of Yu and others demonstrated that this strategy could be extended to meta C@H
arylation, thus providing a unique access to diversified biaryls. To present these achievements in an easy-to-follow way, the contents will be discussed separately according to the type of applied ortho directing group, which include amide, sulfonamide, tertiary amine, pyridine, quinoline, and free carboxylic acid, groups.
3.2.1. Amides or Sulfonamides as Directing Groups
Following the success of meta C@H alkylation of phenylacetic amides by PdII/NBE cooperative catalysis in 2015, Yu and co-workers reported the corresponding selective meta C@H arylation (Scheme 12a).[20] Under slightly modified reaction conditions compared to Scheme 11 a, a series of aryl iodides with ortho-coordinating groups as well as highly reactive 3,5-bis(trifluoromethyl)iodobenzene were found to be competent coupling partners, providing the corresponding biaryls 59 in moderate to high yields. Despite these advances, the arylating reagents involved in the above reaction were mainly limited to aryl iodides with electron-withdrawing ortho-coordinating groups or multiple electron-withdrawing substituents. Therefore, the development of a more general meta arylation procedure compatible
with common aryl iodides was highly desirable. To this end, Yu and co-workers developed an efficient meta-selective C@H arylation process with a broader scope of aryl iodides, which was realized with the aid of quinoline ligand 51 and Yu mediator 52 (Scheme 12b).[21]

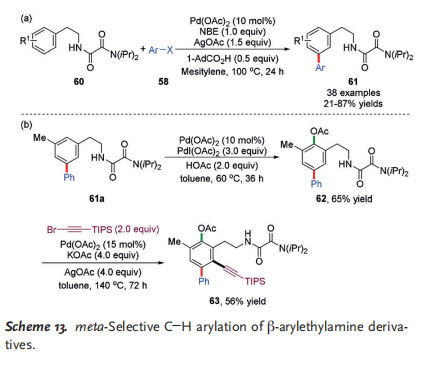
In 2016, Zhao and co-workers reported a selective meta arylation of b-arylethylamine derivatives 60 with a bidentate oxalyl-amide-based directing group and simple NBE as the mediator (Scheme 13).[25a] A variety of aryl iodides bearing ortho, meta, or para substituents reacted well to provide the desired biaryls in moderate to high yields. Notably, the obtained products were further elaborated through sequential ortho C@H functionalizations to afford the polysubstituted arylethylamine derivative 63 in moderate yield (Scheme 13b).[25b]

Later, Shi and co-workers developed a selective interannular meta C@H arylation of biaryl-2-trifluoroacetamides (Scheme 14).[26] In this process, the trifluoroacetyl protecting group is crucial for the interannular selectivity, which may due to its electronic properties and binding ability. It was noted that the reaction proceeded well with aryl iodides bearing an ortho electron-withdrawing group. In contrast, aryl iodides with meta or para substituents gave the corresponding products in particularly low yields. In these cases, switching from NBE to Yu mediator 52 improved the reaction yields.
Remarkably, the authors unambiguously determined that the dimeric palladacycle 66, comprising two cyclopalladated trifluoroacetamino biaryl units linked through trifluoroacetamide, was the key intermediate of this transformation.
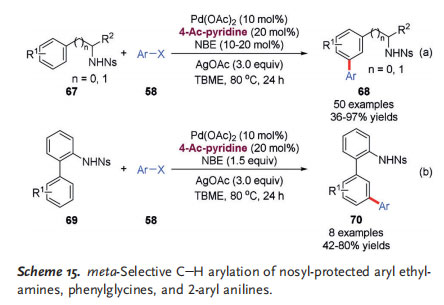
In 2017, Yu and co-workers reported a Pd II/NBE cooperatively catalyzed meta-selective C@H arylation of nosylprotected arylethylamines and phenylglycine esters 67, wherein the common sulfonamide was used as the directing group (Scheme 15a).[27] Subjecting nosyl-protected 2-aryl anilines 69 to this process led to meta C@H arylation at the remote aryl ring (Scheme 15b). Addition of 4-acetylpyridine
as the ligand was key to this process, and the palladium catalyst loading could be reduced to as low as 2.5 mol%. This catalytic system is compatible with various aryl iodides as well as aryl bromides with ortho-coordinating groups, giving the corresponding biaryls in moderate to high yields. It should be mentioned that in the case of nosyl-protected arylethylamines67, a catalytic amount of NBE (10–20 mol%) was sufficient to mediate this meta C@H arylation reaction efficiently, which represented the first example in this field (Scheme 15a).
Utilizing a sulfonamide as the directing group for the meta-selective C@H arylation of benzylsulfonamide derivatives 53 through PdII/NBE cooperative catalysis was also recently reported by the Yu group (Scheme 16).[22] The reaction was carried out by using isoquinoline as the ligand and 52 as the mediator, enabling facile syntheses of metaarylated benzylsulfonamide derivatives 71. Importantly, the
obtained products can be readily transformed into sodium sulfonates, sulfonate esters, sulfonamides, as well as styrenes by Julia-type olefination.

3.2.2. Tertiary Amines as Directing Groups
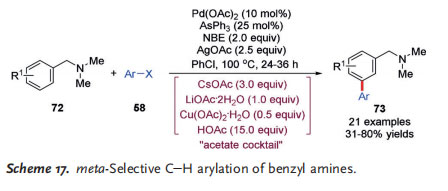
In 2015, Dong and co-workers reported a distinct meta arylation of arenes by PdII/NBE catalysis, using a tertiary amine as the directing group (Scheme 17).[28] It was worth noting that the reaction was promoted by AsPh3 as the ligand and an interesting “acetate cocktail” containing LiOAc·2H2O, CsOAc, Cu(OAc)2·H2O, and acetic acid. A wide range of functional groups, including some heteroarenes, were tolerated under the reaction conditions. However, an ortho electron-withdrawing substituent on the aryl iodide was required to render it a suitable coupling partner. Moreover, the amine directing group can be easily transformed into other common functional groups.
3.2.3. Tethered Pyridine-type Directing Groups
In 2016, meta-selective C@H arylation reactions of anilines, heteroaromatic amines, phenols, and 2-benzylpyridine derivatives 74 were realized by the Yu group (Scheme 18).[29]

By using the versatile 3-acetylamino-2-hydroxypyridine 75 a or its trifluoromethylated derivative 75b as the ligand and NBE as the mediator, a large number of meta-arylated products 76 were afforded in good to excellent yields. This process also exhibited good compatibility with both heteroarene substrates and heteroaryl halide coupling partners. The utility of this method in drug discovery was showcased through the late-stage meta C@H arylation of a lenalidomide derivative in good yield.
Later on, the same group developed an elegant metaselective C@H arylation of benzylamines with a pyridine-type N-substituted directing group. The reaction was promoted by Yu mediator 52 and pyridone-type ligand 78, and the corresponding meta-arylated benzylamine derivatives 79 were obtained in moderate to excellent yields (Scheme 19).[30] Very recently, the same group achieved the meta C@H arylation of masked aromatic aldehydes 80 by employing Yu mediator 52 and pyridone-type ligand 78b (Scheme 20).


The process relied on a pyridine-type directing group, which also served as a masking group for aryl aldehydes, and was readily installed and removed. Control experiments indicated that the correct length of this directing group was crucial for permitting the critical migratory insertion step to proceed efficiently. A wide variety of masked aryl aldehyde substrates and aryl iodide coupling partners were suitable for this reaction, giving the corresponding masked biaryl aldehydes 81 in satisfactory yields. Compounds 81 can be unmasked to yield biaryl aldehydes 82 in heated TBAF solution.
3.2.4. Acetal-Based Quinolines as Directing Groups
In 2017, Ferreira and co-workers reported on the metaselective C@H arylation of benzyl alcohol derived acetal substrates 83 (Scheme 21).[32] The unique acetal-based quinoline-type directing group (QuA), the distinct amino acid derived ligand N-trifluoroacetylglycine (TFA-Gly-OH) as well as Yu mediator 52 were found to be pivotal for the reaction. This transformation exhibited excellent functional
group compatibility, and the corresponding biaryl compounds were afforded in moderate to high yields. In addition, the meta arylation can be combined with ortho arylation or olefination to yield polysubstituted arenes, providing a versatile platform for the diversification of aromatic systems.
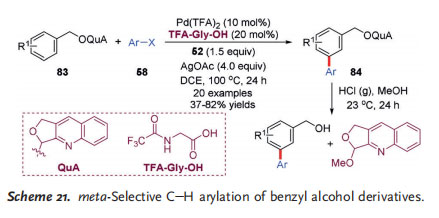
Moreover, the directing group QuA can be readily cleaved and recovered under very mild reaction conditions.
3.2.5. Free Carboxylic Acids as Directing Groups
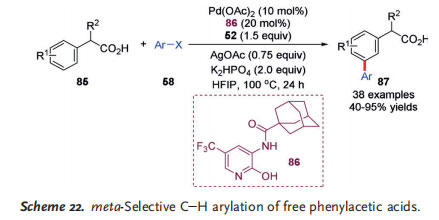
In 2017, the more challenging auxiliary-free meta C@H arylation of free arylacetic acids 85 was realized by the Yu group (Scheme 22).[33] In this reaction, the choice of a suitable monoprotected 3-amino-2-hydroxypyridine-type ligand 86 and the Yu mediator 52 had a significant influence on the reaction efficiency. Notably, a wide range of aryl iodides, including those with non-coordinating substituents (20 examples), displayed good reactivity to give the corresponding products 87 in moderate to high yields.
3.3. meta-Selective C@H Chlorination
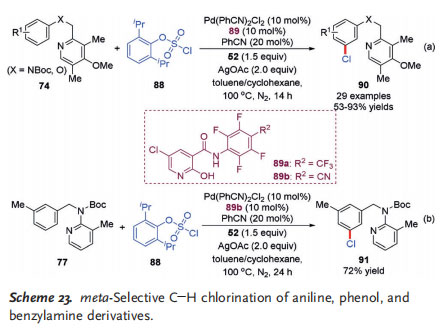
The direct C@H chlorination of arenes is an appealing process to access synthetically versatile aryl chlorides. In 2016, the Yu group developed a meta-selective C@H chlorination of aniline and phenol derivatives with a pyridine-type tether as the directing group that is co-catalyzed by PdII and Yu mediator 52 (Scheme 23a).[34] Aryl chlorosulfate 88 was exploited as the chlorination reagent, and the unique pyridine derivative 89 acted as the ligand of choice. Under the optimized reaction conditions, a large number of substrates bearing various functional groups were tolerated, delivering the corresponding meta-chlorinated products 90 in good to excellent yields. Notably, some medicinally important heterocyclic compounds, such as indole, thiophene, and indazole derivatives, are also competent substrates. Moreover, the chlorinated products were transformed into a wide range of synthetically useful synthons that are difficult to access through direct meta C@H functionalization, for example, borylation and alkoxylation. Using a similar strategy, the meta-selective C@H chlorination of benzylamine derivative 77 with an N-substituted pyridine-type directing group was also
achieved by the same group in 2017 (Scheme 23b).
3.4. meta-Selective C@H Amination
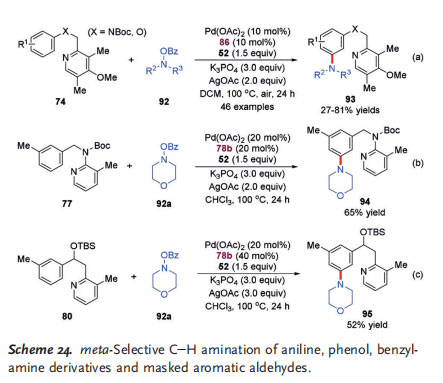
Aromatic amines are important structural motifs that are widely found in bioactive natural products, pharmaceuticals, agrochemicals, and materials.[35] The transition-metal-catalyzed C@H amination of arenes has emerged as a powerful and efficient strategy to access aromatic amines.[36] In 2016, the Yu group reported the first example of a Pd II/NBE cooperatively catalyzed meta C@H amination of aniline and phenol derivatives tethering a specially designed pyridinetype directing group (Scheme 24a).[37] They found that the combination of 3-amino-2-hydroxypyridine-type ligand 86 and Yu mediator 52 was crucial for this transformation, and a large number of meta-aminated products (46 examples) were obtained in moderate to good yields. In a similar fashion, the same group also disclosed the successful meta C@H amination of benzylamine derivative 77 and masked aryl aldehyde 80, both with a pyridine-type directing group(Scheme 24b, c).[30, 31]
3.5. meta-Selective C@H Alkynylation
The only example of a Pd II/NBE co-catalyzed metaselective C@H alkynylation of anilines with a pyridinederived tether as the directing group was reported by Yu and co-workers in 2016 (Scheme 25).[37] By using pyridinebased ligand 97 and LiF as the additive, the reactivity and selectivity (meta versus ortho alkynylation) of the reaction was greatly improved. Under the optimized reaction conditions, aniline substrates with diverse functional groups were tolerated, providing the desired meta-C@H-alkynylated products in moderate to good yields. It should be noted that except for bulky silyl-protected alkynyl bromides, simple alkyl and aryl alkynyl bromides as the alkynylating reagents only gave trace amounts of the corresponding products.

3.6. Enantioselective meta C@H Functionalization
Enantioselective remote C@H functionalization is one of the most challenging yet fascinating directions in organic synthesis. In this context, an impressive breakthrough was recently reported by Yu and co-workers regarding an elegant enantioselective meta C@H arylation and alkylation of diarylmethylamines and homobenzylamines by the cooperative catalysis of PdII and the chiral Yu mediator (+)-52 (Scheme 26).[38] In these processes, the enantioselective differentiation is based on a fast, reversible, and racemic ortho C@H activation followed by stereoselective norbornene insertion and meta C@H activation. (+)-52 served both as a mediator and an efficient chiral source to realize stereoisomeric differentiation of the racemic ortho C@H palladation intermediates to control the enantioselectivity (Scheme 26 a).
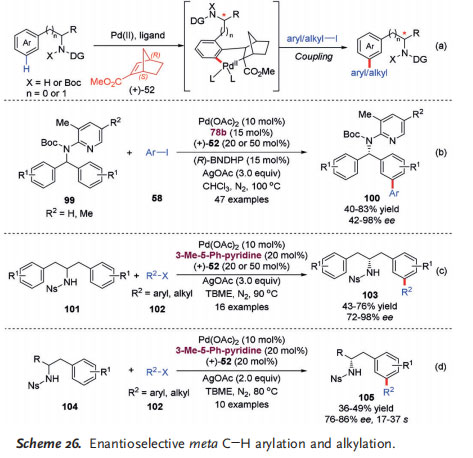
This asymmetric meta C@H arylation reaction exhibited a broad substrate scope and high enantioselectivities. A broad range of prochiral benzylamines 99 with pyridine-type N substituents as the directing group, prochiral N-nosyl homobenzylamines 101, and racemic N-nosyl homobenzylamines 104 as well as diverse aryl iodide coupling reagents were tolerated in this process. In addition to aryl iodides, one
aryl bromide, methyl 2-bromobenzoate, was also identified as a competent arylating reagent, giving the desired product in moderate yield and excellent enantioselectivity. In the case of asymmetric meta C@H arylation of 99 by desymmetrization, it was found that the enantioselectivity can be dramatically enhanced by the use of (R)-BNDHP (1,1’-binaphthyl-2,2’-diyl hydrogen phosphate) as an additive (Scheme 26b). Furthermore, enantioselective meta C@H alkylation was also realized for 101 (by desymmetrization) and 104 (by kinetic resolution), by using ethyl iodoacetate or iodomethane as the reagent
(Scheme 26c,d). These achievements realized through the cooperative catalysis of PdII and an enantiopure NBE-type mediator will open a new avenue for enantioselective remote C@H functionalization—one of the most challenging processes in asymmetric catalysis.
4. Borono-Catellani Reactions
Aryl boronic acids and their derivatives are readily available and very useful organic reagents. In classical Catellani-type reactions, aryl boronic acids and their derivatives mainly act as terminating reagents.
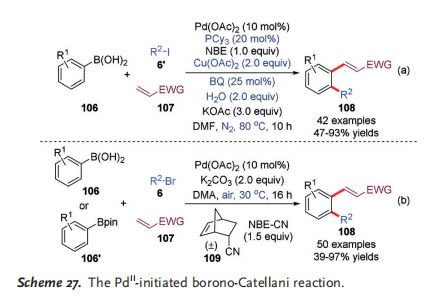
[5] Inspired by previously reported PdII/NBE cooperative catalysis chemistry, the groups of Zhang[39] and Zhou[40] independently reported a novel PdII-initiated Catellani-type reaction that utilized these widely accessible reagents as the substrates instead of conventional aryl halides to react with alkyl halides (mainly bromides and iodides) and olefins, which was named a “borono-Catellani reaction” (Scheme 27). In ZhangQs work, the reaction was performed under N2 atmosphere at 80 8C, with Cu(OAc)2 as the oxidant to regenerate the PdII catalyst. Interestingly, it was found that adding 2 equivalents of water and 25 mol% of benzoquinone (BQ) as additives significantly improved the reaction efficiency (Scheme 27 a). In contrast, the Zhou group provided more practical reaction conditions: The reaction was run at ambient temperature (308C) under air (open flask) while neither phosphine ligand nor additive was needed (Scheme 27b). In addition, the commercially available NBE derivative 109 was introduced as a novel mediator to promote this process for the first time.
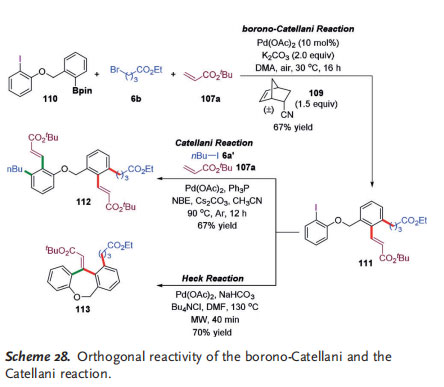
The Zhou group demonstrated the “orthogonal reactivity” of the traditional Pd0-initiated Catellani reaction and the PdII-initiated borono-Catellani reaction, by using a rationally designed bifunctional reagent 110 containing both an iodo and a Bpin group as the model substrate. As shown in Scheme 28, 110 reacted with bromide 6 b and tert-butyl acrylate 107 a under the standard borono-Catellani reaction conditions to provide the borono-Catellani product 111 in 67% yield, with no classical Catellani product observed. The intact iodo group in 111 enabled an ensuing classical Catellani reaction to provide the product 112 in 67% yield. Moreover, 111 underwent a microwave-promoted intramolecular Heck reaction[41] to afford the complex polycyclized product 113 in good yield.
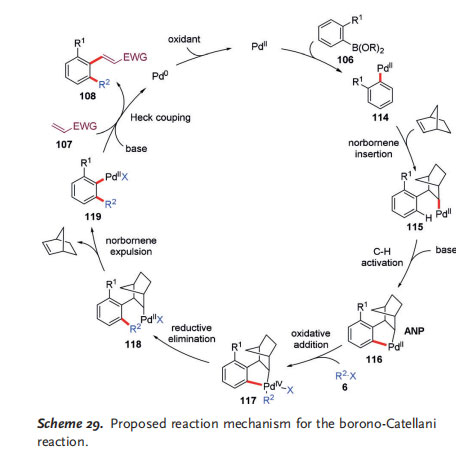
The proposed reaction mechanism of the borono-Catellani reaction is shown in Scheme 29. The catalytic cycle is initiated by the PdII catalyst, which reacts with aryl boronic acid 106 to provide aryl–PdII species 114 by transmetalation. The migratory insertion of NBE and subsequent ortho C@ activation with the aid of a base gives the key ANP complex 116. Oxidative addition of alkyl halide 6 to 116 forms PdIV complex 117, which then undergoes reductive elimination and subsequent expulsion of NBE to deliver PdII species 119.
Finally, 119 couples with olefin 107 to provide the Catellani product 108 and release a Pd 0 species, which is then reoxidized to regenerate the PdII catalyst. Thus an oxidant is needed to promote the catalytic cycle. It should be pointed out that the requirement for a stoichiometric oxidant actually poses a big challenge to the borono-Catellani reaction as multiple possible side reactions of aryl boronic acids have been reported to proceed under the reaction conditions, including a direct oxidative Heck reaction between the aryl boronic acid and the olefin,[42] oxidative homocoupling,[43] oxidation to phenols,[44] and protolytic deboronation.[44] The success of this borono-Catellani reaction relies on the carefully optimized reaction conditions to minimize the possible side reactions.
5. Summary and Outlook
In this Minireview, we have summarized PdII-initiated Catellani-type reactions (PdII/NBE cooperative catalysis) and their application in organic synthesis. As described above, substantial efforts have been devoted to this fascinating process, and significant advancements have been made in the past few years. Pd II/NBE cooperative catalysis has been demonstrated to be a powerful complement to the classical
Catellani-type reactions. Utilizing this strategy, NH-indoles and NH-pyrroles, (hetero)arenes bearing an ortho directing group, as well as aryl boronic acids and derivatives thereof have become suitable substrates that undergo highly selective functionalizations to afford valuable polysubstituted aromatic compounds in a straightforward fashion.
Despite these remarkable advances, the PdII-initiated Catellani-type reaction is still in its infancy for the following reasons. First, only a few NBE-type mediators have been developed thus far. Currently, only NBE and the Yu mediator 52 are widely used. Meanwhile, stoichiometric quantities of mediators are usually required to achieve good reaction efficiency. Therefore, it is highly desirable to develop more
powerful catalytic mediators that allow the Catellani-type processes to occur in a more efficient way. Second, with regard to the development of enantioselective PdII-initiated Catellani-type reaction, only one example has been reported to date by the Yu group. Nevertheless, this inspiring work serves as an excellent demonstration of the power of chiral mediator/PdII cooperative catalysis for remote stereocontrol.
Third, beyond the three types of substrates developed, there is still much room for exploration to expand the applicability of this process. Considering the vast synthetic potential of PdII/NBE
cooperative catalysis, future efforts should be invested in the above directions. We anticipate that palladium(II)-initiated Catellani-type reactions will be flourishing in the near future.
Acknowledgements
We are grateful to the National Natural Science Foundation of China (Grants 21602161, 21871213, 21801193), the National “1000-Youth Talents Plan”, the Innovation Team Program of Wuhan University (Program No. 2042017kf0232), start-up funding from Wuhan University,and the China Postdoctoral Science Foundation (2016M602339, 2018M642894) for financial support.
3-{[(1-methyl-1H-imidazol-2-yl)sulfanyl]methyl}benzoic acidCatalog No.:AA01A8ER CAS No.:1017046-67-1 MDL No.:MFCD09941376 MF:C12H12N2O2S MW:248.3009 |
N-(4-methyl-1,3-thiazol-2-yl)piperidin-4-amineCatalog No.:AA01ACUO CAS No.:1017046-71-7 MDL No.:MFCD09947791 MF:C9H15N3S MW:197.3005 |
6-chloro-N-(prop-2-yn-1-yl)pyridine-3-carboxamideCatalog No.:AA019V90 CAS No.:1017047-71-0 MDL No.:MFCD09944097 MF:C9H7ClN2O MW:194.6177 |
(Z)-N'-Hydroxy-2-(pyrrolidin-1-yl)acetimidamideCatalog No.:AA008VA7 CAS No.:1017047-83-4 MDL No.:MFCD09944126 MF:C6H13N3O MW:143.1869 |
N-(3-aminopropyl)ethane-1-sulfonamideCatalog No.:AA01A837 CAS No.:1017047-84-5 MDL No.:MFCD09941610 MF:C5H14N2O2S MW:166.2419 |
[(2,6-difluorophenyl)methyl](ethyl)amineCatalog No.:AA01A90G CAS No.:1017047-88-9 MDL No.:MFCD09941616 MF:C9H11F2N MW:171.1871 |
6-(1H-1,3-benzodiazol-1-yl)-N'-hydroxypyridine-3-carboximidamideCatalog No.:AA01A7FY CAS No.:1017047-94-7 MDL No.:MFCD14705694 MF:C13H11N5O MW:253.2593 |
4-(2,6-dimethylphenoxy)piperidineCatalog No.:AA01A786 CAS No.:1017048-01-9 MDL No.:MFCD09944168 MF:C13H19NO MW:205.2961 |
3-cyano-N-(prop-2-yn-1-yl)benzamideCatalog No.:AA019W19 CAS No.:1017048-13-3 MDL No.:MFCD09944193 MF:C11H8N2O MW:184.1940 |
1,3,4-Oxadiazol-2-amine, 5-(3-methyl-2-thienyl)-Catalog No.:AA00056N CAS No.:1017048-74-6 MDL No.:MFCD09937859 MF:C7H7N3OS MW:181.2150 |
2-{5-bromo-3-methyl-1H-pyrazolo[3,4-b]pyridin-1-yl}ethan-1-olCatalog No.:AA019YVL CAS No.:1017048-94-0 MDL No.:MFCD09944370 MF:C9H10BrN3O MW:256.0992 |
4-Chloro-6,8-dimethoxyquinoline-3-carbonitrileCatalog No.:AA019VAV CAS No.:1017049-01-2 MDL No.:MFCD09937921 MF:C12H9ClN2O2 MW:248.6651 |
2-[4-(Pyridin-2-yl)-1,3-thiazol-2-yl]acetonitrileCatalog No.:AA019WVG CAS No.:1017049-48-7 MDL No.:MFCD09938083 MF:C10H7N3S MW:201.2477 |
3-amino-1-{4H,5H,6H,7H-thieno[3,2-c]pyridin-5-yl}propan-1-oneCatalog No.:AA01A85G CAS No.:1017049-62-5 MDL No.:MFCD09938104 MF:C10H14N2OS MW:210.2960 |
5-Methyl-2-[(methylsulfonyl)amino]benzoic acidCatalog No.:AA00056M CAS No.:1017051-55-6 MDL No.:MFCD09945630 MF:C9H11NO4S MW:229.2529 |
2-[(5-cyanopyridin-2-yl)amino]acetic acidCatalog No.:AA019WSN CAS No.:1017051-97-6 MDL No.:MFCD09935392 MF:C8H7N3O2 MW:177.1601 |
5-(aminomethyl)-N,N,1-trimethyl-1H-imidazol-2-amineCatalog No.:AA01AHXE CAS No.:1017052-51-5 MDL No.:MFCD09935496 MF:C7H14N4 MW:154.2129 |
4-methoxy-3-(2-methoxy-2-oxoethoxy)benzoic acidCatalog No.:AA019V6G CAS No.:1017053-35-8 MDL No.:MFCD09935716 MF:C11H12O6 MW:240.2094 |
2-[2-chloro-4-(chlorosulfonyl)phenoxy]acetic acidCatalog No.:AA01A89E CAS No.:1017055-32-1 MDL No.:MFCD09938465 MF:C8H6Cl2O5S MW:285.1012 |
1-(aminomethyl)-N,N,3-trimethylcyclohexan-1-amineCatalog No.:AA019WI8 CAS No.:1017057-31-6 MDL No.:MFCD09946464 MF:C10H22N2 MW:170.2951 |
4-Hydroxy-2-(trifluoromethoxy)benzaldehydeCatalog No.:AA00056L CAS No.:1017083-37-2 MDL No.:MFCD06797920 MF:C8H5F3O3 MW:206.1187 |
2-bromo-N-cyclopentyl-3-methylbutanamideCatalog No.:AA019KW4 CAS No.:1017090-31-1 MDL No.:MFCD01070281 MF:C10H18BrNO MW:248.1600 |
4-(3-Formylphenoxy)butanenitrileCatalog No.:AA01A9KT CAS No.:1017090-33-3 MDL No.:MFCD09930002 MF:C11H11NO2 MW:189.2105 |
3-[(3-Bromobenzyl)sulfonyl]propanoic acidCatalog No.:AA019NAJ CAS No.:1017090-62-8 MDL No.:MFCD09941790 MF:C10H11BrO4S MW:307.1609 |
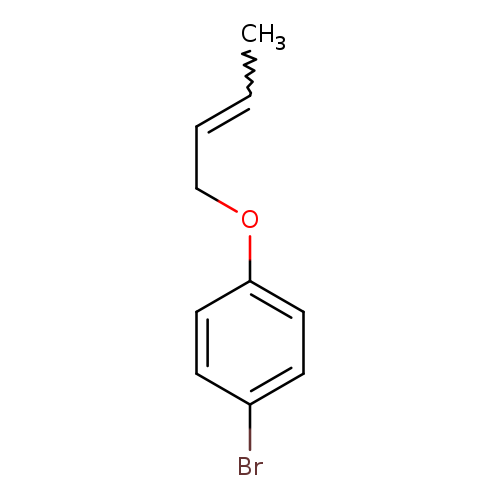
1-bromo-4-(but-2-en-1-yloxy)benzeneCatalog No.:AA01A5Y2 CAS No.:1017091-66-5 MDL No.:MFCD09941965 MF:C10H11BrO MW:227.0977 |

(S)-(+)-2-Methylglutaric acid dimethyl esterCatalog No.:AA000576 CAS No.:10171-92-3 MDL No.:MFCD00671564 MF:C8H14O4 MW:174.1944 |
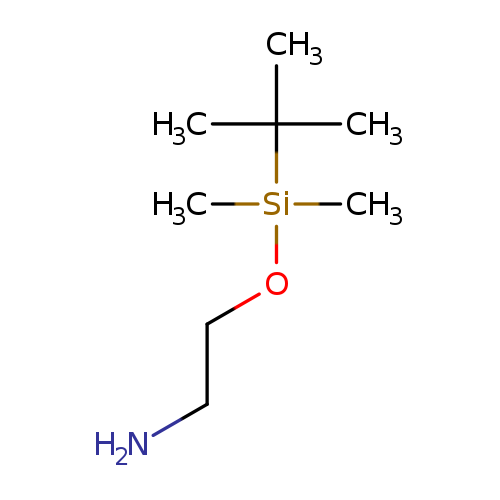
2-(tert-Butyldimethylsilyloxy)ethanamineCatalog No.:AA00057O CAS No.:101711-55-1 MDL No.:MFCD18206132 MF:C8H21NOSi MW:175.3439 |
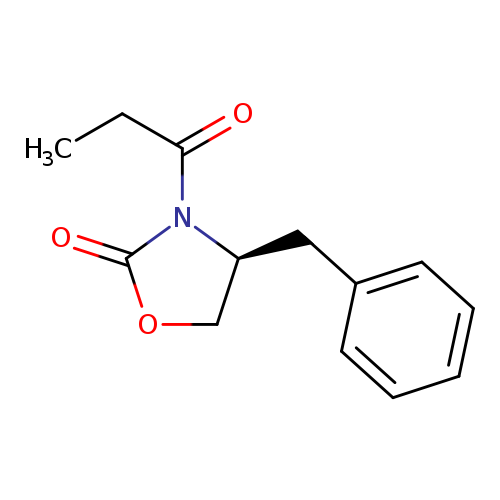
(S)-(+)-4-Benzyl-3-propionyl-2-oxazolidinoneCatalog No.:AA00324L CAS No.:101711-78-8 MDL No.:MFCD00269688 MF:C13H15NO3 MW:233.2631 |
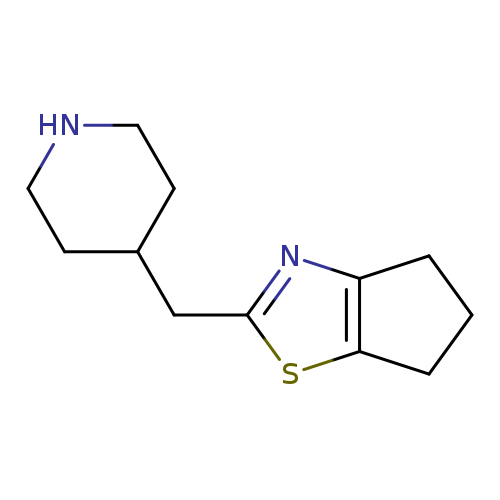
2-(piperidin-4-ylmethyl)-5,6-dihydro-4H-cyclopenta[d]thiazoleCatalog No.:AA01BIU2 CAS No.:1017112-70-7 MDL No.:MFCD09891841 MF:C12H18N2S MW:222.3497 |
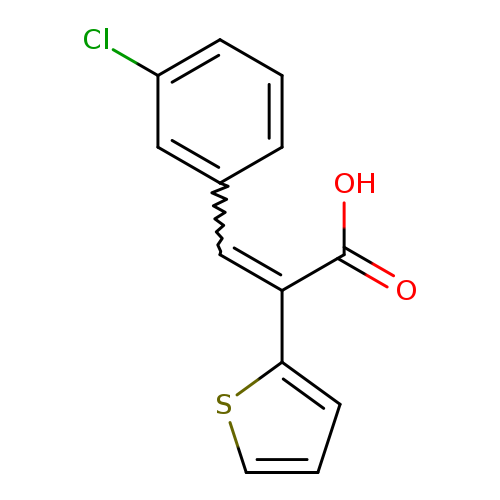
(2E)-3-(3-Chlorophenyl)-2-thien-2-ylacrylic acidCatalog No.:AA00H9HA CAS No.:1017114-62-3 MDL No.:MFCD09885532 MF:C13H9ClO2S MW:264.7274 |
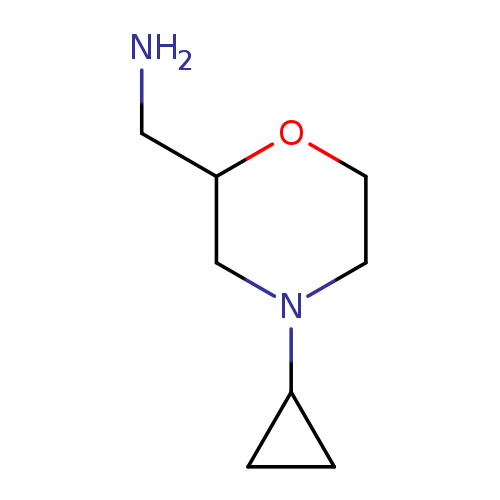
(4-cyclopropylmorpholin-2-yl)methanamineCatalog No.:AA01ABFE CAS No.:1017114-81-6 MDL No.:MFCD09892088 MF:C8H16N2O MW:156.2254 |
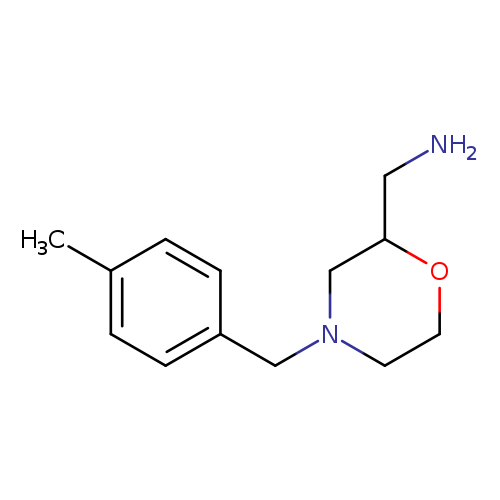
{4-[(4-methylphenyl)methyl]morpholin-2-yl}methanamineCatalog No.:AA01BRX4 CAS No.:1017114-89-4 MDL No.:MFCD09892099 MF:C13H20N2O MW:220.3107 |
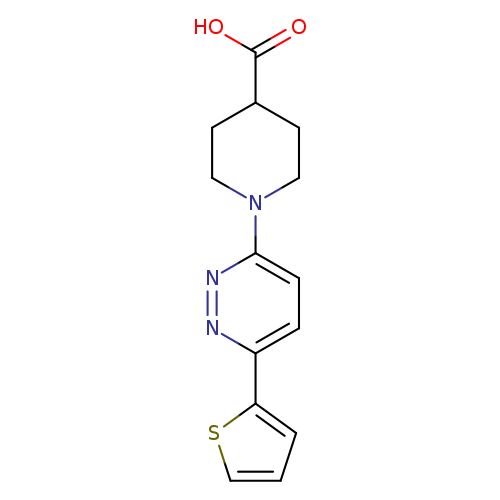
1-(6-(Thiophen-2-yl)pyridazin-3-yl)piperidine-4-carboxylic acidCatalog No.:AA01FLQQ CAS No.:1017117-60-0 MDL No.:MFCD09881929 MF:C14H15N3O2S MW:289.3528 |
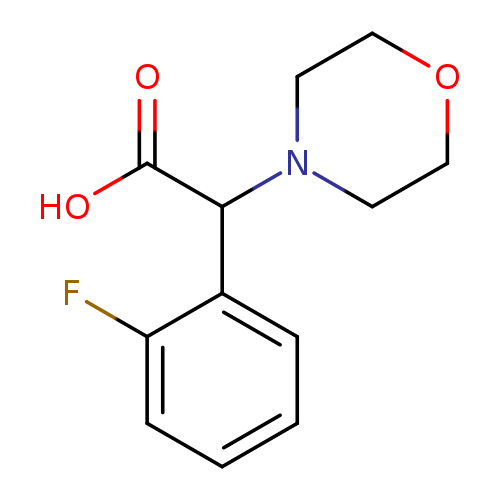
2-(2-Fluorophenyl)-2-morpholinoacetic acidCatalog No.:AA019S9A CAS No.:1017117-65-5 MDL No.:MFCD09888532 MF:C12H14FNO3 MW:239.2429 |
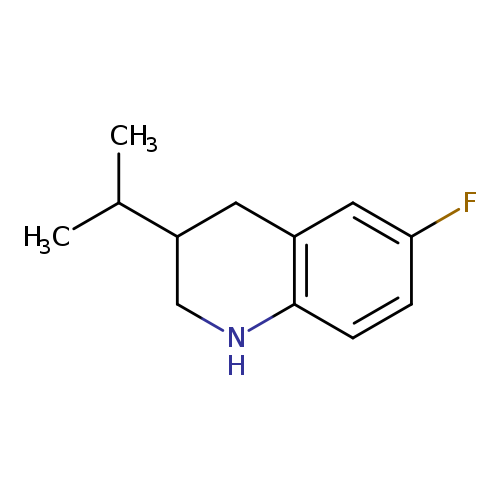
6-fluoro-3-(propan-2-yl)-1,2,3,4-tetrahydroquinolineCatalog No.:AA01EJ46 CAS No.:1017117-98-4 MDL No.:MFCD09896688 MF:C12H16FN MW:193.2605 |
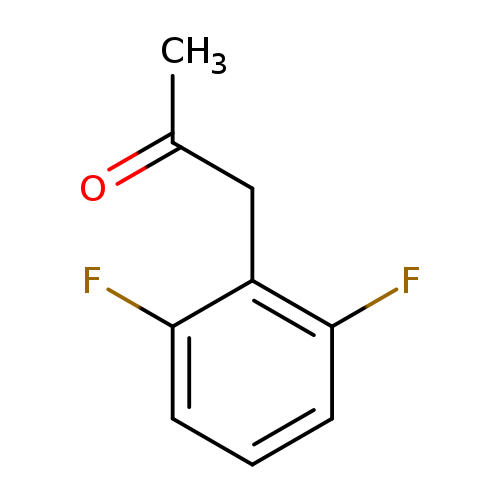
2,6-DIFLUOROPHENYLACETONECatalog No.:AA008RJG CAS No.:101712-20-3 MDL No.:MFCD02258867 MF:C9H8F2O MW:170.1560 |
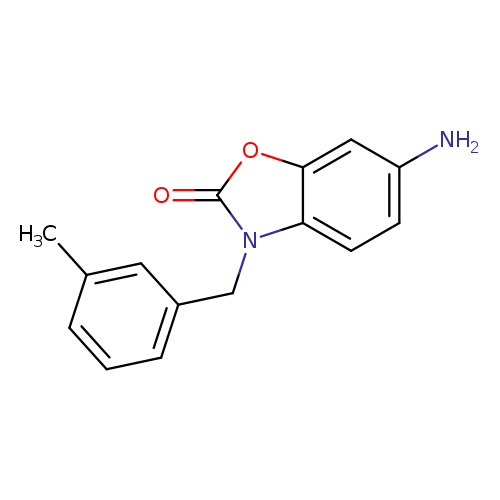
6-amino-3-[(3-methylphenyl)methyl]-2,3-dihydro-1,3-benzoxazol-2-oneCatalog No.:AA01DQPH CAS No.:1017120-20-5 MDL No.:MFCD09894094 MF:C15H14N2O2 MW:254.2839 |
1-(2-Methoxyethyl)-2,6-dimethylpiperazineCatalog No.:AA00VSE0 CAS No.:1017120-41-0 MDL No.:MFCD09894141 MF:C9H20N2O MW:172.2679 |
7-ethyl-1,2,3,4-tetrahydroisoquinolineCatalog No.:AA01AJH6 CAS No.:1017125-75-5 MDL No.:MFCD09886721 MF:C11H15N MW:161.2435 |
3-(Chloromethyl)-7-fluoro-1,2-dihydroquinolin-2-oneCatalog No.:AA01DX3N CAS No.:1017127-42-2 MDL No.:MFCD09895130 MF:C10H7ClFNO MW:211.6201 |
(5-Cyclopropyl-1,3,4-oxadiazol-2-yl)methanamineCatalog No.:AA00057C CAS No.:1017131-06-4 MDL No.:MFCD09883707 MF:C6H9N3O MW:139.1552 |
(6-METHYL-4,5,6,7-TETRAHYDRO-1,3-BENZOTHIAZOL-2-YL)METHANAMINECatalog No.:AA01C9OM CAS No.:1017132-07-8 MDL No.:MFCD09891360 MF:C9H14N2S MW:182.2859 |
[1-(4,5-Dimethyl-1,3-thiazol-2-yl)propyl]amine dihydrochlorideCatalog No.:AA00057B CAS No.:1017132-19-2 MDL No.:MFCD09891375 MF:C8H14N2S MW:170.2752 |
2-(Indolin-1-yl)nicotinic acidCatalog No.:AA01FOCK CAS No.:1017138-83-8 MDL No.: MF:C14H12N2O2 MW:240.2573 |
6-Iodochroman-4-oneCatalog No.:AA00058F CAS No.:101714-35-6 MDL No.:MFCD01319018 MF:C9H7IO2 MW:274.0551 |
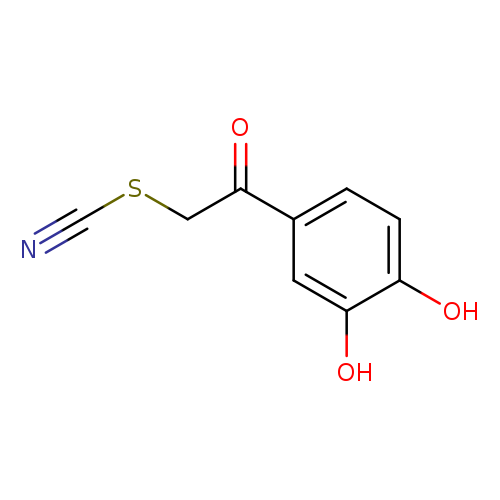
Thiocyanic acid, 2-(3,4-dihydroxyphenyl)-2-oxoethyl esterCatalog No.:AA00058E CAS No.:101714-41-4 MDL No.:MFCD22415334 MF:C9H7NO3S MW:209.2218 |
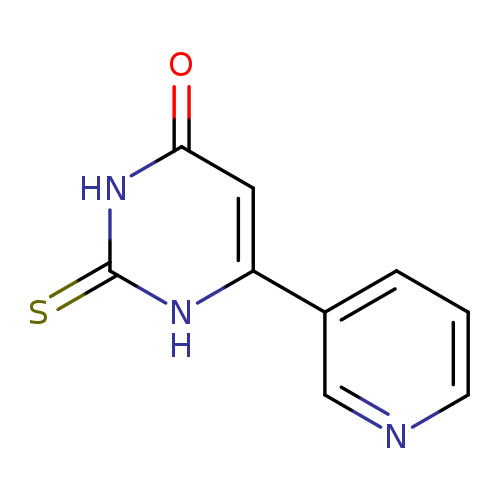
6-PYRIDIN-3-YL-2-THIOXO-2,3-DIHYDROPYRIMIDIN-4(1(H))-ONECatalog No.:AA01AQ0E CAS No.:101714-48-1 MDL No.:MFCD01942634 MF:C9H7N3OS MW:205.2364 |
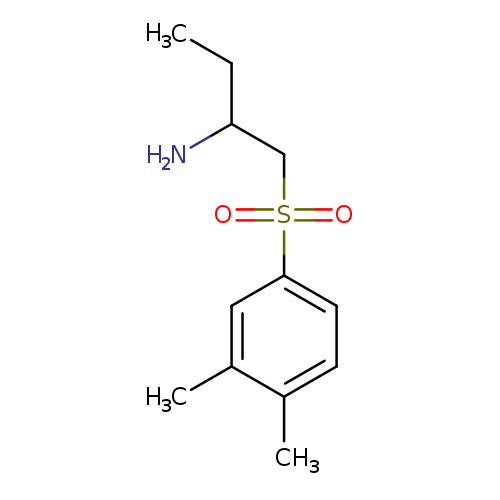
1-(3,4-dimethylbenzenesulfonyl)butan-2-amineCatalog No.:AA01A21Y CAS No.:1017140-34-9 MDL No.:MFCD09893735 MF:C12H19NO2S MW:241.3498 |
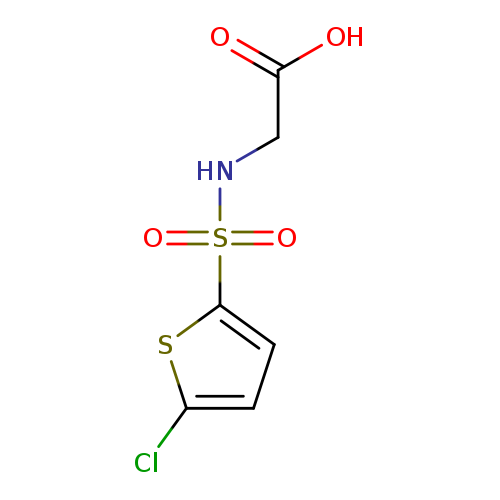
2-(5-Chlorothiophene-2-sulfonamido)acetic acidCatalog No.:AA01A8LS CAS No.:1017141-27-3 MDL No.:MFCD09892560 MF:C6H6ClNO4S2 MW:255.6991 |
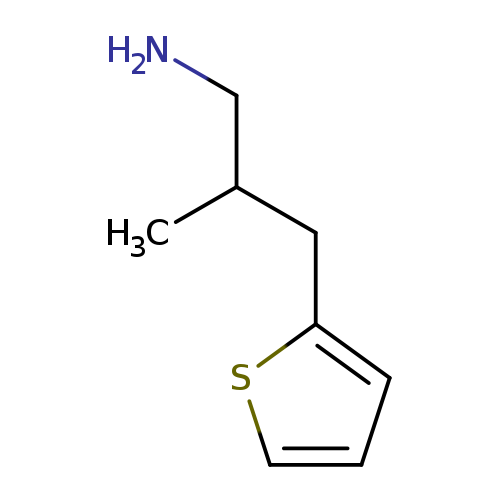
2-methyl-3-(thiophen-2-yl)propan-1-amineCatalog No.:AA019PQ6 CAS No.:1017145-12-8 MDL No.:MFCD09894553 MF:C8H13NS MW:155.2605 |
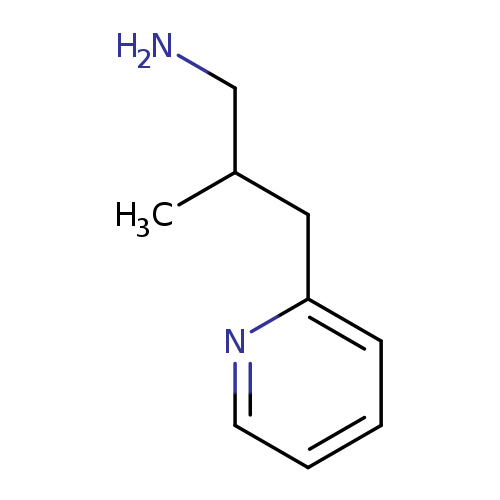
2-methyl-3-(pyridin-2-yl)propan-1-amineCatalog No.:AA01BTGP CAS No.:1017145-15-1 MDL No.:MFCD09894554 MF:C9H14N2 MW:150.2209 |
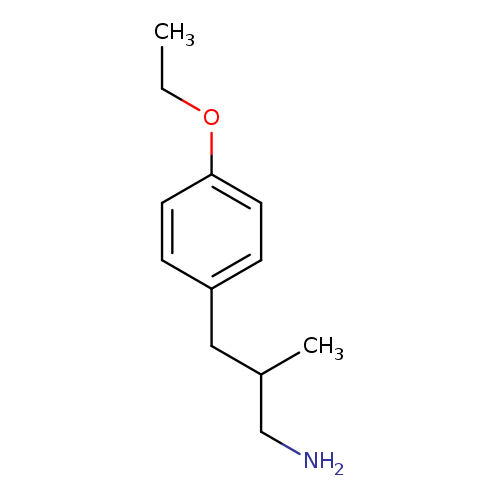
3-(4-ethoxyphenyl)-2-methylpropan-1-amineCatalog No.:AA01C1H6 CAS No.:1017145-67-3 MDL No.:MFCD09894573 MF:C12H19NO MW:193.2854 |
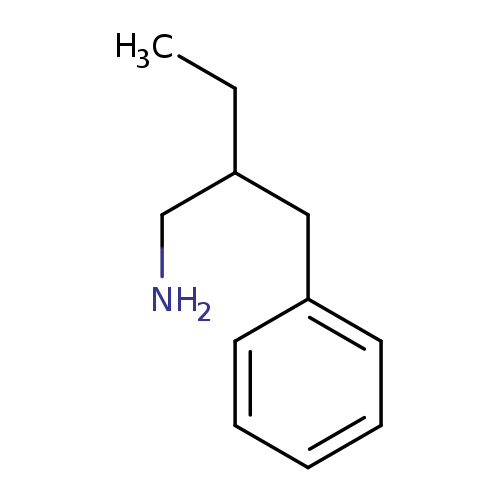
(2-Benzylbutyl)amineCatalog No.:AA000585 CAS No.:1017145-79-7 MDL No.:MFCD09894586 MF:C11H17N MW:163.2594 |
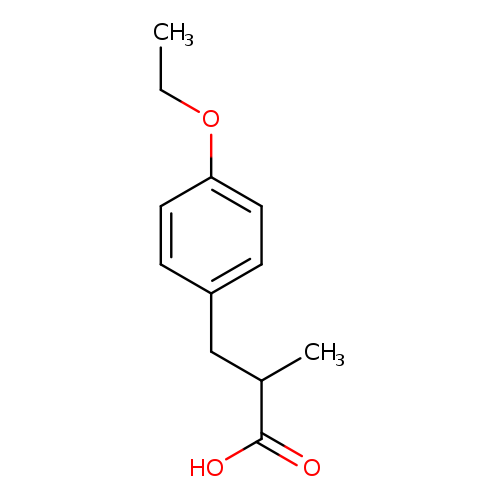
3-(4-ethoxyphenyl)-2-methylpropanoic acidCatalog No.:AA01C366 CAS No.:1017146-71-2 MDL No.:MFCD09894712 MF:C12H16O3 MW:208.2536 |
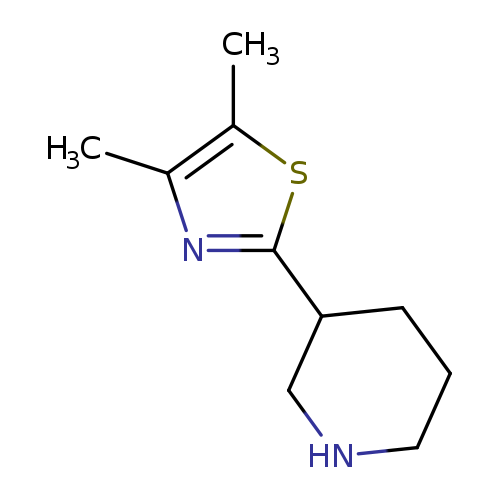
3-(Dimethyl-1,3-thiazol-2-yl)piperidineCatalog No.:AA01A8RS CAS No.:1017153-30-8 MDL No.:MFCD09891525 MF:C10H16N2S MW:196.3124 |
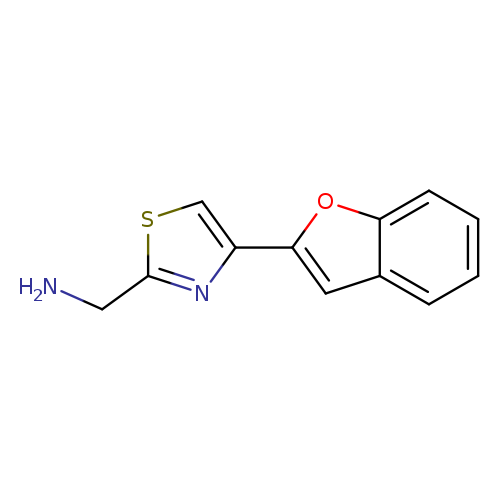
[4-(1-Benzofuran-2-yl)-1,3-thiazol-2-yl]methanamineCatalog No.:AA01ACOU CAS No.:1017156-27-2 MDL No.:MFCD10001788 MF:C12H10N2OS MW:230.2856 |
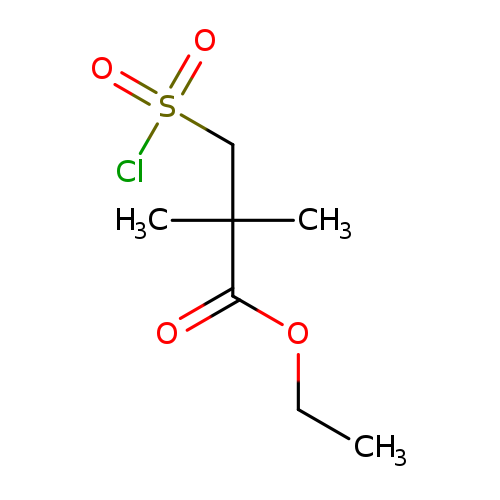
ETHYL 3-(CHLOROSULFONYL)-2,2-DIMETHYLPROPANOATECatalog No.:AA01DX3O CAS No.:1017156-35-2 MDL No.:MFCD09884754 MF:C7H13ClO4S MW:228.6937 |
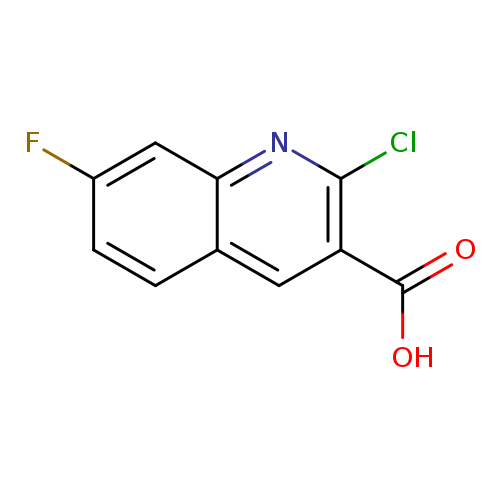
2-Chloro-7-fluoroquinoline-3-carboxylic acidCatalog No.:AA00VSFU CAS No.:1017157-81-1 MDL No.:MFCD09895919 MF:C10H5ClFNO2 MW:225.6036 |
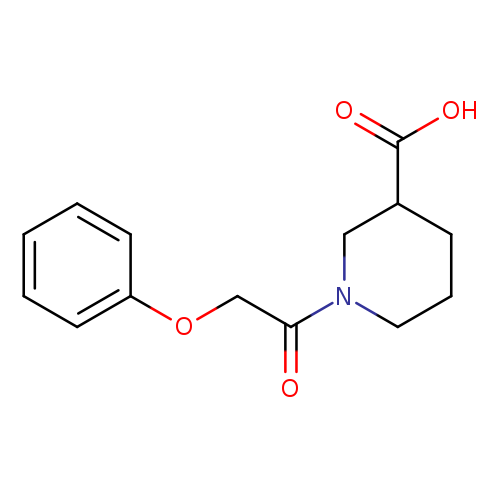
1-(2-Phenoxyacetyl)piperidine-3-carboxylic acidCatalog No.:AA00H9HC CAS No.:1017163-00-6 MDL No.:MFCD09816870 MF:C14H17NO4 MW:263.2891 |
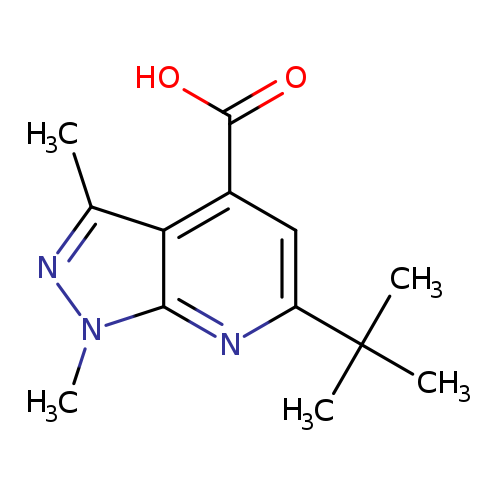
6-(tert-Butyl)-1,3-dimethyl-1h-pyrazolo[3,4-b]pyridine-4-carboxylic acidCatalog No.:AA01C6CT CAS No.:1017163-11-9 MDL No.:MFCD10002294 MF:C13H17N3O2 MW:247.2930 |
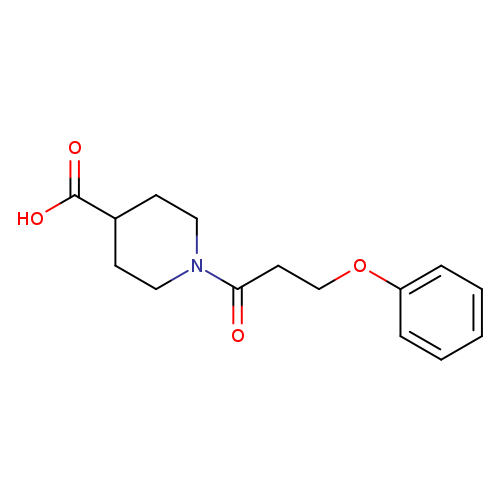
1-(3-Phenoxypropanoyl)piperidine-4-carboxylic acidCatalog No.:AA019VGP CAS No.:1017163-16-4 MDL No.:MFCD09809412 MF:C15H19NO4 MW:277.3157 |
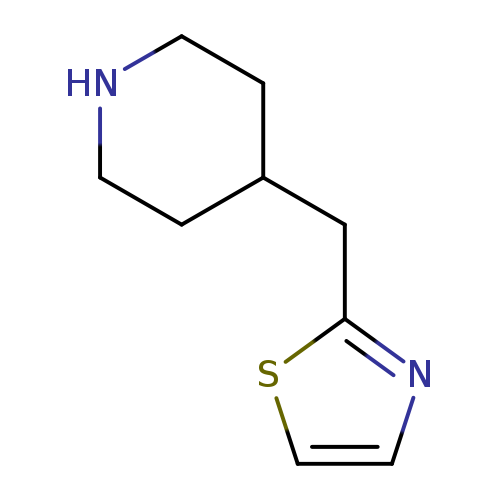
2-(piperidin-4-ylmethyl)thiazoleCatalog No.:AA01C076 CAS No.:1017163-98-2 MDL No.:MFCD09891834 MF:C9H14N2S MW:182.2859 |
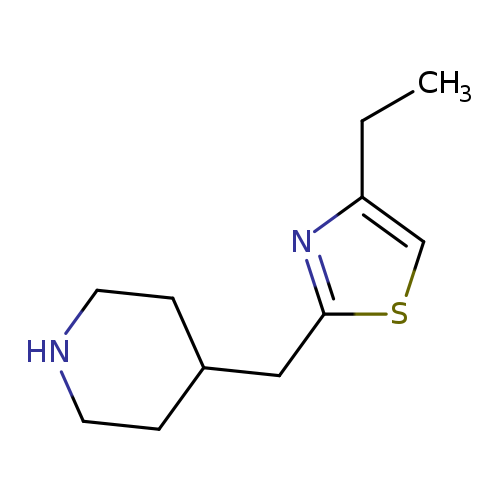
4-[(4-Ethyl-1,3-thiazol-2-yl)methyl]piperidineCatalog No.:AA01B1TQ CAS No.:1017164-02-1 MDL No.:MFCD09891837 MF:C11H18N2S MW:210.3390 |
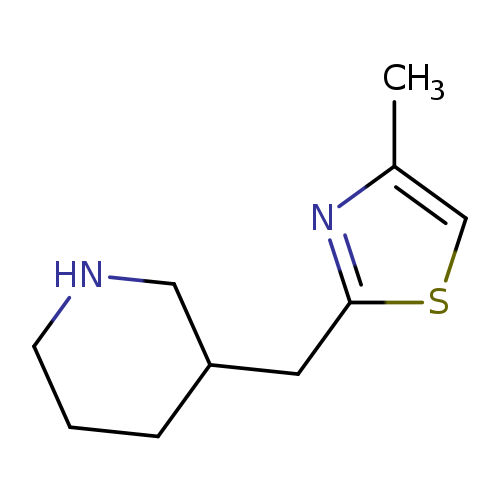
3-[(4-methyl-1,3-thiazol-2-yl)methyl]piperidineCatalog No.:AA01BIF7 CAS No.:1017164-21-4 MDL No.:MFCD09891855 MF:C10H16N2S MW:196.3124 |
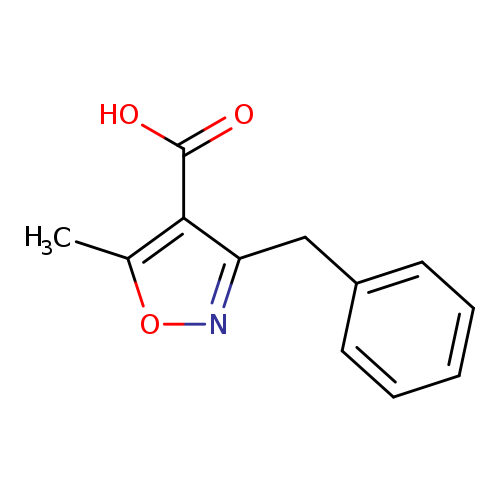
3-benzyl-5-methyl-1,2-oxazole-4-carboxylic acidCatalog No.:AA01C955 CAS No.:1017165-31-9 MDL No.:MFCD07376065 MF:C12H11NO3 MW:217.2206 |
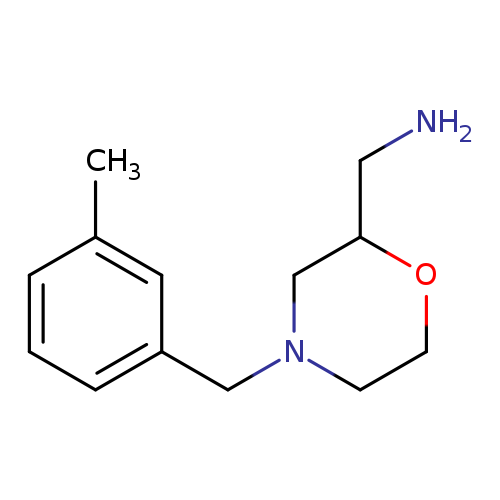
{4-[(3-methylphenyl)methyl]morpholin-2-yl}methanamineCatalog No.:AA01BS0N CAS No.:1017165-81-9 MDL No.:MFCD09892098 MF:C13H20N2O MW:220.3107 |
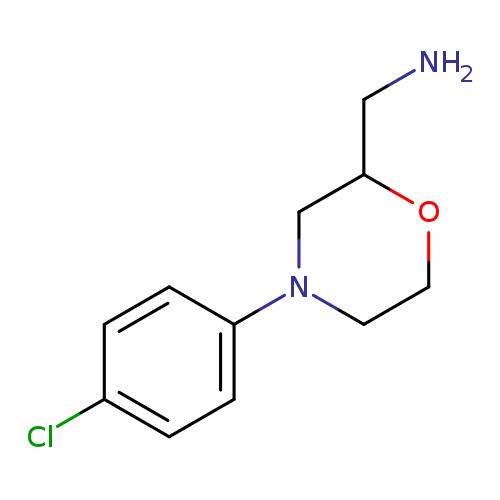
[4-(4-chlorophenyl)morpholin-2-yl]methanamineCatalog No.:AA01BUUM CAS No.:1017166-10-7 MDL No.:MFCD09892142 MF:C11H15ClN2O MW:226.7026 |
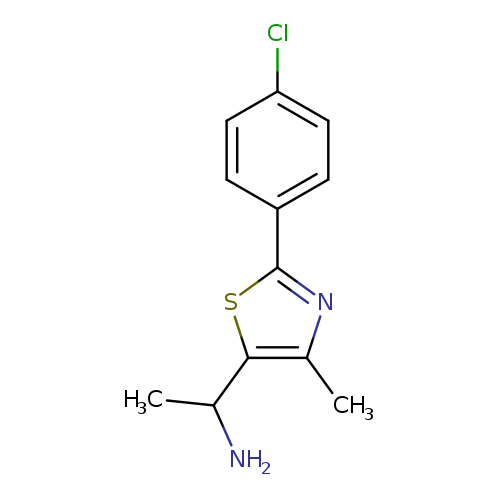
1-[2-(4-Chlorophenyl)-4-methyl-1,3-thiazol-5-yl]ethan-1-amineCatalog No.:AA01ABBR CAS No.:1017170-44-3 MDL No.:MFCD09882222 MF:C12H13ClN2S MW:252.7630 |
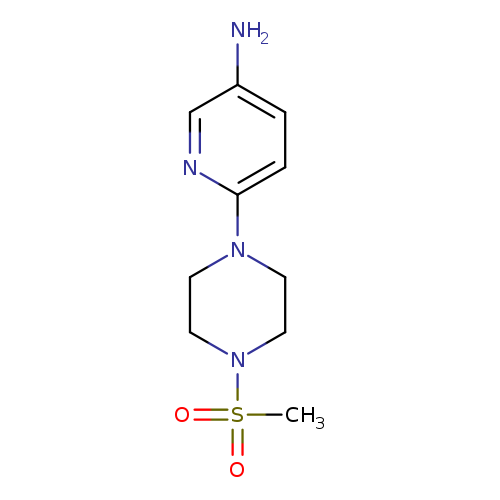
6-(4-(Methylsulfonyl)piperazin-1-yl)pyridin-3-amineCatalog No.:AA01AHJD CAS No.:1017171-54-8 MDL No.:MFCD09882448 MF:C10H16N4O2S MW:256.3246 |
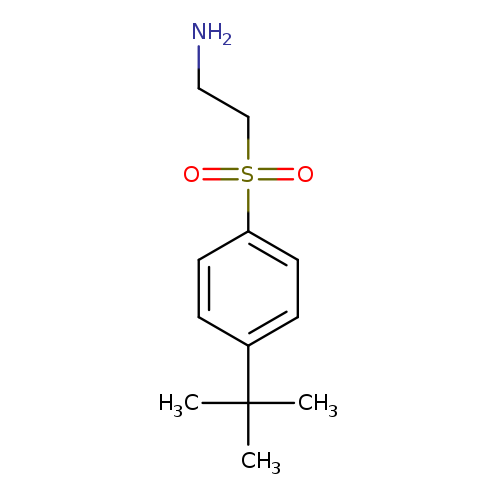
2-[(4-tert-Butylbenzene)sulfonyl]ethan-1-amineCatalog No.:AA01AGKO CAS No.:1017173-46-4 MDL No.:MFCD09893686 MF:C12H19NO2S MW:241.3498 |
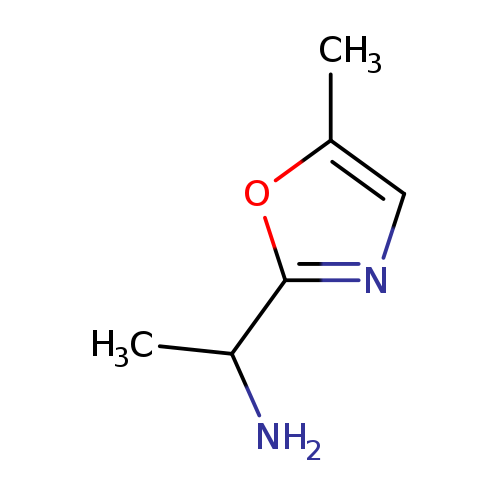
1-(5-methyl-1,3-oxazol-2-yl)ethan-1-amineCatalog No.:AA01A59D CAS No.:1017179-33-7 MDL No.:MFCD09884299 MF:C6H10N2O MW:126.1564 |
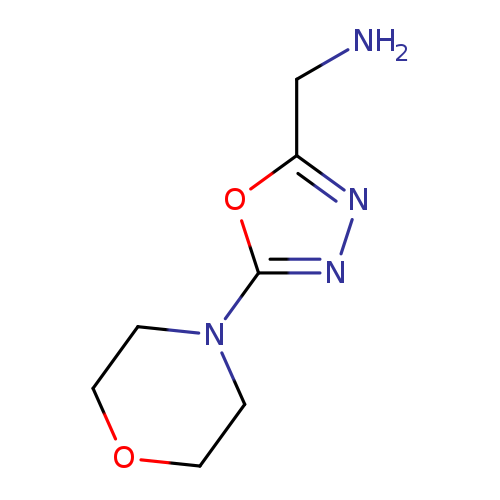
[5-(MORPHOLIN-4-YL)-1,3,4-OXADIAZOL-2-YL]METHANAMINECatalog No.:AA01EI4F CAS No.:1017179-95-1 MDL No.:MFCD09884370 MF:C7H12N4O2 MW:184.1958 |
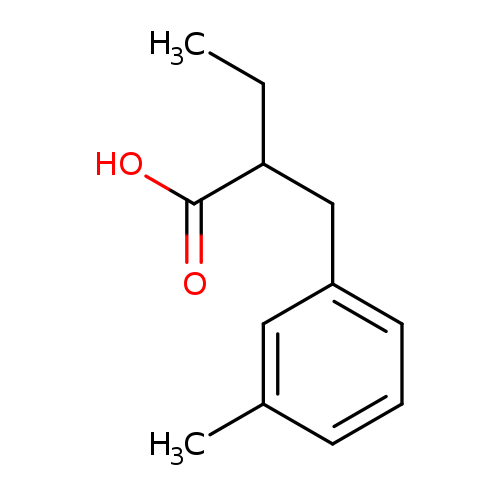
2-[(3-methylphenyl)methyl]butanoic acidCatalog No.:AA01E97B CAS No.:1017183-18-4 MDL No.:MFCD09894725 MF:C12H16O2 MW:192.2542 |
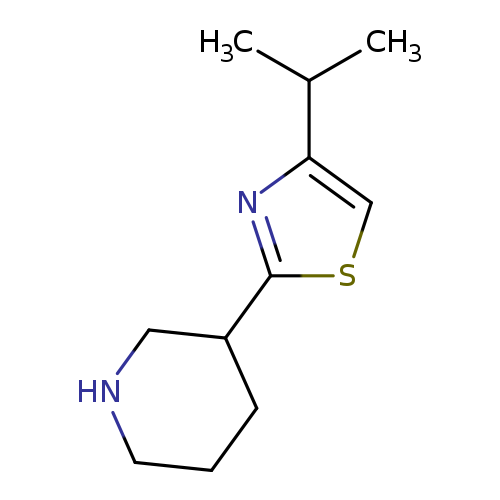
3-[4-(propan-2-yl)-1,3-thiazol-2-yl]piperidineCatalog No.:AA019Q6S CAS No.:1017184-32-5 MDL No.:MFCD09891527 MF:C11H18N2S MW:210.3390 |
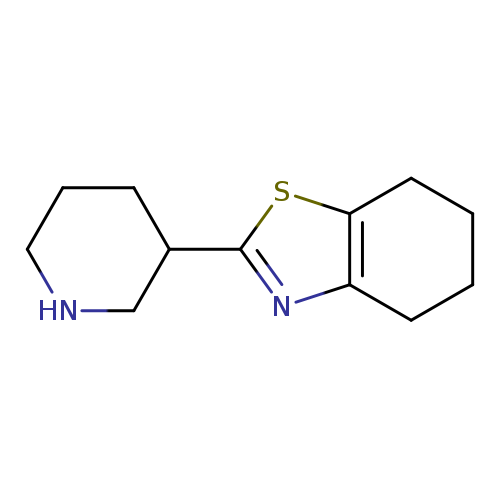
2-(piperidin-3-yl)-4,5,6,7-tetrahydro-1,3-benzothiazoleCatalog No.:AA01A13Q CAS No.:1017184-36-9 MDL No.:MFCD09891531 MF:C12H18N2S MW:222.3497 |

3-amino-2-(pyridin-2-ylmethyl)propan-1-olCatalog No.:AA019Y8U CAS No.:1017184-54-1 MDL No.:MFCD09894873 MF:C9H14N2O MW:166.2203 |
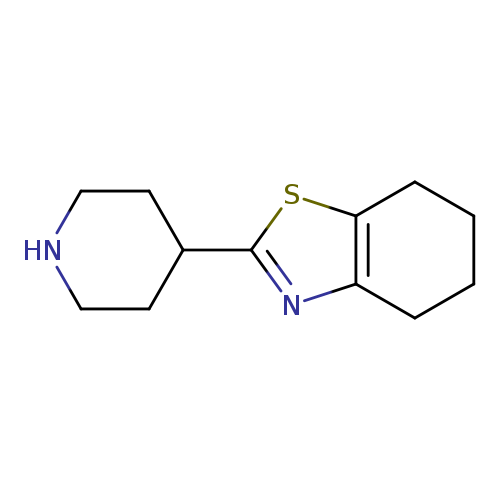
2-(piperidin-4-yl)-4,5,6,7-tetrahydro-1,3-benzothiazoleCatalog No.:AA01C3RM CAS No.:1017184-56-3 MDL No.:MFCD09891555 MF:C12H18N2S MW:222.3497 |
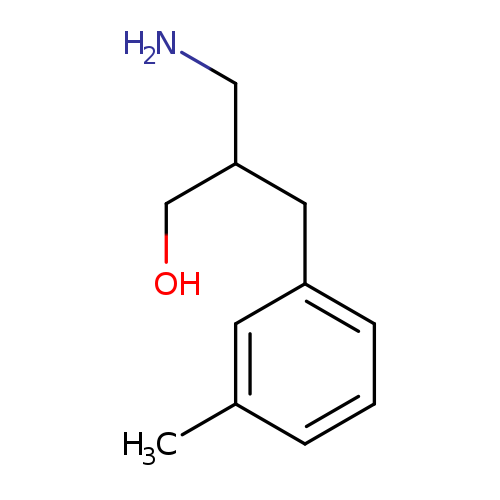
3-amino-2-[(3-methylphenyl)methyl]propan-1-olCatalog No.:AA019MP5 CAS No.:1017184-58-5 MDL No.:MFCD09894877 MF:C11H17NO MW:179.2588 |
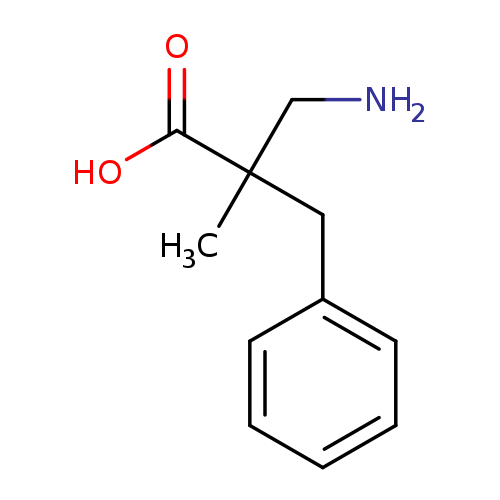
3-amino-2-benzyl-2-methylpropanoic acidCatalog No.:AA01BDKF CAS No.:1017185-07-7 MDL No.:MFCD09894942 MF:C11H15NO2 MW:193.2423 |
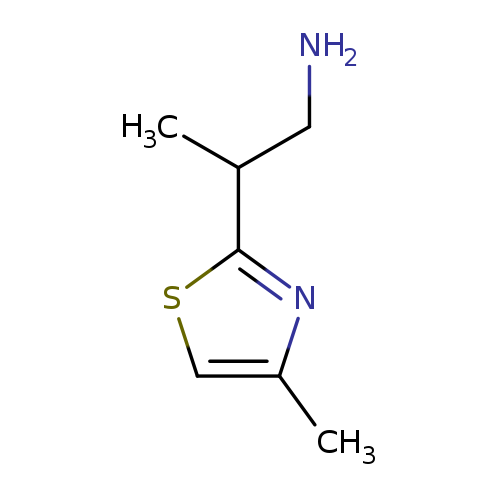
2-(4-Methylthiazol-2-yl)propan-1-amineCatalog No.:AA008V2V CAS No.:1017185-91-9 MDL No.:MFCD09891726 MF:C7H12N2S MW:156.2486 |
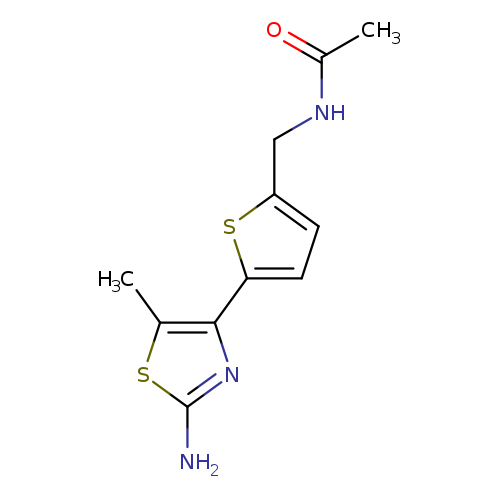
N-([5-(2-Amino-5-methyl-1,3-thiazol-4-yl)thien-2-yl]methyl)acetamideCatalog No.:AA019LM5 CAS No.:1017190-42-9 MDL No.:MFCD09971604 MF:C11H13N3OS2 MW:267.3704 |
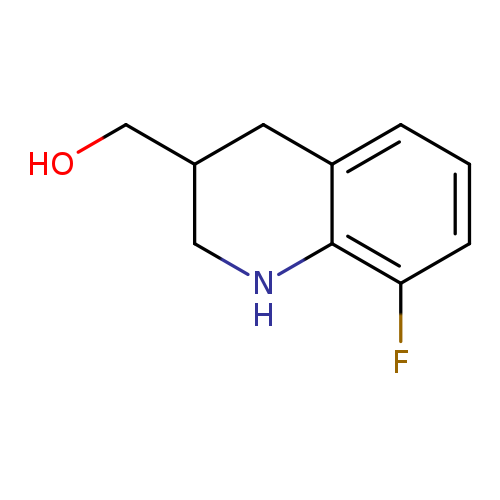
(8-fluoro-1,2,3,4-tetrahydroquinolin-3-yl)methanolCatalog No.:AA01C57U CAS No.:1017191-02-4 MDL No.:MFCD09895662 MF:C10H12FNO MW:181.2068 |
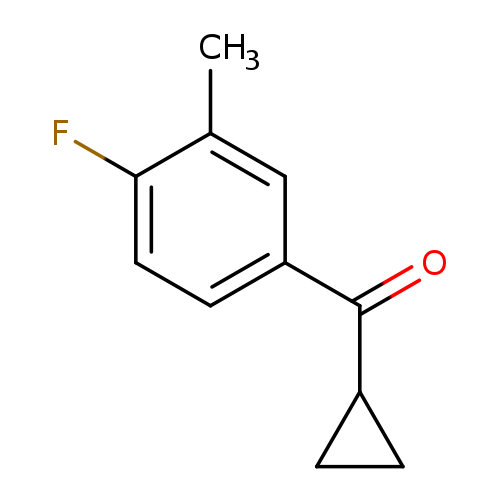
cyclopropyl(4-fluoro-3-methylphenyl)methanoneCatalog No.:AA00VSA5 CAS No.:1017193-22-4 MDL No.:MFCD09889411 MF:C11H11FO MW:178.2028 |
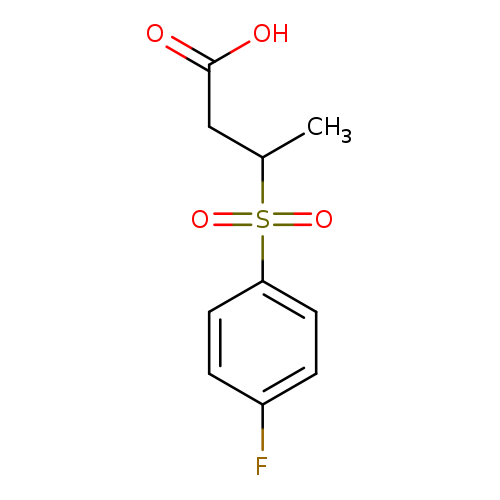
3-[(4-Fluorobenzene)sulfonyl]butanoic acidCatalog No.:AA01AAR8 CAS No.:1017197-73-7 MDL No.:MFCD09893950 MF:C10H11FO4S MW:246.2553 |
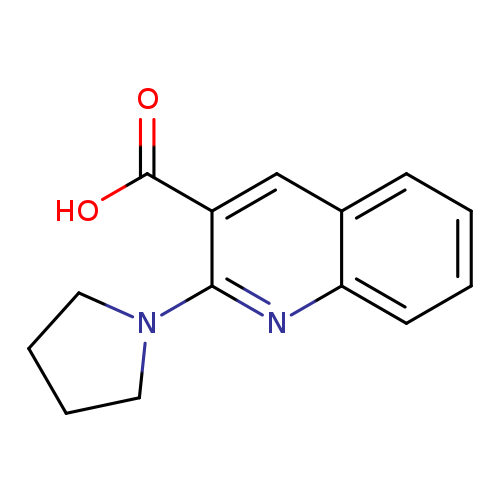
2-(pyrrolidin-1-yl)quinoline-3-carboxylic acidCatalog No.:AA01A9AC CAS No.:1017199-72-2 MDL No.:MFCD09896464 MF:C14H14N2O2 MW:242.2732 |
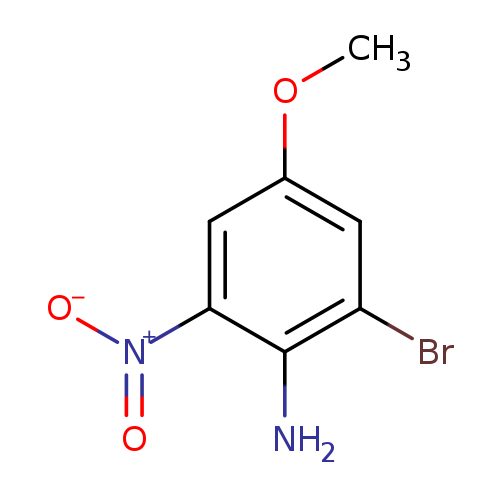
2-Bromo-4-methoxy-6-nitroanilineCatalog No.:AA00058L CAS No.:10172-35-7 MDL No.:MFCD18088521 MF:C7H7BrN2O3 MW:247.0461 |
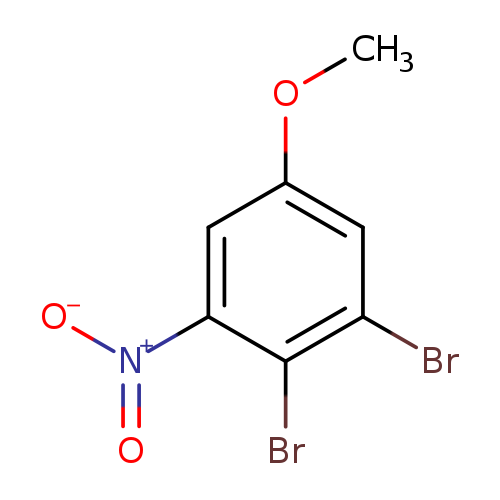
2,3-Dibromo-5-methoxynitrobenzeneCatalog No.:AA00058K CAS No.:10172-36-8 MDL No.:MFCD00094478 MF:C7H5Br2NO3 MW:310.9275 |

N-Acetyl-D-phenylalanineCatalog No.:AA00058G CAS No.:10172-89-1 MDL No.:MFCD00002664 MF:C11H13NO3 MW:207.2258 |
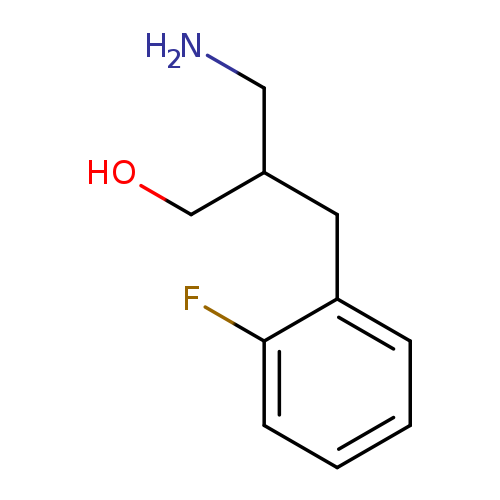
3-amino-2-[(2-fluorophenyl)methyl]propan-1-olCatalog No.:AA019SBQ CAS No.:1017209-73-2 MDL No.:MFCD09894879 MF:C10H14FNO MW:183.2227 |
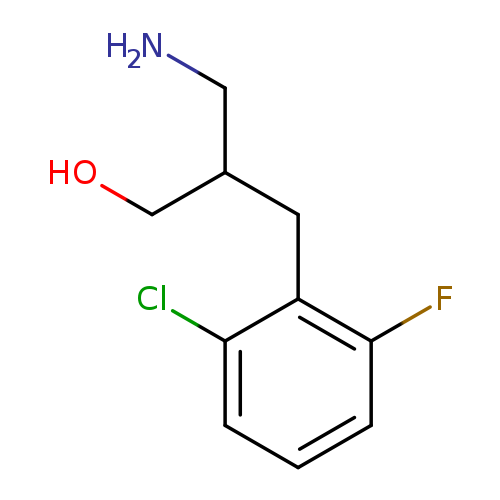
3-amino-2-[(2-chloro-6-fluorophenyl)methyl]propan-1-olCatalog No.:AA019SEZ CAS No.:1017209-82-3 MDL No.:MFCD09894892 MF:C10H13ClFNO MW:217.6677 |
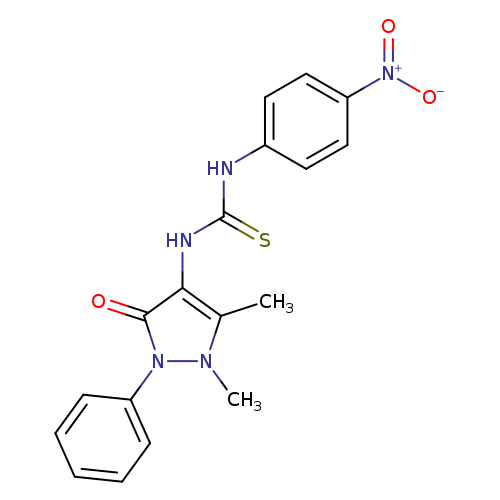
3-(1,5-dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-yl)-1-(4-nitrophenyl)thioureaCatalog No.:AA00ITF6 CAS No.:101721-59-9 MDL No.:MFCD01567589 MF:C18H17N5O3S MW:383.4243 |
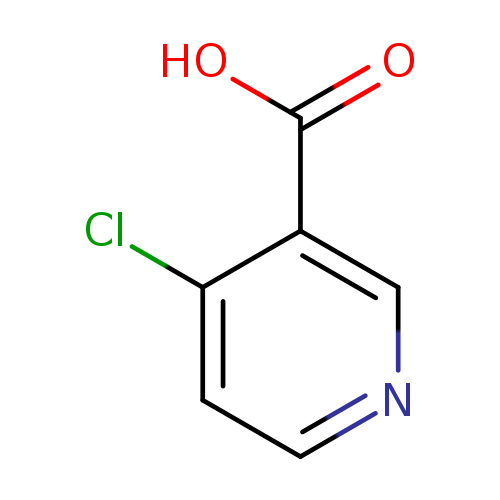
4-Chloronicotinic acidCatalog No.:AA0005D8 CAS No.:10177-29-4 MDL No.:MFCD00128860 MF:C6H4ClNO2 MW:157.5545 |
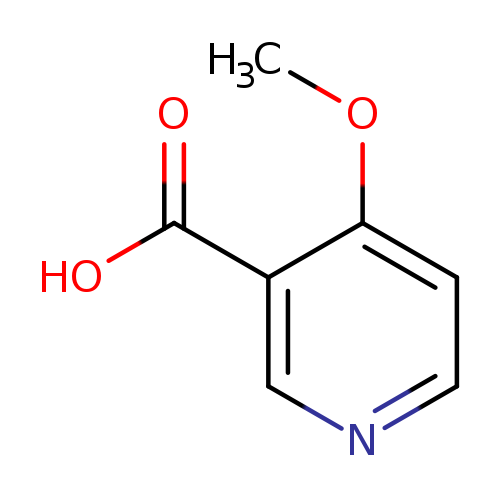
4-Methoxynicotinic acidCatalog No.:AA0005D7 CAS No.:10177-31-8 MDL No.:MFCD04115724 MF:C7H7NO3 MW:153.1354 |
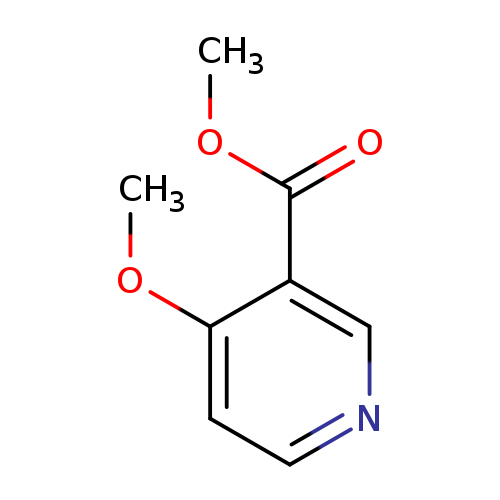
Methyl 4-methoxynicotinateCatalog No.:AA0005D6 CAS No.:10177-32-9 MDL No.:MFCD09864940 MF:C8H9NO3 MW:167.1620 |
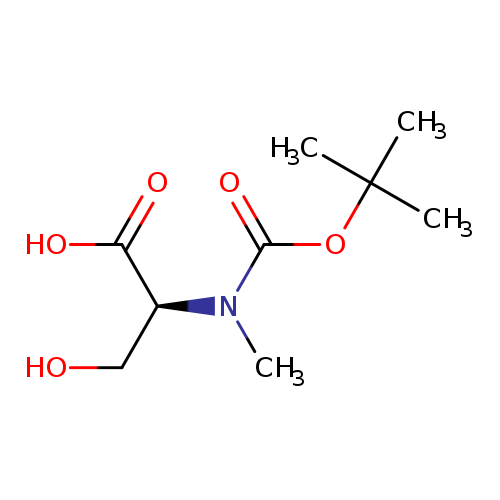
Boc-N-Me-Ser-OHCatalog No.:AA0005E1 CAS No.:101772-29-6 MDL No.:MFCD00037249 MF:C9H17NO5 MW:219.2350 |
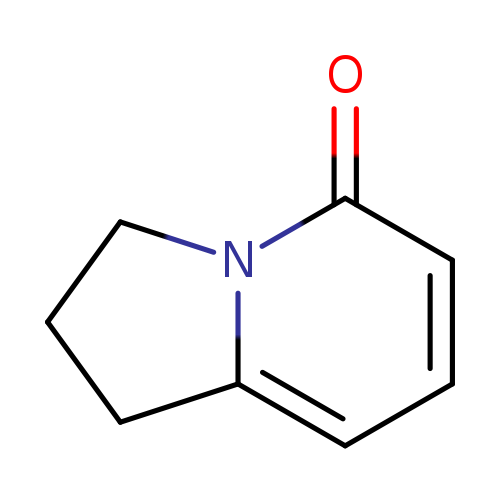
1,2,3,5-tetrahydroindolizin-5-oneCatalog No.:AA0005DT CAS No.:101773-62-0 MDL No.:MFCD19704726 MF:C8H9NO MW:135.1632 |
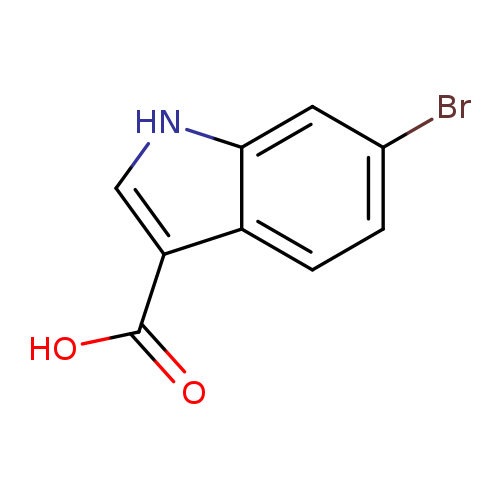
6-Bromo-1h-indole-3-carboxylic acidCatalog No.:AA0005DQ CAS No.:101774-27-0 MDL No.:MFCD05664008 MF:C9H6BrNO2 MW:240.0534 |
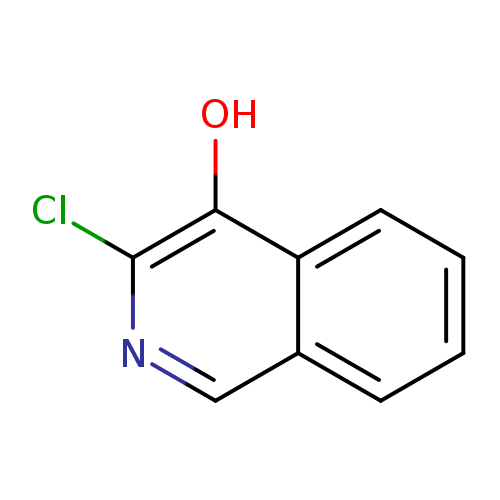
3-Chloroisoquinolin-4-olCatalog No.:AA0005DP CAS No.:101774-33-8 MDL No.:MFCD11846288 MF:C9H6ClNO MW:179.6030 |
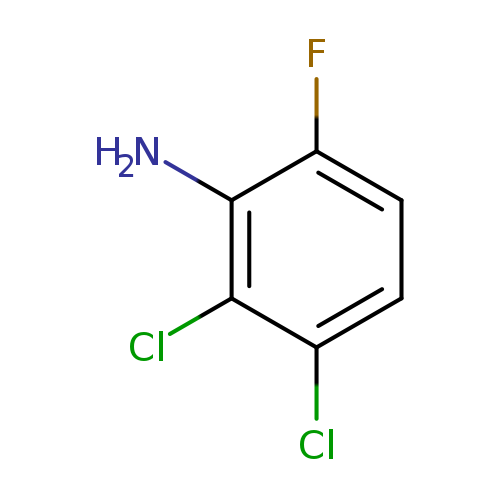
2,3-Dichloro-6-fluoroanilineCatalog No.:AA0090A0 CAS No.:1017777-51-3 MDL No.:MFCD09832261 MF:C6H4Cl2FN MW:180.0071 |
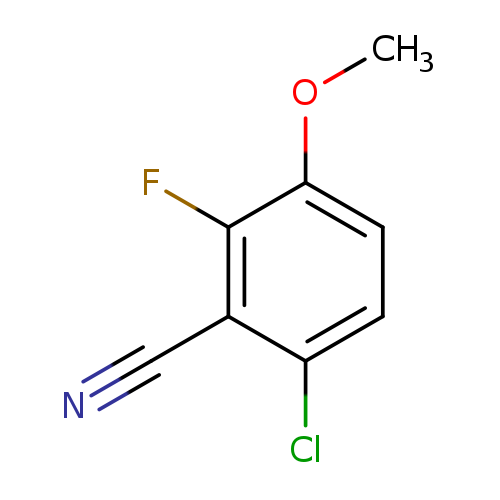
6-Chloro-2-fluoro-3-methoxybenzonitrileCatalog No.:AA0090A7 CAS No.:1017777-72-8 MDL No.:MFCD09832269 MF:C8H5ClFNO MW:185.5828 |
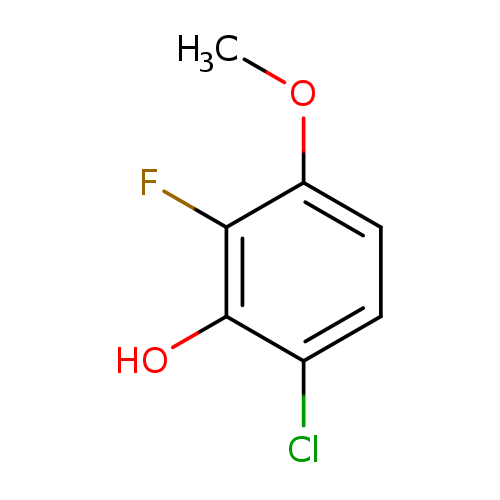
6-Chloro-2-fluoro-3-methoxyphenolCatalog No.:AA0090AM CAS No.:1017777-74-0 MDL No.:MFCD09832270 MF:C7H6ClFO2 MW:176.5727 |
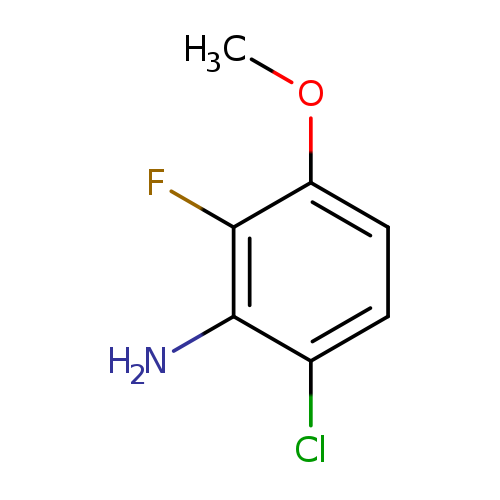
6-Chloro-2-fluoro-3-methoxyanilineCatalog No.:AA008YUY CAS No.:1017777-77-3 MDL No.:MFCD09832271 MF:C7H7ClFNO MW:175.5880 |
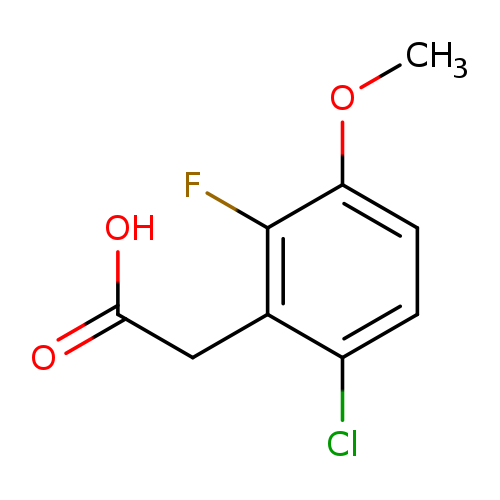
6-Chloro-2-fluoro-3-methoxyphenylacetic acidCatalog No.:AA0005EU CAS No.:1017777-83-1 MDL No.:MFCD09832273 MF:C9H8ClFO3 MW:218.6094 |
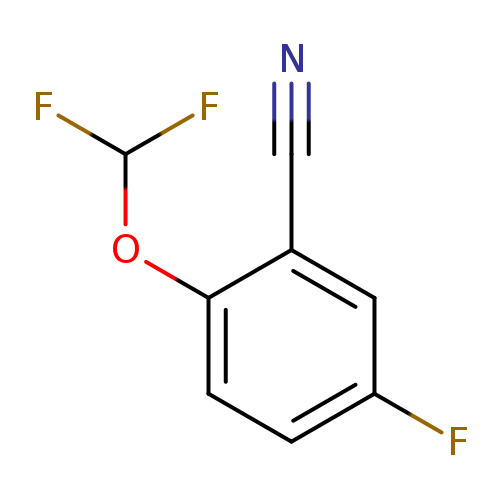
2-(Difluoromethoxy)-5-fluorobenzonitrileCatalog No.:AA009NYJ CAS No.:1017778-48-1 MDL No.:MFCD09832329 MF:C8H4F3NO MW:187.1187 |
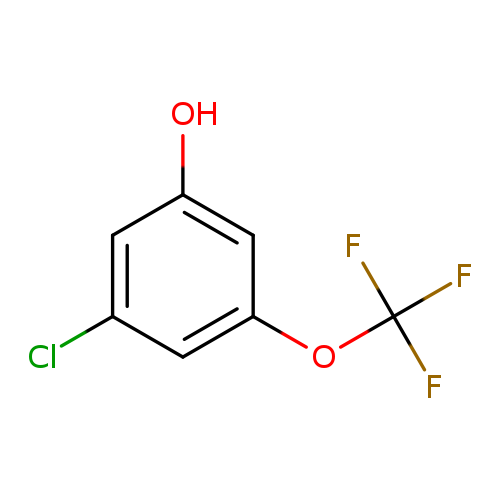
3-Chloro-5-(trifluoromethoxy)phenolCatalog No.:AA00H9IR CAS No.:1017778-52-7 MDL No.:MFCD09832330 MF:C7H4ClF3O2 MW:212.5537 |
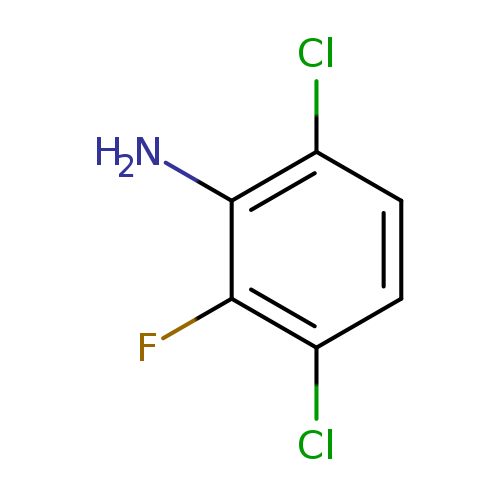
3,6-Dichloro-2-fluoroanilineCatalog No.:AA00VRM9 CAS No.:1017778-56-1 MDL No.:MFCD09832331 MF:C6H4Cl2FN MW:180.0071 |
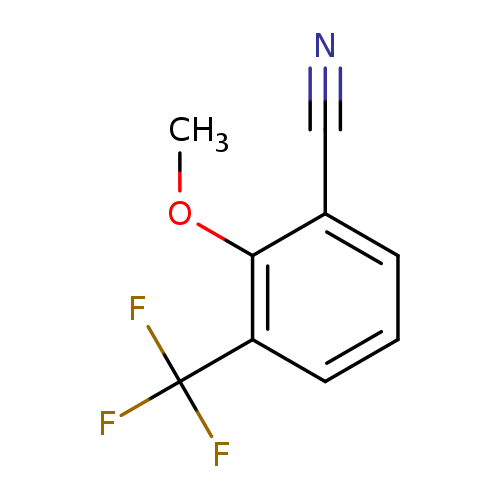
2-Methoxy-3-(trifluoromethyl)benzonitrileCatalog No.:AA00VRM7 CAS No.:1017778-62-9 MDL No.:MFCD09832310 MF:C9H6F3NO MW:201.1452 |
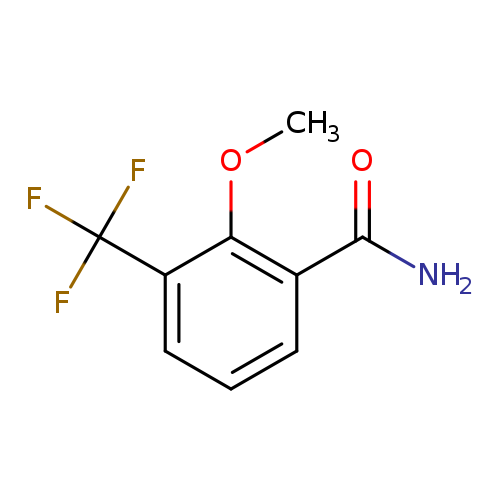
2-Methoxy-3-(trifluoromethyl)benzamideCatalog No.:AA0099TQ CAS No.:1017778-70-9 MDL No.:MFCD09832313 MF:C9H8F3NO2 MW:219.1605 |
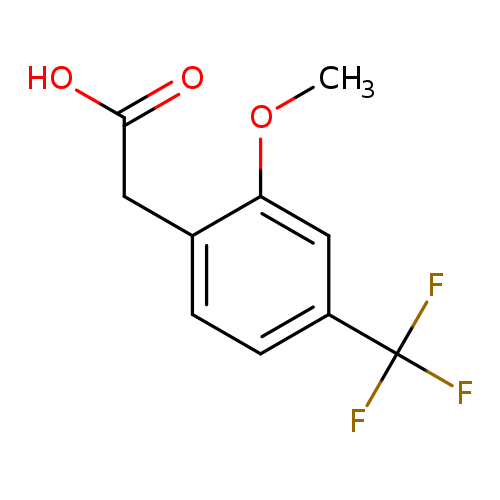
2-(2-Methoxy-4-(trifluoromethyl)phenyl)acetic acidCatalog No.:AA0095C0 CAS No.:1017779-22-4 MDL No.:MFCD09832353 MF:C10H9F3O3 MW:234.1719 |
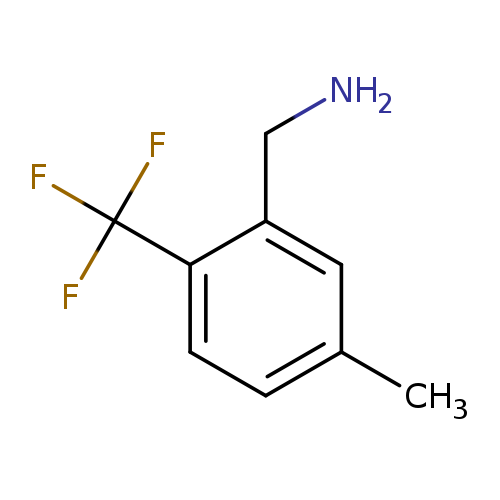
(5-Methyl-2-(trifluoromethyl)phenyl)methanamineCatalog No.:AA00H9JB CAS No.:1017779-30-4 MDL No.:MFCD09832358 MF:C9H10F3N MW:189.1776 |
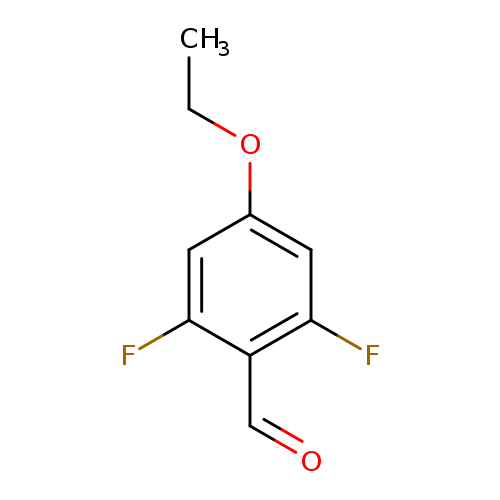
4-Ethoxy-2,6-difluorobenzaldehydeCatalog No.:AA0005EM CAS No.:1017779-48-4 MDL No.:MFCD09258695 MF:C9H8F2O2 MW:186.1554 |
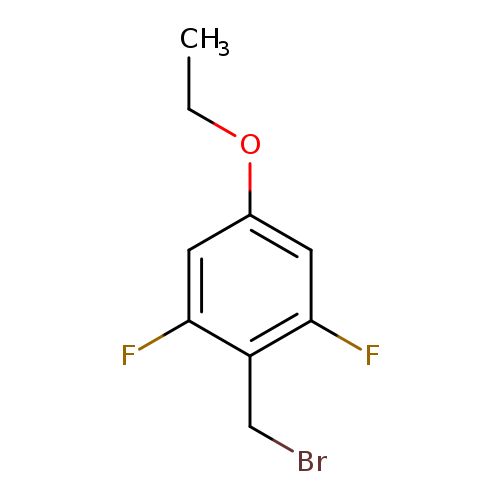
2-(Bromomethyl)-5-ethoxy-1,3-difluorobenzeneCatalog No.:AA0005EL CAS No.:1017779-51-9 MDL No.:MFCD09258697 MF:C9H9BrF2O MW:251.0680 |
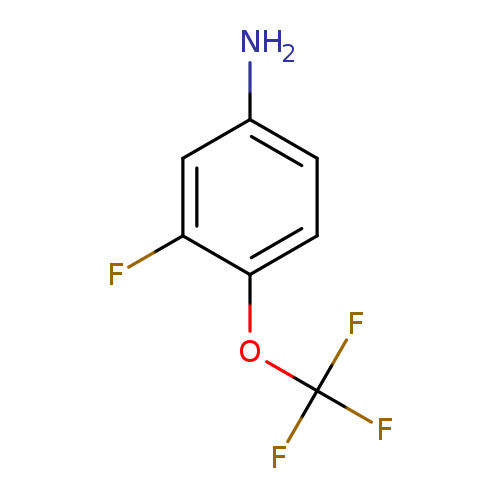
3-Fluoro-4-(trifluoromethoxy)anilineCatalog No.:AA0005EI CAS No.:1017779-69-9 MDL No.:MFCD09832383 MF:C7H5F4NO MW:195.1143 |
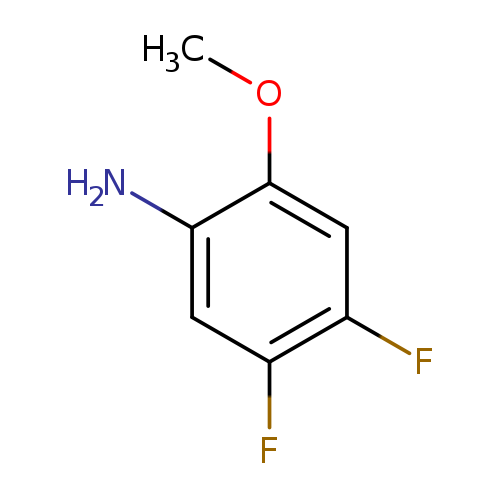
4,5-Difluoro-2-methoxyanilineCatalog No.:AA0005EH CAS No.:1017779-71-3 MDL No.:MFCD09832387 MF:C7H7F2NO MW:159.1334 |

2,3,6-Trifluorobenzenesulfonyl chlorideCatalog No.:AA0005EG CAS No.:1017779-75-7 MDL No.:MFCD09832391 MF:C6H2ClF3O2S MW:230.5921 |
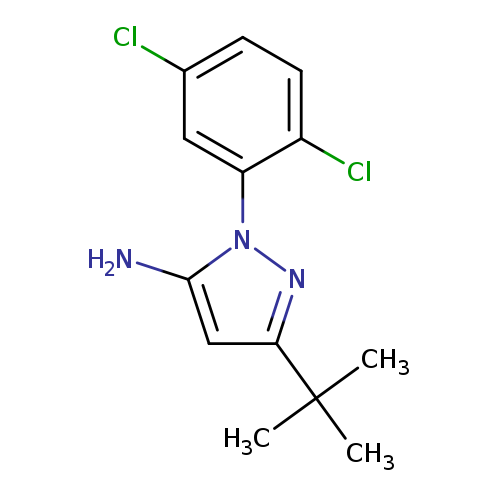
5-tert-Butyl-2-(2,5-dichloro-phenyl)-2h-pyrazol-3-ylamineCatalog No.:AA0005EB CAS No.:1017781-20-2 MDL No.:MFCD04115089 MF:C13H15Cl2N3 MW:284.1843 |
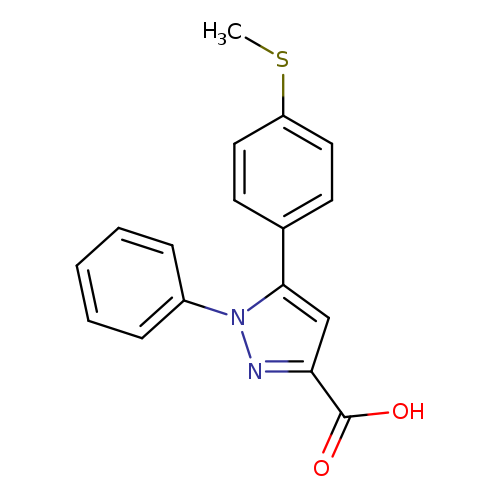
5-(4-Methylsulfanyl-phenyl)-1-phenyl-1h-pyrazole-3-carboxylic acidCatalog No.:AA008X6T CAS No.:1017781-22-4 MDL No.:MFCD04115100 MF:C17H14N2O2S MW:310.3703 |
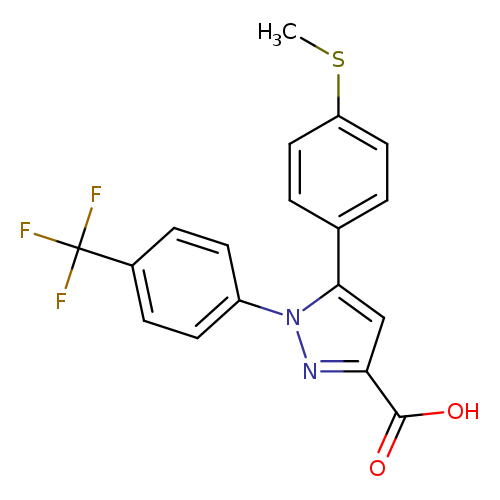
5-(4-Methylsulfanyl-phenyl)-1-(4-trifluoromethyl-phenyl)-1h-pyrazole-3-carboxylic acidCatalog No.:AA0005EA CAS No.:1017781-23-5 MDL No.:MFCD04115084 MF:C18H13F3N2O2S MW:378.3682 |
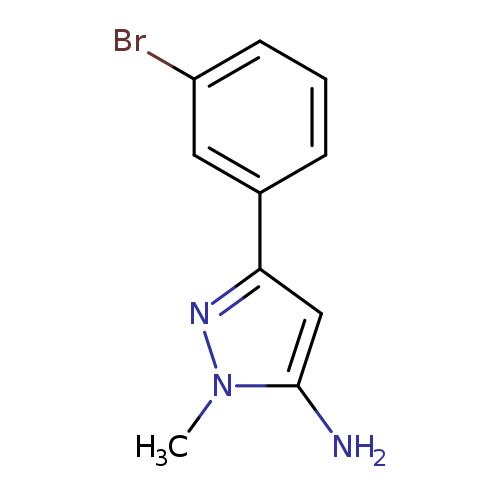
5-(3-Bromophenyl)-2-methylpyrazol-3-amineCatalog No.:AA0005E6 CAS No.:1017781-27-9 MDL No.:MFCD06738293 MF:C10H10BrN3 MW:252.1105 |
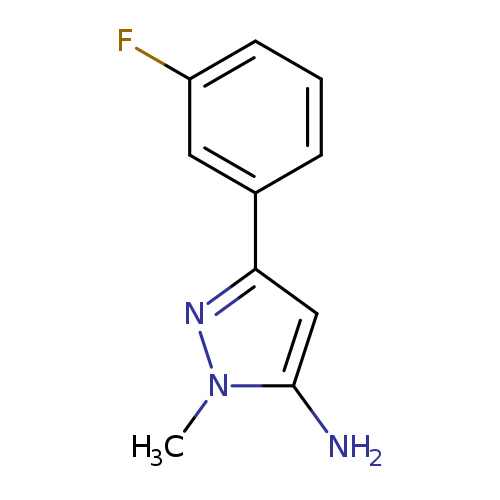
5-Amino-3-(3-fluorophenyl)-1-methylpyrazoleCatalog No.:AA0005FJ CAS No.:1017781-28-0 MDL No.:MFCD06738294 MF:C10H10FN3 MW:191.2049 |
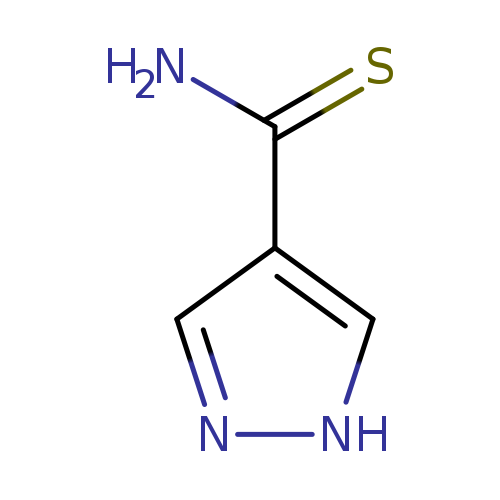
1H-Pyrazole-4-carbothioamideCatalog No.:AA0005FH CAS No.:1017781-31-5 MDL No.:MFCD06738945 MF:C4H5N3S MW:127.1676 |
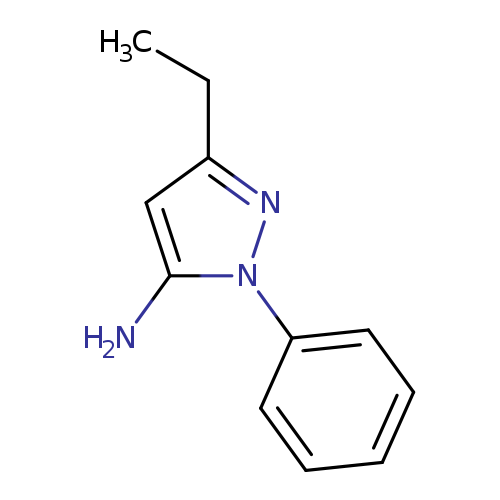
3-Ethyl-1-phenyl-1h-pyrazol-5-amineCatalog No.:AA0005FD CAS No.:1017781-37-1 MDL No.:MFCD02255835 MF:C11H13N3 MW:187.2410 |
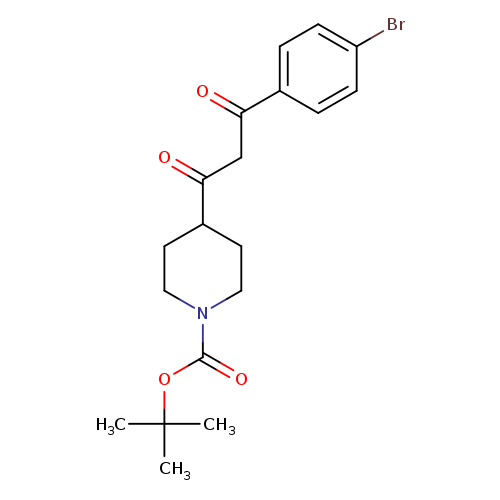
tert-Butyl 4-(3-(4-bromophenyl)-3-oxopropanoyl)piperidine-1-carboxylateCatalog No.:AA0005F5 CAS No.:1017781-49-5 MDL No.:MFCD09997718 MF:C19H24BrNO4 MW:410.3022 |
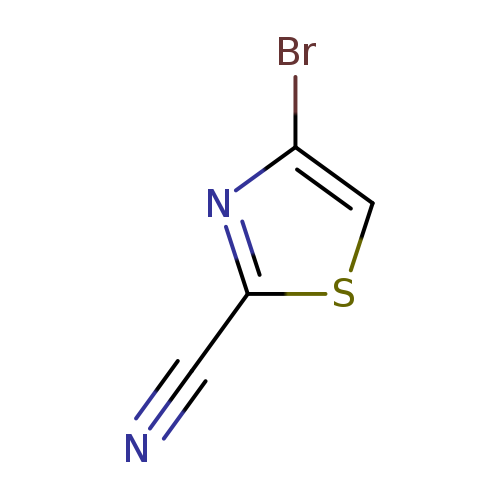
4-Bromo-2-cyanothiazoleCatalog No.:AA0005F2 CAS No.:1017781-52-0 MDL No.:MFCD09607690 MF:C4HBrN2S MW:189.0331 |
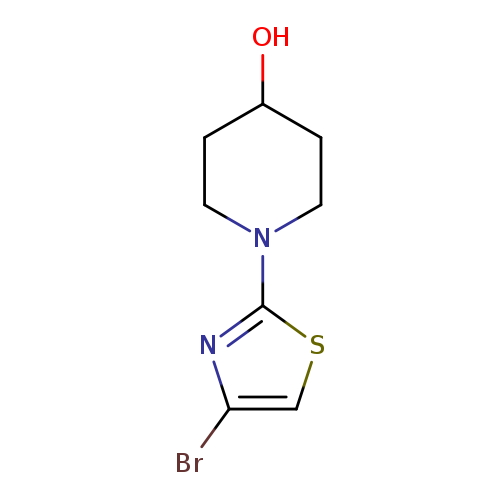
4-Bromo-2-(4-hydroxypiperidino)thiazoleCatalog No.:AA0005EY CAS No.:1017781-58-6 MDL No.:MFCD09607693 MF:C8H11BrN2OS MW:263.1547 |
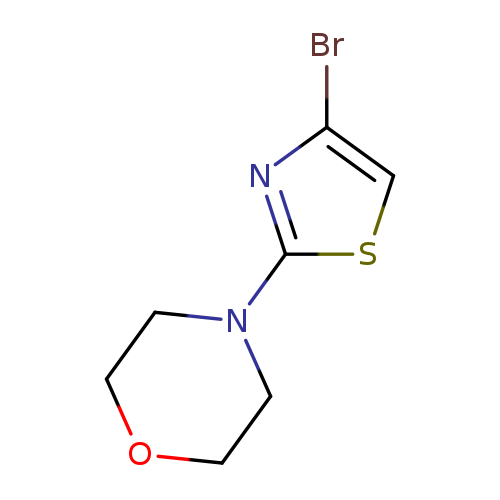
4-(4-Bromothiazol-2-yl)morpholineCatalog No.:AA0005EW CAS No.:1017781-60-0 MDL No.:MFCD09607696 MF:C7H9BrN2OS MW:249.1282 |
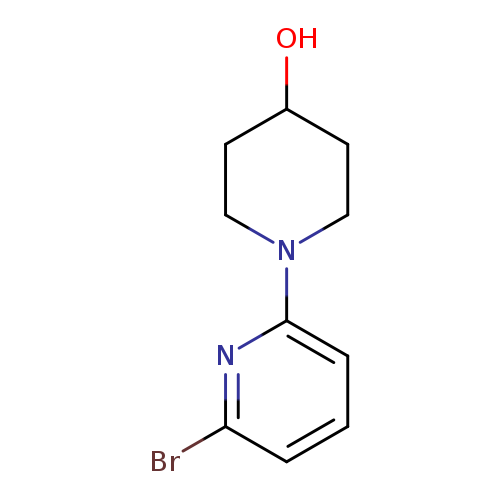
1-(6-Bromopyridin-2-yl)piperidin-4-olCatalog No.:AA0005G7 CAS No.:1017781-64-4 MDL No.:MFCD09607698 MF:C10H13BrN2O MW:257.1270 |
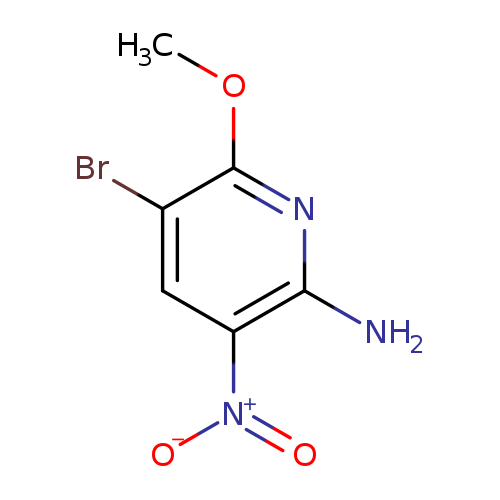
5-Bromo-6-methoxy-3-nitro-pyridin-2-ylamineCatalog No.:AA0005FL CAS No.:1017782-09-0 MDL No.:MFCD09997888 MF:C6H6BrN3O3 MW:248.0341 |
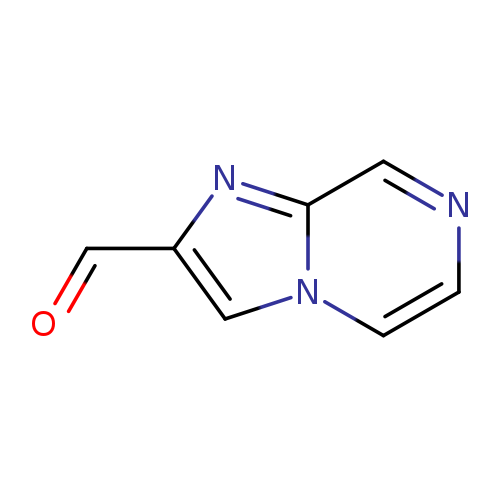
Imidazo[1,2-a]pyrazine-2-carbaldehydeCatalog No.:AA0005GW CAS No.:1017782-15-8 MDL No.:MFCD09995736 MF:C7H5N3O MW:147.1341 |
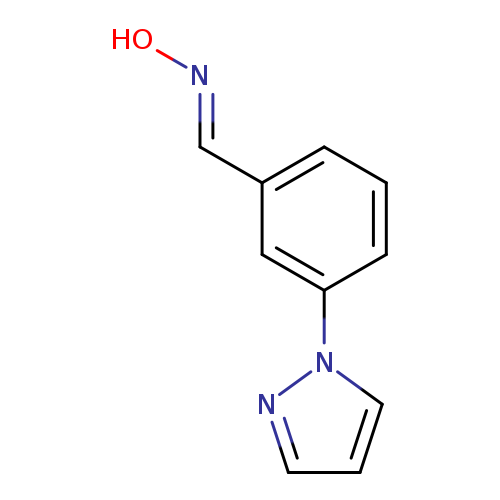
3-(1H-Pyrazol-1-yl)benzenecarbaldehyde oximeCatalog No.:AA00ITIT CAS No.:1017782-43-2 MDL No.:MFCD09152715 MF:C10H9N3O MW:187.1980 |
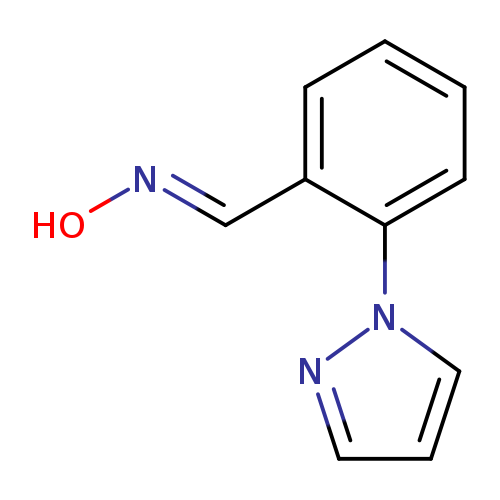
2-(1H-Pyrazol-1-yl)benzaldehyde oximeCatalog No.:AA00ITIV CAS No.:1017782-44-3 MDL No.:MFCD09152718 MF:C10H9N3O MW:187.1980 |
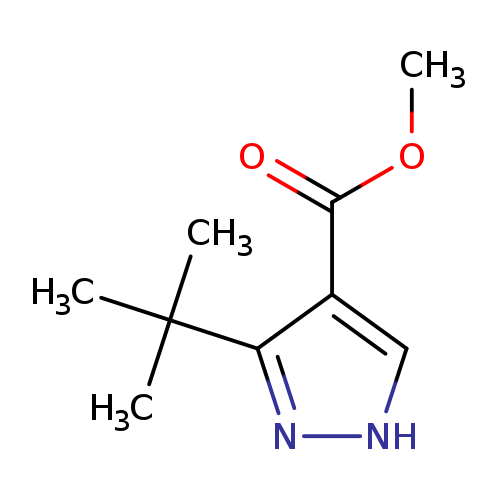
Methyl 3-(tert-butyl)-1h-pyrazole-4-carboxylateCatalog No.:AA0005GU CAS No.:1017782-45-4 MDL No.:MFCD00829900 MF:C9H14N2O2 MW:182.2197 |
N-(2-hydroxyethyl)-4-(1H-pyrazol-1-yl)benzamideCatalog No.:AA00IWBS CAS No.:1017782-46-5 MDL No.:MFCD09607919 MF:C12H13N3O2 MW:231.2505 |
4-((3,4-Difluorobenzyl)oxy)benzaldehydeCatalog No.:AA00IZ51 CAS No.:1017782-47-6 MDL No.:MFCD09972208 MF:C14H10F2O2 MW:248.2248 |
Ethyl 1-(4-amino-3-methoxyphenyl)piperidine-4-carboxylateCatalog No.:AA0090WL CAS No.:1017782-48-7 MDL No.:MFCD09972211 MF:C15H22N2O3 MW:278.3468 |
6-Chloro-n-methyl-2-phenylpyrimidin-4-amineCatalog No.:AA00IRIP CAS No.:1017782-49-8 MDL No.:MFCD00697490 MF:C11H10ClN3 MW:219.6702 |
(4-((3,4-Dichlorophenoxy)methyl)phenyl)methanolCatalog No.:AA008ZDS CAS No.:1017782-50-1 MDL No.:MFCD09972212 MF:C14H12Cl2O2 MW:283.1499 |
1-(5-Chlorobenzo[d]oxazol-2-yl)-N-methylmethanamineCatalog No.:AA00IRIR CAS No.:1017782-51-2 MDL No.:MFCD09972215 MF:C9H9ClN2O MW:196.6336 |
{[5-(4-chlorophenyl)-1,3,4-oxadiazol-2-yl]methyl}(methyl)amineCatalog No.:AA00IRIS CAS No.:1017782-52-3 MDL No.:MFCD09972216 MF:C10H10ClN3O MW:223.6589 |
2-(2-(Methylsulfonyl)phenoxy)acetic acidCatalog No.:AA00IW3Q CAS No.:1017782-54-5 MDL No.:MFCD09972219 MF:C9H10O5S MW:230.2377 |
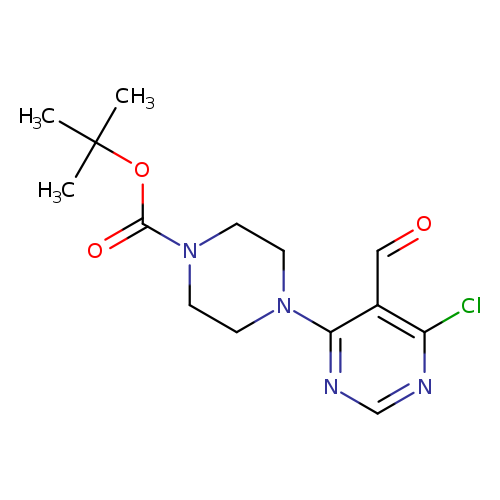
tert-Butyl 4-(6-chloro-5-formylpyrimidin-4-yl)piperazine-1-carboxylateCatalog No.:AA00IRIU CAS No.:1017782-55-6 MDL No.:MFCD09972225 MF:C14H19ClN4O3 MW:326.7787 |
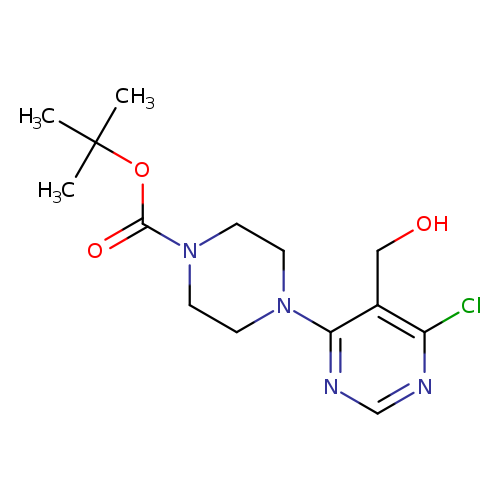
tert-Butyl 4-(6-chloro-5-(hydroxymethyl)pyrimidin-4-yl)piperazine-1-carboxylateCatalog No.:AA00ITJI CAS No.:1017782-56-7 MDL No.:MFCD09972226 MF:C14H21ClN4O3 MW:328.7945 |
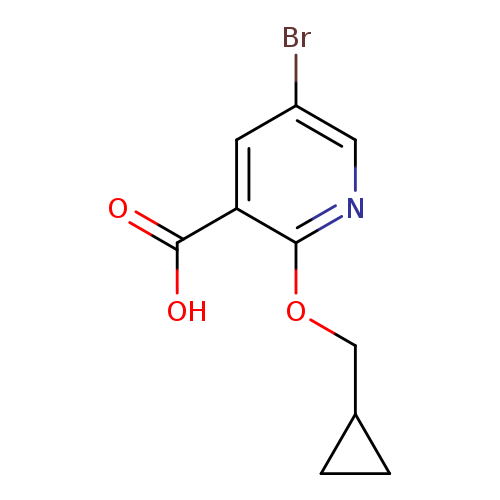
5-Bromo-2-(cyclopropylmethoxy)nicotinic acidCatalog No.:AA00IZ72 CAS No.:1017782-57-8 MDL No.:MFCD09972229 MF:C10H10BrNO3 MW:272.0953 |
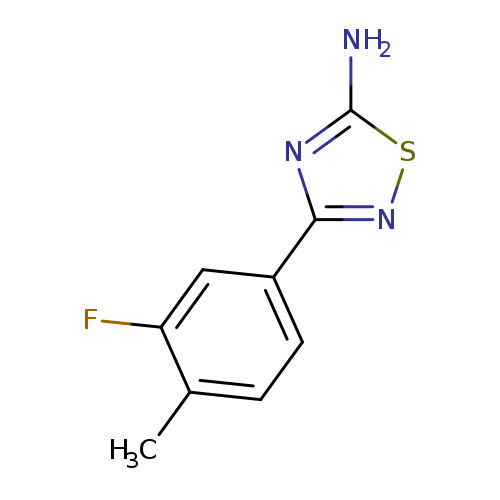
3-(3-fluoro-4-methylphenyl)-1,2,4-thiadiazol-5-amineCatalog No.:AA00ISIO CAS No.:1017782-58-9 MDL No.:MFCD09865007 MF:C9H8FN3S MW:209.2433 |
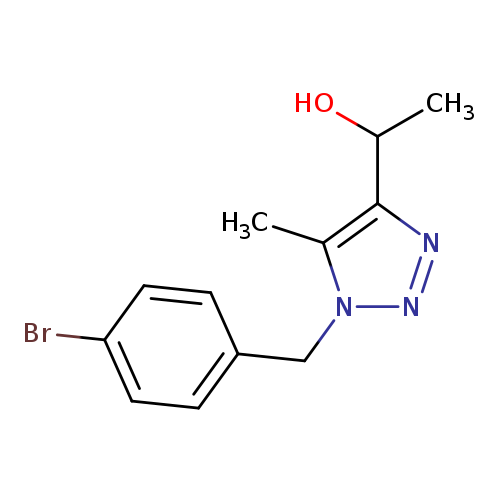
1-(1-(4-Bromobenzyl)-5-methyl-1H-1,2,3-triazol-4-yl)ethanolCatalog No.:AA00ISR4 CAS No.:1017782-60-3 MDL No.:MFCD09865012 MF:C12H14BrN3O MW:296.1631 |
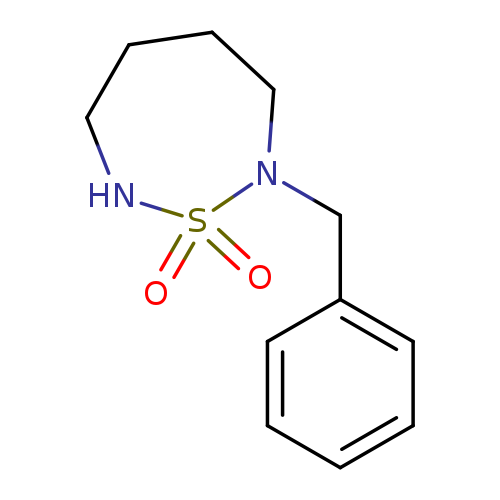
2-Benzyl-1,2,7-thiadiazepane 1,1-dioxideCatalog No.:AA00IZO2 CAS No.:1017782-61-4 MDL No.:MFCD09972235 MF:C11H16N2O2S MW:240.3219 |
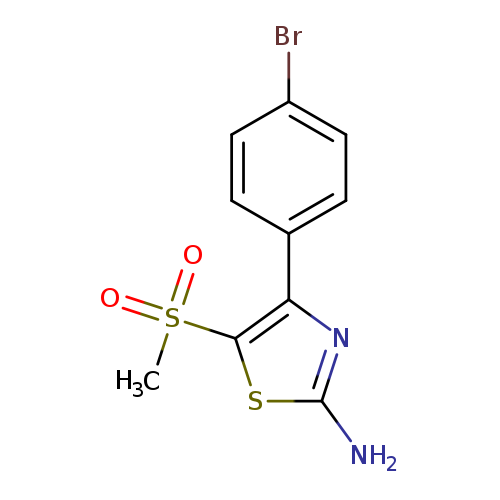
4-(4-Bromophenyl)-5-(methylsulfonyl)thiazol-2-amineCatalog No.:AA00IUSV CAS No.:1017782-62-5 MDL No.:MFCD09972236 MF:C10H9BrN2O2S2 MW:333.2247 |
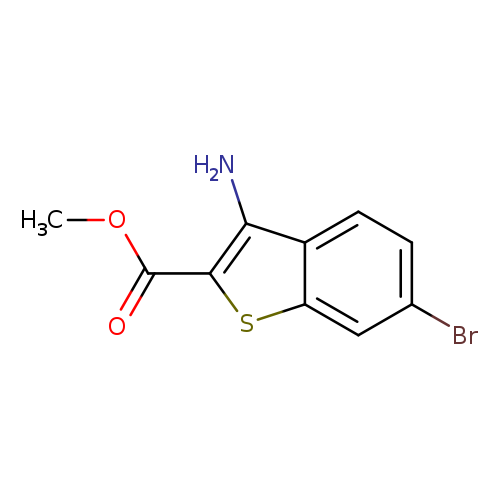
Methyl 3-amino-6-bromo-1-benzothiophene-2-carboxylateCatalog No.:AA0093JE CAS No.:1017782-63-6 MDL No.:MFCD09865017 MF:C10H8BrNO2S MW:286.1450 |
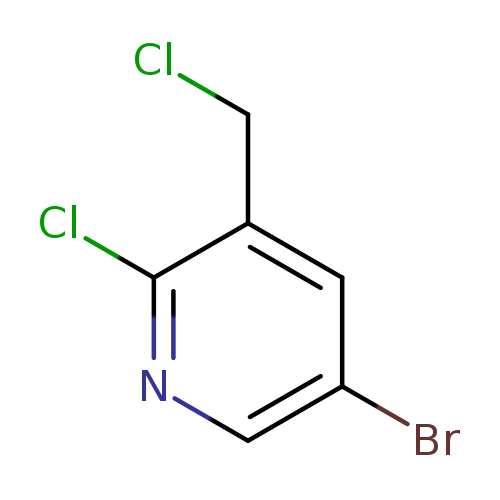
5-Bromo-2-chloro-3-(chloromethyl)pyridineCatalog No.:AA0005GT CAS No.:1017782-64-7 MDL No.:MFCD09865020 MF:C6H4BrCl2N MW:240.9127 |
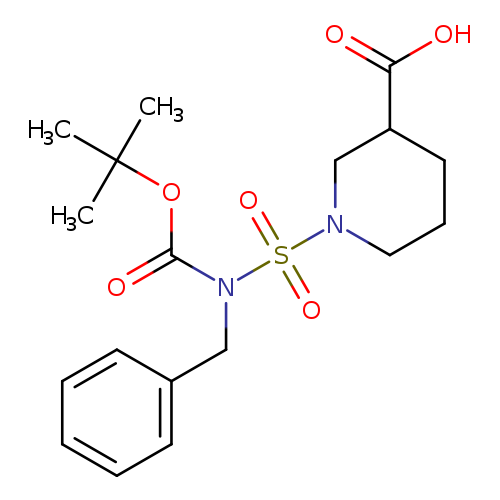
1-(N-Benzyl-N-(tert-butoxycarbonyl)sulfamoyl)piperidine-3-carboxylic acidCatalog No.:AA0005GS CAS No.:1017782-65-8 MDL No.:MFCD09972238 MF:C18H26N2O6S MW:398.4738 |
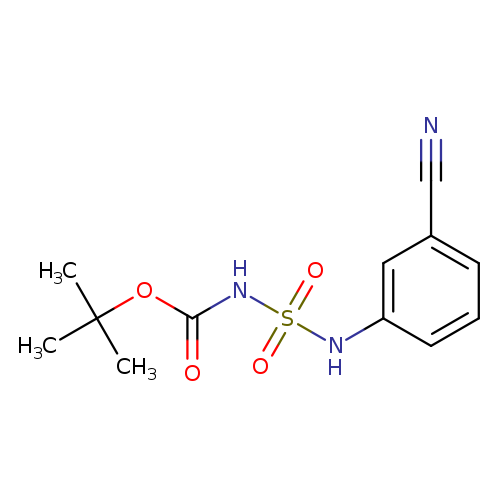
tert-butyl N-[(3-cyanophenyl)sulfamoyl]carbamateCatalog No.:AA00IUSX CAS No.:1017782-66-9 MDL No.:MFCD09865021 MF:C12H15N3O4S MW:297.3302 |
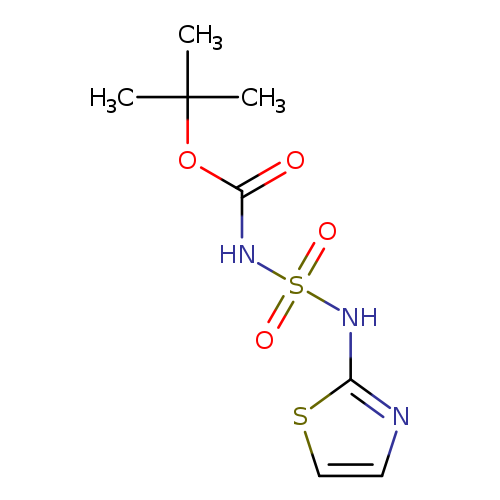
tert-Butyl 2,2-dioxo-3-(1,3-thiazol-2-yl)-2lambda(6)-diazathiane-1-carboxylateCatalog No.:AA00IUSY CAS No.:1017782-67-0 MDL No.:MFCD09865022 MF:C8H13N3O4S2 MW:279.3365 |
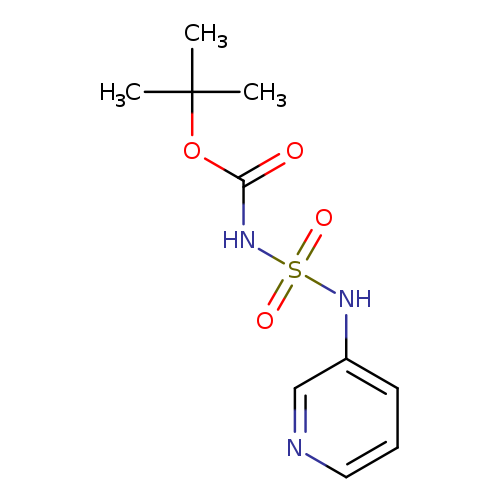
tert-butyl N-[(pyridin-3-yl)sulfamoyl]carbamateCatalog No.:AA00ISYV CAS No.:1017782-68-1 MDL No.:MFCD09865023 MF:C10H15N3O4S MW:273.3088 |
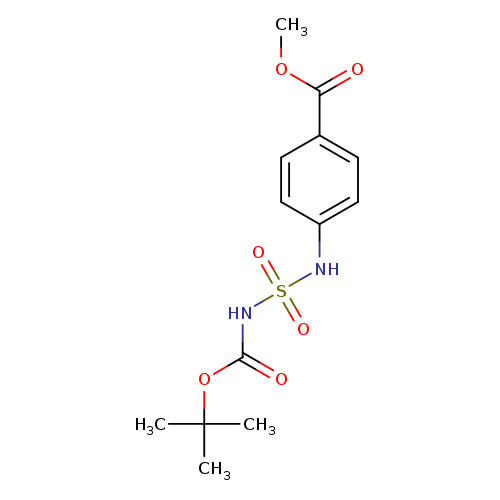
methyl 4-[({[(tert-butoxy)carbonyl]amino}sulfonyl)amino]benzoateCatalog No.:AA00ISYW CAS No.:1017782-69-2 MDL No.:MFCD09865024 MF:C13H18N2O6S MW:330.3568 |
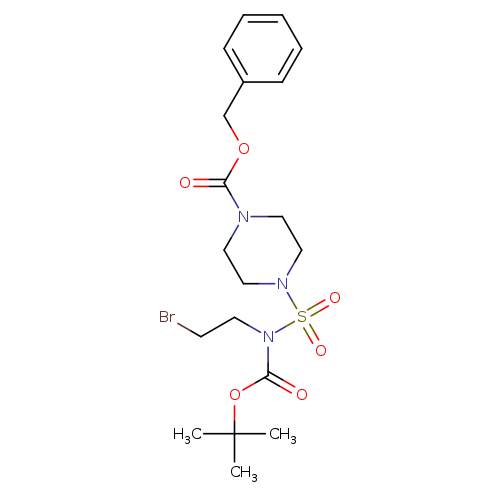
Benzyl 4-(N-(2-bromoethyl)-N-(tert-butoxycarbonyl)sulfamoyl)piperazine-1-carboxylateCatalog No.:AA0090WI CAS No.:1017782-70-5 MDL No.:MFCD09972239 MF:C19H28BrN3O6S MW:506.4111 |
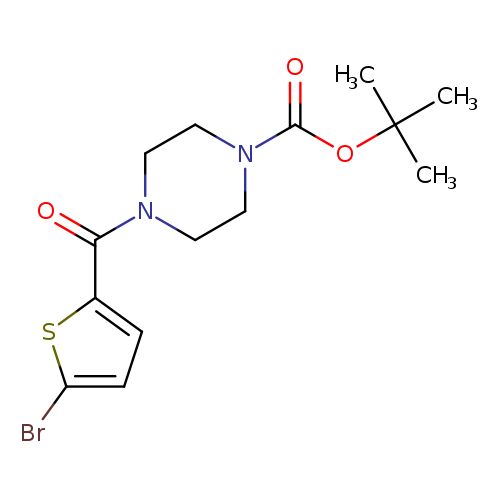
tert-Butyl 4-(5-bromothiophene-2-carbonyl)piperazine-1-carboxylateCatalog No.:AA00ISYX CAS No.:1017782-71-6 MDL No.:MFCD09972240 MF:C14H19BrN2O3S MW:375.2813 |
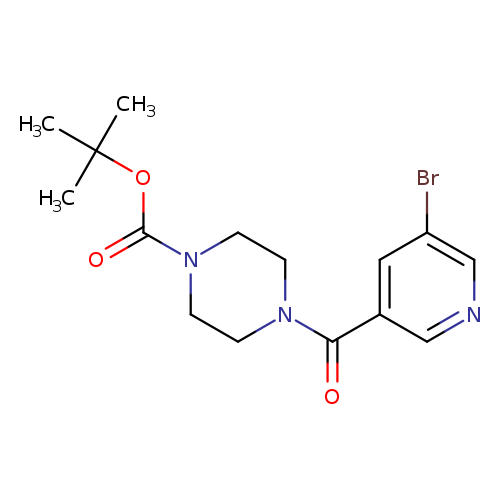
tert-Butyl 4-[(5-bromo-3-pyridinyl)carbonyl]tetrahydro-1(2h)-pyrazinecarboxylateCatalog No.:AA00IUT0 CAS No.:1017782-72-7 MDL No.:MFCD09972241 MF:C15H20BrN3O3 MW:370.2416 |
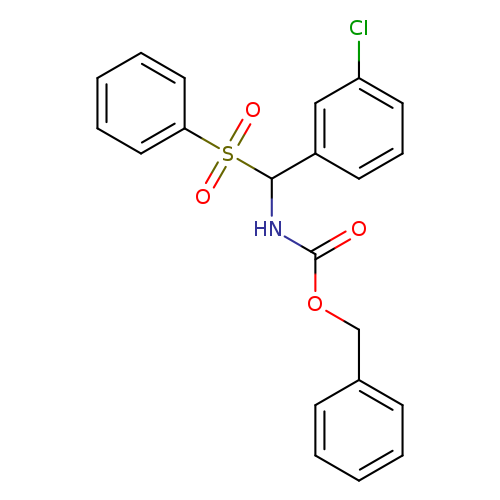
benzyl N-[(benzenesulfonyl)(3-chlorophenyl)methyl]carbamateCatalog No.:AA00IZQ6 CAS No.:1017782-73-8 MDL No.:MFCD09972242 MF:C21H18ClNO4S MW:415.8899 |
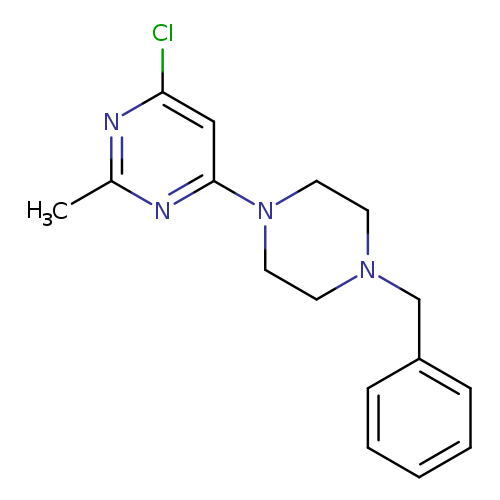
4-(4-Benzylpiperazin-1-yl)-6-chloro-2-methylpyrimidineCatalog No.:AA00IZQ7 CAS No.:1017782-74-9 MDL No.:MFCD09972243 MF:C16H19ClN4 MW:302.8019 |
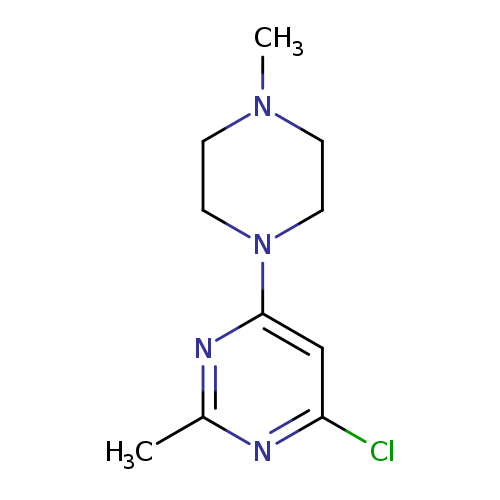
4-chloro-2-methyl-6-(4-methylpiperazin-1-yl)pyrimidineCatalog No.:AA00IT1B CAS No.:1017782-75-0 MDL No.:MFCD09972245 MF:C10H15ClN4 MW:226.7059 |
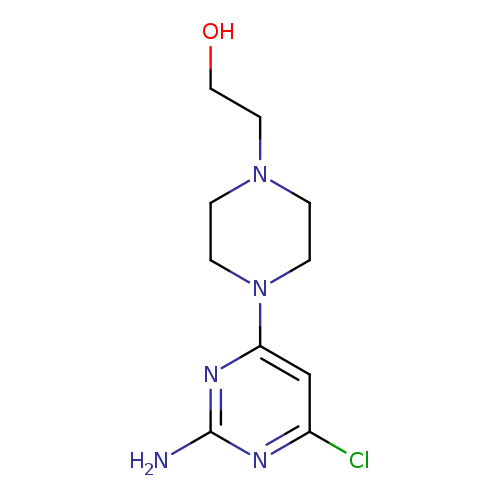
2-[4-(2-amino-6-chloropyrimidin-4-yl)piperazin-1-yl]ethan-1-olCatalog No.:AA00IZQ8 CAS No.:1017782-76-1 MDL No.:MFCD09972248 MF:C10H16ClN5O MW:257.7199 |
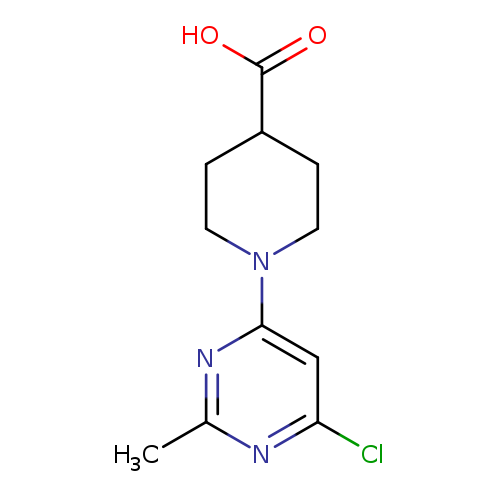
1-(6-Chloro-2-methylpyrimidin-4-yl)piperidine-4-carboxylic acidCatalog No.:AA00IWX2 CAS No.:1017782-77-2 MDL No.:MFCD09972249 MF:C11H14ClN3O2 MW:255.7008 |
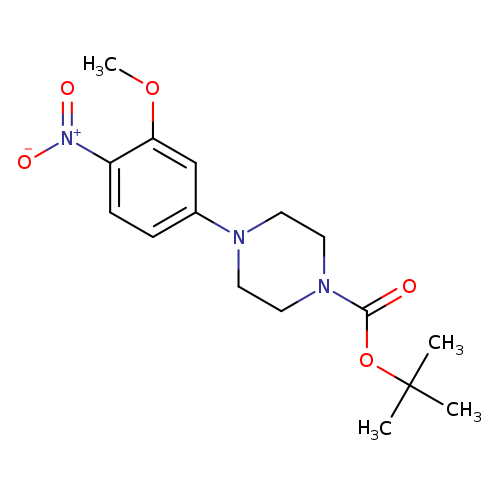
5-(4-BOC-piperazino)-2-nitroanisoleCatalog No.:AA0005GR CAS No.:1017782-79-4 MDL No.:MFCD09972251 MF:C16H23N3O5 MW:337.3709 |
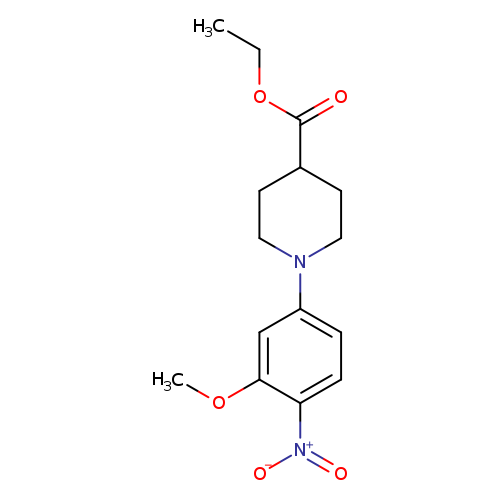
Ethyl 1-(3-methoxy-4-nitrophenyl)piperidine-4-carboxylateCatalog No.:AA00IUVT CAS No.:1017782-81-8 MDL No.:MFCD09972253 MF:C15H20N2O5 MW:308.3297 |
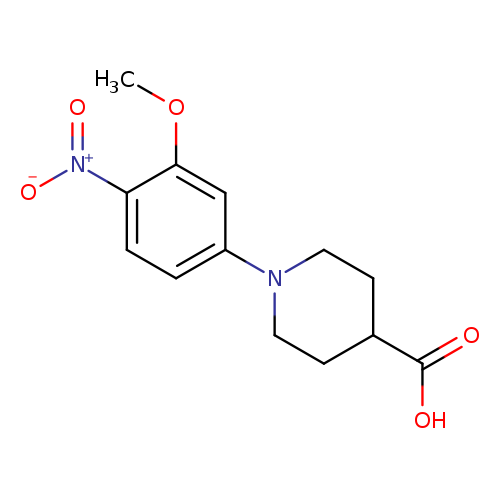
1-(3-Methoxy-4-nitrophenyl)piperidine-4-carboxylic acidCatalog No.:AA00IWX3 CAS No.:1017782-83-0 MDL No.:MFCD09972254 MF:C13H16N2O5 MW:280.2765 |
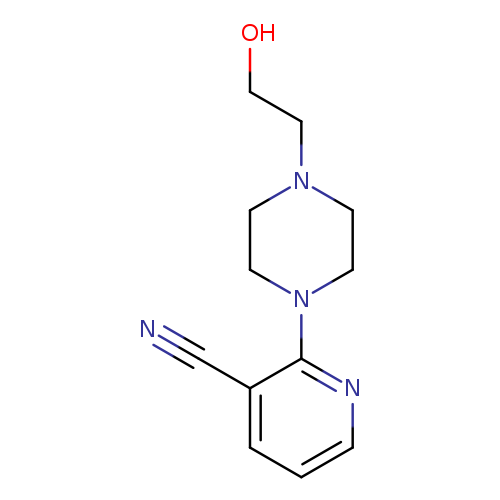
2-(4-(2-Hydroxyethyl)piperazin-1-yl)nicotinonitrileCatalog No.:AA00IUVU CAS No.:1017782-85-2 MDL No.:MFCD09972255 MF:C12H16N4O MW:232.2816 |
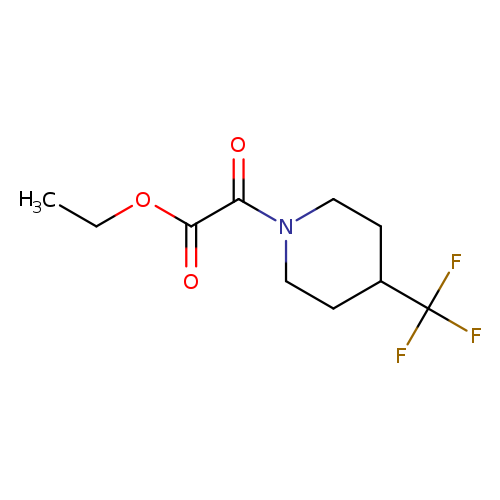
Ethyl 2-oxo-2-(4-(trifluoromethyl)piperidin-1-yl)acetateCatalog No.:AA00IT1D CAS No.:1017782-87-4 MDL No.:MFCD09972256 MF:C10H14F3NO3 MW:253.2183 |
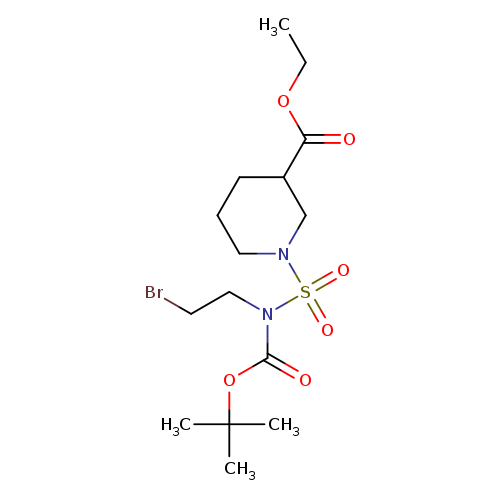
3-Piperidinecarboxylic acid, 1-[[(2-bromoethyl)[(1,1-dimethylethoxy)carbonyl]amino]sulfonyl]-, ethyl esterCatalog No.:AA0005GQ CAS No.:1017782-89-6 MDL No.:MFCD09972257 MF:C15H27BrN2O6S MW:443.3537 |
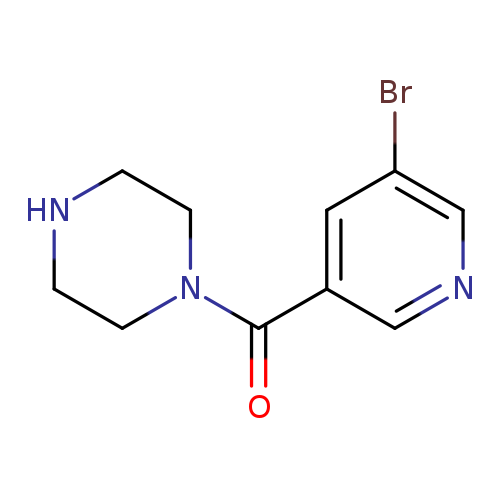
(5-Bromopyridin-3-yl)(piperazin-1-yl)methanoneCatalog No.:AA0005GP CAS No.:1017782-91-0 MDL No.:MFCD09972258 MF:C10H12BrN3O MW:270.1258 |
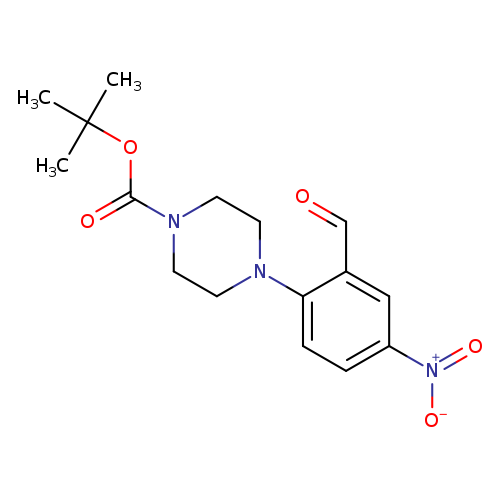
tert-Butyl 4-(2-formyl-4-nitrophenyl)piperazine-1-carboxylateCatalog No.:AA00IUVV CAS No.:1017782-93-2 MDL No.:MFCD09972259 MF:C16H21N3O5 MW:335.3550 |
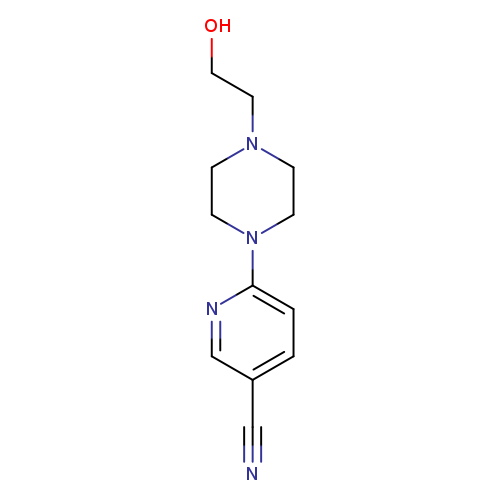
6-(4-(2-Hydroxyethyl)piperazin-1-yl)nicotinonitrileCatalog No.:AA00J05C CAS No.:1017782-95-4 MDL No.:MFCD09972262 MF:C12H16N4O MW:232.2816 |
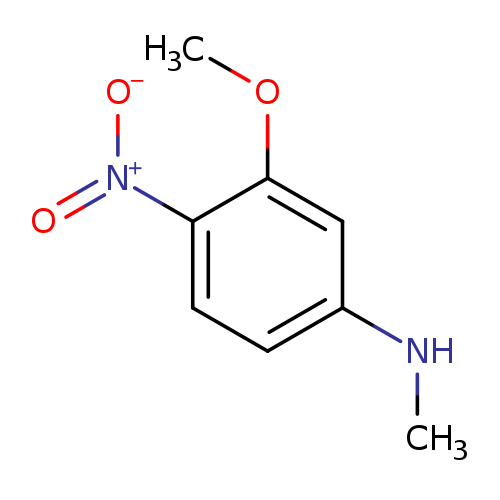
3-Methoxy-N-methyl-4-nitroanilineCatalog No.:AA00IXEC CAS No.:1017782-97-6 MDL No.:MFCD09972263 MF:C8H10N2O3 MW:182.1766 |