2019-11-13 09:42:24
Navjeet Kaur
To cite this article: Navjeet Kaur (2019) Nickel catalysis: six membered heterocycle syntheses,
Synthetic Communications, 49:9, 1103-1133, DOI: 10.1080/00397911.2019.1568499
To link to this article: https://doi.org/10.1080/00397911.2019.1568499
Introduction
The heterocyclic compounds are used in chemical, biological, and industrial fields.[1–3] Heterocyclic compounds form the central core of many biologically active natural products and pharmaceutical agents (such as anti-viral, anti-inflammatory, anti-bacterial, anti-tumoral, and anti-oxidant activities agents), and are used in herbicides and corrosion inhibitors. Heterocyclic compounds, due to their presence in natural products and biologically active compounds are the most explored compounds.[4–6] They are significant not only for the synthesis of drugs, pharmaceuticals, and pigments but also for the development of organic functional materials. Many biologically active pharmaceuticals, agrochemicals, modifiers, and additives used in industrial applications like plastics, cosmetics reprography, and information storage are heterocyclic in nature.[7–10]
Many protocols have been investigated for the synthesis of heterocycles. These protocols for the formation of heterocyclic compounds involve carbon–nitrogen bond-forming reactions such as nucleophilic substitution, reductive amination, or dipolar cycloaddition for ring closure.[11–13] Although various protocols have been developed, their functional group tolerance and substrate scope are often limited. In recent years, for the synthesis of heterocycles, many new protocols have been developed which involve metal and nonmetal assisted carbon–nitrogen bond-forming reactions. These
reactions tolerated a number of functional groups, occur under mild conditions, and proceed with high stereoselectivity. The utilization of metal and nonmetal allows highly convergent multi-component coupling approaches, which form many bonds and/or stereocenters in the one-pot protocol.[14–16] This review describes the approaches for the preparation of several six-membered heterocyclic compounds through nickel assisted reactions.
Nickel-catalyzed synthesis of six-membered heterocycles
Synthesis of six-membered N-heterocycles
An azazirconacyclopentenone was formed by the coupling of isocyanate and alkyne with Zr. The nickelcyclopentenone was produced when Ni(PPh3)2Cl2 was reacted with azazirconacyclopentenone. The pyridine was formed upon insertion of the second alkyne. This was the first example where nickel was utilized for the preparation of pyridines. Unfortunately, this reaction required both Zr and nickel in stoichiometric amounts (Scheme 1).[17]

The nickel and N-heterocyclic carbene, IPr, were utilized in combination with both unactivated nitriles to afford excellent yields of pyridines at room temperature. The nitriles and alkynes were combined in an intermolecular fashion by this reaction.[18] Pyridines were obtained from nitriles by an effective Ni-catalyzed cycloaddition. The desired pyridines were also formed from untethered alkynes and the unsymmetrical diyne furnished a single regioisomer (Scheme 2). Louie[19] synthesized pyridones using metal complexes as catalysts. High yields of pyridones were obtained when isocyanates and diynes were reacted at ambient temperature employing a catalytic system Ni/SIPr (1,3-bis(2,6-diisopropylphenyl)-imidazolin-2-ylidene). In contrast, high yields of a series of 2-pyridones were reported by a [2 þ 2 þ 2] cycloaddition reaction of isocyanates and diynes using similar catalytic system(Scheme 3).


The catalytic reactions were varied involving carbon–carbon triple and double bonds. For instance, the 1,3-dienes and aldehydes were coupled with HSiEt3 in the presence of nickel catalyst (Schemes 4 and 5).[20] The BuLi was added to a mixture of imidazolium salt and NiCl2 for the in situ generation of nickel N-heterocyclic carbene complex. Moderate to high yield of (Z)-alkene was obtained. The (E)-alkene was obtained when a phosphane ligand was utilized in place of N-heterocyclic carbene. Jamison et al. performed the coupling of terminal olefins with isocyanates and olefins with aldehydes to afford acrylamides using in situ mixture of IPr (1,3-(2,6-diisopropylphenyl)imidazol-2-ylidene) and Ni(COD)2 as a catalyst. Two isocyanates were coupled with alkynes under similar conditions to provide pyrimidine diones.[23] Variously substituted pyridines were obtained when diynes were reacted with nitriles in the presence of in situ produced nickel N-heterocyclic carbene complex catalyst under basic conditions. Vinyl cyclopropanes were isomerized to cyclopentenes with a mixture of a nickel(0) precursor.
Other reactions with C–C double bonds, for example, the polymerization of styrene[24] and dimerization of ethane[25,26] were carried out with nickel N-heterocyclic carbene complexes. A variety of imines were treated with NaOi-Pr in the presence of IMes and nickel(0) to provide amines by transfer hydrogenation.[27] The dihydrogen gas was produced rapidly by dehydrogenation when a triazol-5-ylidene was reacted with catalytic amounts of Ni(0) and H3NBH3, through hydrogen transfer of ammonia–borane to the carbene carbon, followed by C–H activation by the nickel species.[28]
Longer tethers between the olefin and imine were used to probe the generality of this insertion cascade. Only 20% yield of six-membered lactam was obtained along with five-membered exo-cyclization product in high amounts (60%) when the length of tether was increased by one carbon. No (<5%) seven-membered lactam was obtained and a six-membered lactam was obtained with almost complete selectivity (72% yield) upon extending the tether to three methylene units[29] (Schemes 6 and 7).

Hoberg[30–32] for first time reported the use of nickel for the preparation of pyridones. Pyridine was formed selectively when an alkyne was reacted with an isocyanate in stoichiometric amounts of Ni (Scheme 8). The oxidative coupling of phenyl isocyanate and alkyne provided nickel acyclopentenone which was coupled with one more equivalent of an alkyne moiety to afford moderate yields of pyridone through nickelacycle. Although this was the first successful method to synthesize pyridones employing nickel, the reaction to construct the nickelacycle needed long reaction time (3 d) and low temperatures (50 C).

Moore and coworkers[33] synthesized unsymmetrical and symmetrical bipyridines by cross-coupling reactions using heterogeneous palladium and nickel catalysts under MWI(Scheme 9). No phosphine ligand was required when nickel/aluminum oxide-silicon oxide (50 mol% loading) was utilized in the case of nickel catalysis. Under palladium-catalysis moderate yields were obtained; however, only 5 mol% catalyst loading was needed.[6e] Pyridine underwent metalation and functionalization reactions ideally in the presence of metal reagent in catalytic amounts. For the preparation of pyridine derivatives,
various synthetic methods were enabled by transition-metal catalysis. For example, cycloaddition reactions in the presence of transition metal catalysts are highly versatile for the synthesis of substituted pyridine from commercially available unsaturated compounds (Scheme 10).

The polyhydroquinoline derivatives were synthesized in high yields by MMS in the absence of solvent using heterogeneous catalyst nanosized Ni particles through Hantzsch protocol (Scheme 11).[36–37]
The tert-butyl imines of 2-iodobenzaldehydes and several alkynes were annulated efficiently in the presence of Ni catalyst to afford a variety of substituted isoquinolines[38] (Scheme 12). Two different alkyne insertion routes were there for catalytic reactions as indicated by the regiochemistry of isoquinolines.
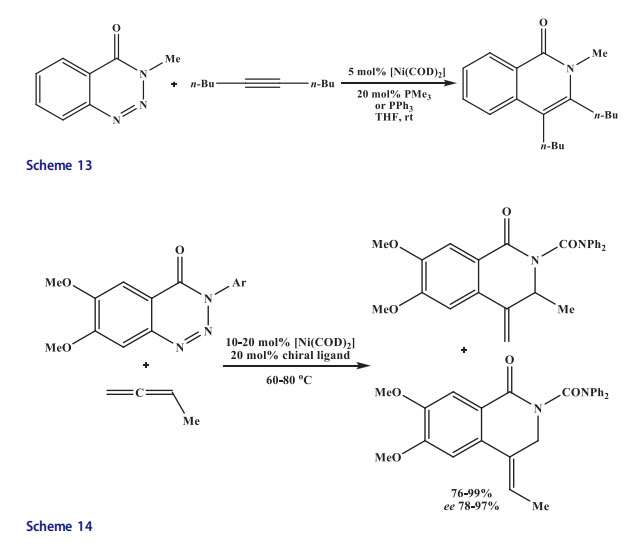
Terminal alkynes, as well as various unsymmetrical and symmetrical internal alkynes, provided isoquinolones in very high yields. However, varied regioselectivity was shown by unsymmetrical alkynes. Murakami et al.[39] reported that 1,2,3-benzotriazinones also served as good substrates in denitrogenative transannulation reactions. Thus, 1,2,3-benzotriazinones were reacted with alkynes in the presence of Ni catalyst to synthesize isoquinolones. Initially, nickel(0) was inserted into the N,N linkage of 1,2,3-benzotriazinones, which lost dinitrogen to afford azanickelacycle.[40] The alkyne was inserted into the nickel–carbon bond to produce a seven-membered nickelacycle intermediate, which provided the final product isoquinolones after the reductive elimination and the regeneration of the nickel(0) catalyst (Scheme 13). This reaction did not occur with N-unsubstituted benzotriazinone.
The 1,2,3-benzotriazinones were transannulated with a wide range of monosubstituted allenes by a catalytic version of this reaction (20 mol% PMe3, 5 mol% [Ni(cod)2], 60 C, THF). Both the electron-donating and electron-withdrawing substituents present at the aromatic ring of the benzotriazinone and at the N atom of the triazole moiety worked well and a major regioisomer of differently substituted isoquinolones were provided.[41] The regiochemistry was reversed completely in the reaction with trialkylsilyland tert-butyl-substituted allenes due to sterics. The use of phosphinooxazoline ligand (S,S)-iPr-foxap[42] afforded very high enantio- and regioselectivities (Scheme 14).
Murakami and coworkers[43] reported that 1,2,3-benzotriazinones underwent denitrogenative transannulation with allenes in the presence of Ni catalyst. A five-membered azanickelacycle intermediate was produced when triazene was reacted with [Ni(cod)2] and dppbenz in stoichiometric amounts. Treatment of azanickelacycle intermediate with an allene at 60 C in tetrahydrofuran provided 3,4- dihydroisoquinolin-1(2H)-ones(54:46) in 99% yield as an isomeric mixture (Scheme 15).

Various N-protected isoquinolones were synthesized when differently substituted benzotriazinones were reacted with symmetrical 1,3-dienes using 1,1’-bis(diphenylphosphino)ferrocene (10 mol%) and [Ni(cod)2] (10 mol%) at 60 C in tetrahydrofuran (Scheme 16). The yields were high with all other substrates with the exception of N-benzyl-substituted benzotriazinone (24%). Benzotriazinones with both electron-withdrawing and electron-donating groups at the benzene ring were equally competent in this reaction. Isoquinolones were obtained as major regioisomers using unsymmetrical dienes.[43]
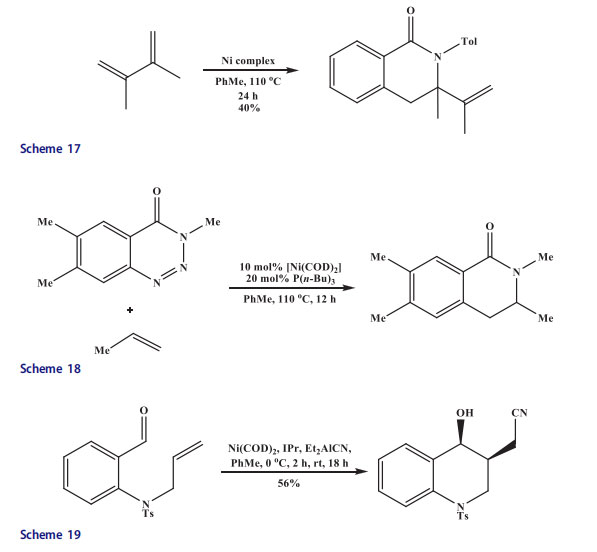
The benzotriazinones were transannulated with activated alkenes and 1,3-dienes in the presence of Ni catalyst.[44] The quinoline was obtained in only trace amounts when Ni complex was mixed with 1,3-diene without a phosphine ligand. However, quinoline was formed in 40% yield upon addition of 1,1’-bis(diphenylphosphino)ferrocene ligand(Scheme 17).
The benzotriazinones underwent transannulation with activated alkenes in the presence of P(n-Bu)3 and [Ni(cod)2]. Thus, excellent yields of dehydroisoquinolinones were obtained when electron-deficient alkenes, like acrylonotrile, acrylamide, and methyl acrylate underwent transannulation smoothly with benzotriazinones. Pyridyl-possessing alkenes were efficient, whereas with styrene product was formed in a low yield. Electron-rich and electron-neutral alkenes did not participate in the reaction at all (Scheme 18).
Ho[45] reported that N-allyl aniline through cyanative alkene aldehyde coupling afforded quinoline derivative with the help of nickel(0) catalyst-N-heterocyclic carbene/Et2AlCN (Scheme 19). The isoquinoline derivatives were produced in good yields by a [2 þ 2 þ 2] co-cyclization in the presence of Ni(0) catalyst at room temperature[46] (Scheme 20). But steric hindrance influenced the cyclization. No cyclized isoquinoline products were obtained when disubstituted alkynes were used in place of acetylene.
The diphenylacetylene and azazirconacyclopentadiene were coupled in the presence of NiCl2(PPh3)2 catalyst to afford a substituted isoquinoline derivative[47] (Scheme 21). The coupling reaction occurred in high regioselectivity and furnished only single products when unsymmetrical alkynes like 1-phenyl-1-butyne and 1-phenyl-1-propyne were utilized as the second alkyne. Louie[48] reported successfully that there was no need of stoichiometric amounts of nickel to prepare pyridines. Alkyne trimerization with nickel catalysts delivered mixtures of highly functionalized benzene derivatives. One alkyne was replaced with a nitrile to synthesize pyridines and various other nitrogen-containing heterocycles through similar intermediates. A metallocyclopentadiene was prepared when diyne was stitched together,
the resulting compound was reacted with a third alkyne in a Diels–Alder reaction. The formed metal-bridged norbornadiene was aromatized with the loss of nickel catalyst and the cycle begins again(Scheme 22). The 2-aminopyridine is important, therefore authors developed an efficient protocol for the synthesis of this motif from an easily available and simple starting material in a single step. Authors reported a [2 þ 2 þ 2] cycloaddition of readily available cyanamides and diynes. The cycloaddition reaction of various cyanamides and diynes was also reported employing a nickel-N-heterocyclic carbene catalyst system.[49] A Fe-catalyzed protocol to obtain 2-aminopyridines has not yet been reported (Scheme 23).

The ynals by reductive cyclization reactions provided fused azabicycle in the presence of nickel catalyst.[50] The reaction occurred with high stereoselectivity due to the formation of highly organized oxametallacycles, which underwent reduction with triethylsilane to synthesize diverse indolizidine and pyrrolizidine products. This protocol was applicable in Montgomery’s total preparation of indolizidine alkaloid allopumiliotoxin through the stereoselective reductive cyclization followed by two subsequent protecting group manipulations (Scheme 24).[51]

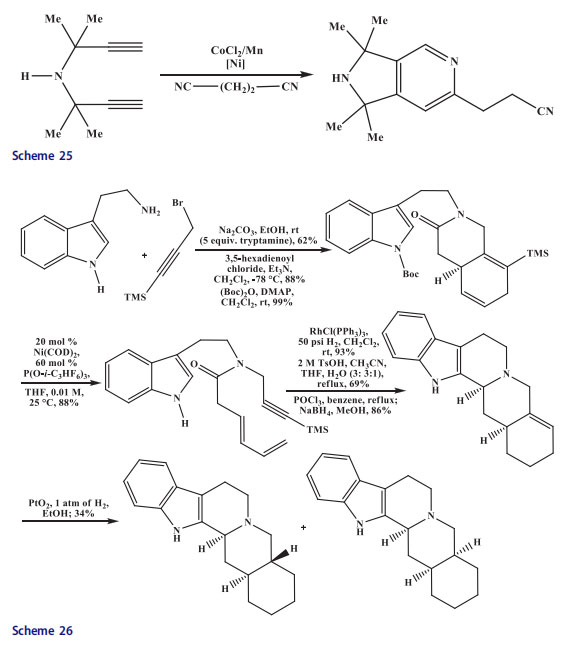
The gem-dialkyl groups bearing dipropargyl amines [52,53] were transformed in the presence of cobalt(II) chloride/manganese catalytic system. The reaction temperature was decreased to 80 C and yield of dihydropyrrolopyridines were increased up to 90–98% due to the presence of gem-dialkyl groups in diacetylene (Scheme 25).[54] This method was extended for the construction of DE-ring skeleton of the yohimbine(Scheme 26) by initiating the synthesis of tryptophylsubstituted amide in three steps by sequential alkylation,[55] acylation, and Boc protection [56] of tryptamine. The cycloaddition of tryptophylsubstituted amide in the presence of Ni catalyst afforded quinoline in 88% yield at room temperature, while the alternative synthetic thermal method needed 150 C temperature to cleave the Boc protecting group and to give 1,4-cyclohexadiene product in 45% yield only. The resulting product was elaborated when the sterically less encumbered olefin was hydrogenated selectively, the vinylsilane underwent protiodesilylation[57] with concomitant removal of the Boc protecting group, and the C-ring underwent Bischler–Napieralski closure to afford the pentacyclic 19,20-didehydroyohimban in
86% yield. The 19,20-didehydroyohimban was hydrogenated catalytically in platinum oxide to afford alloyohimban and yohimban in a 1:1 ratio.[58–60] Cook and coworkers[61,62] synthesized opioid agonistic alkaloid mitragynine through the formation of allylamine intermediate. An asymmetric Pictet–Spengler reaction and a cyclization in the presence of Ni(cod)2 provided allylamine intermediate (Scheme 27).

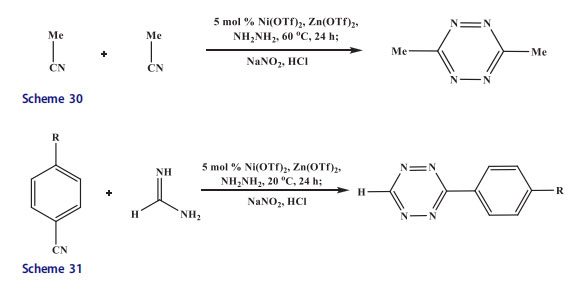
The 2-(2-nitrophenyl)benzimidazole was reduced to afford 2-(2-aminophenyl)benzimidazole. The 2-(2-aminophenyl)benzimidazole was reacted with aldehydes in acetic acid/ethanol mixture to synthesize benzimidazo[1,2-c]quinazolines in high yield (Scheme 28).[63] The phenazine derivatives were constructed when substituted 2,2’-dinitrodiphenyl amines underwent reductive cyclization in one-step in alkaline media under mild reduction conditions.[64] Raney-nickel or Ru/C[65] were utilized as catalysts. Similarly, 1,6-dimethoxy-phenazines[66] were also synthesized (Scheme 29). The Zn and Ni triflates afforded high yields and their influence on the yields of various other tetrazine syntheses was evaluated where an alkyl nitrile was at least one component. Higher yields were obtained with zinc salts and less active nitriles like those that were influenced by electron-donating groups or sterically hindered. In contratst, more reactive nitriles were advantageous with Ni salts. However, there were exceptions
where both catalysts were utilized for the synthesis of new tetrazines. For the preparation of symmetric 3,6-dialkyl 1,2,4,5-tetrazines, yields ranged from 95% for 3,6-dibenzyl-1,2,4,5-tetrazine and 24% for the sterically hindered 3,6-di-tert-butyl-1,2,4,5-tetrazine. The former tetrazine was formed in impressive moderate yield as all previous attempts to prepare the molecule led to trace isolated yield.[67,68] The scope of this protocol was extended to the most challenging synthesis of tetrazines i.e. asymmetric 3,6-disubstituted 1,2,4,5-tetrazines.[69] The synthesis of 6-methyl-terminated tetrazines in 40–70% yield from aromatic nitriles and acetonitrile was promoted in the presence of metal ions. The 6-methyl terminated alkyl tetrazines were formed in 36–40% yield when alkyl nitriles and acetonitrile were combined with hydrazine. For instance, it was reported that in bioorthogonal cycloadditions methyl-terminated tetrazines were highly stable candidates and were utilized in a mutually orthogonal fashion with azide-alkyne reactive substrates like amidine salts and imidates in lower yield.[71] The isolation of dialkyl asymmetric tetrazines with bulkier substituents was extremely difficult, even from amidines and imidates. On the other hand, excess hexanenitrile and benzyl cyanide afforded 3-benzyl-6-pentyl-1,2,4,5-tetrazine albeit in lower yield (12%)(Scheme 30).[72]

Many researchers have measured the rate of cycloaddition between strained dienophiles like trans-cyclooctene and norbornene and various tetrazines.[72,73] The kinetics of reaction was significantly affected by the substituents present in the third and sixth positions of 1,2,4,5-tetrazines. While 6-methyl-terminated tetrazines were benefited from stability, tetrazines terminated with hydrogen at the sixth position reacted much faster and were applicable in live animal and live cell where the labeling agent was used in lowered concentrations.[74–76] For the synthesis of hydrogen-terminated asymmetric tetrazine, the trimethylsilyl cyanide was used in excess together with an aromatic nitrile.
This was the first example where trimethylsilyl cyanide was utilized for the synthesis of tetrazines and was possible because of the addition of nitrile-activating metal catalysts. Additionally, the influence of Zn(OTf)2 and Ni(OTf)2 on the preparation of tetrazine from formamidine salts and aromatic nitriles was explored. Although these reactions occurred without catalysis, yields were low (10–20%).[77,78] The reaction was promoted with metal ions and increased the yield of tetrazine ranging from 60–74% depending on the catalyst and precursors utilized (Scheme 31).
The hydrazine was added to aromatic nitriles followed by oxidation of the 1,2-dihydrotetrazine product and proved to be the most convenient pathway to 1,2,4,5-tetrazines.[79–81] Unfortunately, this protocol was not viable for the synthesis of tetrazines from unactivated nitriles like alkyl nitriles. As per earlier reports regarding the direct generation of tetrazines from alkyl nitriles proved to be difficult, due to confusion between the intermediate isomeric 4-amino-1,2,4-triazoles and 1,2-dihydrotetrazines.[82,83] Many methods have been reported to synthesize dialkyl tetrazines from alternative substrates, like amidine salts, imidates, and aldehydes, but these processes suffer from limited substrate scope, need of additional synthetic steps, and the low yields.[84] Therefore, a robust and general protocol to form asymmetric and symmetric 1,2,4,5-tetrazines directly from unactivated nitriles was needed. The reaction started with nucleophilic attack of the nitrile by hydrazine to form an amidrazone.[85]
This reaction was promoted with the addition of Lewis acid catalysts by binding to the hydrazine and/or nitrile. The nitriles were activated with metal ions for nucleophilic addition.[86–91] However, there was no report of utilizing homogenous transition metal catalysis for the promotion of synthesis of 1,2,4,5-tetrazines.[92,93] A variety of Lewis acid catalysts at 5 mol% loading was surveyed by the reaction of
benzyl cyanide with neat hydrazine. Tetrazine products were not obtained without a catalyst. Remarkably, 3,6-dibenzyl-1,2,4,5-tetrazine was synthesized in quantitative yield upon addition of 5 mol% Ni triflate (Ni(OTf)2). Zn salts also afforded good yields, the desired tetrazine was formed in 70% yield with the addition of 5 mol% zinc triflate (Zn(OTf)2). Lower yields were obtained with Zn and Ni salts
containing stronger coordinating anions because these salts have lowered solubility in aprotic media and the strength of Lewis acid decreased compared to the triflates(Scheme 32).
Synthesis of six-membered O-heterocycles
Takahashi[95] reported the reaction of alkynes and halopropenoates with the help of halogen-dependent catalytic reaction, where (Z)-3-iodopropenoate afforded pyrones and (Z)-3-bromopropenoate gave cyclopentadienes (Scheme 33). cycloadditions.

Unsaturated alkyl halides afforded high yields of oxa-, aza-, and carbocycles by a convenient and mild free-radical cyclization of organohalides with 1,2-dimethoxyethane/Pybox complex as a catalyst, nickel chloride, and zinc powder in methanol (Scheme 34).[97] Pyrans were synthesized by nickel-catalyzed cycloaddition reactions. The aldehydes and diynes were coupled with combined utilization of a phosphine ligand and a nickel(0) catalyst; however, the reaction temperature was exceeded to 130 C.[98] Room temperature conditions provided cycloadducts and catalytic activity was increased when the phosphine ligand was replaced with SIPr.[99] Both aliphatic and aryl aldehydes were introduced into the dienones. Despite the decreased reactivity of unactivated ketones, the cyclohexanone was cyclized smoothly to afford a good yield of pyran. The catalytic activity increased due to the ability of N-heterocyclic carbene ligand to increase the carbon–oxygen bond-forming reductive elimination
(Scheme 35).

Good yields of pyrans were obtained when enynes were reacted with ketones. On the other hand with aldehyde reactions, the outcome of the reaction was little influenced by substitution pattern present on the substrate and the carbonyl carbon was bound to the olefin, regardless of the alkyne substitution (Scheme 36).[13,100,101] The alkynes and phthalic anhydride by cycloaddition reaction provided isochromones through the formation of nickelacycle intermediate through decarbonylation.[102] The heterocyclic compounds were prepared from thiophthalic anhyderide and phthalic imide through the addition to alkynes under nickel catalysis.[103,104] A coumarine derivative was synthesized efficiently when o-acyloxybenzonitrile was treated with a bulky Lewis acid, MAD (methylaluminium bis(2,6-di-tert-butyl-4-methylphenoxide) and nickel(0) catalyst in the presence of alkynes. The reaction occurred through the formation of a nickelacycle by elimination of aryl cyanide. The cleavage of two carbon–carbon bonds afforded this key intermediate (Scheme 37).[105]
An interesting outcome of the reaction was observed using cyclic 1,3-disubstituted allene. The type of phosphine ligand affected the nature of the product. The imino ester was formed in 75% yield when trimethylphosphine was used in tetrahydrofuran at 60 C, whereas benzopyran was obtained as a sole product in 99% yield with bidentate phosphine ligand (R,R)-Me-duphos at 100 C in toluene. The control experiment has shown that imino ester was completely isomerized into benzopyran in the presence of (R,R)-Me-duphos and [Ni(cod)2] at 100 C in toluene (Scheme 38).

Louie and coworkers[107] developed a hetero-[2 þ 2 þ 2]-cycloaddition of carbon dioxide with diynes in the presence of Ni catalyst. The pyrones were formed in high yields when diynes were reacted with carbon dioxide under atmospheric pressures in the presence of N-heterocyclic carbine ligand and bis(1,5-cyclooctadiene)nickel.[108–110] Inoue, and further developed by Tsuda,[111–116] reported a nickel/phosphine-catalyzed coupling of two alkynes with carbon dioxide to provide pyrones. These reactions needed elevated temperatures and high pressures of carbon dioxide. Additionally, only some
diynes were successfully transformed into pyrone. Like with various cycloaddition reactions, the major side reaction was oligomerization of diyne. These obstacles were overcome when IPr was utilized as a ligand in lieu of phosphines. The oligomerization of diyne was suppressed with steric bulk of this ligand. As a result, high yields of many bicyclic pyrones were obtained. All pyrones were formed using relatively low reaction temperatures and ambient pressures. The coupling of diynes and carbon dioxide was performed with nickel/IPr as a general catalyst system. To date, this catalyst has not
afforded pyrones from either sterically hindered diynes or terminal diynes. The oligomerization of terminal diynes occurred at a faster rate than the incorporation of carbon dioxide. On the other hand, sterically hindered diynes did not react under any condition (pressure and elevated temperature). Asymmetrical diynes such as diynes containing one sterically demanding substituent were cleanly converted into pyrones.[117] However, one isomer was preferentially formed and the regioselectivity of the reaction was improved as the relative difference between the two terminal groups was increased.
Furthermore, only one regioisomer was formed when a diyne possessing a very bulky group (such as tert-butyl or trimethylsilyl) and a methyl group was used(Scheme 39).[118,119]
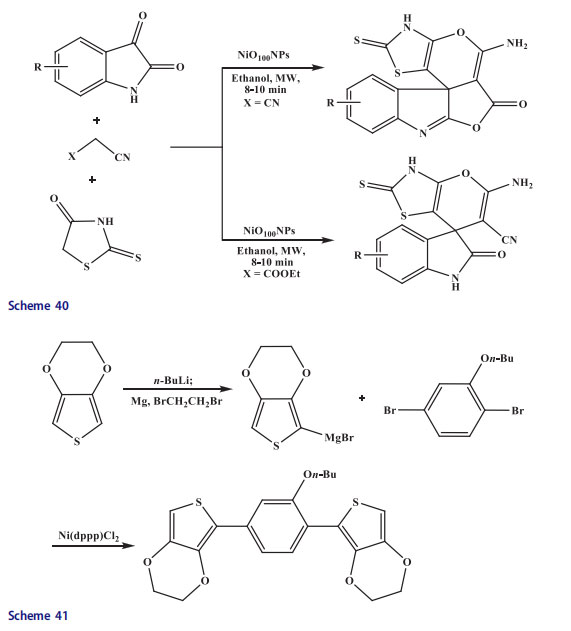
A one-pot 3-CR reaction of activated methylene reagent, 2-thioxo-4-thiazolidinone and substituted 1H-indole-2,3-diones afforded condensed spiro[indoline-3,40-pyrano[2,3-c]thiazolecarbonitrile and thiazolo[500,400:50,60]pyrano[40,30:3,4]furo[2,3-b]indole derivatives under microwave irradiation. Knoevenagel condensation followed by Michael addition with the assistance of nickel oxide nanoparticle catalyst in absolute ethanol furnished novel condensed and spiro indole derivatives. The reaction was performed in the absence of a catalyst in order to confirm the effective involvement of nickel oxide nanoparticles.

An incomplete reaction was reported under microwave irradiation even after 30 min though the compound was formed in small amounts without nickel oxide nanoparticle. The reaction was conducted under conventional heating in the absence of a catalyst to see the specific effect of microwaves. The product was synthesized in 25–35% yields under reflux for 30 h while 32–40% yields of the product were obtained in 30 min under microwave irradiation (Scheme 40).[13c,120]
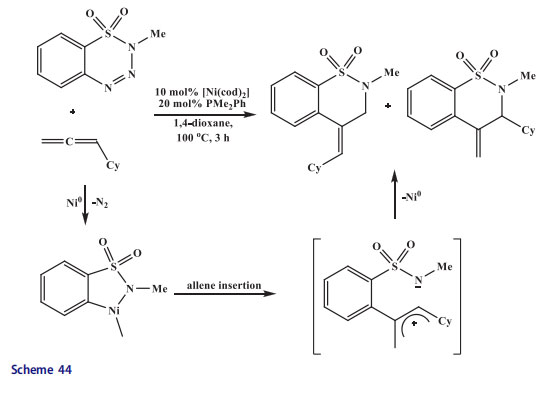
The traditional halogen-metal exchange with magnesium turnings afforded 2-thienylmagnesium bromide which was utilized in addition reactions as a nucleophile to afford vinylogous acyl triflates,[121] decahydroisoquinoline-derived aldehyde [122] or lactones.[123] Ring-opening of azabicyclic alkenes was performed with this heteromagnesium reagent in the presence of copper catalyst [124] as well as metalated counterpart in cross-coupling of alkyl halides with a cobalt catalyst.[125,126] The 2-thienylmagnesium bromide and some alkylated derivatives were used for the synthesis of highly conjugated compounds having electro-optical properties through cross-coupling reactions of haloarenes in the presence of palladium,[127] or more frequently, Ni-catalysts.[128–130] These couplings were employed to 2-thienylmagnesiums as shown in Scheme 41. Other metalation protocols were more convenient when other moieties were existing. The Grignard reagent was derived by lithium-magnesium transmetalation of 2,3-dihydrothieno[3,4-b][1,4]dioxine.
The Ni-catalyzed coupling of thienyl Grignard reagent with aryl dibromide provided bisthiophene.[131,132] The 4-benzyloxy-3-methoxybenzoic acid provided N-allyl anhydride in six steps[133–135] (Scheme 42). Esterification of 4-benzyloxy-3-methoxybenzoic acid produced methyl ester which afforded methyl 4-benzyloxy-5-methoxy-2-nitrobenzoate in 83% yield upon nitration with fuming nitric acid. The nitro group present in methyl 4-benzyloxy-5-methoxy-2-nitrobenzoate was reduced with sodium borohydride in the presence of NiCl2 catalyst to produce aniline, which was transformed into acid upon
hydrolysis. The 7-benzyloxy-6-methoxyisatoic anhydride was formed directly when sodium salt of acid was treated with the phosgene-toluene solution. The 7-benzyloxy-6-methoxyisatoic anhydride was treated with NaH followed by allyl bromide in N,N-dimethylacetamide to afford desired N-allyl anhydride.[136,137]

The readily available 2-aminophenols under microwave irradiation by a one-pot synthesis afforded 3,4-dihydro-3-oxo-2H-1,4-benzoxazines regioselectively. Acyclic intermediates were formed by base-assisted regioselective O-alkylation with 2-bromoalkanoates. The desired products were afforded in good yields by a subsequent intramolecular amidation reaction (Scheme 43).
Synthesis of six-membered S-heterocycles
Murakami et al.[139] reported that 1,2,3,4-benzothiatriazine-1,2(2H)-dioxide underwent Ni-catalyzed enantioselective transannulation reaction with mono-substituted allenes to afford 1,2,3,4-benzothiazine-1,1(2H)-dioxide. The five-membered intermediate was produced by oxidative addition of nickel(0) into the N–N bond followed by elimination of dinitrogen molecule. The p-allylnickel intermediate was formed by a subsequent allene insertion into five-membered intermediate. An allylic amidation occurred at the moresubstituted carbon atom[140,141] in the p-allylnickel intermediate to afford the reaction
product and the nickel(0) catalyst was regenerated (Scheme 44). It was reported that C2-symmetric bidentate bisphosphine ligands like (S,S’, R,R’)-tangphos, (S)-binap,[142] and (R,R)-Me-duphos[143] were not competent in this reaction. However, both excellent enantioselectivity and good yield were obtained using unsymmetrical P,N-type bidentate ligands like quinap and (S,S)-iPr-foxap.[144] This reaction was general with respect to the alkyl substituent at the N atom of the triazine functionality and afforded thiazine in good enantioselectivity as the major regioisomer. The thiazine was produced as a major regioisomer when tert-butyl possessing substrate triazine moiety was reacted because of steric reasons. This reaction with p-tolyl-substituted substrate was much less efficient. Many mono-substituted allenes were equally effective in transannulation with triazine functionality, thus furnishing good enantioselectivities and high yields of products. Allenes containing benzyloxy, siloxy, and N-phthalimidoyl groups at the alkyl chains also reacted well, although slightly lower enantioselectivities were reported.
3-(1,5-dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-yl)-1-(4-nitrophenyl)thioureaCatalog No.:AA00ITF6 CAS No.:101721-59-9 MDL No.:MFCD01567589 MF:C18H17N5O3S MW:383.4243 |
1-(4-Methyl-1,3-thiazol-2-yl)propan-1-amineCatalog No.:AA01AAXL CAS No.:1017211-92-5 MDL No.:MFCD09891374 MF:C7H12N2S MW:156.2486 |
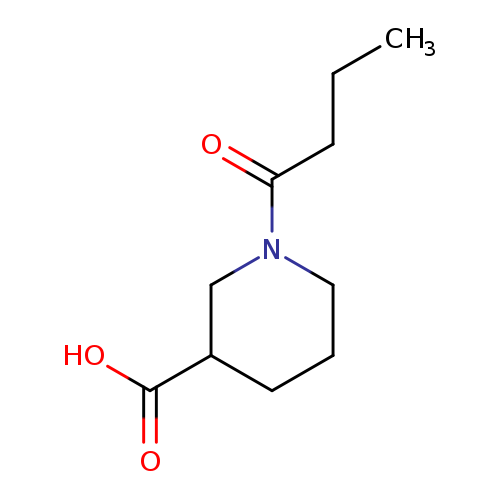
1-Butanoylpiperidine-3-carboxylic acidCatalog No.:AA00H9HF CAS No.:1017213-97-6 MDL No.:MFCD09813958 MF:C10H17NO3 MW:199.2469 |
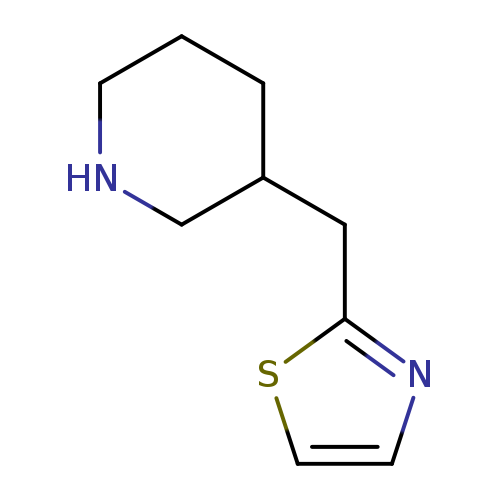
3-(1,3-thiazol-2-ylmethyl)piperidineCatalog No.:AA01BT01 CAS No.:1017215-27-8 MDL No.:MFCD09891854 MF:C9H14N2S MW:182.2859 |
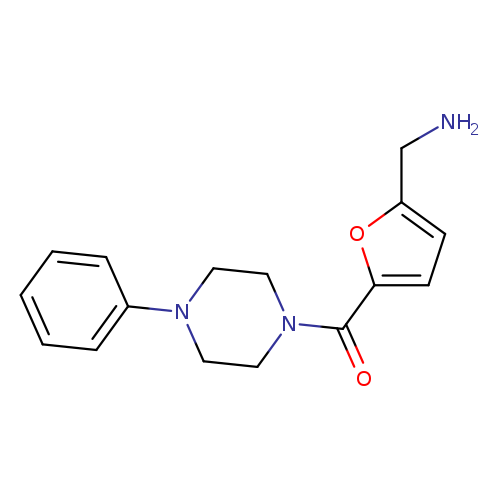
[5-(4-phenylpiperazine-1-carbonyl)furan-2-yl]methanamineCatalog No.:AA01BS5J CAS No.:1017217-14-9 MDL No.:MFCD09889139 MF:C16H19N3O2 MW:285.3410 |
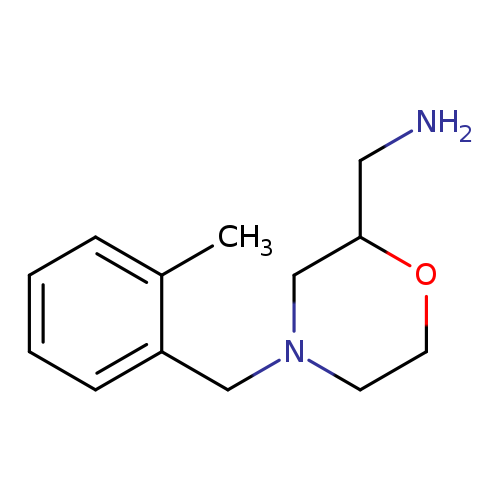
{4-[(2-methylphenyl)methyl]morpholin-2-yl}methanamineCatalog No.:AA01BRX5 CAS No.:1017217-24-1 MDL No.:MFCD09892097 MF:C13H20N2O MW:220.3107 |
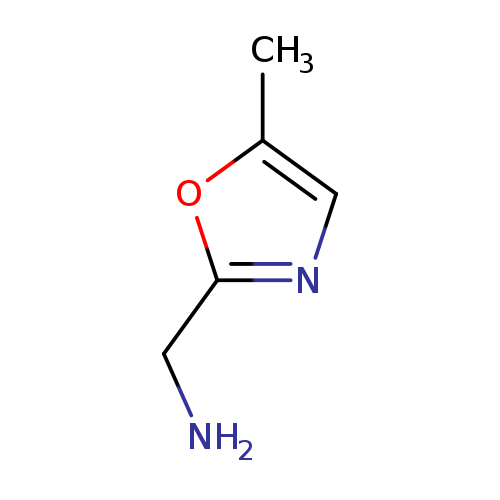
(5-Methyl-1,3-oxazol-2-yl)methanamineCatalog No.:AA00VS7T CAS No.:1017228-56-6 MDL No.:MFCD09884236 MF:C5H8N2O MW:112.1298 |
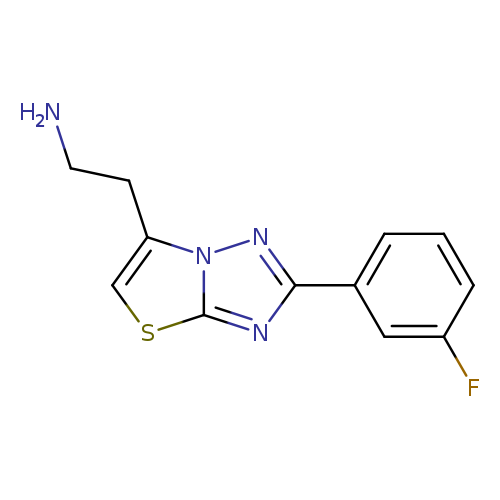
2-[2-(3-fluorophenyl)-[1,2,4]triazolo[3,2-b][1,3]thiazol-6-yl]ethan-1-amineCatalog No.:AA01C6CU CAS No.:1017232-65-3 MDL No.:MFCD09883670 MF:C12H11FN4S MW:262.3059 |
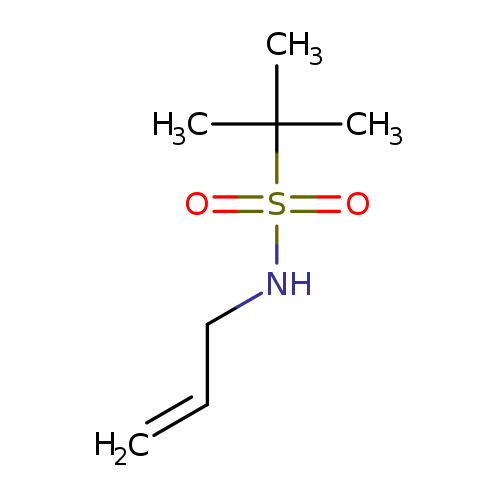
2-methyl-N-(prop-2-en-1-yl)propane-2-sulfonamideCatalog No.:AA01A4DT CAS No.:1017238-86-6 MDL No.:MFCD20366535 MF:C7H15NO2S MW:177.2645 |
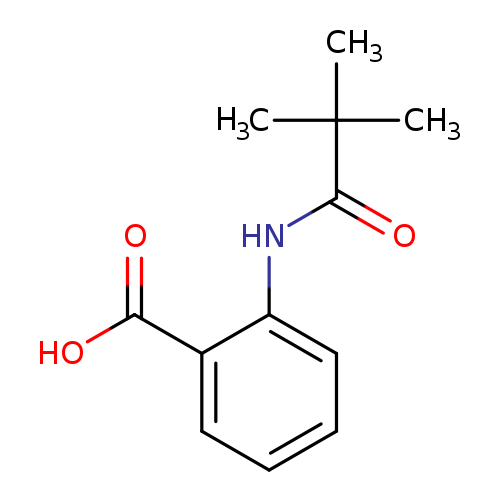
2-Pivalamidobenzoic acidCatalog No.:AA00058Y CAS No.:101724-84-9 MDL No.:MFCD01006384 MF:C12H15NO3 MW:221.2524 |

2H-1,5-Benzodiazepin-2-one, 1,3-dihydro-4-(4-methyl-1-piperazinyl)-3-(2-oxopropylidene)-, (3Z)-Catalog No.:AA00058U CAS No.:1017241-34-7 MDL No.:MFCD21363737 MF:C17H20N4O2 MW:312.3663 |

2-Propanone, 1-[4,5-dihydro-2-(4-methyl-1-piperazinyl)-4-thioxo-3H-1,5-benzodiazepin-3-ylidene]-, (1Z)-Catalog No.:AA00058T CAS No.:1017241-36-9 MDL No.:MFCD18379579 MF:C17H20N4OS MW:328.4319 |
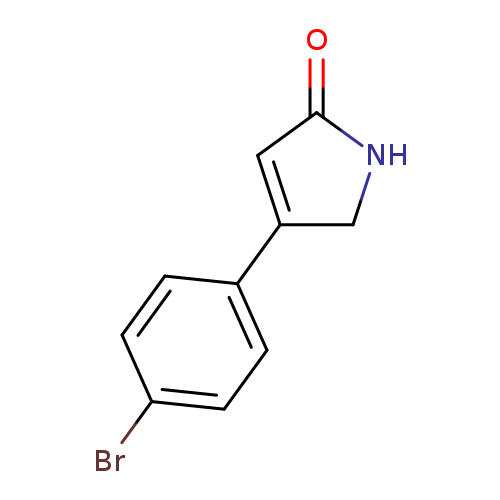
4-(4-bromophenyl)-2,5-dihydro-1h-pyrrol-2-oneCatalog No.:AA01C599 CAS No.:1017246-65-9 MDL No.:MFCD29763368 MF:C10H8BrNO MW:238.0806 |
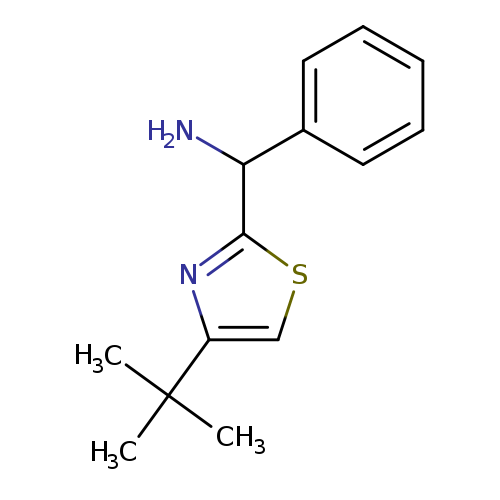
(4-tert-butyl-1,3-thiazol-2-yl)(phenyl)methanamineCatalog No.:AA01A9KY CAS No.:1017250-43-9 MDL No.:MFCD09891459 MF:C14H18N2S MW:246.3711 |
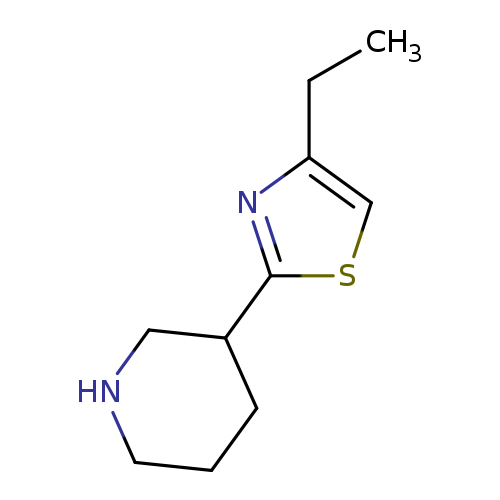
3-(4-ethyl-1,3-thiazol-2-yl)piperidineCatalog No.:AA01A8RR CAS No.:1017250-60-0 MDL No.:MFCD09891526 MF:C10H16N2S MW:196.3124 |
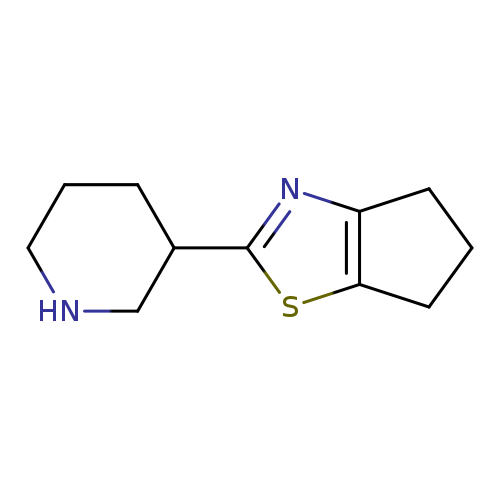
2-(piperidin-3-yl)-5,6-dihydro-4H-cyclopenta[d]thiazoleCatalog No.:AA01BIY8 CAS No.:1017250-61-1 MDL No.:MFCD09891530 MF:C11H16N2S MW:208.3231 |
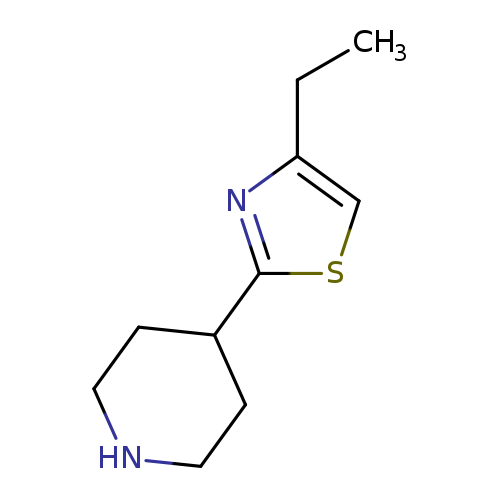
4-(4-Ethyl-1,3-thiazol-2-yl)piperidineCatalog No.:AA01A8RV CAS No.:1017250-65-5 MDL No.:MFCD09891550 MF:C10H16N2S MW:196.3124 |
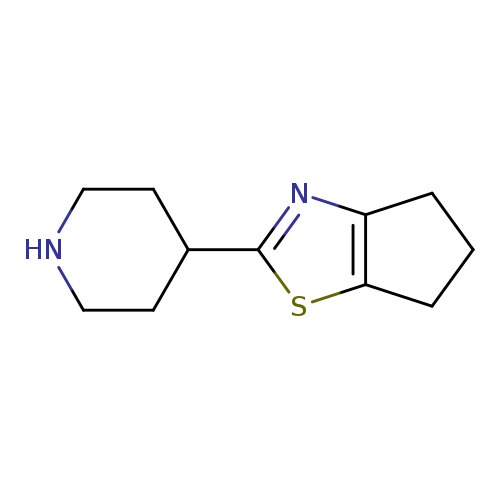
4-{4H,5H,6H-cyclopenta[d][1,3]thiazol-2-yl}piperidineCatalog No.:AA01BJHD CAS No.:1017250-66-6 MDL No.:MFCD09891554 MF:C11H16N2S MW:208.3231 |
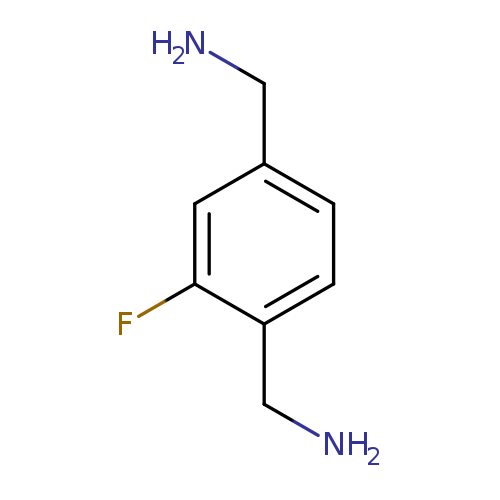
[4-(aminomethyl)-2-fluorophenyl]methanamineCatalog No.:AA01BH6R CAS No.:1017264-58-2 MDL No.:MFCD16669391 MF:C8H11FN2 MW:154.1847 |
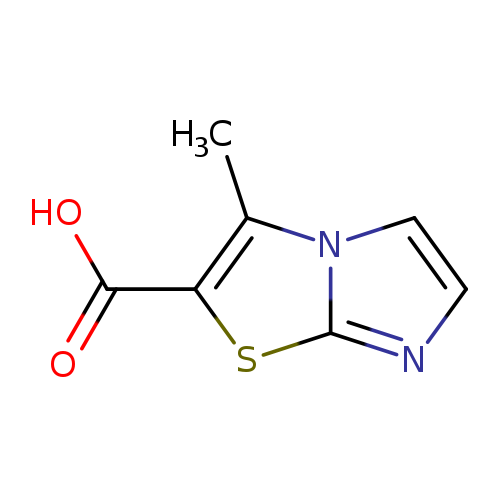
3-Methylimidazo[2,1-b]thiazole-2-carboxylic acidCatalog No.:AA00058Q CAS No.:1017273-59-4 MDL No.:MFCD11889936 MF:C7H6N2O2S MW:182.1997 |
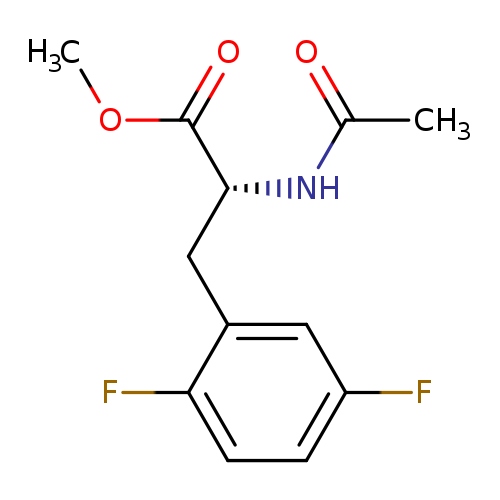
Methyl n-acetyl-3-(2,5-difluorophenyl)-d-alaninateCatalog No.:AA00058P CAS No.:1017294-05-1 MDL No.:MFCD08436171 MF:C12H13F2NO3 MW:257.2333 |
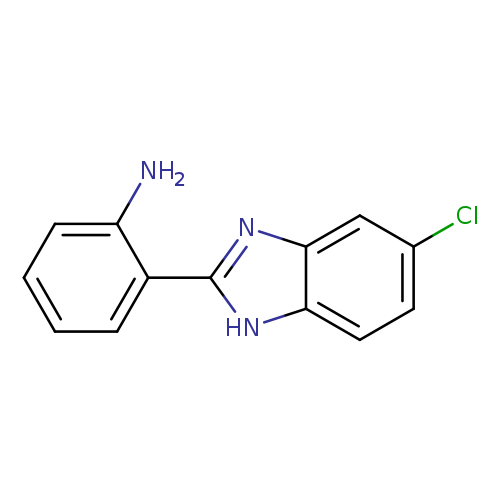
2-(5-chloro-1H-1,3-benzodiazol-2-yl)anilineCatalog No.:AA01C552 CAS No.:10173-52-1 MDL No.:MFCD10692537 MF:C13H10ClN3 MW:243.6916 |
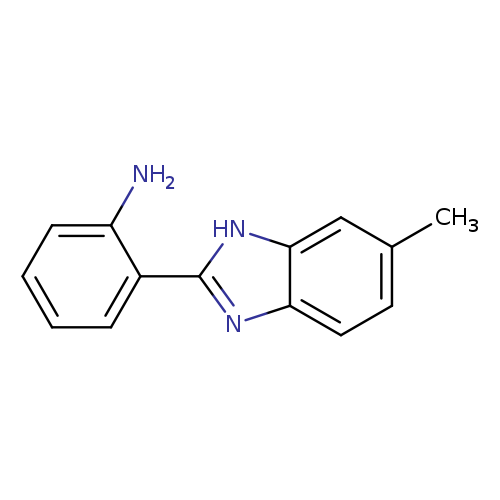
Benzenamine, 2-(6-methyl-1H-benzimidazol-2-yl)-Catalog No.:AA000594 CAS No.:10173-53-2 MDL No.:MFCD03148293 MF:C14H13N3 MW:223.2731 |
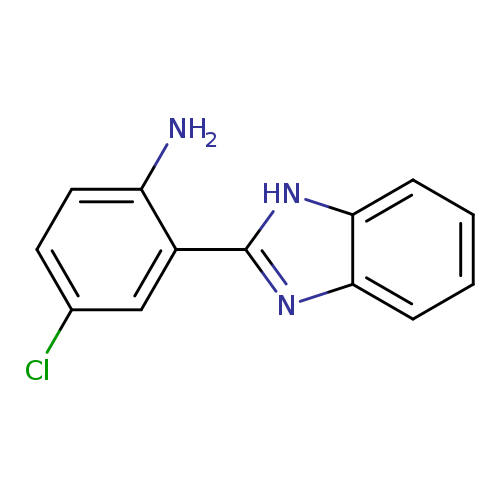
2-(1H-1,3-Benzodiazol-2-yl)-4-chloroanilineCatalog No.:AA01AGF0 CAS No.:10173-56-5 MDL No.:MFCD00829970 MF:C13H10ClN3 MW:243.6916 |
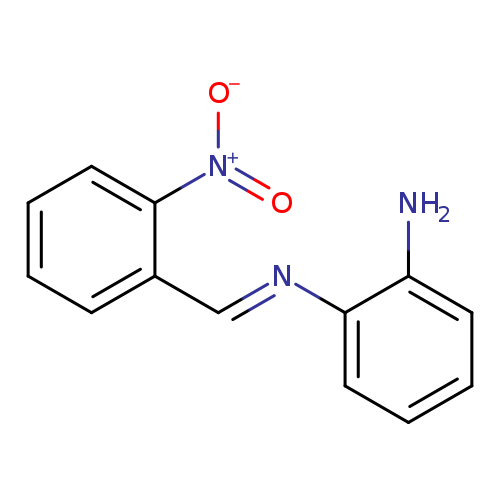
(1E)-N1-[(2-nitrophenyl)methylidene]benzene-1,2-diamineCatalog No.:AA00IVPM CAS No.:10173-60-1 MDL No.:MFCD00665097 MF:C13H11N3O2 MW:241.2453 |
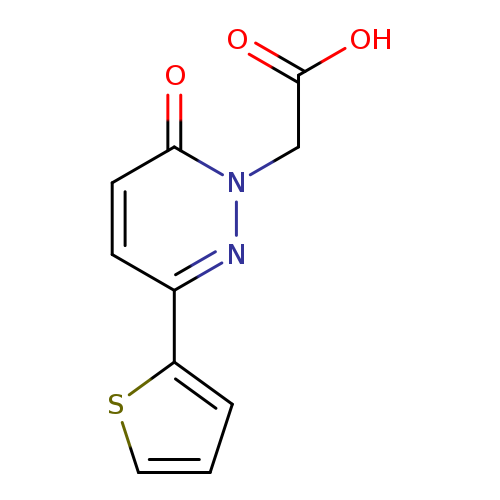
2-[6-Oxo-3-(thiophen-2-yl)-1,6-dihydropyridazin-1-yl]acetic acidCatalog No.:AA00H9HL CAS No.:1017326-15-6 MDL No.:MFCD09881873 MF:C10H8N2O3S MW:236.2471 |
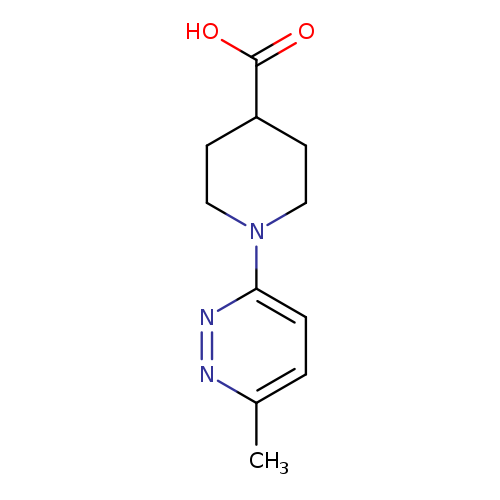
1-(6-Methylpyridazin-3-yl)piperidine-4-carboxylic acidCatalog No.:AA018MSK CAS No.:1017326-43-0 MDL No.:MFCD09881913 MF:C11H15N3O2 MW:221.2557 |
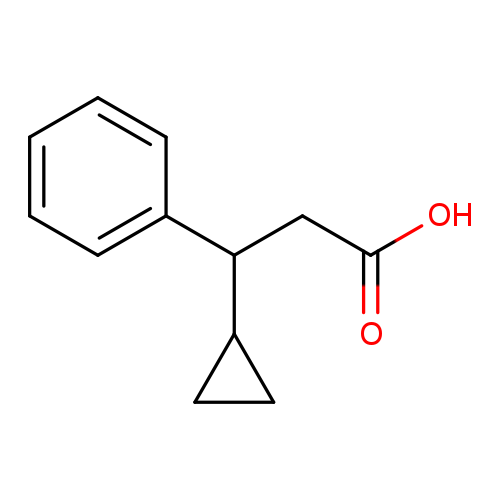
3-cyclopropyl-3-phenylpropanoic acidCatalog No.:AA01E7O1 CAS No.:1017329-96-2 MDL No.:MFCD09889681 MF:C12H14O2 MW:190.2384 |
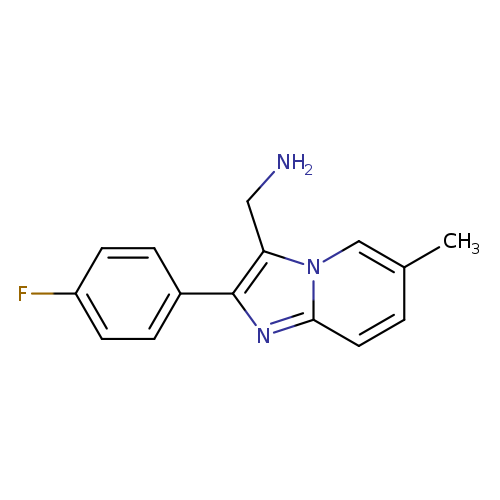
[2-(4-fluorophenyl)-6-methylimidazo[1,2-a]pyridin-3-yl]methanamineCatalog No.:AA01EJEP CAS No.:1017330-88-9 MDL No.:MFCD09886126 MF:C15H14FN3 MW:255.2902 |
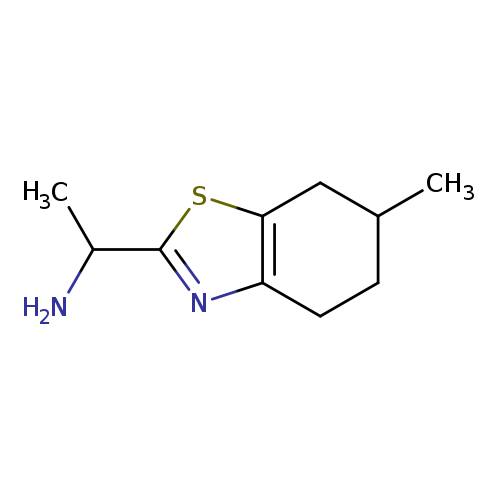
1-(6-METHYL-4,5,6,7-TETRAHYDRO-1,3-BENZOTHIAZOL-2-YL)ETHAN-1-AMINECatalog No.:AA01DX3S CAS No.:1017345-80-0 MDL No.:MFCD09891369 MF:C10H16N2S MW:196.3124 |
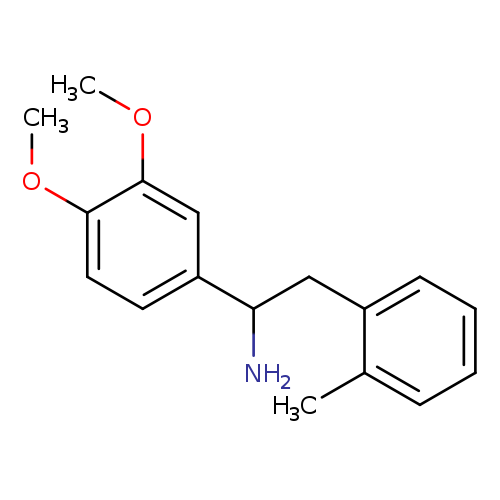
1-(3,4-dimethoxyphenyl)-2-(2-methylphenyl)ethan-1-amineCatalog No.:AA019Y43 CAS No.:1017346-27-8 MDL No.:MFCD09810563 MF:C17H21NO2 MW:271.3541 |
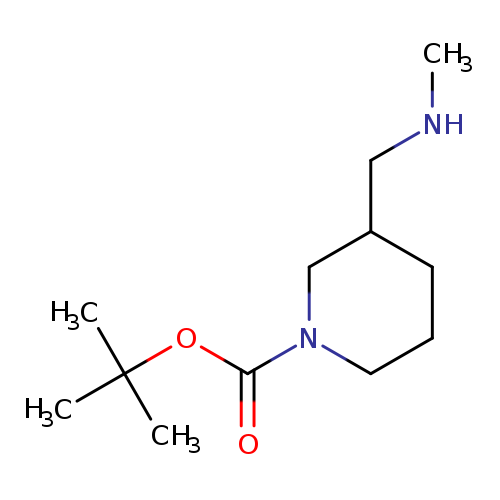
tert-Butyl 3-((methylamino)methyl)piperidine-1-carboxylateCatalog No.:AA00059E CAS No.:1017356-25-0 MDL No.:MFCD08061952 MF:C12H24N2O2 MW:228.3312 |
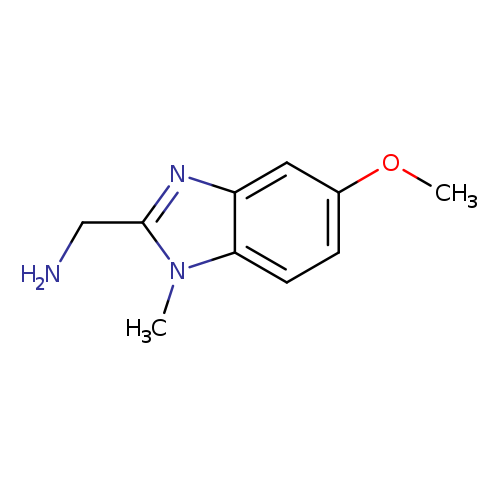
(5-methoxy-1-methyl-1H-1,3-benzodiazol-2-yl)methanamineCatalog No.:AA01ACUZ CAS No.:1017357-82-2 MDL No.:MFCD09882443 MF:C10H13N3O MW:191.2297 |

2-(5-Methyl-2-phenylthiazole-4-yl)acetic acidCatalog No.:AA00059N CAS No.:101736-22-5 MDL No.:MFCD00277218 MF:C12H11NO2S MW:233.2862 |
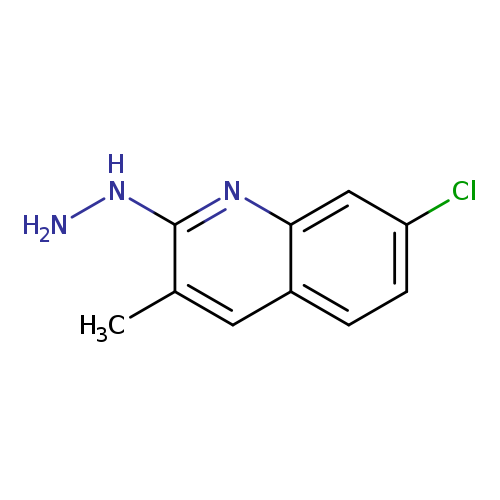
7-chloro-2-hydrazinyl-3-methylquinolineCatalog No.:AA008XRO CAS No.:1017360-44-9 MDL No.:MFCD23135594 MF:C10H10ClN3 MW:207.6595 |
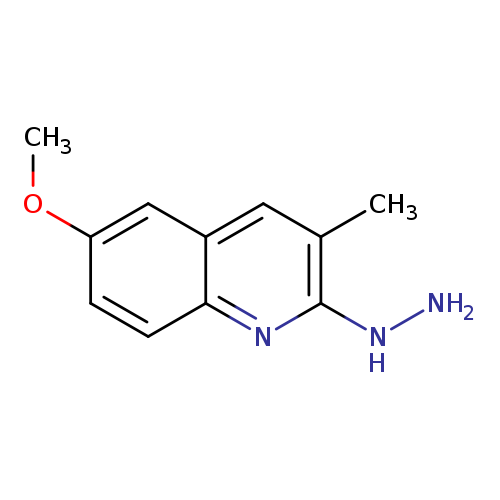
2-Hydrazino-6-methoxy-3-methylquinoline hydrochlorideCatalog No.:AA008XRM CAS No.:1017360-52-9 MDL No.:MFCD11505163 MF:C11H13N3O MW:203.2404 |
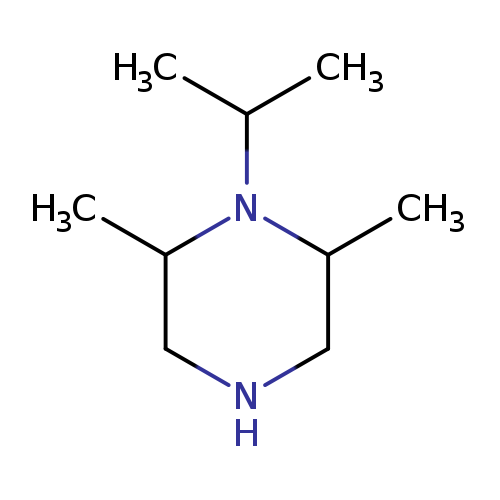
2,6-dimethyl-1-(propan-2-yl)piperazineCatalog No.:AA01C52T CAS No.:1017366-56-1 MDL No.:MFCD09894132 MF:C9H20N2 MW:156.2685 |
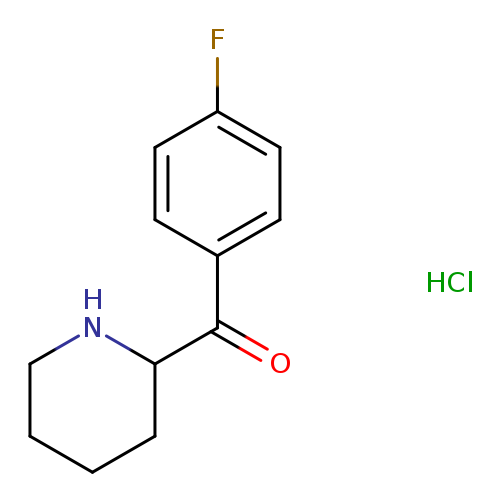
2-[(4-Fluorophenyl)carbonyl]piperidine HClCatalog No.:AA009492 CAS No.:1017366-82-3 MDL No.:MFCD11974884 MF:C12H15ClFNO MW:243.7050 |
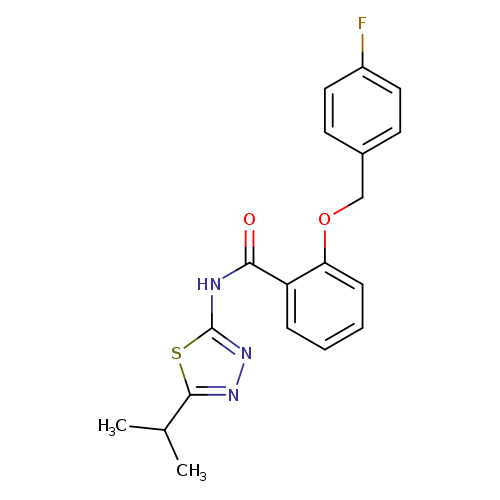
2-[(4-fluorophenyl)methoxy]-N-[5-(propan-2-yl)-1,3,4-thiadiazol-2-yl]benzamideCatalog No.:AA01DX3V CAS No.:1017370-54-5 MDL No.:MFCD10562520 MF:C19H18FN3O2S MW:371.4285 |
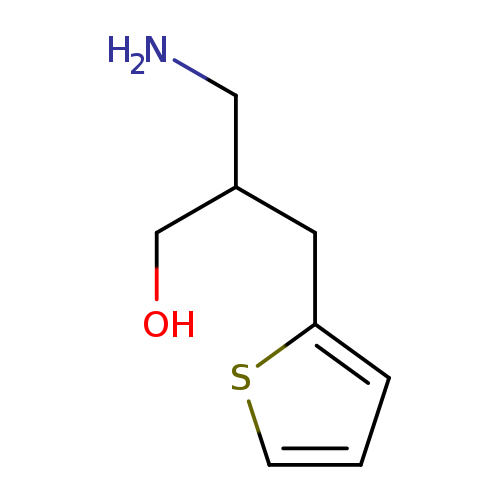
3-Amino-2-(2-thienylmethyl)-1-propanolCatalog No.:AA008V9O CAS No.:1017371-11-7 MDL No.:MFCD09894872 MF:C8H13NOS MW:171.2599 |
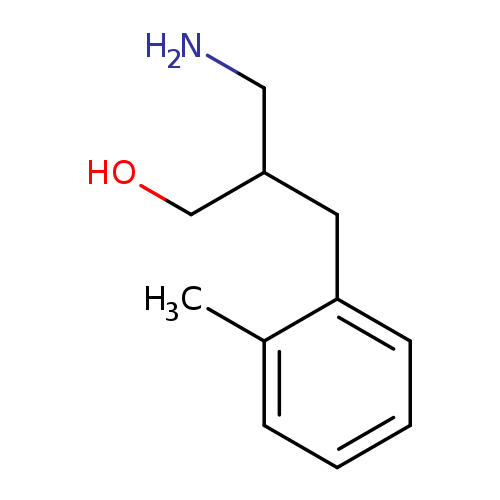
3-amino-2-[(2-methylphenyl)methyl]propan-1-olCatalog No.:AA019MP3 CAS No.:1017371-15-1 MDL No.:MFCD09894876 MF:C11H17NO MW:179.2588 |
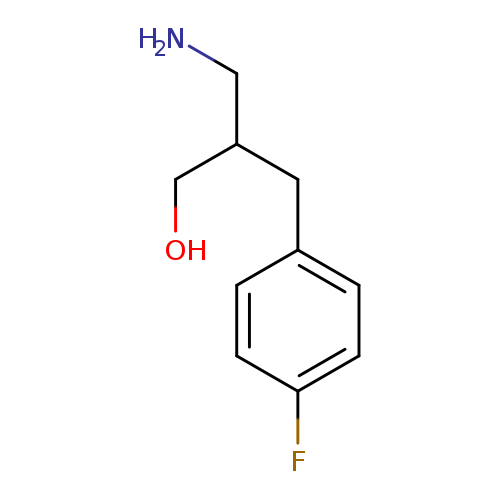
3-amino-2-[(4-fluorophenyl)methyl]propan-1-olCatalog No.:AA019MP6 CAS No.:1017371-19-5 MDL No.:MFCD09894880 MF:C10H14FNO MW:183.2227 |
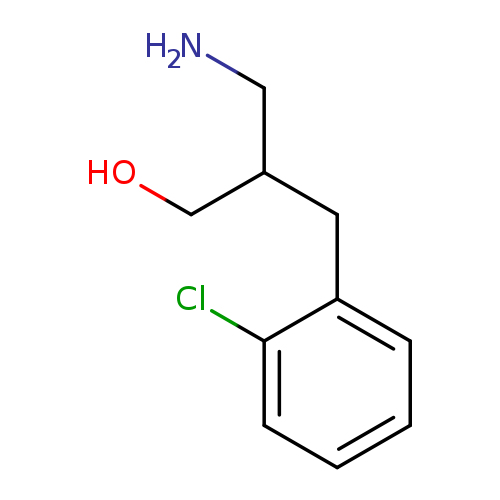
3-amino-2-[(2-chlorophenyl)methyl]propan-1-olCatalog No.:AA019SBP CAS No.:1017371-23-1 MDL No.:MFCD09894885 MF:C10H14ClNO MW:199.6773 |
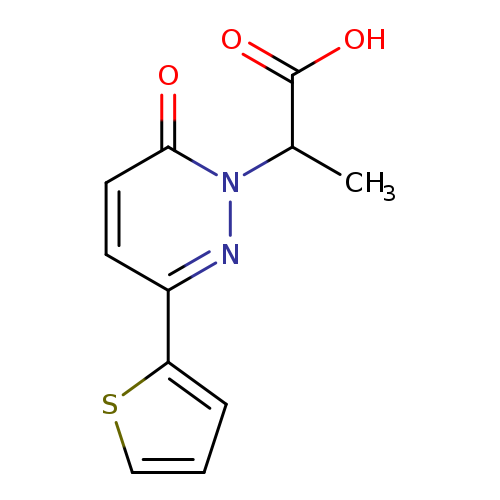
"2-[6-oxo-3-(thiophen-2-yl)-1,6-dihydropyridazin-1-yl]propanoic acid"Catalog No.:AA01FMVK CAS No.:1017378-02-7 MDL No.:MFCD09881889 MF:C11H10N2O3S MW:250.2737 |
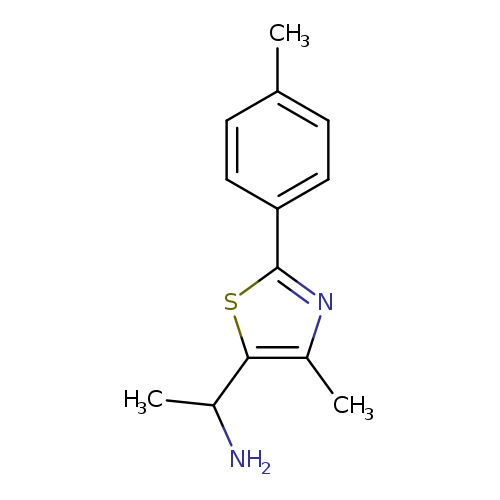
1-[4-Methyl-2-(4-methylphenyl)-1,3-thiazol-5-yl]ethan-1-amineCatalog No.:AA01AGLE CAS No.:1017379-98-4 MDL No.:MFCD09882220 MF:C13H16N2S MW:232.3445 |
3-((4-Methoxyphenyl)sulfonyl)propanoyl chlorideCatalog No.:AA01ARYL CAS No.:1017381-16-6 MDL No.:MFCD09893539 MF:C10H11ClO4S MW:262.7099 |
1-(2-Phenylacetyl)-3-piperidinecarboxylic acidCatalog No.:AA00H9HQ CAS No.:1017381-24-6 MDL No.:MFCD09814410 MF:C14H17NO3 MW:247.2897 |
1-[(Dimethylamino)sulfonyl]piperidine-4-carboxylic acidCatalog No.:AA00H9HR CAS No.:1017381-49-5 MDL No.:MFCD02055741 MF:C8H16N2O4S MW:236.2886 |
2-(2,6-difluorophenyl)-5-methyl-1,3-thiazole-4-carboxylic acidCatalog No.:AA00VSMD CAS No.:1017385-07-7 MDL No.:MFCD10007665 MF:C11H7F2NO2S MW:255.2406 |
4-aminoquinazoline-7-carboxylic acidCatalog No.:AA01ALO0 CAS No.:1017385-11-3 MDL No.:MFCD07754182 MF:C9H7N3O2 MW:189.1708 |
1-(2-methyl-1,3-thiazol-5-yl)ethan-1-amineCatalog No.:AA01ANBL CAS No.:1017386-12-7 MDL No.:MFCD10007039 MF:C6H10N2S MW:142.2220 |
7-FLUORO-3-(HYDROXYMETHYL)-1,2-DIHYDROQUINOLIN-2-ONECatalog No.:AA01DX3W CAS No.:1017386-68-3 MDL No.:MFCD10004917 MF:C10H8FNO2 MW:193.1744 |
1-(4-Methoxyphenyl)cyclobutan-1-amine hydrochlorideCatalog No.:AA0096QN CAS No.:1017387-07-3 MDL No.:MFCD09903763 MF:C11H15NO MW:177.2429 |
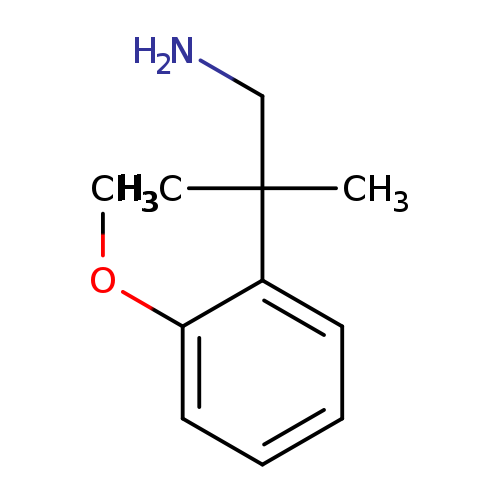
2-(2-methoxyphenyl)-2-methylpropan-1-amineCatalog No.:AA01A7LF CAS No.:1017387-13-1 MDL No.:MFCD09903778 MF:C11H17NO MW:179.2588 |
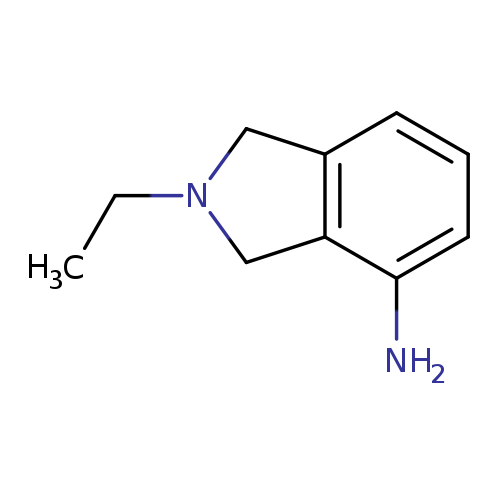
2-ethyl-2,3-dihydro-1H-isoindol-4-amineCatalog No.:AA01A4PS CAS No.:1017388-28-1 MDL No.:MFCD10007251 MF:C10H14N2 MW:162.2316 |
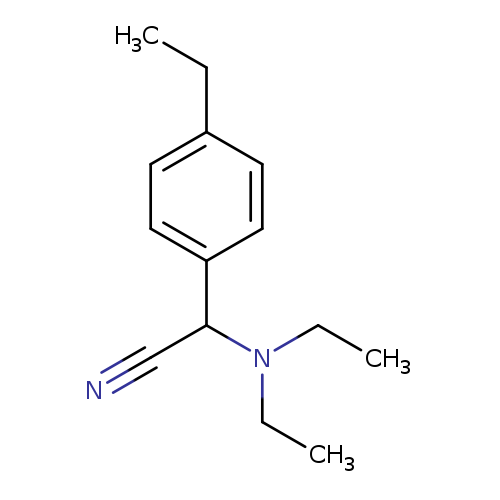
2-(Diethylamino)-2-(4-ethylphenyl)acetonitrileCatalog No.:AA01BB51 CAS No.:1017388-30-5 MDL No.:MFCD10005132 MF:C14H20N2 MW:216.3220 |
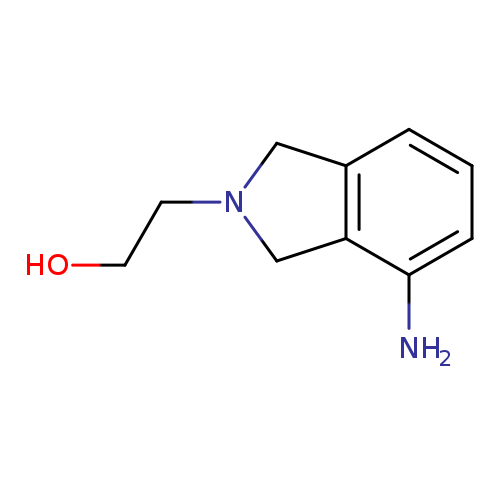
2-(4-Amino-2,3-dihydro-1h-isoindol-2-yl)ethan-1-olCatalog No.:AA01A4SN CAS No.:1017388-32-7 MDL No.:MFCD10007254 MF:C10H14N2O MW:178.2310 |
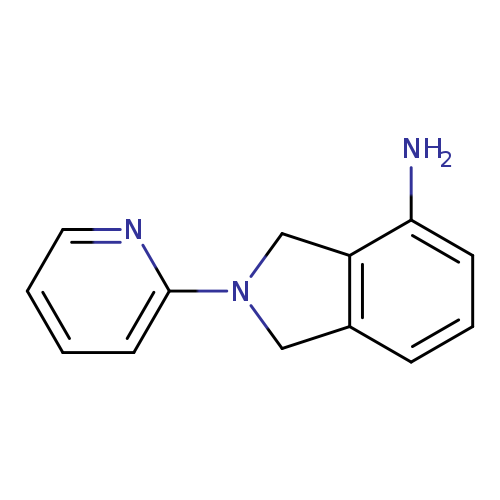
2-(Pyridin-2-yl)-2,3-dihydro-1h-isoindol-4-amineCatalog No.:AA01A2SI CAS No.:1017388-43-0 MDL No.:MFCD10007264 MF:C13H13N3 MW:211.2624 |
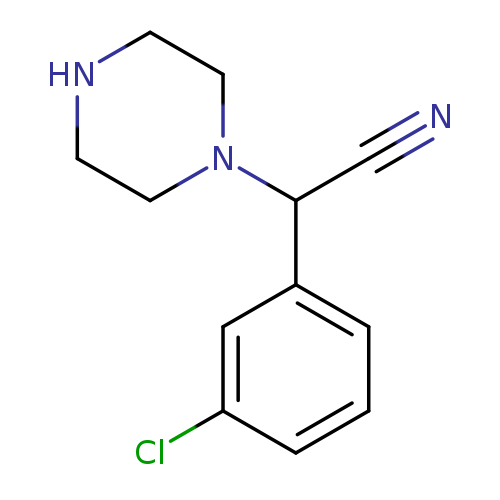
2-(3-chlorophenyl)-2-(piperazin-1-yl)acetonitrileCatalog No.:AA01BGCR CAS No.:1017388-57-6 MDL No.:MFCD09817086 MF:C12H14ClN3 MW:235.7127 |
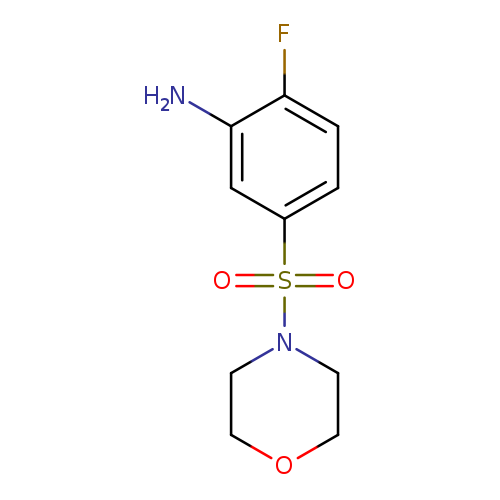
2-fluoro-5-(morpholine-4-sulfonyl)anilineCatalog No.:AA01AKHB CAS No.:1017390-45-2 MDL No.:MFCD09900651 MF:C10H13FN2O3S MW:260.2852 |
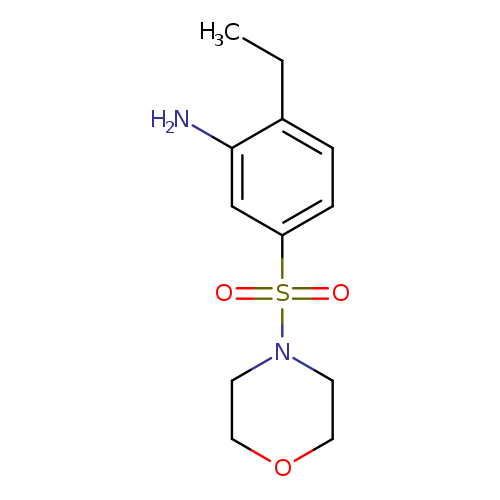
2-ethyl-5-(morpholine-4-sulfonyl)anilineCatalog No.:AA01C4H7 CAS No.:1017390-48-5 MDL No.:MFCD09900654 MF:C12H18N2O3S MW:270.3479 |
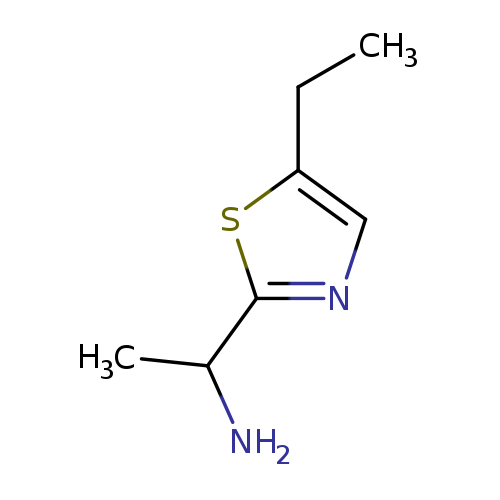
1-(5-ethyl-1,3-thiazol-2-yl)ethan-1-amineCatalog No.:AA019Y7J CAS No.:1017391-90-0 MDL No.:MFCD09904508 MF:C7H12N2S MW:156.2486 |
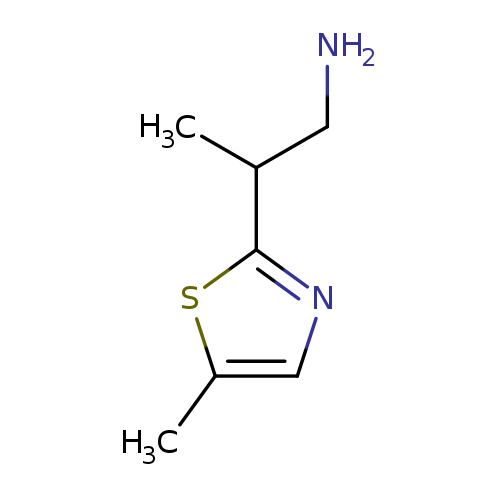
2-(5-methyl-1,3-thiazol-2-yl)propan-1-amineCatalog No.:AA01A70G CAS No.:1017392-90-3 MDL No.:MFCD09904595 MF:C7H12N2S MW:156.2486 |
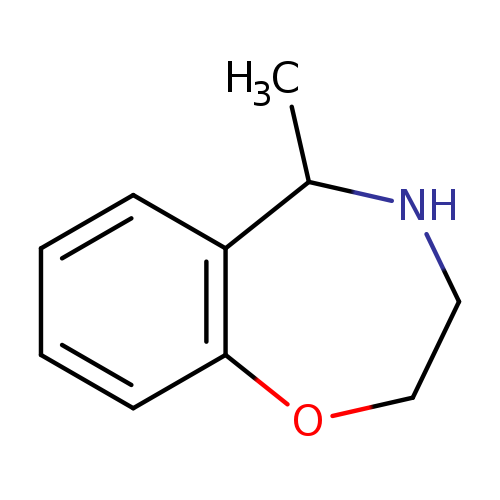
5-methyl-2,3,4,5-tetrahydro-1,4-benzoxazepineCatalog No.:AA019VYG CAS No.:1017394-91-0 MDL No.:MFCD09901382 MF:C10H13NO MW:163.2163 |
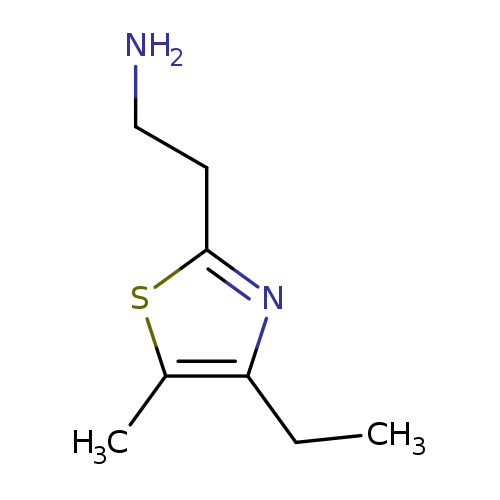
2-(4-ethyl-5-methyl-1,3-thiazol-2-yl)ethan-1-amineCatalog No.:AA01A99H CAS No.:1017394-96-5 MDL No.:MFCD09904759 MF:C8H14N2S MW:170.2752 |
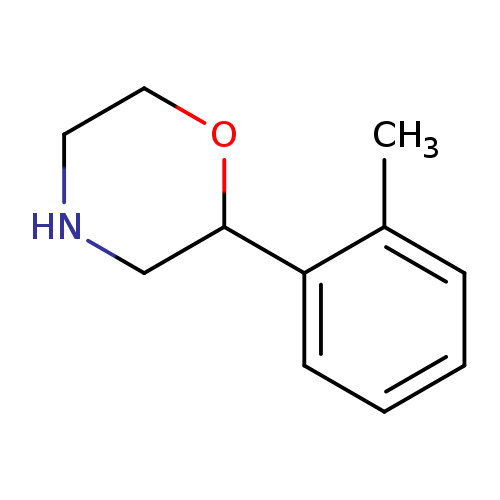
2-(2-Methylphenyl)morpholineCatalog No.:AA00059C CAS No.:1017395-56-0 MDL No.:MFCD09901447 MF:C11H15NO MW:177.2429 |
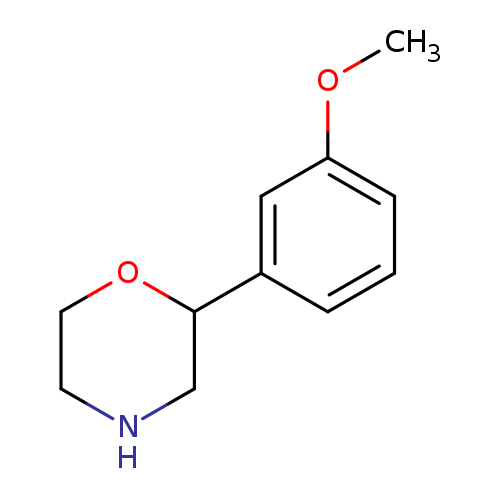
Morpholine, 2-(3-methoxyphenyl)-Catalog No.:AA0005AF CAS No.:1017395-60-6 MDL No.:MFCD09901452 MF:C11H15NO2 MW:193.2423 |
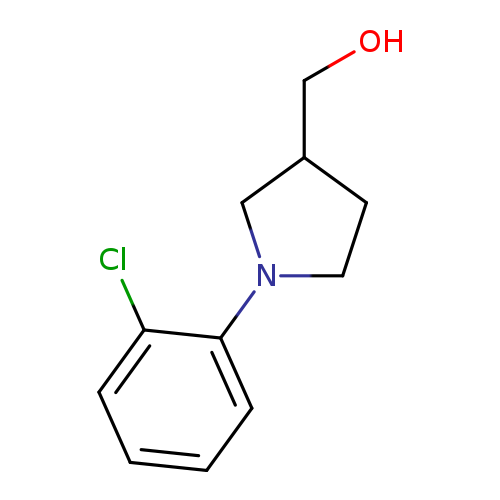
[1-(2-chlorophenyl)pyrrolidin-3-yl]methanolCatalog No.:AA01BAOG CAS No.:1017396-23-4 MDL No.:MFCD10003936 MF:C11H14ClNO MW:211.6880 |
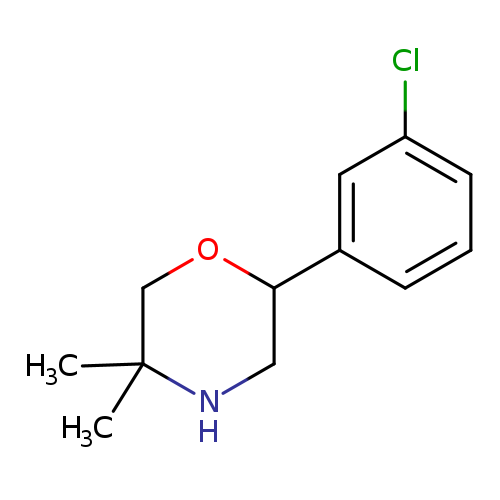
2-(3-Chlorophenyl)-5,5-dimethylmorpholineCatalog No.:AA01B63D CAS No.:1017396-25-6 MDL No.:MFCD09901507 MF:C12H16ClNO MW:225.7145 |
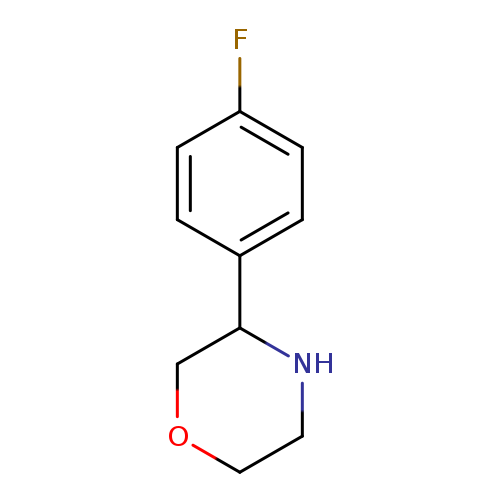
3-(4-FLUOROPHENYL)MORPHOLINECatalog No.:AA009647 CAS No.:1017396-52-9 MDL No.:MFCD09901528 MF:C10H12FNO MW:181.2068 |
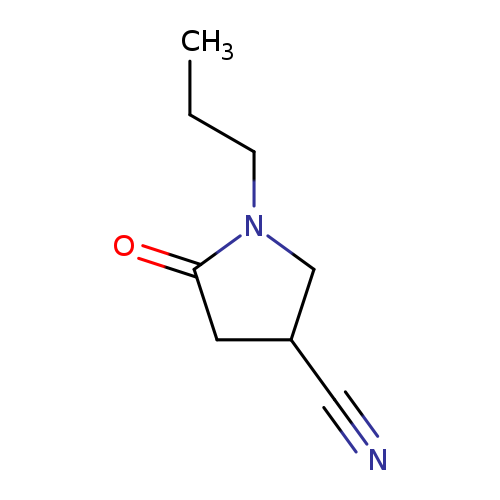
5-Oxo-1-propylpyrrolidine-3-carbonitrileCatalog No.:AA01ALZ4 CAS No.:1017397-60-2 MDL No.:MFCD10004073 MF:C8H12N2O MW:152.1937 |
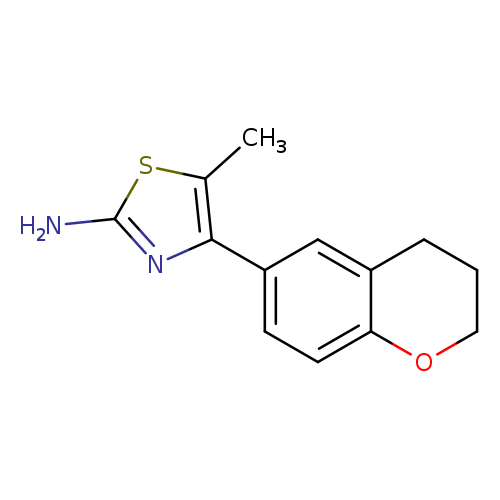
4-(3,4-dihydro-2H-1-benzopyran-6-yl)-5-methyl-1,3-thiazol-2-amineCatalog No.:AA01A2F8 CAS No.:1017398-32-1 MDL No.:MFCD10006115 MF:C13H14N2OS MW:246.3281 |
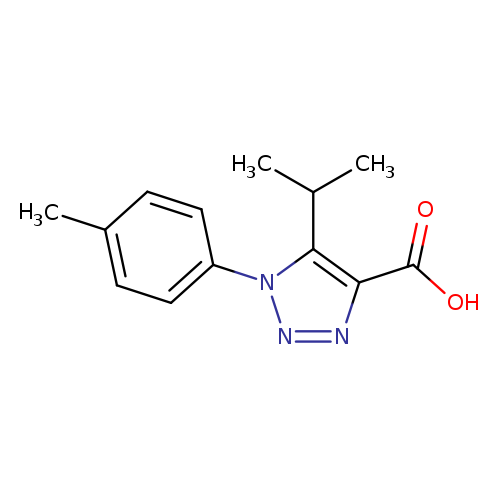
1-(4-methylphenyl)-5-(propan-2-yl)-1H-1,2,3-triazole-4-carboxylic acidCatalog No.:AA019V2Z CAS No.:1017399-09-5 MDL No.:MFCD10003277 MF:C13H15N3O2 MW:245.2771 |
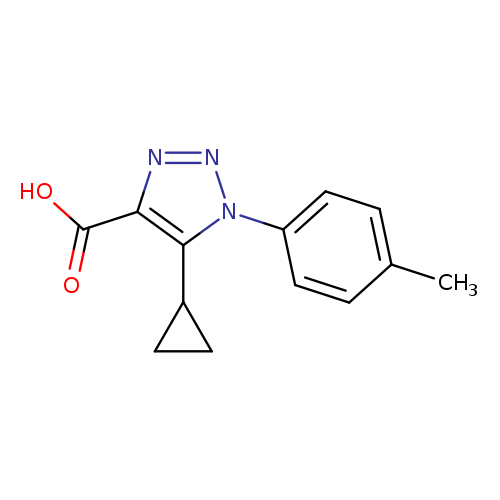
5-Cyclopropyl-1-(p-tolyl)-1H-1,2,3-triazole-4-carboxylic acidCatalog No.:AA01A89O CAS No.:1017399-13-1 MDL No.:MFCD10003280 MF:C13H13N3O2 MW:243.2612 |
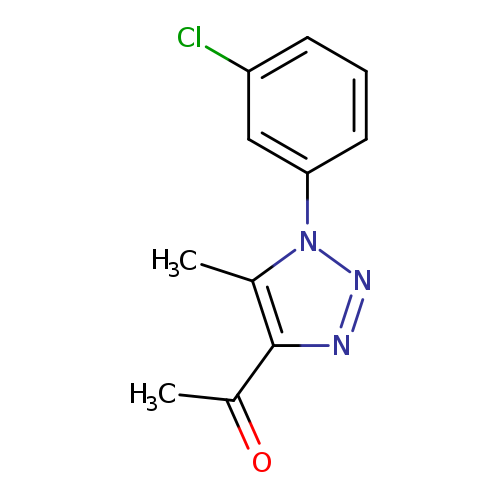
1-[1-(3-chlorophenyl)-5-methyl-1H-1,2,3-triazol-4-yl]ethan-1-oneCatalog No.:AA019WCS CAS No.:1017399-61-9 MDL No.:MFCD10003319 MF:C11H10ClN3O MW:235.6696 |
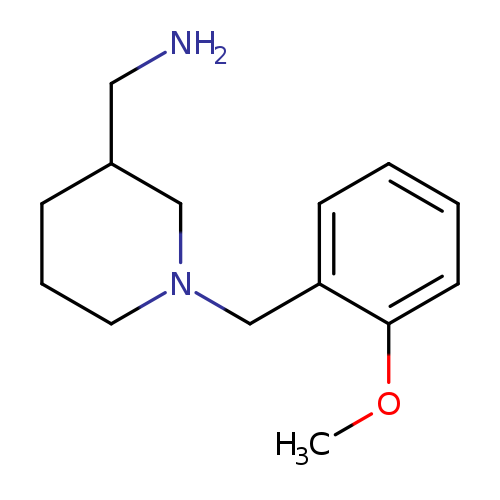
(1-(2-methoxybenzyl)piperidin-3-yl)methanamineCatalog No.:AA01FMF8 CAS No.:1017399-89-1 MDL No.:MFCD10003337 MF:C14H22N2O MW:234.3373 |
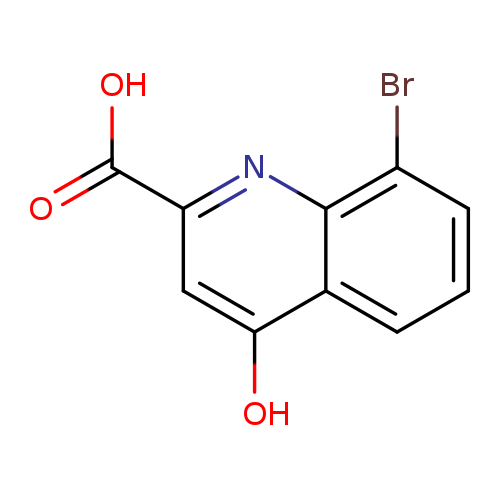
8-Bromo-4-hydroxyquinoline-2-carboxylic acidCatalog No.:AA0005AL CAS No.:10174-71-7 MDL No.:MFCD26634232 MF:C10H6BrNO3 MW:268.0635 |
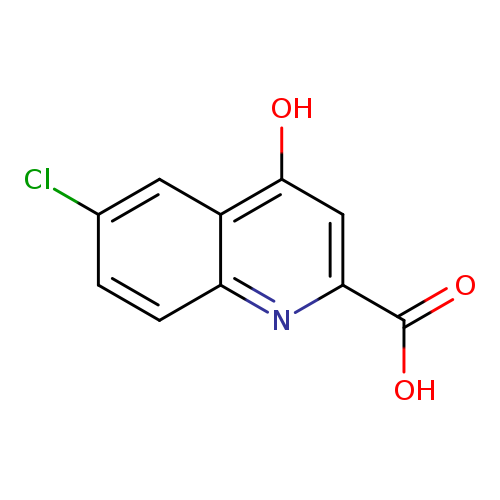
6-Chloro-4-hydroxyquinoline-2-carboxylic acidCatalog No.:AA0005AK CAS No.:10174-72-8 MDL No.:MFCD00673835 MF:C10H6ClNO3 MW:223.6125 |
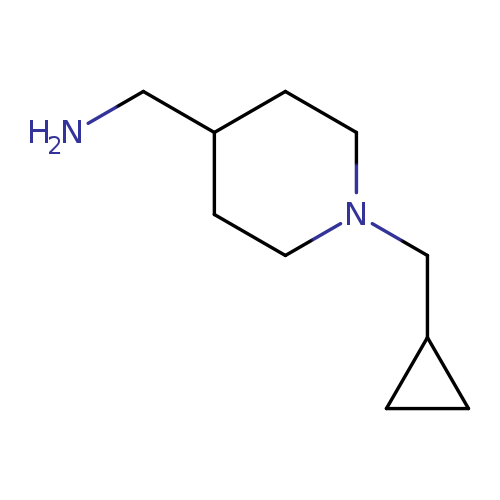
1-[1-(cyclopropylmethyl)-4-piperidinyl]methanamineCatalog No.:AA008V59 CAS No.:1017400-92-8 MDL No.:MFCD10003421 MF:C10H20N2 MW:168.2792 |
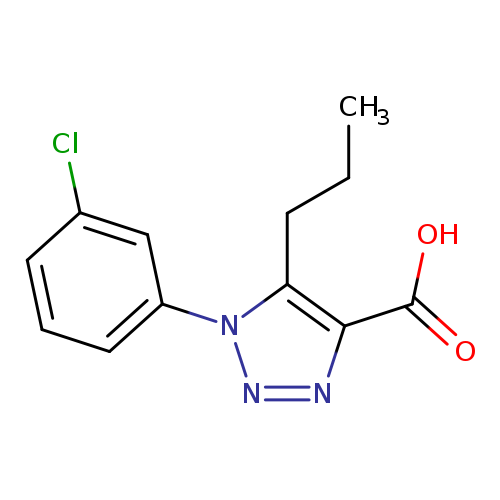
1-(3-Chlorophenyl)-5-propyl-1h-1,2,3-triazole-4-carboxylic acidCatalog No.:AA01AGXA CAS No.:1017402-13-9 MDL No.:MFCD10006696 MF:C12H12ClN3O2 MW:265.6956 |
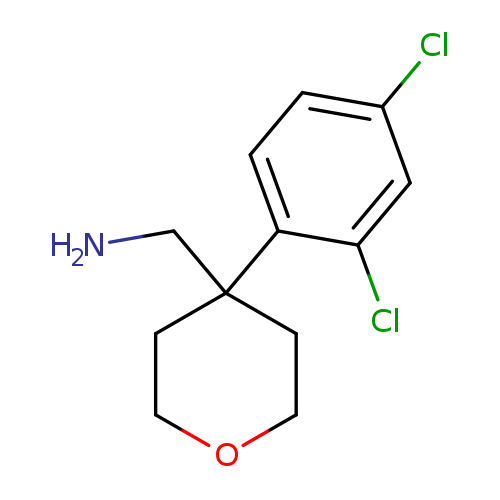
[4-(2,4-dichlorophenyl)oxan-4-yl]methanamineCatalog No.:AA019X3L CAS No.:1017407-65-6 MDL No.:MFCD09904139 MF:C12H15Cl2NO MW:260.1596 |
3-(4-Fluorophenyl)-3-methylbutan-2-oneCatalog No.:AA01AJ11 CAS No.:1017409-06-1 MDL No.:MFCD09904319 MF:C11H13FO MW:180.2187 |
3-Amino-N-tert-butyl-4-methylbenzenesulfonamideCatalog No.:AA00946X CAS No.:1017410-65-9 MDL No.:MFCD09900854 MF:C11H18N2O2S MW:242.3378 |
5-Bromoquinoline-2-carboxylic acidCatalog No.:AA0005A9 CAS No.:1017412-53-1 MDL No.:MFCD19689976 MF:C10H6BrNO2 MW:252.0641 |
1-phenyl-5-(pyridin-4-yl)-1H-1,2,3-triazole-4-carboxylic acidCatalog No.:AA00J0IK CAS No.:1017415-16-5 MDL No.:MFCD10006446 MF:C14H10N4O2 MW:266.2548 |
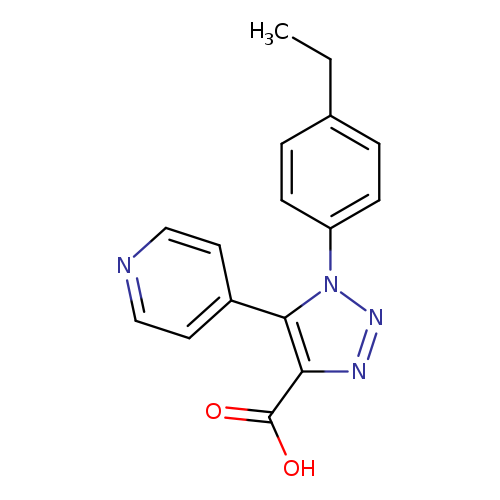
1-(4-ethylphenyl)-5-(pyridin-4-yl)-1H-1,2,3-triazole-4-carboxylic acidCatalog No.:AA018RMT CAS No.:1017415-74-5 MDL No.:MFCD10006508 MF:C16H14N4O2 MW:294.3080 |
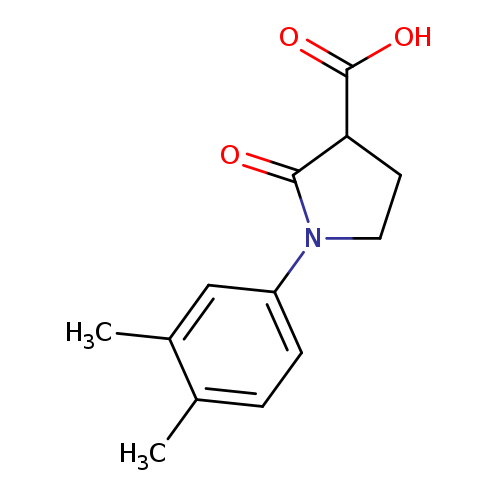
1-(3,4-Dimethylphenyl)-2-oxopyrrolidine-3-carboxylic acidCatalog No.:AA019WV4 CAS No.:1017417-05-8 MDL No.:MFCD09815095 MF:C13H15NO3 MW:233.2631 |
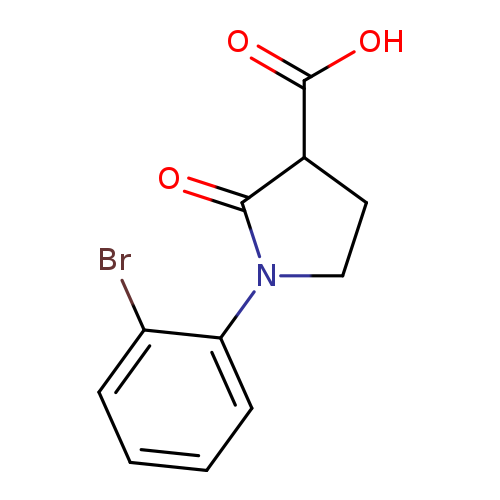
1-(2-bromophenyl)-2-oxopyrrolidine-3-carboxylic acidCatalog No.:AA019V7Z CAS No.:1017417-29-6 MDL No.:MFCD09814386 MF:C11H10BrNO3 MW:284.1060 |
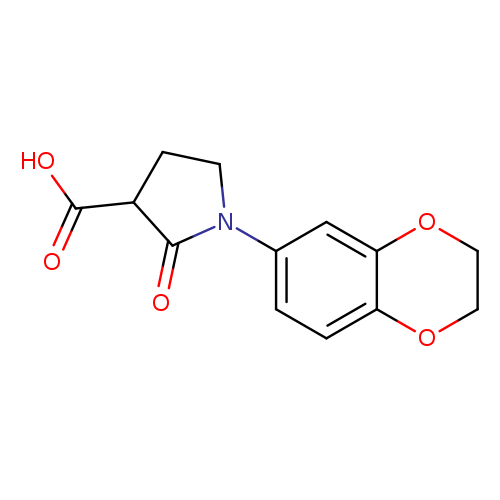
1-(2,3-dihydro-1,4-benzodioxin-6-yl)-2-oxopyrrolidine-3-carboxylic acidCatalog No.:AA01AGF2 CAS No.:1017417-35-4 MDL No.:MFCD09947168 MF:C13H13NO5 MW:263.2460 |
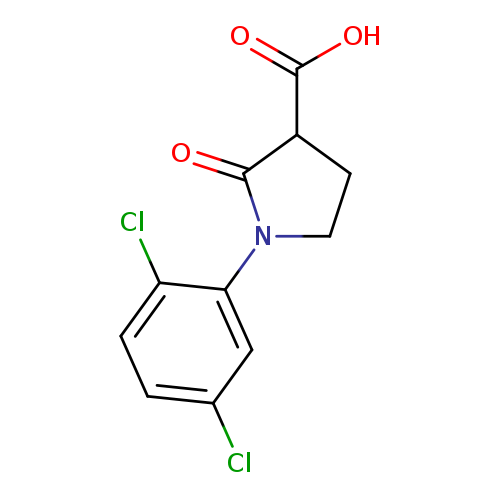
1-(2,5-Dichlorophenyl)-2-oxopyrrolidine-3-carboxylic acidCatalog No.:AA019WWI CAS No.:1017417-38-7 MDL No.:MFCD09814355 MF:C11H9Cl2NO3 MW:274.1001 |
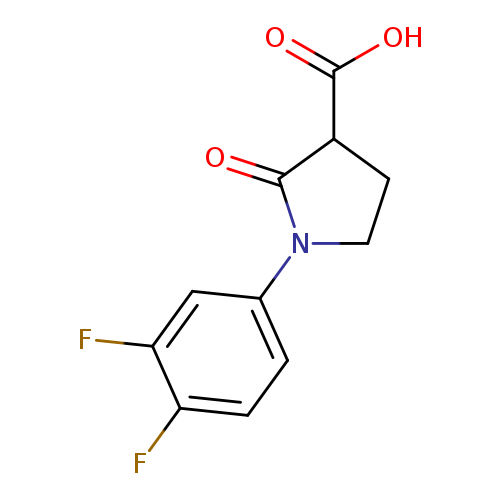
1-(3,4-difluorophenyl)-2-oxopyrrolidine-3-carboxylic acidCatalog No.:AA019WNA CAS No.:1017417-44-5 MDL No.:MFCD09812105 MF:C11H9F2NO3 MW:241.1909 |
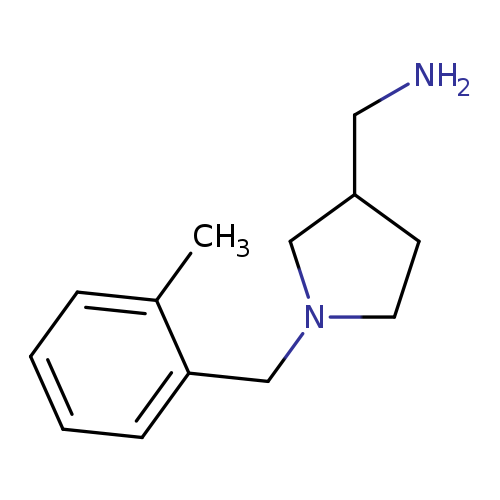
3-Pyrrolidinemethanamine, 1-[(2-methylphenyl)methyl]-Catalog No.:AA0005A7 CAS No.:1017417-47-8 MDL No.:MFCD10003769 MF:C13H20N2 MW:204.3113 |
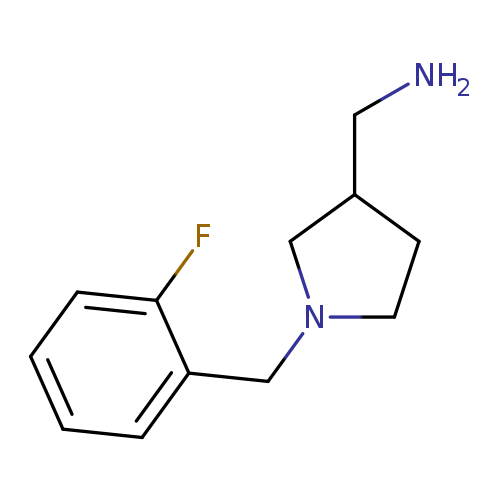
{[1-(2-fluorobenzyl)-3-pyrrolidinyl]methyl}amine dihydrochloride hydrateCatalog No.:AA00J1O0 CAS No.:1017417-50-3 MDL No.:MFCD09941662 MF:C12H17FN2 MW:208.2752 |
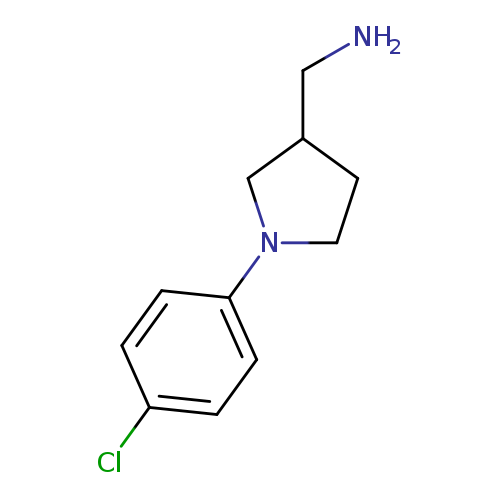
[1-(4-Chlorophenyl)pyrrolidin-3-yl]methanamineCatalog No.:AA00J61Q CAS No.:1017417-73-0 MDL No.:MFCD09947945 MF:C11H15ClN2 MW:210.7032 |
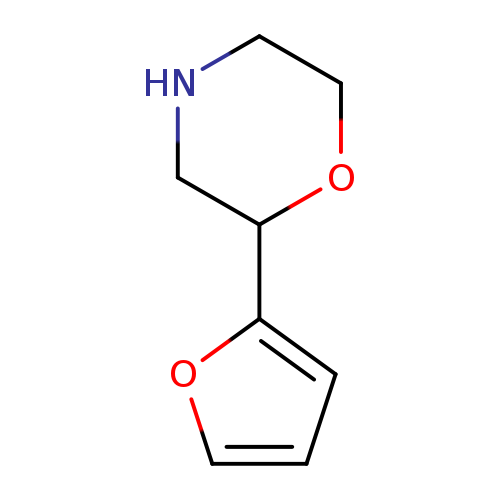
2-(Furan-2-yl)morpholineCatalog No.:AA0005A6 CAS No.:1017417-81-0 MDL No.:MFCD09901445 MF:C8H11NO2 MW:153.1784 |
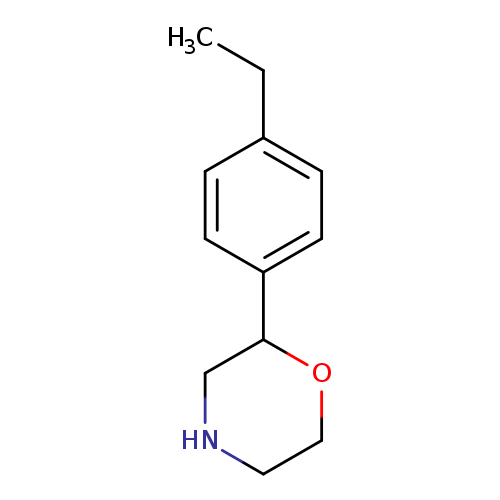
2-(4-ethylphenyl)morpholineCatalog No.:AA00VSH9 CAS No.:1017417-84-3 MDL No.:MFCD09901450 MF:C12H17NO MW:191.2695 |
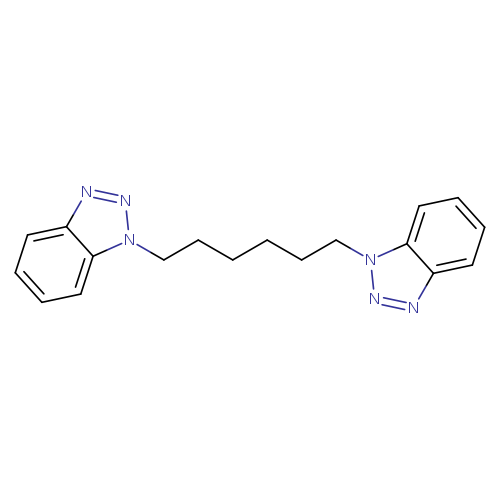
1-[6-(1H-1,2,3-Benzotriazol-1-yl)hexyl]-1H-1,2,3-benzotriazoleCatalog No.:AA01EQIR CAS No.:101742-45-4 MDL No.:MFCD00967061 MF:C18H20N6 MW:320.3916 |
[2-(([4-(Acetylamino)phenyl]sulfonyl)amino)-1,3-thiazol-4-yl]acetic acidCatalog No.:AA00H9HZ CAS No.:1017421-54-3 MDL No.:MFCD10007619 MF:C13H13N3O5S2 MW:355.3894 |
2-{3-methyl-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazin-6-yl}acetic acidCatalog No.:AA00VSK1 CAS No.:1017422-44-4 MDL No.:MFCD09998389 MF:C7H8N4O2S MW:212.2290 |
2-[5-methyl-1-(4-methylphenyl)-1H-1,2,3-triazol-4-yl]acetic acidCatalog No.:AA019WCM CAS No.:1017423-18-5 MDL No.:MFCD10003257 MF:C12H13N3O2 MW:231.2505 |
5-cyclopropyl-1-(4-fluorophenyl)-1H-1,2,3-triazole-4-carboxylic acidCatalog No.:AA01A89C CAS No.:1017423-32-3 MDL No.:MFCD10003269 MF:C12H10FN3O2 MW:247.2251 |
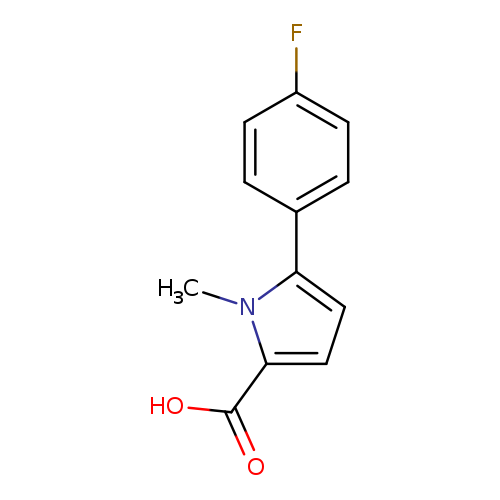
5-(4-fluorophenyl)-1-methyl-1H-pyrrole-2-carboxylic acidCatalog No.:AA01A8E6 CAS No.:1017427-35-8 MDL No.:MFCD10006411 MF:C12H10FNO2 MW:219.2117 |
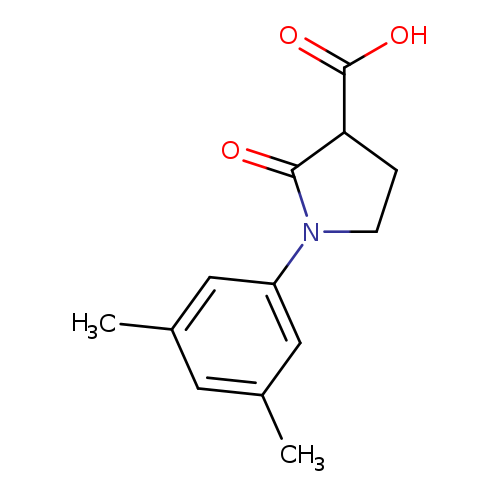
1-(3,5-dimethylphenyl)-2-oxopyrrolidine-3-carboxylic acidCatalog No.:AA019WNI CAS No.:1017427-36-9 MDL No.:MFCD09809113 MF:C13H15NO3 MW:233.2631 |
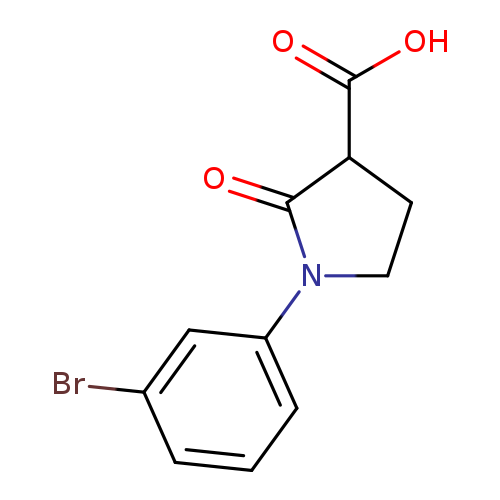
1-(3-Bromophenyl)-2-oxopyrrolidine-3-carboxylic acidCatalog No.:AA019VD6 CAS No.:1017427-72-3 MDL No.:MFCD09803028 MF:C11H10BrNO3 MW:284.1060 |
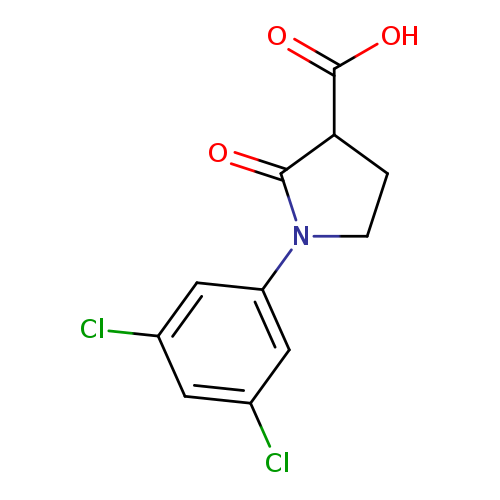
1-(3,5-Dichlorophenyl)-2-oxopyrrolidine-3-carboxylic acidCatalog No.:AA019MBY CAS No.:1017427-84-7 MDL No.:MFCD09815849 MF:C11H9Cl2NO3 MW:274.1001 |
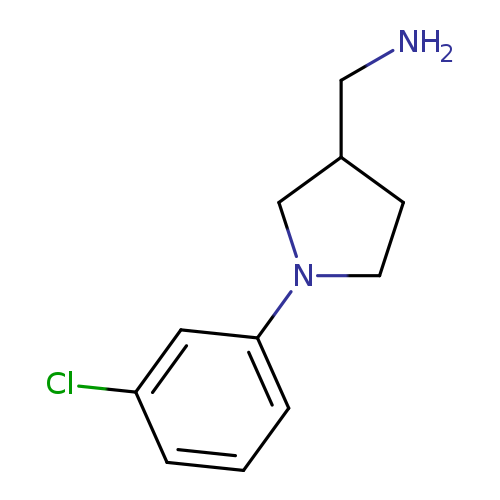
1-[1-(3-chlorophenyl)pyrrolidin-3-yl]methanamineCatalog No.:AA008V6B CAS No.:1017428-33-9 MDL No.:MFCD09948211 MF:C11H15ClN2 MW:210.7032 |
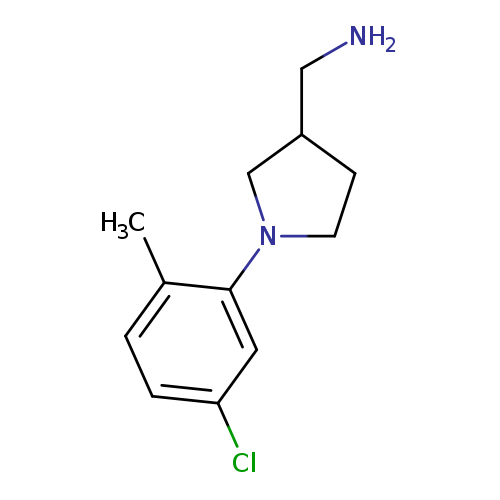
[1-(5-chloro-2-methylphenyl)pyrrolidin-3-yl]methanamineCatalog No.:AA01A9BI CAS No.:1017428-37-3 MDL No.:MFCD09934776 MF:C12H17ClN2 MW:224.7298 |
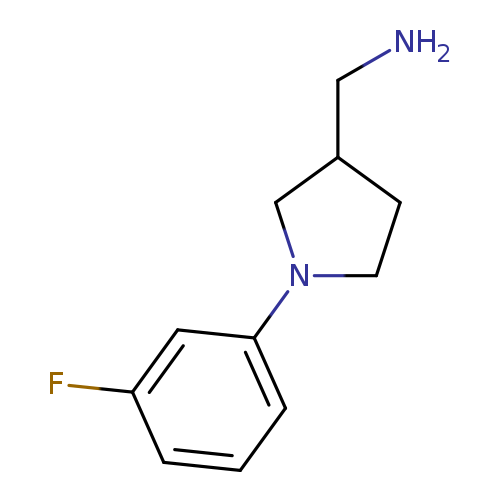
[1-(3-fluorophenyl)pyrrolidin-3-yl]methanamineCatalog No.:AA01AA9U CAS No.:1017428-57-7 MDL No.:MFCD09939491 MF:C11H15FN2 MW:194.2486 |
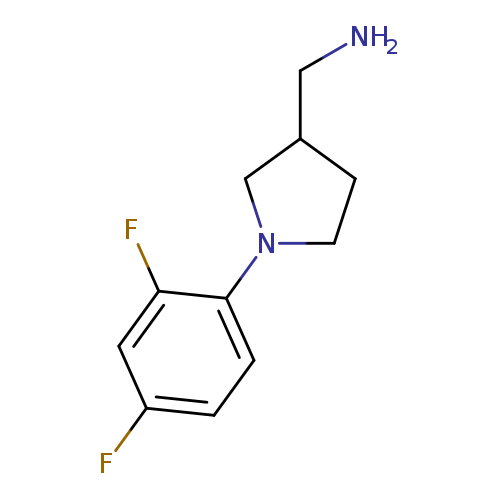
(1-(2,4-difluorophenyl)pyrrolidin-3-yl)methanamineCatalog No.:AA01DX3X CAS No.:1017428-61-3 MDL No.:MFCD09932882 MF:C11H14F2N2 MW:212.2391 |
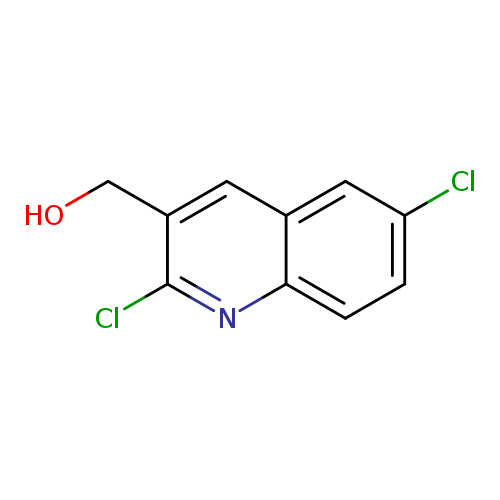
2,6-Dichloroquinoline-3-methanolCatalog No.:AA0005B3 CAS No.:1017429-35-4 MDL No.:MFCD09997988 MF:C10H7Cl2NO MW:228.0747 |
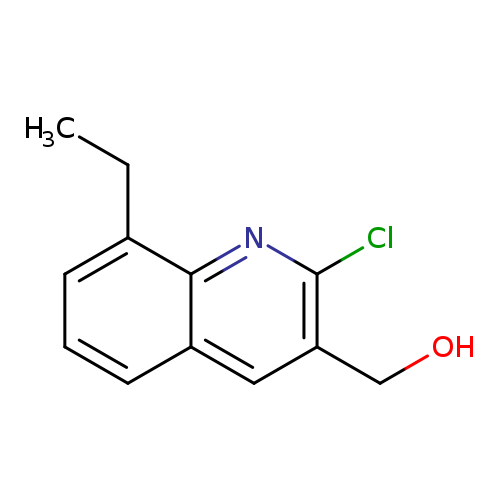
2-Chloro-8-ethylquinoline-3-methanolCatalog No.:AA0005B2 CAS No.:1017429-39-8 MDL No.:MFCD09998024 MF:C12H12ClNO MW:221.6828 |
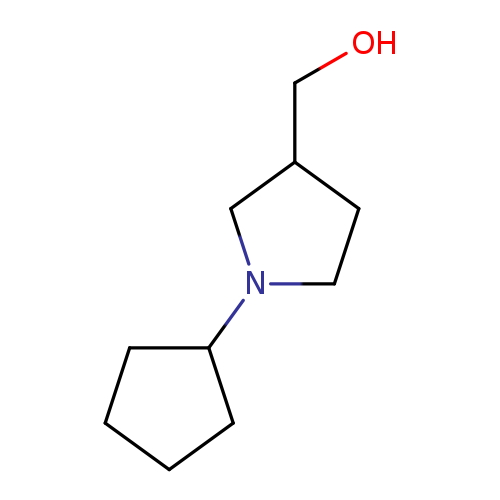
(1-Cyclopentylpyrrolidin-3-yl)methanolCatalog No.:AA00H9I1 CAS No.:1017429-88-7 MDL No.:MFCD10003884 MF:C10H19NO MW:169.2640 |