2019-12-18 14:09:48
The present invention relates to the preparation of N-trityl-imidazole and its salts and such compounds. More particularly, the present invention relates to N-trityl-imidazole and salts thereof of the formula: wherein R, R 1 and R 2 are hydrogen, lower alkyl or phenyl, or R 1 and R 2 together form a banded aromatic The benzene ring of the radical, X, X and X "are alkyl or electronegative moieties having 1 to 12 carbon atoms, and n, n and n" are integers of 0 to 2, or a pharmaceutically acceptable acid thereof salt. When R1, R2 or R3 is an alkyl moiety, those having 1 to 4 carbon atoms are preferred. When X, X or X "is an alkyl moiety, a moiety having 1 to 12 carbon atoms is preferred, and a moiety having 1 to 4 carbon atoms is particularly preferred. A particularly preferred electronegative substituent is halogen, ie, fluorine, chlorine , Bromine and iodine, NO CF CN, and S-lower alkyl and O-lower alkyl; preferably the alkyl portion has 1-4 carbon atoms, the term alkyl and lower alkyl also includes straight chain as branched alkyl Some also include those containing double bonds.
N-Trityl-imidazole salt (I) is a pharmaceutically acceptable non-toxic acid salt. Examples of suitable acids are hydrohalic acid (especially preferably hydrochloric acid), phosphoric acid, mono- and difunctional carboxylic acids such as acetic acid, propionic acid, maleic acid, succinic acid, fumaric acid, tartaric acid, citric acid, salicylic acid , Sorbic acid. Acid, lactic acid and 1, S-naphthalene-disulfonic acid. Hydrohalides, especially the hydrochloride, lactate and salicylate, are of particular value.
In a particularly preferred embodiment of the present invention, N-trityl-imidazole has the formula: wherein X, X 'and X "are alkyl or electronegative substituents having 1 to 12 carbon atoms, And n, n 'and n "are .1 or 2. With regard to formula IIa, particularly preferred substituents are those in which X "is fluorine, chlorine, bromine, iodine, NCFCN, SCHOCH and its" ". The compounds of the invention can be prepared according to techniques known per se, for example by Reacting a silver or alkali metal salt, especially a potassium salt of an imidazole of formula HI, with a trityl halide of formula IV: R, R and RX, X and X "and n, n" n "and" n " Has the above meaning, and Hal is chlorine, bromine or iodine in an inert solvent such as benzene, toluene, hexane, cyclohexane or diethyl ether at a temperature of about 1 ° C to about 110 ° C. Chem. 92, 92 (1959); 93, 570 (1960)].
The compounds of the invention can also be prepared according to techniques known per se by reacting an imidazole derivative of formula III with trityl methanol (see reaction of methanol corresponding to halide IV with a secondary amine). In this case, imidazole is usually used in excess of up to about 100%. If the method is carried out under pressure, a molar amount can be used. Furthermore, it may be advantageous to azeotropically remove water at the boiling point of the solvent used in the presence of a high boiling point inert organic solvent such as xylene, chlorobenzene, and the like. In the absence of a solvent, the process is performed in a temperature range of about 1 ° C to about 230 ° C, preferably about 170 ° C to about 190 ° C.
It may be more advantageous to promote water removal by working in the presence of a dehydrating agent such as magnesium sulfate. The number of moles of alkaline earth metal oxides (MgO, BaO, CaO) and Al is usually about 0, but it may be excessive to about 2-3 moles.
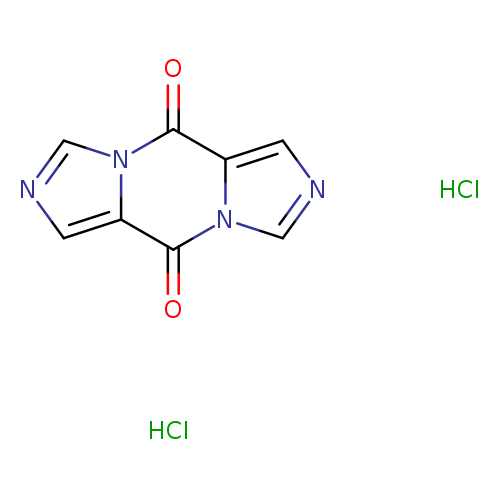
Diimidazo[1,5-a:1',5'-d]pyrazine-5,10-dione, DiHClCatalog No.:AA00157C CAS No.:1215646-82-4 MDL No.:MFCD10699721 MF:C8H6Cl2N4O2 MW:261.0648 |
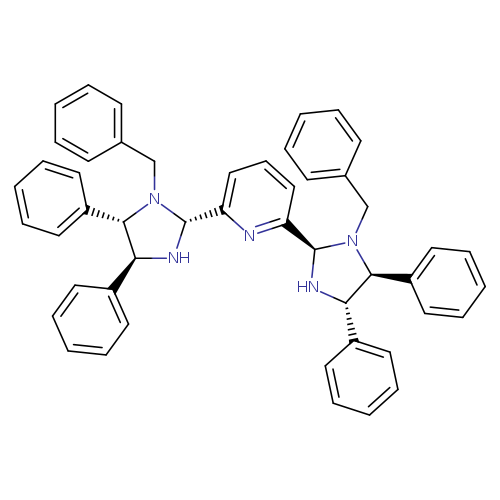
2,6-Bis[(2r,4s,5s)-1-benzyl-4,5-diphenylimidazolidin-2-yl]pyridineCatalog No.:AA0016ZG CAS No.:1223020-29-8 MDL No.:MFCD22581437 MF:C49H45N5 MW:703.9151 |
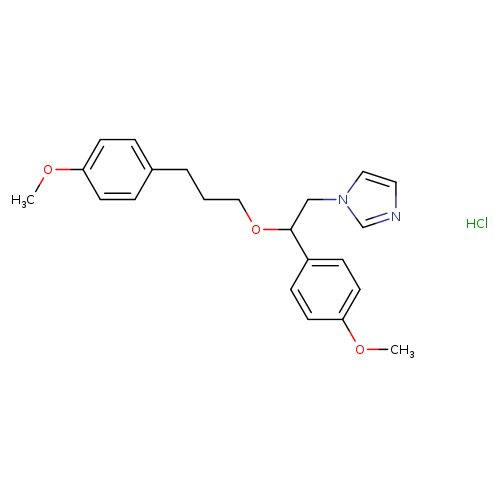
SKF-96365 hydrochlorideCatalog No.:AA000UFG CAS No.:130495-35-1 MDL No.:MFCD00236407 MF:C22H27ClN2O3 MW:402.9144 |
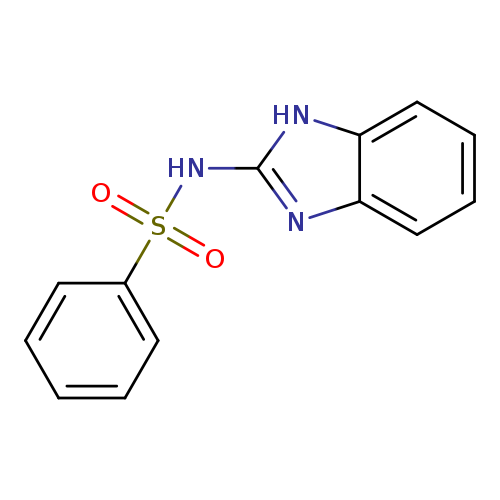
Benzenesulfonamide, N-1H-benzimidazol-2-yl-Catalog No.:AA000UQ8 CAS No.:13068-57-0 MDL No.:MFCD01350346 MF:C13H11N3O2S MW:273.3103 |
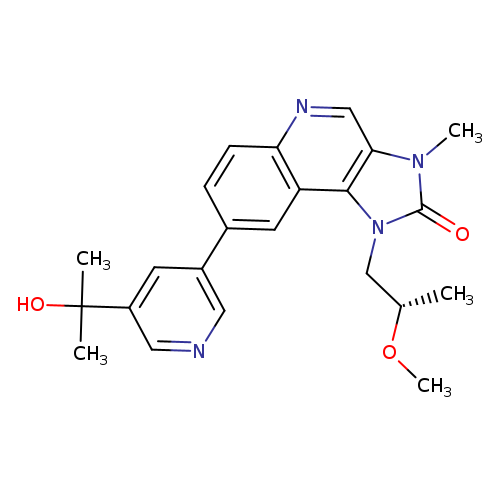
LY3023414Catalog No.:AA0018X8 CAS No.:1386874-06-1 MDL No.:MFCD28411368 MF:C23H26N4O3 MW:406.4775 |
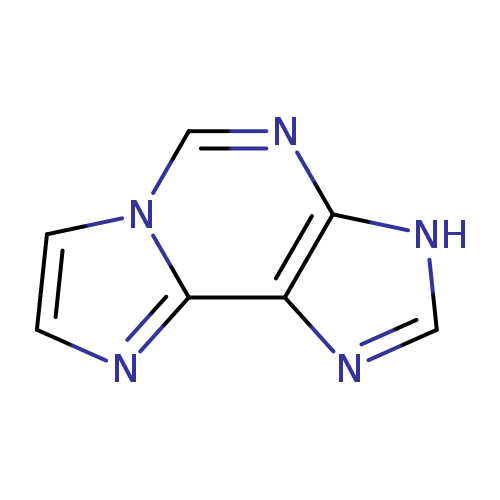
3H-Imidazo[2,1-i]purineCatalog No.:AA0019EF CAS No.:13875-63-3 MDL No.:MFCD00056763 MF:C7H5N5 MW:159.1481 |
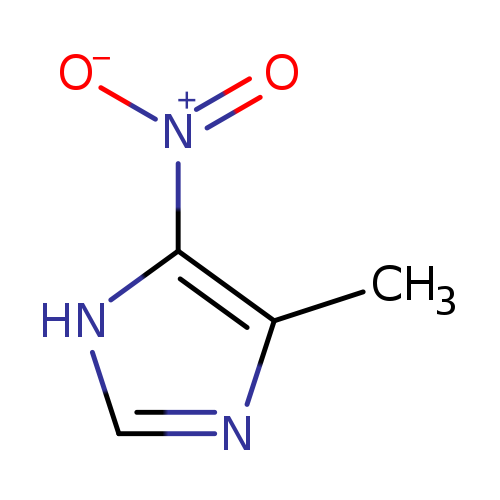
4-Methyl-5-nitroimidazoleCatalog No.:AA001BVW CAS No.:14003-66-8 MDL No.:MFCD01721395 MF:C4H5N3O2 MW:127.1014 |
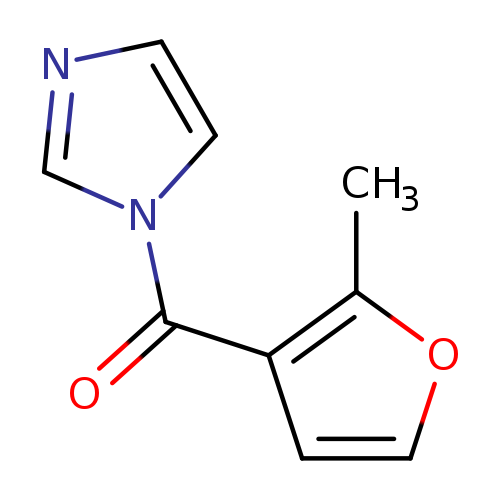
(1H-Imidazol-1-yl)(2-methylfuran-3-yl)methanoneCatalog No.:AA001GH3 CAS No.:1415238-77-5 MDL No.:MFCD01795769 MF:C9H8N2O2 MW:176.1720 |
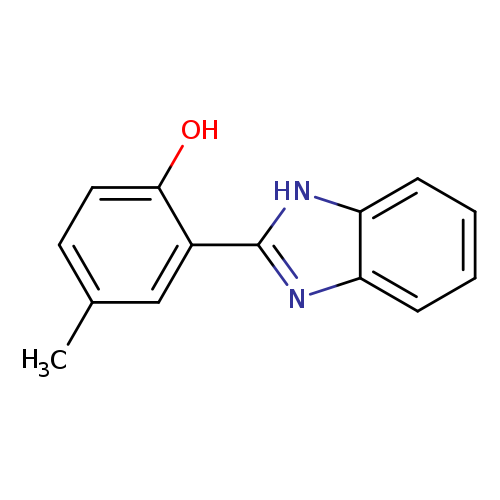
Phenol, 2-(1H-benzimidazol-2-yl)-4-methyl-Catalog No.:AA001HZ7 CAS No.:14225-73-1 MDL No.:MFCD11180238 MF:C14H12N2O MW:224.2579 |
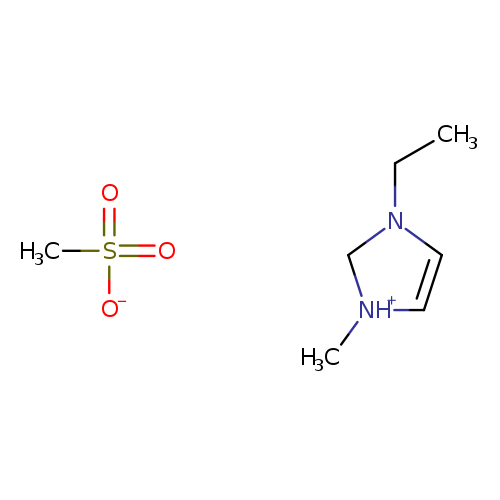
1-Ethyl-3-methylimidazolium methanesulfonateCatalog No.:AA001KEL CAS No.:145022-45-3 MDL No.:MFCD06798171 MF:C7H16N2O3S MW:208.2785 |
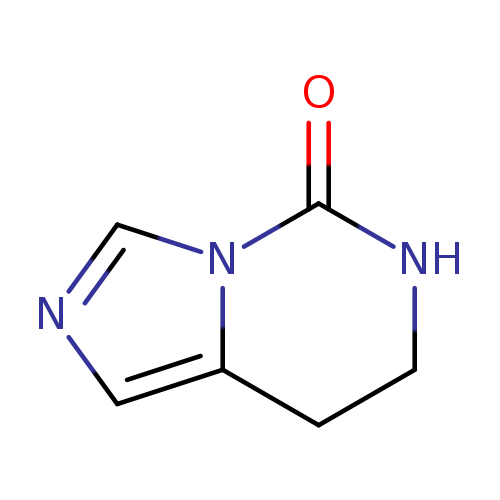
7,8-Dihydroimidazo[1,5-c]pyrimidin-5(6H)-oneCatalog No.:AA001KHK CAS No.:14509-66-1 MDL No.:MFCD01443896 MF:C6H7N3O MW:137.1393 |
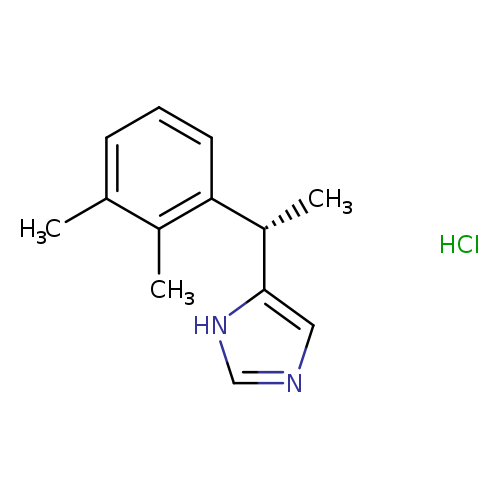
(S)-5-(1-(2,3-Dimethylphenyl)ethyl)-1h-imidazole hydrochlorideCatalog No.:AA001KI3 CAS No.:145108-58-3 MDL No.:MFCD07772269 MF:C13H17ClN2 MW:236.7405 |
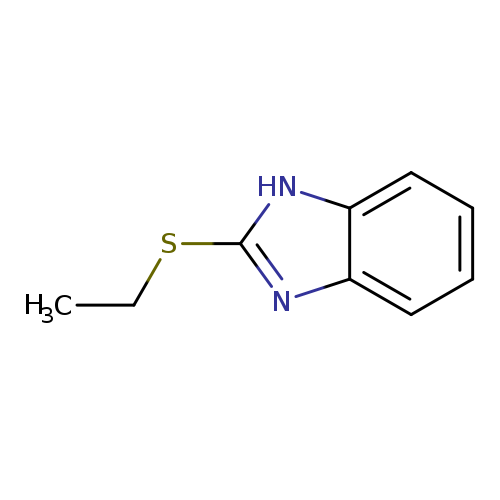
2-Ethylsulfanyl-1h-benzoimidazoleCatalog No.:AA001DMR CAS No.:14610-11-8 MDL No.:MFCD00223154 MF:C9H10N2S MW:178.2541 |
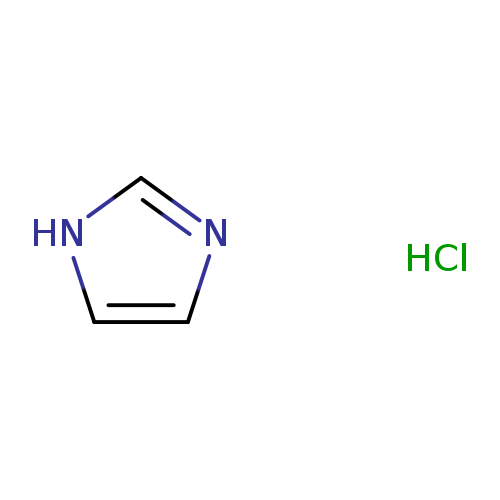
Imidazole hydrochlorideCatalog No.:AA001E5V CAS No.:1467-16-9 MDL No.:MFCD00012695 MF:C3H5ClN2 MW:104.5382 |
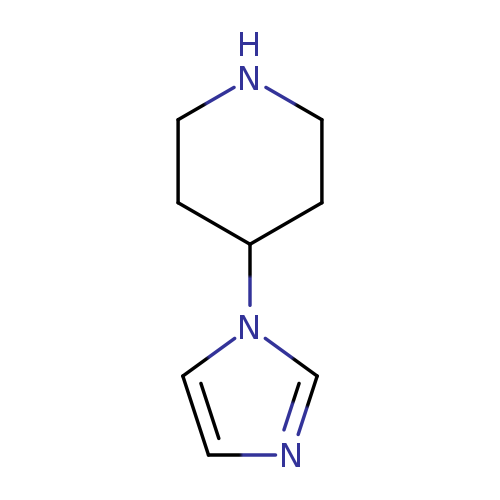
4-(1H-imidazol-1-yl)piperidineCatalog No.:AA001EHQ CAS No.:147081-85-4 MDL No.:MFCD09834721 MF:C8H13N3 MW:151.2089 |
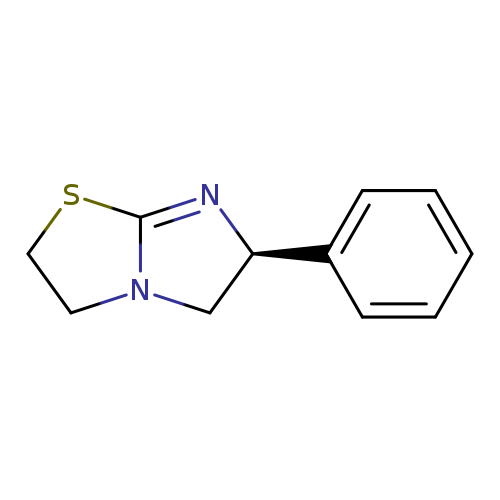
(6S)-6-phenyl-2H,3H,5H,6H-imidazo[2,1-b][1,3]thiazoleCatalog No.:AA001F0S CAS No.:14769-73-4 MDL No.:MFCD00792481 MF:C11H12N2S MW:204.2914 |
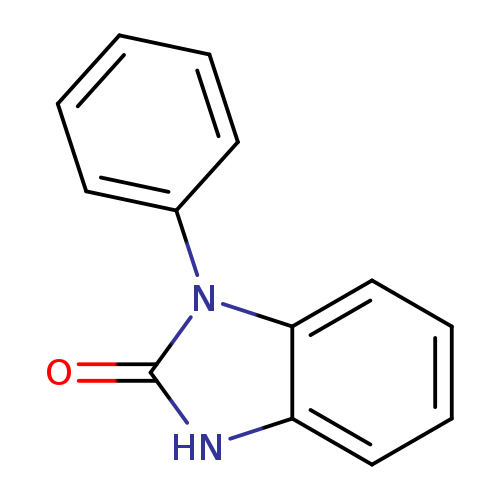
1-Phenyl-1H-benzo[d]imidazol-2(3H)-oneCatalog No.:AA001FE7 CAS No.:14813-85-5 MDL No.:MFCD01658803 MF:C13H10N2O MW:210.2313 |
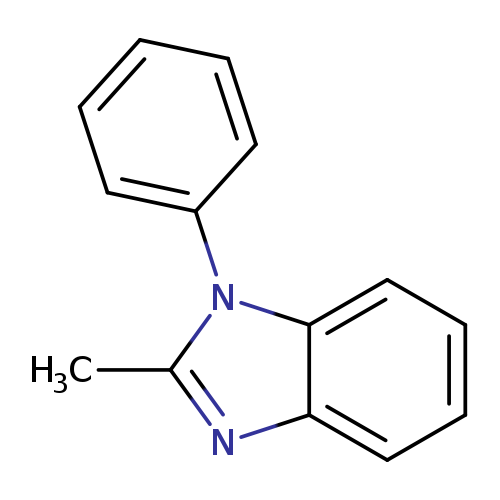
1H-Benzimidazole, 2-methyl-1-phenyl-Catalog No.:AA001KTZ CAS No.:1484-39-5 MDL No.:MFCD00227220 MF:C14H12N2 MW:208.2585 |
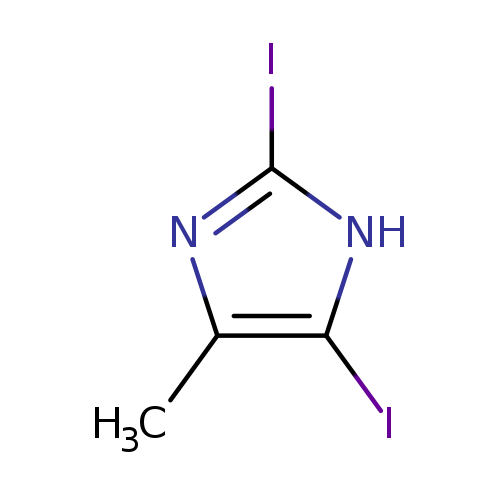
2,5-Diiodo-4-methyl-1H-imidazoleCatalog No.:AA001LPG CAS No.:149510-85-0 MDL No.:MFCD02179520 MF:C4H4I2N2 MW:333.8969 |
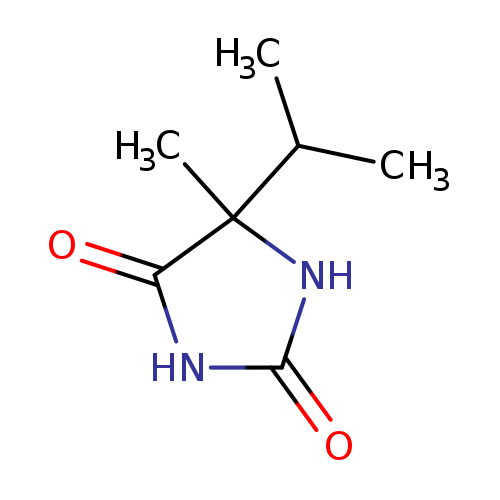
5-Isopropyl-5-methylimidazolidine-2,4-dioneCatalog No.:AA001MCK CAS No.:150226-22-5 MDL No.:MFCD00033554 MF:C7H12N2O2 MW:156.1824 |
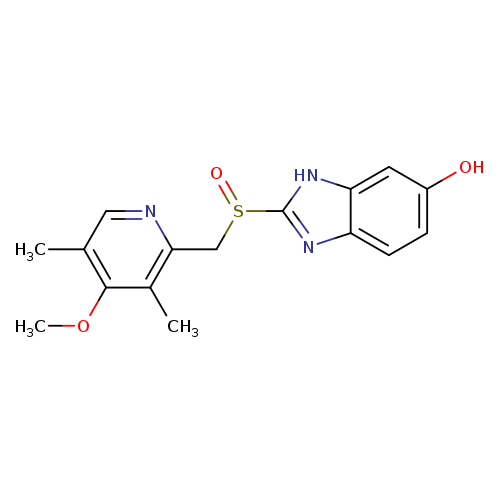
1H-Benzimidazol-6-ol, 2-[[(4-methoxy-3,5-dimethyl-2-pyridinyl)methyl]sulfinyl]-Catalog No.:AA001NDG CAS No.:151602-49-2 MDL No.:MFCD08063681 MF:C16H17N3O3S MW:331.3895 |
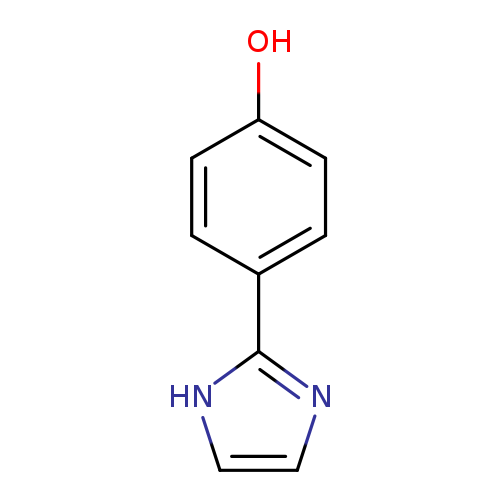
4-(1H-Imidazol-2-yl)phenolCatalog No.:AA001NVP CAS No.:15548-89-7 MDL No.:MFCD08668843 MF:C9H8N2O MW:160.1726 |
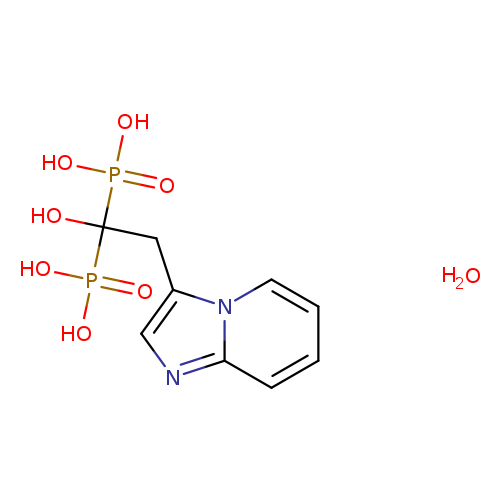
(1-Hydroxy-2-(imidazo[1,2-a]pyridin-3-yl)ethane-1,1-diyl)diphosphonic acid hydrateCatalog No.:AA001O0N CAS No.:155648-60-5 MDL No.:MFCD00890772 MF:C9H14N2O8P2 MW:340.1636 |
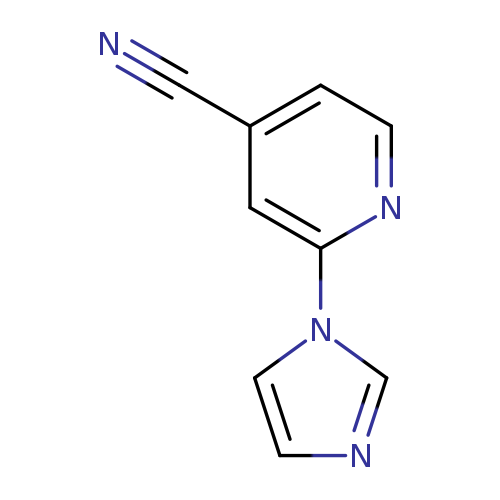
2-(1H-Imidazol-1-yl)pyridine-4-carbonitrileCatalog No.:AA001PUL CAS No.:158020-84-9 MDL No.:MFCD09929166 MF:C9H6N4 MW:170.1707 |
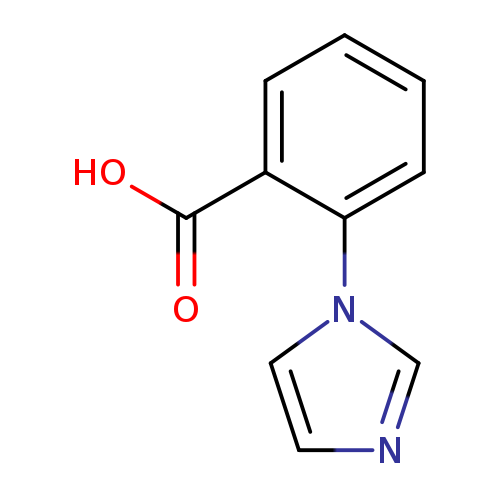
2-(1H-Imidazol-1-yl)benzoic acidCatalog No.:AA001R3H CAS No.:159589-67-0 MDL No.:MFCD06254507 MF:C10H8N2O2 MW:188.1827 |
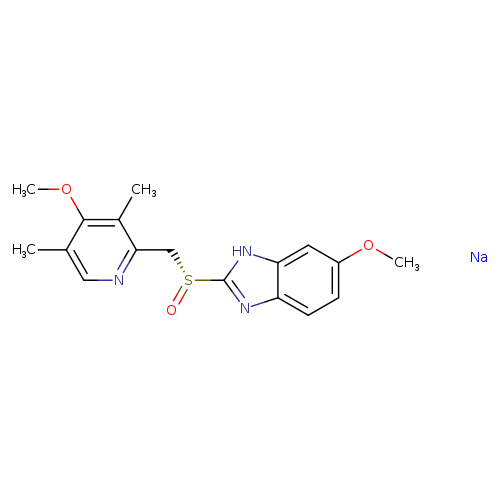
1H-Benzimidazole, 6-methoxy-2-[(R)-[(4-methoxy-3,5-dimethyl-2-pyridinyl)methyl]sulfinyl]-, sodium salt (1:1)Catalog No.:AA001VT8 CAS No.:161796-77-6 MDL No.:MFCD23160332 MF:C17H19N3NaO3S MW:368.4058 |