2020-01-11 10:58:27
Peng Yang, Li Yang,Yan Wang, Lixian Song, Junxiao Yanga and Guanjun Chang
Introduction
Water is the source of life and one of the most indispensable and precious natural resources. However, with the rapid development of industry, heavy metal ions in surface water are of great concern from an environmental perspective because of their toxicity and bioaccumulation.1–4 Moreover, excessive intake of heavy metal ions, for example, Ni(II),5 Cu(II),6 Zn(II)7 and Cr(III),8 is harmful to humans and other species. A wide range of technologies have been used for the metal ion removal from water including chemical precipitation,9 biological removal,10 organic chelation,11 ion-exchange,12,13 membrane separation14 and adsorption.15,16 Among these approaches, adsorption is found to be the most attractive way due to its advantages of relative simplicity, easy scale-up and high efficiency over a wide concentration range.17,18 Recently a new form of activated carbon has been developed: carbon aerogels, which are attractive as adsorbents because of their promise of low cost, high surface area, and hydrophilicity.19,20 However, a comprehensive consideration of their adsorption capacities and selectivities still remains a challenge.
To achieve high uptake capacity, fast kinetics, and excellent selectivity, the use of complexing functionalities, which are surface modifted functional groups (e.g., –NH2, –COOH, –OH,–SH etc.), on adsorbents has come into play to combat heavy- metal pollution. Complexing functionalities have been developed based on the covalent graving of coordination groups to the surface of porous materials.22–24 Li et al.22 reported a biomass-based hydrogel showing high and fast adsorption capacities for Zn(II), Fe(III), Cu(II) and Cr(III), which were 121.2, 78.5, 75.4 and 41.7 mg g—1, respectively. Panic et al.23 designed PMCNa hybrid hydrogels which are rich in peptide bonds, and carboxylic, hydroxyl and amino pendant groups with excellent removal properties toward Ni2+ in a wide range of concentra- tions (0.05–200 ppm). In short, it has been shown that the incorporation of functional groups into the pore wall of porous materials had a profound impact on the adsorption capacity and removal efficiency of metal ions by enhancing the complexation of functional groups with heavy metals. None- theless, using new forces between adsorbents and heavy metals to enhance uptake capacity is an important challenge.
As a new type of noncovalent binding interaction, in recent years, cation–p interactions have received considerable theo- retical and experimental attention, owing to the pivotal roles they play in multidisciplinary areas of research, such as biology, organic synthesis and the design of host–guest assemblies.25–27 As a typical aromatic compound, indole possesses a structure more electron-rich than a general aromatic structure,28 which makes it more easily form cation–p interactions with cations. Herein, we explore a facile and easily controllable condensation technique for the synthesis of a novel aerogel (4-HIFA) that contains both hydroxyl and indole groups via sol–gel technology followed by CO2 supercritical drying (see the Experimental part for details) (Scheme 1). For comparison, a resorcinol–formal- dehyde aerogel (RFA) was prepared as well (Scheme S1 and Fig. S3–S5†). Taking advantage of the synergistic effects of the complexation and cation–p interactions of the hydroxyl and indole groups with heavy metals, the resulting 4-HIFA exhibits enhanced heavy metal uptake. Meanwhile, the amount of 4-HIFA, the sorption pH, kinetics, isotherms, selectivity and reusability were also investigated.
Experimental Materials
4-Hydroxyindole was purchased from J & K Technology Co., Ltd., and was purifted by column chromatography on a 100–200 mesh silica gel with petroleum ether (PE) : ethyl acetate (EA) ¼ 3.5 : 1. Resorcinol and formaldehyde were purchased from J & K Technology Co., Ltd., and used without further puriftcation. Nickel(II) chloride hexahydrate (NiCl2$6H2O), copper(II) sulfate pentahydrate (CuSO4$5H2O), chromium(III) chloride hexahy- drate (CrCl3$6H2O), zinc(II) sulfate hexahydrate (ZnSO4$6H2O), mercury(II) chloride (HgCl2), cadmium(II) chloride (CdCl2) and lead(II) nitrate (Pb(NO3)2) were supplied by Aladdin. The rest of the materials and reagents were obtained from different commercial sources and used without further puriftcation.
Measurements
FT-IR spectra were recorded on a Nicolet 6700 FTIR spectrom- eter. 1H NMR spectroscopy was performed on a Bruker AV-400 spectrometer at 400 MHz, while 13C NMR spectra were also recorded on a Bruker AV-400 spectrometer at 100 MHz. A solid- state cross-polarization magic-angle-spinning (CP/MAS) NMR spectrum was recorded on a Bruker Avance III 400 NMR spec- trometer. UV-Visible spectroscopy was performed on a Solid- spec-3700 instrument. Scanning electron microscopy (SEM) was performed on an S-4800 (Hitachi Ltd) fteld emission scanning electron microscope. Digital photos were taken using a Canon 600D camera. N2 sorption isotherms were measured at 196 ○C by using an Autosorb IQ instrument, where the speciftc surface area (SBET) and pore size distribution were evaluated using the Brunauer–Emmett–Teller (BET) method (P/P0 ¼ 0.05–0.30)29 and Barrett–Joyner–Halenda (BJH) method30 (desorption branches of nitrogen isotherms), respectively. The metal ion concentrations in solutions before and aer adsorption were measured using ICP-AES (Jarrell-ASH, ICAP-9000).
Preparation of the 4-hydroxyindole–formaldehyde aerogel (4-HIFA)
The 4-HIFA was prepared by mixing 4-hydroxyindole with formaldehyde in CH3CN in a molar ratio of 1 : 2. The total solid content in the pre-gel solution was kept at 20%. Hydrochloric acid was used as the catalyst with a molar ratio of 4-hydrox- yindole : HCl ¼ 100 : 1. And the above solution was stirred for 20 min and then poured into ampoule bottles, followed by heat treatment at 60 ○C for 60 min and was thus made capable of gelling. Before supercritical drying, CH3CN in the wet gel was replaced by solvent exchange with acetone 3 times once in 24 h. Aer that, the gel was supercritically dried at 45 ○C for 7 days, and the 4-HIFA was obtained, ftnally.
Heavy metal uptake experiments
The heavy metal uptakes from aqueous solutions with various concentrations were studied using the batch method. 0.02 g 4-HIFA solid was mixed with 25 mL (V/m ¼ 1250 mL g—1) of each solution with a concentration of z100 ppm at pH ¼ 6, under stirring for 24 h. Aer the adsorption experiments completed, centrifugation was performed, and the solid samples were dried in air for further characterization. Meanwhile, the metal concentrations in the supernatant solutions and their mother solutions were determined using inductively coupled plasma- atomic emission spectroscopy (ICP-AES). The experiments were repeated three times, and the results are presented as averages at room temperature. The pH values of Ni(II), Cu(II), Cr(III), Zn(II), Hg(II), Cd(II) and Pb(II) solutions were adjusted using 0.1 mol L—1 HNO3 or 0.1 mol L—1 NaOH solutions. Freshly prepared solutions were utilized for all experiments.
The effect of the amount of 4-HIFA on the removal of Ni2+, Cu2+, Cr3+ and Zn2+: 0.002, 0.005, 0.01, 0.015 or 0.02 g 4-HIFA
solid was mixed with 25 mL of each solution with a concentra- tion of z100 ppm at pH 6, under stirring for 24 h.
pH effect on the removal of Ni2+, Cu2+, Cr3+ and Zn2+ by 4-HIFA: 0.02 g 4-HIFA solid was mixed with 25 mL (V/m 1250 mL g—1) of each solution with a concentration of z100 ppm at pH ¼ 2, 3, 4, 5 or 6, under stirring for 24 h.
Equilibrium adsorption isotherm studies for Ni(II), Cu(II), Cr(III) and Zn(II): the concentrations of Ni(II), Cu(II), Cr(III) and Zn(II) ions were in the range of 10–500 ppm to ensure that adsorption equilibrium was achieved. 0.02 g 4-HIFA or RAF added to the 25 mL solution was used to maintain a V/m value of 1250 mL g—1. The contact time was about 24 h. Adsorption kinetics of Ni(II), Cu(II), Cr(III) and Zn(II): an amount of 0.04 g 4-HIFA or RFA was added to the 50 mL (V/m 1250 mL g—1) solution with a concentration of z10 ppm, undergoing vigorous stirring continuously for 5 min, 30 min, 1 h, 2 h, 3 h, 4 h and 5 h. The contact time was varied to check the adsorption kinetics.
Adsorption in mixed metal ion solutions: the competitive adsorption of Ni(II), Cu(II), Cr(III) and Zn(II) ions on the 4-HIFA in binary, ternary and quaternary mixtures was investigated. Each ion concentration in the mixed solution was the same, and the total concentration of all the ions was 2 mmol L—1 (pH ¼ 6, contact time ¼ 24 h, m ¼ 0.02 g, V ¼ 25 mL, V/m ¼ 1250 mL g—1). Regeneration study: aer the equilibrium study, the metal- loaded 4-HIFA was collected and washed with deionized water and dried to constant weight. Then the adsorbent (0.1 g) was shaken with 50 mL of HCl (1 mol L—1) in 250 mL Erlenmeyer asks at 150 rpm on an orbital shaker at 25 ○C for 2 h. Aer washing with deionized water, the treated 4-HIFA was ready for the next use.
Data treatment: the distribution coefficient (Kd) is deftned by the equation of Kd (V[(C0 Cf)/Cf])/m, where C0 and Cf are, respectively, the initial and equilibrium concentrations of Mn+ (ppm, mg mL—1) aer the contact, V is the solution volume (mL), and m is the solid amount (g). The % removal is calculated with the equation 100 × (C0 — Cf)/C0. The removal capacity (qm) is given by the equation: qm ¼ 10—3 × (C0 — Cf) × V/m.
Results and discussion
Preparation and characterization
Traditional indole-based microporous polymers were synthe- sized by the catalyst-free nucleophilic substitution poly- condensation via C–N and C–O coupling reactions,31 which means the sacriftce of phenolic-OH. As a metal-philic active site, the phenolic-OH is crucial for achieving a high metal loading. Keeping this in mind, in this paper, we describe a simple, highly efficient way to fabricate a OH-containing indole-based porous architecture by polycondensation curing of the mixed acetoni- trile solution of 4-hydroxyindole and formaldehyde (see the Experimental part for details) (Scheme 1). The resultant sample is in the form of an aerogel with a brown color (inset in Fig. 1c). The successful formation of the indole-based aerogel was conftrmed by Fourier Transform Infrared (FT-IR) spectrometry and solid-state cross-polarization magic-angle-spinning (CP/ MAS) NMR spectrometry. The FTIR spectrum of the 4-HIFA is shown in Fig. 1a, with bands at 2927 and 1458 cm—1 associated with the CH2 stretching and bending vibrations, whereas the broadband at 3409 cm—1 is indexed to the NH and the aromatic OH groups of 4-hydroxyindole, respectively.33 The band at 1352 cm—1 comes from O–H bending vibrations.23 The band at 1637 cm—1 comes from aromatic ring stretches, whereas medium to weak absorption bands at 1256 and 1046 cm—1 indicate that methylene ether linkages between 4-hydroxyindole rings are present but not dominant.33 There are no bands at about 2128 cm—1 associated with the stretching vibrations of the terminal cyano groups of CH3CN,32 which indicates the complete removal of CH3CN from the ftnal products (Fig. 1a). The RFA was also characterized by Fourier transform infrared spectroscopy, and the results were in good agreement with the proposed structures (Fig. S3†). The structural information of the prepared 4-HIFA was also obtained by 13C CP/MAS NMR spec- troscopy (Fig. 1b). There are three broad peaks at 155–98, 68, and 25 ppm. The peak at about 147 ppm is ascribed to the phenolic carbons of the substituted 4-hydroxyindole (Fig. 1b; 6), and the broad peaks at 155–98 ppm are ascribed to the indole group carbons (Fig. 1b; 3, 4, 5, 7, 8, 9, and 10). Consistent with the IR spectrum, the smaller peak at 68 ppm is assigned to the small amount of CH2–O–CH2 bridges, and the broad peak at about 25 ppm is assigned to the different types of CH2 bridges, which is perfectly consistent with a previous study about acid- catalyzed resorcinol-formaldehyde aerogels.33 In conclusion, the characterization data can conftrm that the desired aerogel has been synthesized successfully.
Fig. 1c and S2† show the scanning electron microscopy (SEM) images of the 4-HIFA. The structure, which is very loose, contains mesopores and macropores. It consists of very small spheres of approximately 20 nm diameter, arranged in a ftla- ment-like structure to form a three-dimensional matrix, pretty similar to the structure of the RF gel.34 As shown in Fig. S4,† the RFA has a similar porous structure with minor differences in the size and shape of the aggregated particles.
Porosity parameters of the resulting material were investi- gated by sorption analysis using nitrogen as the sorbate molecule. The nitrogen adsorption–desorption isotherms of the 4-HIFA measured at —196 ○C are shown in Fig. 1d, displaying a typical type IV curve.35 There are hysteresis loops at relative pressures of 0.7–0.95, suggesting the presence of mesopores and macropores in the sample,36 in good agreement with those observed with the SEM technique (Fig. 1c). Correspondingly, the pore size is distributed at 46 nm, as calculated by the nonlocal density functional theory method (NLDFT). The BET speciftc surface area and pore volume of the 4-HIFA are esti- mated to be 130 m2 g—1 and 1.01 cm3 g—1, respectively (inset in Fig. 1d). As shown in Fig. S5,† the RFA exhibits a similar nitrogen adsorption–desorption isotherm and pore size distribution (inset in Fig. S5†). The BET speciftc surface area of the RFA was estimated to be 151 m2 g—1, and the pore size was distributed at 40 nm.
The 4-HIFA and RFA were used as adsorbent materials without any pretreatment. However, their physicochemical properties were characterized for obtaining relevant parameters before conducting experiments (Table S1†). The zeta potential of the RFA and 4-HIFA is —7.1 and —10.5 mV at pH ¼ 6, respectively. This suggests that the surface of the RFA and 4- HIFA is electronegative which beneftts the heavy metal ion adsorption. The charge density of the RFA and 4-HIFA is —0.314 and —0.458 meq g—1, respectively. The contact angle of the RFA and 4-HIFA is 5○ and 9○, respectively, which suggests that the RFA and 4-HIFA are hydrophilic due to the hydroxyls on the surface of the RFA and 4-HIFA. The main functional groups of the RFA and 4-HIFA are hydroxyls as characterized by Fourier Transform Infrared (FT-IR) spectrometry (Fig. S3† and 1a).
Heavy metal removal using the 4-HIFA
It was expected that the resulting 4-HIFA with an OH-containing indole-based porous architecture may attract small heavy metal ions through an improved non-covalent interaction. In addition to the complexation of hydroxyls with metal ions, the high charge density of the indole units of the aerogel might facilitate the cation–p interactions between the heavy metal ions and the adsorbent, which inspired us to investigate its heavy metal ion adsorption capacity.
The amount of 4-HIFA is one of the most important parameters affecting the sorption process. As shown in Fig. S1a,† notably, the removal of Ni2+, Cu2+, Cr3+ and Zn2+ ions was increased with the increase of the amount of 4-HIFA from 0.002 to 0.02 g. This phenomenon could be explained by the fact that the increase of the amount of 4-HIFA would increase the total surface area and the number of adsorbent sites, correspondingly, also increasing their chance to come into contact with the heavy metal ions in the solution, resulting in high adsorption capacity. The removal of Ni2+, Cu2+, Cr3+ and Zn2+ ions was practically unchanged with the increase of the amount of 4-HIFA from 0.02 to 0.03 g, which could be explained by the fact that the concentration of Ni2+, Cu2+, Cr3+ and Zn2+ gradually decreased with the adsorption proceeding, resulting in reaching equilibrium. 0.02 g 4-HIFA was selected as the optimal amount for the following batch experiments.
The pH of a solution is one of the most important parame- ters affecting the sorption process. Fig. S1b† shows the depen- dencies of the adsorption capacity for Ni2+, Cu2+, Cr3+ and Zn2+ on the pH value (2.0–6.0) over the 4-HIFA in order to eliminate the effect of precipitation at higher pH values. Notably, it can be observed that the adsorption of Ni2+, Cu2+, Cr3+ and Zn2+ ions was increased with the increase of pH value from 2.0 to 6.0. This phenomenon could be explained by the fact that at lower pH, the relatively high H+ concentration would strongly compete with metal ions for adsorption sites, resulting in low adsorption capacity. Meanwhile, the hydroxyls of the 4-HIFA would be protonated to form –OH2+ groups, which lead to electrostatic repulsion between the metal cations and the protonated groups and prevent the uptake of the metal ions. With the increase of pH, the competition between H+ and other cations could be neglected, and the hydroxyl groups would be deprotonated to form –O— groups, thereby enhancing the electrostatic attraction between the adsorbent sites and meal cations. This justiftcation was also in accordance with surface complex formation theory, according to which an increase in the pH decreases the competition between metal ions and protons favouring the metal ion adsorption.37 In view of the fact that the partial hydrolysis of M+ (e.g., Ni2+, Cu2+, Cr3+, Zn2+ etc.) takes place resulting in the formation of MOH+ and M(OH)2 at pH > 6,19 pH 6.0 was selected as the optimum pH for the following batch experiments to eliminate the effect of hydrolysis.
The uptake of heavy metal ions by the 4-HIFA from aqueous solutions was studied with the batch method at room temper- ature. The affinity of the 4-HIFA for Mn+ ions can be expressed in terms of the distribution coefficient KM (for deftnition, see the Experimental section). The data of the adsorption behavior toward single ions of Ni2+, Cu2+, Cr3+, Zn2+, Hg2+, Cd2+ and Pb2+ (at z10 ppm initial concentration) are shown in Table 1. The removal ability is poor for Hg2+, Cd2+ and Pb2+ but excellent for Ni2+, Cu2+, Cr3+ and Zn2+. For Cr3+ and Zn2+, 96.74% and 97.23% removal rates were reached, respectively, and for Ni2+ and Cu2+, 99.61% and 99.53% removal rates were achieved, all of which reect high capture ability for these ions.
The adsorption kinetics of the Ni2+, Cu2+, Cr3+ and Zn2+ ions by the 4-HIFA was also investigated in order to study the adsorption rate and pathways of adsorption until equilibrium was reached. The results (Tables 2 and S2–S4†) and sorption kinetics curves (Fig. 2) show rapid uptake rates and high removal efficiencies. Within 5 min, the 4-HIFA achieved $97% removal rates and Kd values of >104 mL g—1 for Ni2+ (Table 2) and Cu2+ (Table S2†). Within 30 min, the 4-HIFA achieved
$98% removal rates and Kd values of >104 mL g—1 for Ni2+ (Table 2) and >105 mL g—1 for Cu2+ (Table S2†). Within 1 h, the 4-HIFA achieved $99% removal rates and Kd values of>105 mL g—1 for Ni2+ (Table 2) and for Cu2+ (Table S2†). For the Cr3+ and Zn2+ ions (Tables S3 and S4†), the adsorption is slightly slow but still 96.7% and 97.8% removal rates are achieved in 1 h, respectively. The adsorptions for all of the four ions reach equilibrium within ~5 min (Fig. 2b and c).
The removal rate can be determined in two different ways, pseudo-ftrst-order and pseudo-second-order mechanisms, which are deftned as follows: where qe (mg g—1) is the adsorbed amount per unit mass of adsorbent at equilibrium and qt (mg g— ) is the adsorbed mass at time t, while k1 (min—1) and k2 (g mg—1 min—1) are the corresponding equilibrium rate constants. The k1 value was obtained by plotting ln(qe — qt) against t and the k2 by plotting t/qt against t. The linear relationship of t/qt versus t is presented in Fig. 2d. From the kinetic parameters of Ni2+, Cu2+, Cr3+ and Zn2+ (Table S5†), the calculated sorption capacities derived from the pseudo-second-order model are quite close to the corresponding experimental values (qe,exp). The ftt coeffi- cient (R2) of 1 indicates that the adsorption is well fttted with the pseudo-second-order kinetic model, suggesting a chemisorp- tion process.
Uptake capacity toward Ni2+, Cu2+, Cr3+ and Zn2+ by the 4-HIFA from aqueous solutions was studied with the batch method at room temperature. The maximum adsorption capacity of the material was determined from an adsorption equilibrium study. The Ni2+ capture by the 4-HIFA was found to increase successively with increasing concentration (10– 500 ppm, Table 3). Over a wide range of the initial concentration (10–100 ppm), the Ni2+ removal rates reached values of >97%, with the KNi values ranging from 4.8 × 104 to 3.3 × 105 mL g—1. The maximum removal capacity (qm) for Ni2+ reached~240 mg g—1. This is an exceptionally high capacity competing with those of the best adsorbents such as PMCNa hybrid hydrogels (224 mg g—1)23 and the polyvinyl alcohol/corn starch hydrogel,40 and adsorption capacities of various adsorbents are shown in Table 4.
We also checked the adsorption of the 4-HIFA material for Cu2+ in the range of concentrations from 10 ppm to 500 ppm (Table S6†) and found that there are >99% removal rates in the initial concentration range (10–50 ppm) and ~265 mg g—1 maximum adsorption capacity; this value is still very high compared to that of reported adsorbents.44–48 For Cr3+ and Zn2+, the maximum adsorption capacities are found to be relatively lower at ~92.5 mg g—1 and 125 mg g—1 (Tables S7 and S8†); however these values are still very high compared to those of reported adsorbents.22,49–52 In comparison to the reported materials as shown in Table 4, the present 4-HIFA demonstrates superior removal capacities for the target ions, which is attrib- uted to the function of the introduced indole groups in the sample, which is very helpful for the cation–p interaction between the indole and heavy metals in the framework of the adsorbent.
2,3-Difluorophenylboronic acid pinacol esterCatalog No.:AA009522 CAS No.:1073339-17-9 MDL No.:MFCD12405347 MF:C12H15BF2O2 MW:240.0541 |
2-(5-Bromo-2,3,4-trifluorophenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolaneCatalog No.:AA00HAWQ CAS No.:1073339-18-0 MDL No.:MFCD12405350 MF:C12H13BBrF3O2 MW:336.9406 |

5-Bromo-2,3,4-trifluorophenolCatalog No.:AA0094EA CAS No.:1073339-19-1 MDL No.:MFCD11857741 MF:C6H2BrF3O MW:226.9787 |
2-Trifluoromethylphenylboronic acid, pinacol esterCatalog No.:AA008WMN CAS No.:1073339-21-5 MDL No.:MFCD06795676 MF:C13H16BF3O2 MW:272.0711 |
2-(4-Methoxythiophen-2-yl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolaneCatalog No.:AA009987 CAS No.:1073339-22-6 MDL No.:MFCD12405477 MF:C11H17BO3S MW:240.1269 |
3-Chloromethylphenylboronic acid pinacol esterCatalog No.:AA007EKX CAS No.:1073353-44-2 MDL No.:MFCD11053847 MF:C13H18BClO2 MW:252.5448 |
N-Methyl-4-benzenesulfonamideboronic acid pinacol esterCatalog No.:AA007EKW CAS No.:1073353-47-5 MDL No.:MFCD06657895 MF:C13H20BNO4S MW:297.1782 |
4-(2-Hydroxyethylcarbamoyl)phenylboronic acid, pinacol esterCatalog No.:AA007EKU CAS No.:1073353-51-1 MDL No.:MFCD06795648 MF:C15H22BNO4 MW:291.1505 |
4-Pyrrolidinylcarbonylphenylboronic acid, pinacol esterCatalog No.:AA007WF1 CAS No.:1073353-55-5 MDL No.:MFCD09027085 MF:C17H24BNO3 MW:301.1884 |
4-(N-Benzylaminocarbonyl)phenylboronic acid, pinacol esterCatalog No.:AA003SOM CAS No.:1073353-57-7 MDL No.:MFCD09266184 MF:C20H24BNO3 MW:337.2205 |
4-(N,O-Dimethylhydroxylaminocarbonyl)phenylboronic acid, pinacol esterCatalog No.:AA003T2P CAS No.:1073353-58-8 MDL No.:MFCD09953501 MF:C15H22BNO4 MW:291.1505 |
4-(Furfurylaminocarbonyl)phenylboronic acid, pinacol esterCatalog No.:AA003K5M CAS No.:1073353-59-9 MDL No.:MFCD09266168 MF:C18H22BNO4 MW:327.1826 |
4-(2-Methoxyethylcarbamoyl)phenylboronic acid, pinacol esterCatalog No.:AA0090CG CAS No.:1073353-60-2 MDL No.:MFCD09266188 MF:C16H24BNO4 MW:305.1771 |
3-Pyrrolidinylcarbonylphenylboronic acid, pinacol esterCatalog No.:AA007WF0 CAS No.:1073353-61-3 MDL No.:MFCD09027086 MF:C17H24BNO3 MW:301.1884 |
3-(Piperidine-1-carbonyl)phenylboronic acid, pinacol esterCatalog No.:AA0084T3 CAS No.:1073353-62-4 MDL No.:MFCD09027084 MF:C18H26BNO3 MW:315.2149 |
3-(Furfurylaminocarbonyl)phenylboronic acid, pinacol esterCatalog No.:AA003I5J CAS No.:1073353-63-5 MDL No.:MFCD09266173 MF:C18H22BNO4 MW:327.1826 |
3-(2-Methoxyethylcarbamoyl)phenylboronic acid, pinacol esterCatalog No.:AA003HY8 CAS No.:1073353-64-6 MDL No.:MFCD09266187 MF:C16H24BNO4 MW:305.1771 |
2,4-Bis(trifluoromethyl)phenylboronic acid, pinacol esterCatalog No.:AA007EKT CAS No.:1073353-65-7 MDL No.:MFCD09953467 MF:C14H15BF6O2 MW:340.0691 |
2-Fluoro-4-trifluoromethylphenylboronic acid, pinacol esterCatalog No.:AA008SFX CAS No.:1073353-68-0 MDL No.:MFCD08458187 MF:C13H15BF4O2 MW:290.0616 |
3-Chloro-2-fluoropyridine-4-boronic acid pinacol esterCatalog No.:AA003J3V CAS No.:1073353-71-5 MDL No.:MFCD09037476 MF:C11H14BClFNO2 MW:257.4968 |
1,3-Bis(4-boronophenyl)urea, bispinacol esterCatalog No.:AA0090D4 CAS No.:1073353-72-6 MDL No.:MFCD09972179 MF:C25H34B2N2O5 MW:464.1699 |
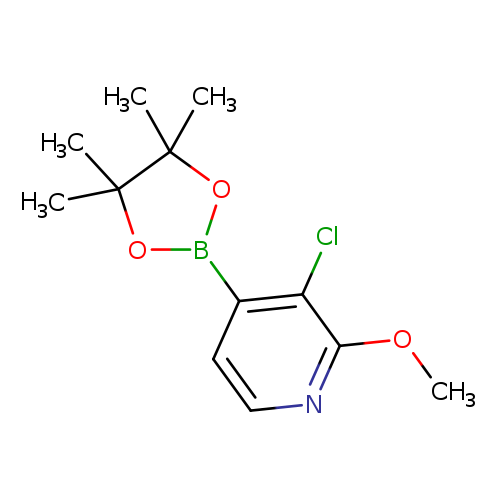
3-Chloro-2-methoxypyridine-4-boronic acid, pinacol esterCatalog No.:AA003J4A CAS No.:1073353-73-7 MDL No.:MFCD06798258 MF:C12H17BClNO3 MW:269.5323 |
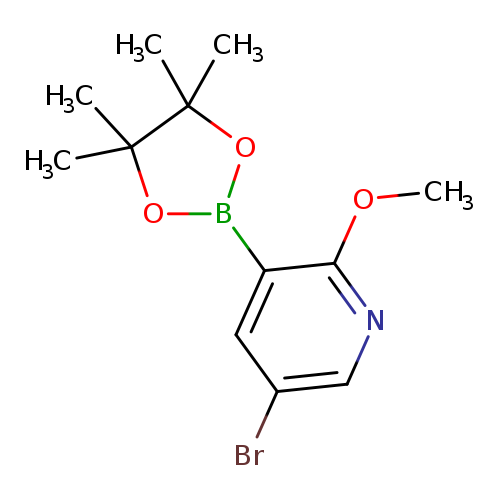
5-Bromo-2-methoxypyridine-3-boronic acid, pinacol esterCatalog No.:AA008RUV CAS No.:1073353-75-9 MDL No.:MFCD07781157 MF:C12H17BBrNO3 MW:313.9833 |
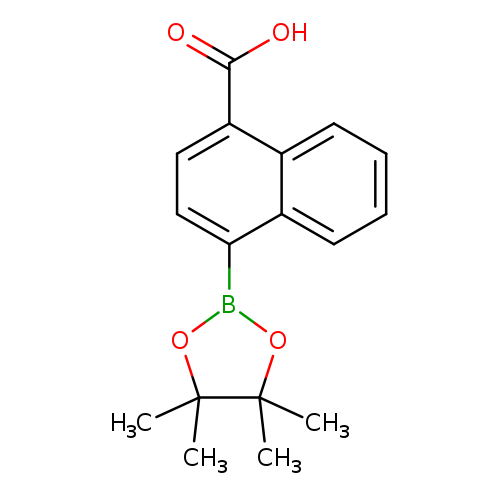
4-Carboxynaphthalene-1-boronic acid, pinacol esterCatalog No.:AA003JZ3 CAS No.:1073353-77-1 MDL No.:MFCD09972180 MF:C17H19BO4 MW:298.1414 |
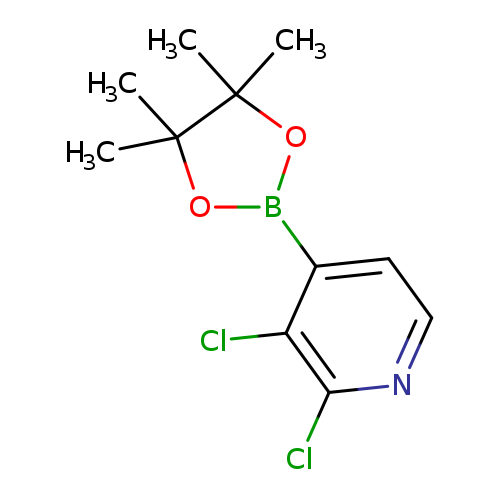
2,3-Dichloropyridine-4-boronic acid, pinacol esterCatalog No.:AA007WEZ CAS No.:1073353-78-2 MDL No.:MFCD06798257 MF:C11H14BCl2NO2 MW:273.9514 |
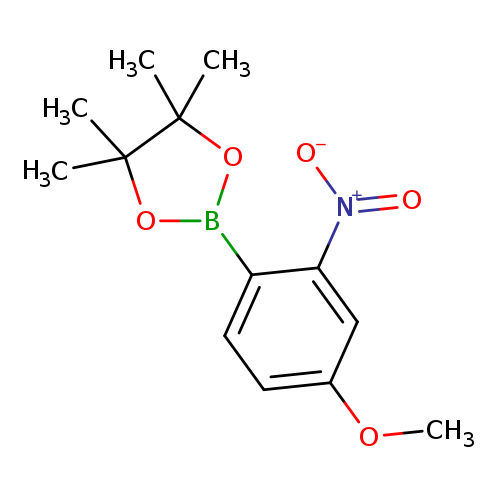
4-Methoxy-2-nitrophenylboronic acid, pinacol esterCatalog No.:AA003LJO CAS No.:1073353-81-7 MDL No.:MFCD10699701 MF:C13H18BNO5 MW:279.0967 |
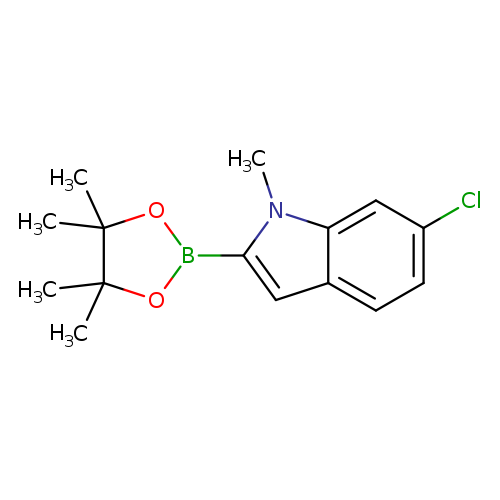
6-Chloro-1-methylindole-2-boronic acid, pinacol esterCatalog No.:AA003N2G CAS No.:1073353-82-8 MDL No.:MFCD11504964 MF:C15H19BClNO2 MW:291.5809 |
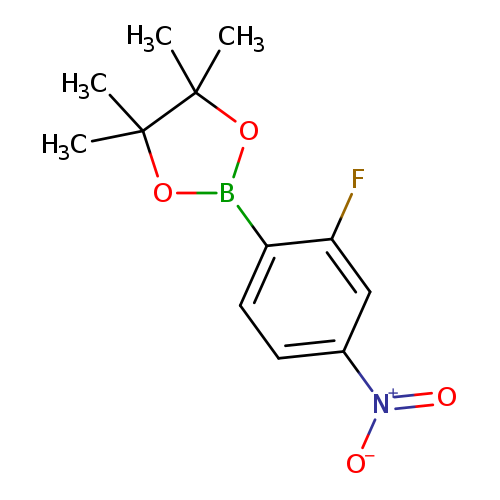
2-Fluoro-4-nitrophenylboronic acid, pinacol esterCatalog No.:AA008SR8 CAS No.:1073353-89-5 MDL No.:MFCD09264075 MF:C12H15BFNO4 MW:267.0612 |
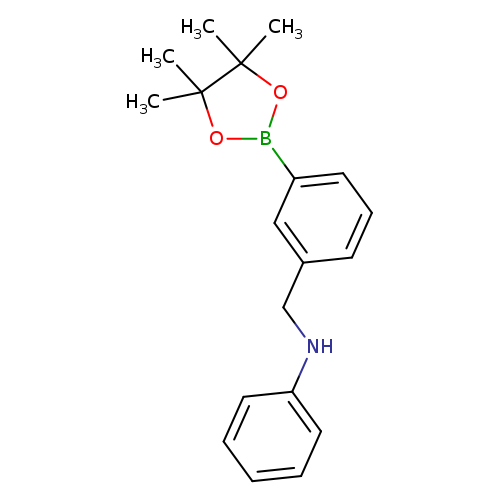
3-((Phenylamino)methyl)phenylboronic acid, pinacol esterCatalog No.:AA003HW7 CAS No.:1073353-90-8 MDL No.:MFCD09266198 MF:C19H24BNO2 MW:309.2104 |
3-(2-Bromoethoxy)phenylboronic acid, pinacol esterCatalog No.:AA0084T1 CAS No.:1073353-91-9 MDL No.:MFCD11044883 MF:C14H20BBrO3 MW:327.0218 |
3,3'-(Ethane-1,2-diylbis(oxy))bis(3,1-phenylene)diboronic acid, pinacol esterCatalog No.:AA003I8Q CAS No.:1073353-94-2 MDL No.:MFCD10699702 MF:C26H36B2O6 MW:466.1824 |
2,5-Dichloropyridine-4-boronic acid, pinacol esterCatalog No.:AA007WEY CAS No.:1073353-98-6 MDL No.:MFCD06798256 MF:C11H14BCl2NO2 MW:273.9514 |
5-Chloro-2-nitrophenylboronic acid, pinacol esterCatalog No.:AA007EKS CAS No.:1073353-99-7 MDL No.:MFCD11053894 MF:C12H15BClNO4 MW:283.5158 |
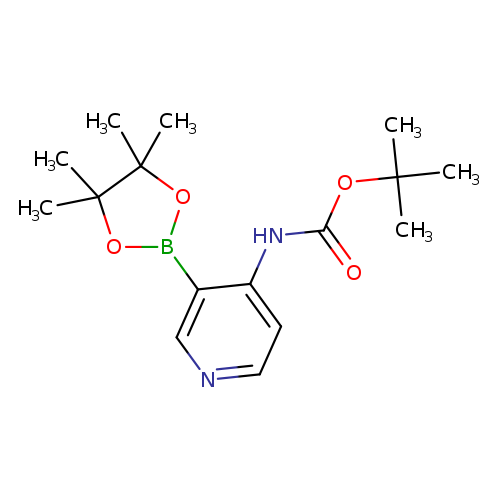
4-Boc-Aminopyridine-3-boronic acid, pinacol esterCatalog No.:AA003UG2 CAS No.:1073354-02-5 MDL No.:MFCD08063074 MF:C16H25BN2O4 MW:320.1917 |
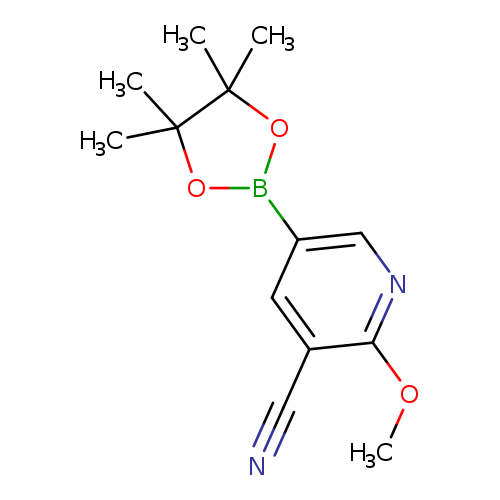
3-Cyano-2-methoxypyridine-5-boronic acid, pinacol esterCatalog No.:AA0084T0 CAS No.:1073354-05-8 MDL No.:MFCD11504968 MF:C13H17BN2O3 MW:260.0967 |
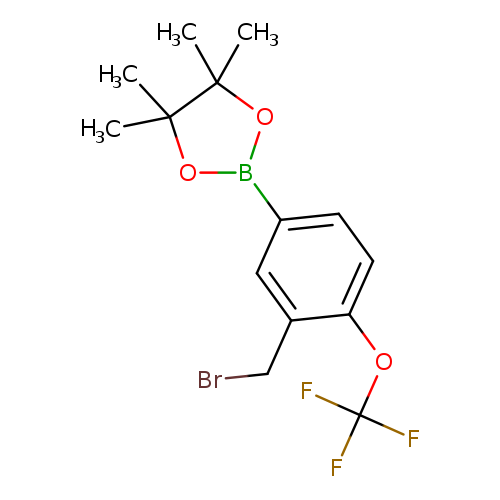
(3-Bromomethyl-4-trifluoromethoxyphenylboronic acid, pinacol esterCatalog No.:AA003BON CAS No.:1073354-06-9 MDL No.:MFCD11504969 MF:C14H17BBrF3O3 MW:380.9932 |
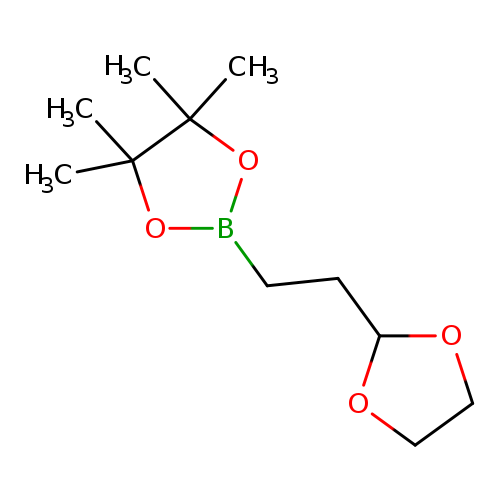
2-(1,3-Dioxolan-2-yl)-1-ethylboronic acid pinacol esterCatalog No.:AA003EN6 CAS No.:1073354-07-0 MDL No.:MFCD03788722 MF:C11H21BO4 MW:228.0930 |
N-(2-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)pivalamideCatalog No.:AA00HAWT CAS No.:1073354-10-5 MDL No.:MFCD05663849 MF:C17H26BNO3 MW:303.2042 |
2-Formylpyridine-5-boronic acid pinacolateCatalog No.:AA007WEX CAS No.:1073354-14-9 MDL No.:MFCD06659508 MF:C12H16BNO3 MW:233.0713 |
4-(4-Isopropylpiperizinyl)phenylboronic acid, pinacol esterCatalog No.:AA007EKR CAS No.:1073354-18-3 MDL No.:MFCD06795656 MF:C19H31BN2O2 MW:330.2726 |
1-((4-Chlorophenyl)sulfonyl)-4-(5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyridin-2-yl)piperazineCatalog No.:AA007EKQ CAS No.:1073354-20-7 MDL No.:MFCD06797988 MF:C21H27BClN3O4S MW:463.7858 |
6-(Benzylamino)pyridine-3-boronic acid pinacol esterCatalog No.:AA003SOP CAS No.:1073354-27-4 MDL No.:MFCD06798270 MF:C18H23BN2O2 MW:310.1984 |
N-Benzyl-N-methyl-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyridin-2-amineCatalog No.:AA003SPF CAS No.:1073354-30-9 MDL No.:MFCD06798271 MF:C19H25BN2O2 MW:324.2250 |
6-(Cyclohexylamino)pyridine-3-boronic acid pinacol esterCatalog No.:AA003SUA CAS No.:1073354-34-3 MDL No.:MFCD06798277 MF:C17H27BN2O2 MW:302.2195 |
6-(4-Morpholineamino)pyridine-3-boronic acid pinacol esterCatalog No.:AA003SAO CAS No.:1073354-38-7 MDL No.:MFCD06798279 MF:C15H24BN3O3 MW:305.1804 |
2-Pyrrolidinopyridine-3-boronic acid, pinacol esterCatalog No.:AA003F1R CAS No.:1073354-41-2 MDL No.:MFCD06798280 MF:C15H23BN2O2 MW:274.1663 |
2-(4-BOC-Piperazino)pyridine-3-boronic acid, pinacol esterCatalog No.:AA003UH4 CAS No.:1073354-42-3 MDL No.:MFCD07781131 MF:C20H32BN3O4 MW:389.2968 |
2-Cyclohexyloxypyridine-3-boronic acid, pinacol esterCatalog No.:AA003EY1 CAS No.:1073354-43-4 MDL No.:MFCD07781171 MF:C17H26BNO3 MW:303.2042 |
4-(4'-Allyloxycarbonylpiperizino)phenylboronic acid, pinacol esterCatalog No.:AA003JZB CAS No.:1073354-49-0 MDL No.:MFCD07781206 MF:C20H29BN2O4 MW:372.2663 |
3,5-Difluoro-2-methoxyphenylboronic acid, pinacol esterCatalog No.:AA003IJN CAS No.:1073354-50-3 MDL No.:MFCD07781207 MF:C13H17BF2O3 MW:270.0801 |
1-(Toluene-4-sulfonyl)-1H-indole-3-boronic acid pinacol esterCatalog No.:AA003I00 CAS No.:1073354-51-4 MDL No.:MFCD08063118 MF:C21H24BNO4S MW:397.2956 |
2-(4-Boc-piperazin-1-yl)-3-methylpyridine-5-boronic acid pinacol esterCatalog No.:AA003UH7 CAS No.:1073354-54-7 MDL No.:MFCD08064052 MF:C21H34BN3O4 MW:403.3234 |
trans-3-Cyclopentylpropen-1-ylboronic acid, pinacol esterCatalog No.:AA003EQZ CAS No.:1073354-57-0 MDL No.:MFCD08276877 MF:C14H25BO2 MW:236.1581 |
2-[4-(N-Boc)piperazin-1-yl]phenylboronic acid pinacol esterCatalog No.:AA007EKP CAS No.:1073354-59-2 MDL No.:MFCD08669542 MF:C21H33BN2O4 MW:388.3087 |
3-Cyanothiophene-4-boronic acid pinacol esterCatalog No.:AA003J9T CAS No.:1073354-61-6 MDL No.:MFCD08669563 MF:C11H14BNO2S MW:235.1104 |
2-(5-Chloro-2,4-difluorophenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolaneCatalog No.:AA007EKO CAS No.:1073354-65-0 MDL No.:MFCD08669564 MF:C12H14BClF2O2 MW:274.4992 |
4-Formyl-2-methylphenylboronic acid pinacol esterCatalog No.:AA003JMY CAS No.:1073354-66-1 MDL No.:MFCD08669583 MF:C14H19BO3 MW:246.1099 |
1-Boc-3,5-dimethylpyrazole-4-boronic acid pinacol esterCatalog No.:AA003UGS CAS No.:1073354-70-7 MDL No.:MFCD09027070 MF:C16H27BN2O4 MW:322.2076 |
6-[Cyclohexyl(methyl)amino]pyridine-3-boronic acid pinacol esterCatalog No.:AA003SUG CAS No.:1073354-73-0 MDL No.:MFCD09027071 MF:C18H29BN2O2 MW:316.2461 |
3-Fluoro-4-(N-morpholinomethyl)phenylboronic acid, pinacol esterCatalog No.:AA003JWX CAS No.:1073354-74-1 MDL No.:MFCD09027076 MF:C17H25BFNO3 MW:321.1947 |
6-(4-Fluorophenyl)pyridine-3-boronic acid pinacol esterCatalog No.:AA003ETI CAS No.:1073354-81-0 MDL No.:MFCD09037494 MF:C17H19BFNO2 MW:299.1477 |
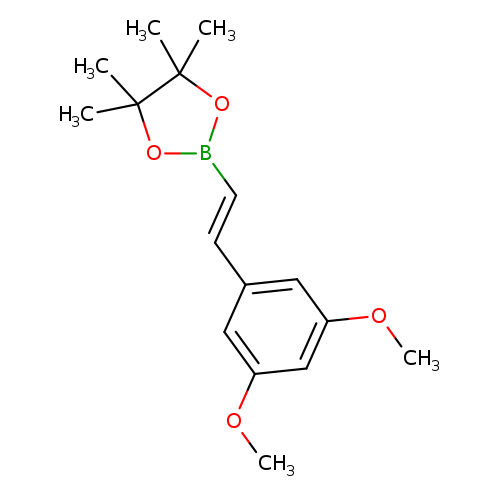
E-2-(3,5-Dimethoxyphenyl)vinylboronic acid pinacol esterCatalog No.:AA003BXG CAS No.:1073354-86-5 MDL No.:MFCD09038437 MF:C16H23BO4 MW:290.1624 |
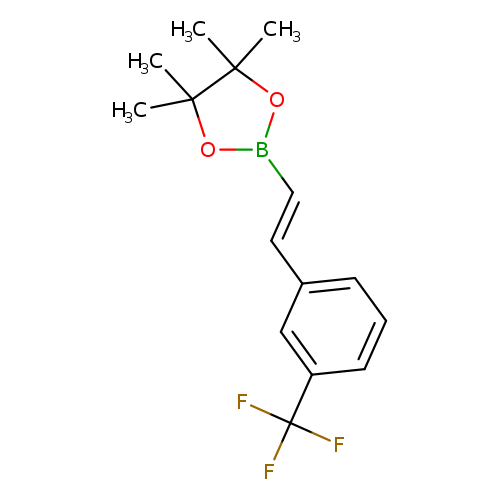
E-2-(3-Trifluoromethylphenyl)vinylboronic acid pinacol esterCatalog No.:AA003PU3 CAS No.:1073354-88-7 MDL No.:MFCD09038444 MF:C15H18BF3O2 MW:298.1084 |
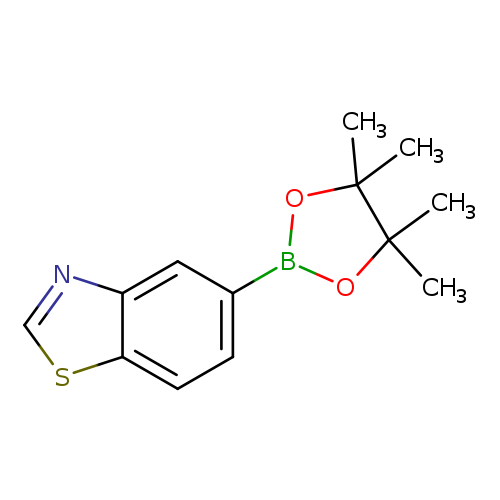
Benzothiazole-5-boronic acid pinacol esterCatalog No.:AA003M26 CAS No.:1073354-91-2 MDL No.:MFCD09260439 MF:C13H16BNO2S MW:261.1476 |
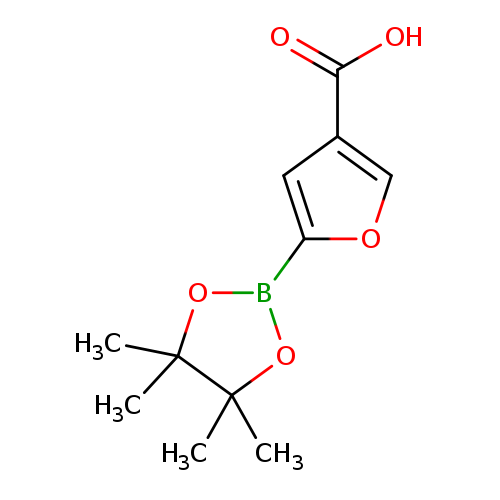
4-Carboxyfuran-2-boronic acid pinacol esterCatalog No.:AA003M28 CAS No.:1073354-94-5 MDL No.:MFCD09260445 MF:C11H15BO5 MW:238.0448 |
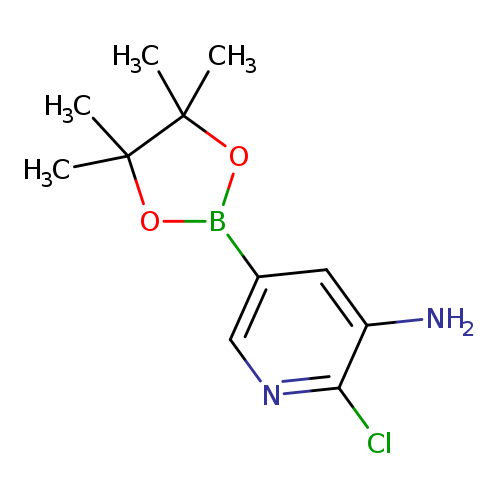
3-Amino-2-chloropyridine-5-boronic acid, pinacol esterCatalog No.:AA003GV8 CAS No.:1073354-96-7 MDL No.:MFCD09260487 MF:C11H16BClN2O2 MW:254.5209 |
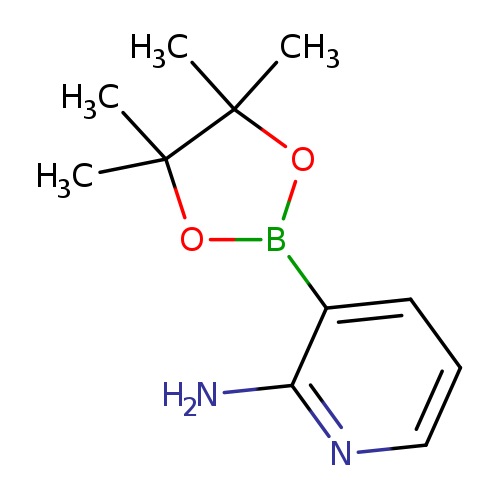
2-Aminopyridine-3-boronic acid, pinacol esterCatalog No.:AA008SDL CAS No.:1073354-97-8 MDL No.:MFCD09260488 MF:C11H17BN2O2 MW:220.0759 |
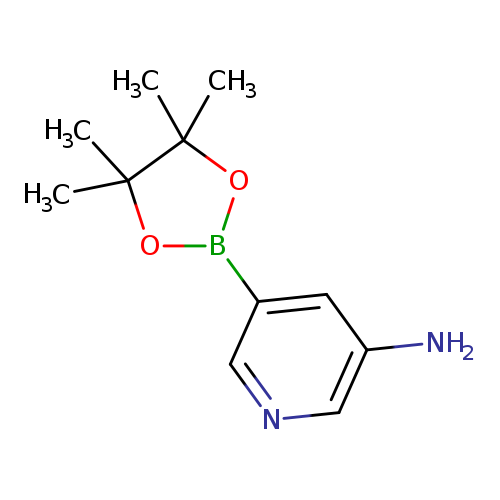
3-Aminopyridine-5-boronic acid, pinacol esterCatalog No.:AA003IVB CAS No.:1073354-99-0 MDL No.:MFCD09260489 MF:C11H17BN2O2 MW:220.0759 |
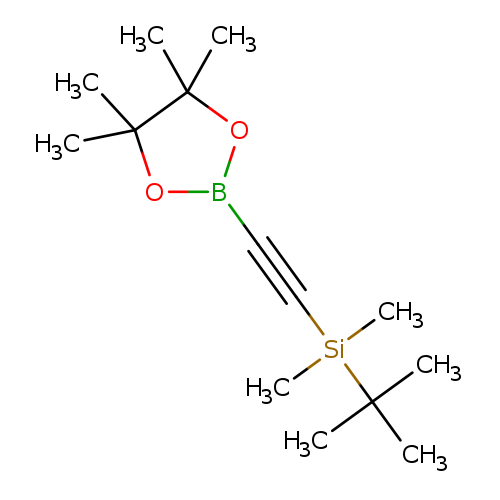
2-((tert-Butyldimethylsilanyl)ethynyl) boronic acid pinacol esterCatalog No.:AA003UIU CAS No.:1073355-02-8 MDL No.:MFCD09265092 MF:C14H27BO2Si MW:266.2595 |
3-[3-(Ethoxycarbonyl)piperidine-1-carbonyl]phenylboronic acid pinacol esterCatalog No.:AA007WEU CAS No.:1073355-04-0 MDL No.:MFCD09266178 MF:C21H30BNO5 MW:387.2776 |
3-Benzylphenylboronic acid pinacol esterCatalog No.:AA007W8Q CAS No.:1073355-05-1 MDL No.:MFCD09266179 MF:C19H23BO2 MW:294.1957 |
3-(Tetrahydrofurfurylaminocarbonyl)phenylboronic acid pinacol esterCatalog No.:AA0090C0 CAS No.:1073355-06-2 MDL No.:MFCD09266189 MF:C18H26BNO4 MW:331.2143 |
4-(Tetrahydrofurfurylaminocarbonyl)phenylboronic acid pinacol esterCatalog No.:AA0090C4 CAS No.:1073355-09-5 MDL No.:MFCD09266200 MF:C18H26BNO4 MW:331.2143 |
2-(4-Boc-piperazin-1-yl)-4-methylpyridine-5-boronic acid pinacol esterCatalog No.:AA003UH9 CAS No.:1073355-13-1 MDL No.:MFCD09271789 MF:C21H34BN3O4 MW:403.3234 |
Benzofurazan-5-boronic acid, pinacol esterCatalog No.:AA007W8O CAS No.:1073355-14-2 MDL No.:MFCD09842717 MF:C12H15BN2O3 MW:246.0701 |
3-Benzyloxy-4-methoxycarbonylphenylboronic acid, pinacol esterCatalog No.:AA0084SZ CAS No.:1073355-16-4 MDL No.:MFCD09864186 MF:C21H25BO5 MW:368.2312 |
3-Acetoxy-4-methoxycarbonylphenylboronic acid, pinacol esterCatalog No.:AA003RJG CAS No.:1073355-18-6 MDL No.:MFCD09864187 MF:C16H21BO6 MW:320.1453 |
2-Cyano-4-(trifluoromethyl)phenylboronic acid pinacol esterCatalog No.:AA007EKN CAS No.:1073355-21-1 MDL No.:MFCD09878539 MF:C14H15BF3NO2 MW:297.0806 |
3-(2-Hydroxyphenyl)propylboronic acid, pinacol esterCatalog No.:AA003EQ0 CAS No.:1073355-25-5 MDL No.:MFCD09953481 MF:C15H23BO3 MW:262.1523 |
4-(N-Boc-phenylaminomethyl)phenylboronic acid pinacol esterCatalog No.:AA0090XV CAS No.:1073371-71-7 MDL No.:MFCD09953500 MF:C24H32BNO4 MW:409.3262 |
4-(Hydroxy(thiazol-2-yl)methyl)phenylboronic acid, pinacol esterCatalog No.:AA00906S CAS No.:1073371-73-9 MDL No.:MFCD09953504 MF:C16H20BNO3S MW:317.2109 |
2-(t-Butyldimethylsilyl)thiophene-5-boronic acid pinacol esterCatalog No.:AA003UIV CAS No.:1073371-74-0 MDL No.:MFCD10567054 MF:C16H29BO2SSi MW:324.3618 |
4-(3-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)benzyl)morpholine hydrochlorideCatalog No.:AA008UMU CAS No.:1073371-76-2 MDL No.:MFCD06657893 MF:C17H27BClNO3 MW:339.6652 |
2-Amino-5-chlorophenylboronic acid, pinacol esterCatalog No.:AA007W8M CAS No.:1073371-77-3 MDL No.:MFCD06795672 MF:C12H17BClNO2 MW:253.5329 |
4-((N,N-Dimethylamino)methyl)phenylboronic acid pinacol ester HClCatalog No.:AA0090D7 CAS No.:1073371-85-3 MDL No.:MFCD07771993 MF:C15H25BClNO2 MW:297.6285 |
5-(Morpholine-4-carbonyl)pyridine-3-boronic acid, pinacol esterCatalog No.:AA003S3H CAS No.:1073371-92-2 MDL No.:MFCD07781248 MF:C16H23BN2O4 MW:318.1758 |
2-Nitropyridine-5-boronic acid, pinacol esterCatalog No.:AA007W8L CAS No.:1073371-93-3 MDL No.:MFCD08063079 MF:C11H15BN2O4 MW:250.0588 |
2-Fluoro-5-methylpyridine-3-boronic acid pinacol esterCatalog No.:AA0084SW CAS No.:1073371-96-6 MDL No.:MFCD08063092 MF:C12H17BFNO2 MW:237.0783 |
2,5-Dichloropyridine-3-boronic acid pinacol esterCatalog No.:AA003FU7 CAS No.:1073371-98-8 MDL No.:MFCD08063094 MF:C11H14BCl2NO2 MW:273.9514 |
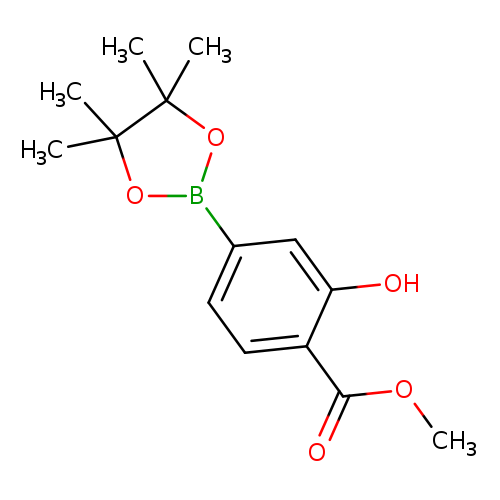
3-Hydroxy-4-methoxycarbonylphenylboronic acid, pinacol esterCatalog No.:AA007W8K CAS No.:1073371-99-9 MDL No.:MFCD08458200 MF:C14H19BO5 MW:278.1087 |
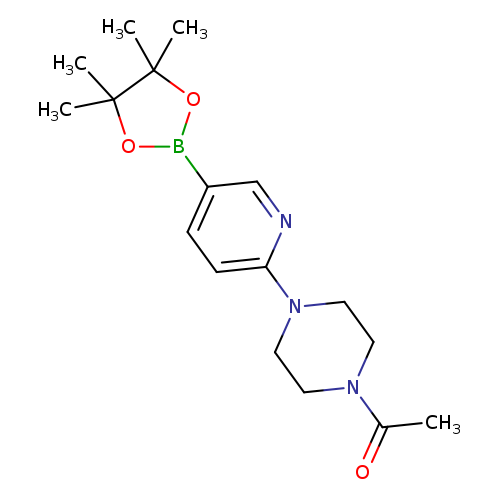
2-(4-Acetylpiperazin-1-yl)pyridine-5-boronic acid, pinacol esterCatalog No.:AA007W8J CAS No.:1073372-01-6 MDL No.:MFCD09027063 MF:C17H26BN3O3 MW:331.2176 |
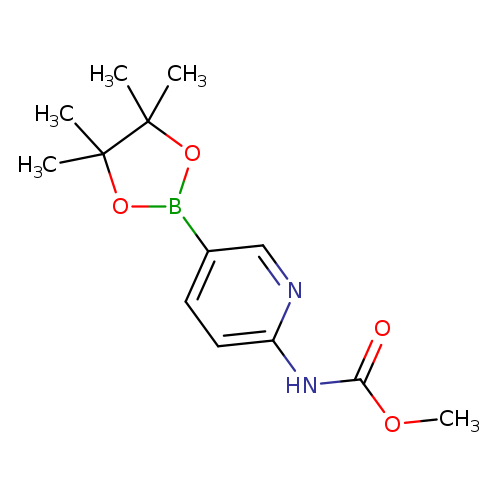
2-Methoxycarbonylaminopyridine-5-boronic acid, pinacol esterCatalog No.:AA008TRP CAS No.:1073372-02-7 MDL No.:MFCD09027078 MF:C13H19BN2O4 MW:278.1120 |
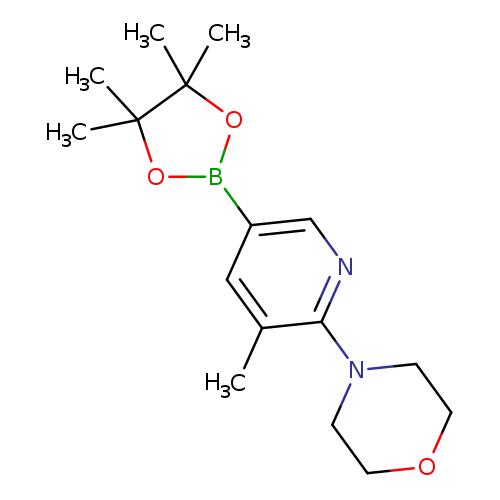
5-Methyl-6-morpholinylpyridine-3-boronic acid pinacol esterCatalog No.:AA003JYI CAS No.:1073372-03-8 MDL No.:MFCD09037483 MF:C16H25BN2O3 MW:304.1923 |
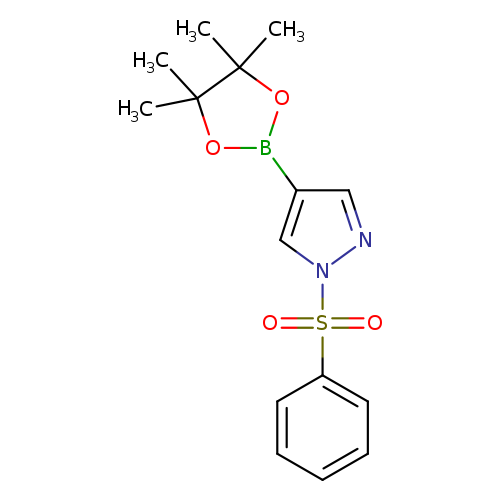
1-(Phenylsulfonyl)pyrazole-4-boronic acid, pinacol esterCatalog No.:AA0032GF CAS No.:1073372-04-9 MDL No.:MFCD09801236 MF:C15H19BN2O4S MW:334.1984 |
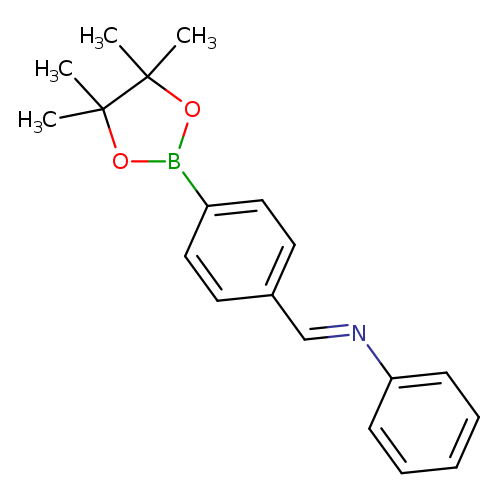
4-((Phenylimino)methyl)phenylboronic acid pinacol esterCatalog No.:AA0090XY CAS No.:1073372-06-1 MDL No.:MFCD09266199 MF:C19H22BNO2 MW:307.1945 |
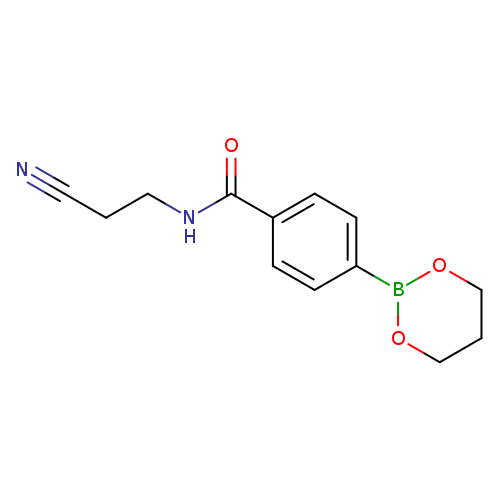
N-(2-Cyanoethyl)-4-(1,3,2-dioxaborinan-2-yl)benzamideCatalog No.:AA003S6E CAS No.:1073372-08-3 MDL No.:MFCD11053850 MF:C13H15BN2O3 MW:258.0808 |
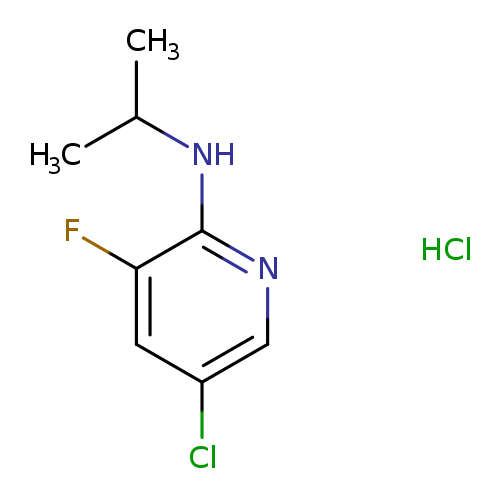
5-Chloro-3-fluoro-2-(N-isopropylamino)pyridine, HClCatalog No.:AA003ML5 CAS No.:1073372-10-7 MDL No.:MFCD09972198 MF:C8H11Cl2FN2 MW:225.0907 |
Benzyl 4-chloropyridin-2-ylcarbamateCatalog No.:AA003O0I CAS No.:1073372-14-1 MDL No.:MFCD11504979 MF:C13H11ClN2O2 MW:262.6916 |
Benzyl 4-methoxypyridin-2-ylcarbamateCatalog No.:AA003O0J CAS No.:1073372-17-4 MDL No.:MFCD11504982 MF:C14H14N2O3 MW:258.2726 |
4,5-Dichloro-n-methyl-2-nitroanilineCatalog No.:AA007W8H CAS No.:107342-18-7 MDL No.:MFCD00186241 MF:C7H6Cl2N2O2 MW:221.0407 |
2-BROMO-4-CHLORO-5-NITROPHENOLCatalog No.:AA019EKP CAS No.:1073437-11-2 MDL No.:MFCD30534472 MF:C6H3BrClNO3 MW:252.4499 |
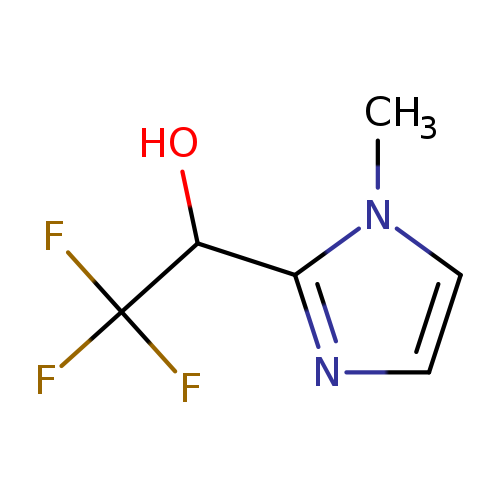
2,2,2-trifluoro-1-(1-methyl-1H-imidazol-2-yl)ethan-1-olCatalog No.:AA019K1L CAS No.:107344-63-8 MDL No.:MFCD08264986 MF:C6H7F3N2O MW:180.1278 |
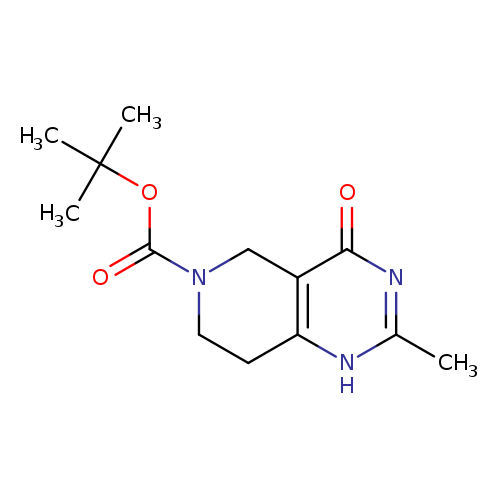
tert-Butyl 4-hydroxy-2-methyl-5H,7H,8H-pyrido[4,3-d]pyrimidine-6-carboxylateCatalog No.:AA0093HG CAS No.:1073440-84-2 MDL No.:MFCD13189665 MF:C13H19N3O3 MW:265.3083 |
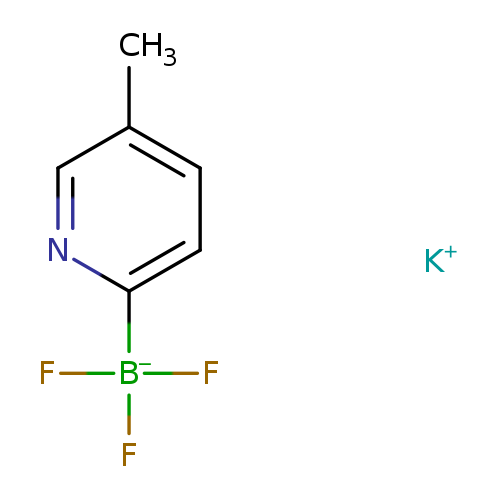
Potassium 5-methylpyridine-2-trifluoroborateCatalog No.:AA003TSW CAS No.:1073468-31-1 MDL No.:MFCD09992972 MF:C6H6BF3KN MW:199.0230 |
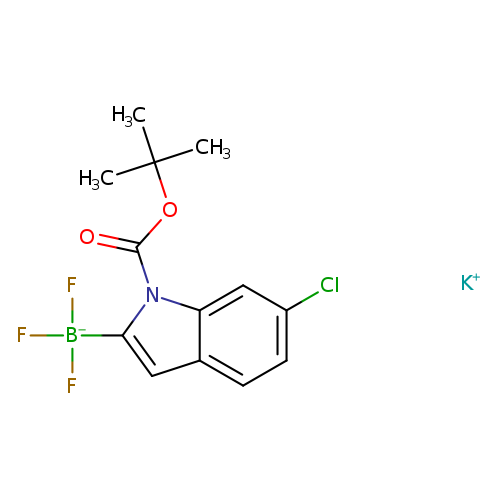
Potassium 1-Boc-6-chloroindole-2-trifluoroborateCatalog No.:AA008SRK CAS No.:1073468-33-3 MDL No.:MFCD11054034 MF:C13H13BClF3KNO2 MW:357.6053 |
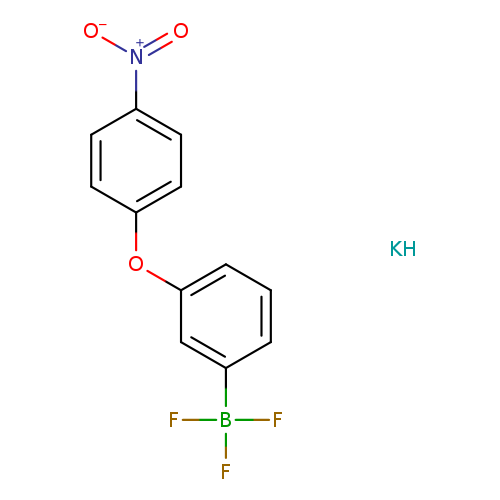
Potassium 3-(4-nitrophenoxy)phenyltrifluoroborateCatalog No.:AA008SR4 CAS No.:1073468-34-4 MDL No.:MFCD09972205 MF:C12H9BF3KNO3 MW:322.1093 |
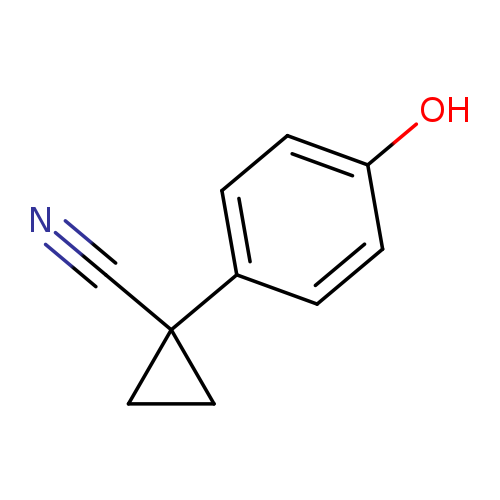
1-(4-Hydroxyphenyl)cyclopropane-1-carbonitrileCatalog No.:AA0093K8 CAS No.:1073477-06-1 MDL No.:MFCD19692083 MF:C10H9NO MW:159.1846 |
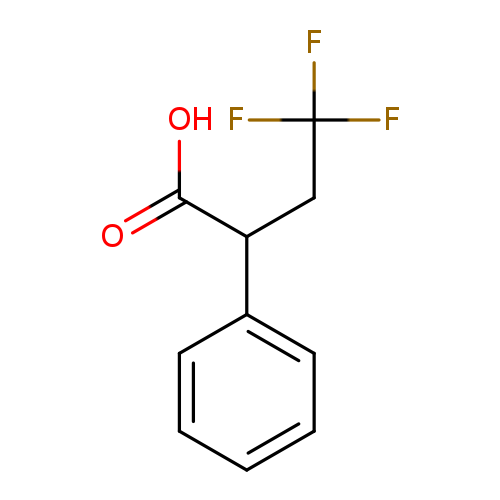
4,4,4-trifluoro-2-phenylbutanoic acidCatalog No.:AA01DX76 CAS No.:1073477-18-5 MDL No.:MFCD21922772 MF:C10H9F3O2 MW:218.1725 |
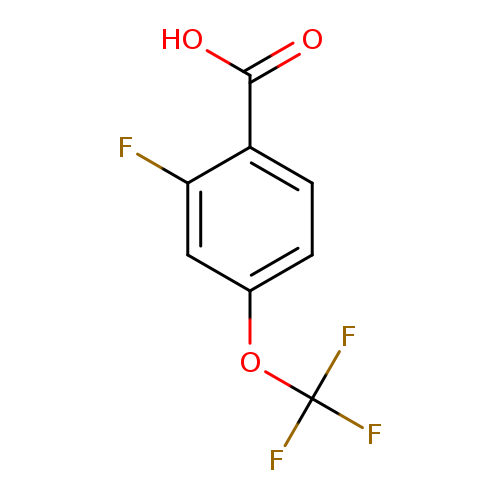
2-Fluoro-4-(trifluoromethoxy)benzoic acidCatalog No.:AA00971U CAS No.:1073477-22-1 MDL No.:MFCD16652439 MF:C8H4F4O3 MW:224.1092 |
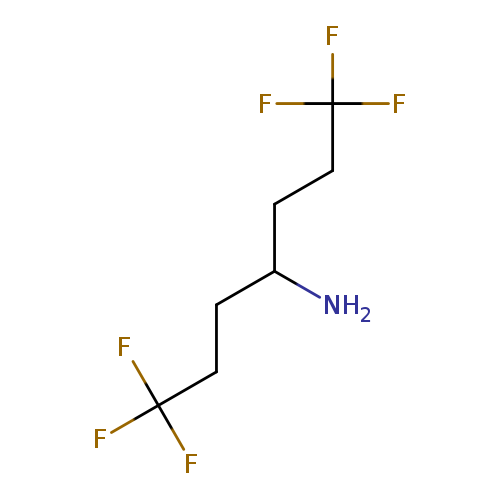
1,1,1,7,7,7-hexafluoroheptan-4-amineCatalog No.:AA01B625 CAS No.:1073477-67-4 MDL No.:MFCD23801863 MF:C7H11F6N MW:223.1594 |
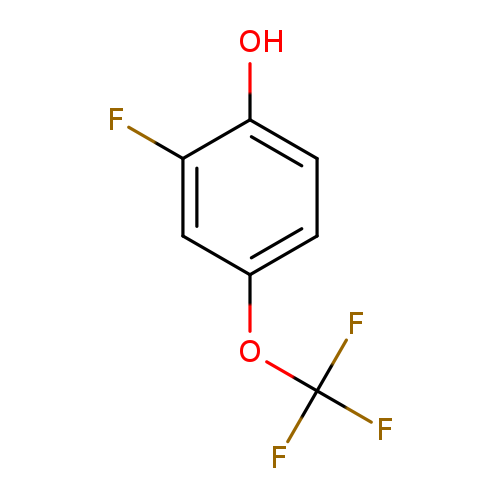
2-Fluoro-4-(trifluoromethoxy)phenolCatalog No.:AA0095B0 CAS No.:1073477-74-3 MDL No.:MFCD23111095 MF:C7H4F4O2 MW:196.0991 |
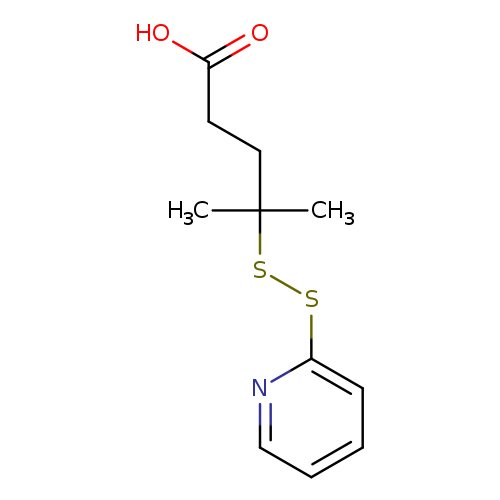
4-methyl-4-(pyridin-2-yldisulfanyl)pentanoic acidCatalog No.:AA01DHU3 CAS No.:107348-49-2 MDL No.:MFCD27935396 MF:C11H15NO2S2 MW:257.3723 |
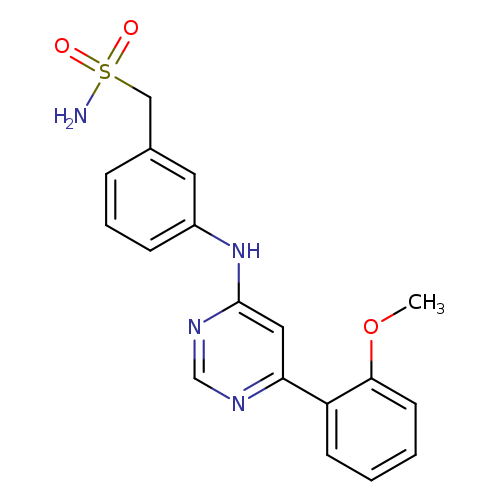
Ldc000067Catalog No.:AA008TEH CAS No.:1073485-20-7 MDL No.:MFCD28137788 MF:C18H18N4O3S MW:370.4255 |
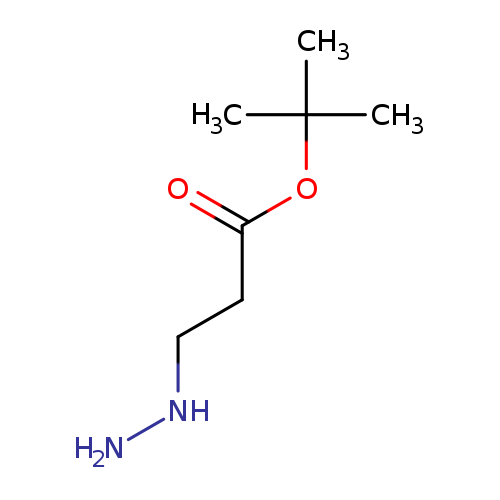
Propanoic acid, 3-hydrazinyl-, 1,1-diMethylethyl esterCatalog No.:AA0092WQ CAS No.:107349-78-0 MDL No.:MFCD14707427 MF:C7H16N2O2 MW:160.2141 |
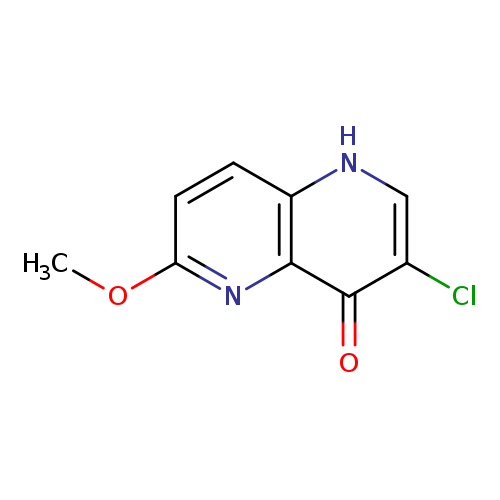
3-Chloro-6-methoxy-1,5-naphthyridin-4(1h)-oneCatalog No.:AA0096V8 CAS No.:1073495-87-0 MDL No.:MFCD24396067 MF:C9H7ClN2O2 MW:210.6171 |
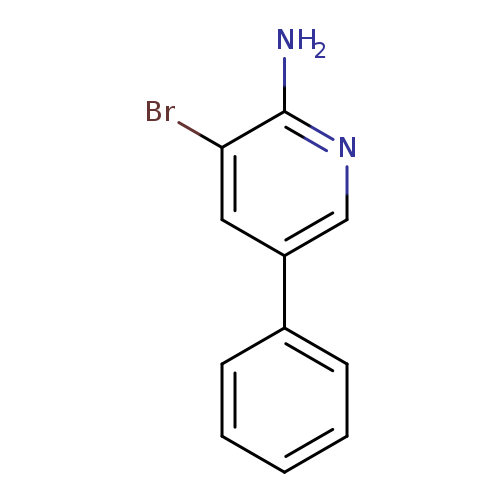
3-Bromo-5-phenylpyridin-2-amineCatalog No.:AA007W8C CAS No.:107351-80-4 MDL No.:MFCD02183569 MF:C11H9BrN2 MW:249.1066 |
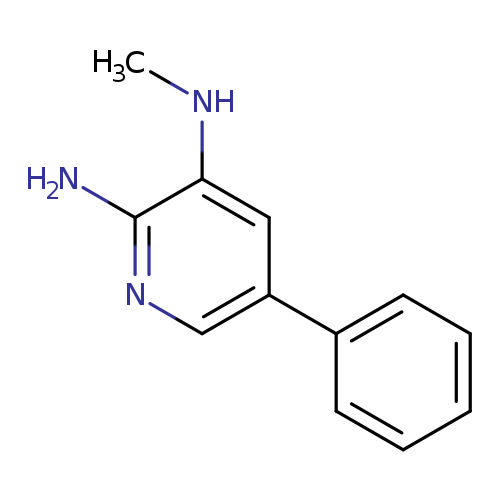
2-AMINO-3-METHYLAMINO-5-PHENYLPYRIDINECatalog No.:AA007W8B CAS No.:107351-81-5 MDL No.:MFCD03701121 MF:C12H13N3 MW:199.2517 |
2-Bromo-5-phenylpyridineCatalog No.:AA0084N5 CAS No.:107351-82-6 MDL No.:MFCD00869668 MF:C11H8BrN MW:234.0919 |
4-OXO-3,3-DIPHENYL-[1,2]DIAZETIDINE-1,2-DICARBOXYLIC ACID DIMETHYL ESTERCatalog No.:AA01DU0Q CAS No.:1073529-41-5 MDL No.:MFCD28053514 MF:C17H20N2O5 MW:332.3511 |
3-Cyclopropyl-3-(4-hydroxyphenyl)propanoic acidCatalog No.:AA00HAX1 CAS No.:1073545-88-6 MDL No.:MFCD22574044 MF:C12H14O3 MW:206.2378 |
3-butyl-1-methylpiperazin-2-oneCatalog No.:AA008VHC CAS No.:1073556-04-3 MDL No.:MFCD08060031 MF:C9H18N2O MW:170.2520 |
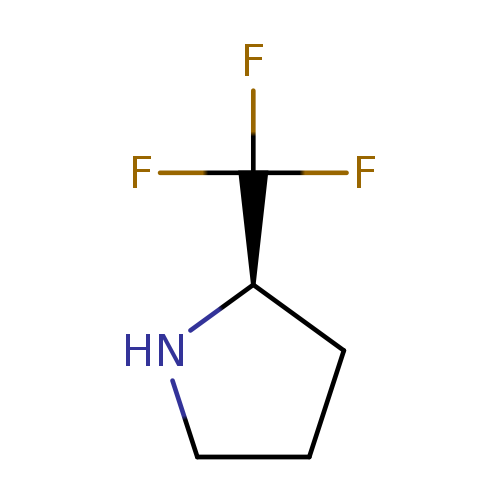
(R)-2-(Trifluoromethyl)pyrrolidineCatalog No.:AA008SCZ CAS No.:1073556-31-6 MDL No.:MFCD07784180 MF:C5H8F3N MW:139.1189 |
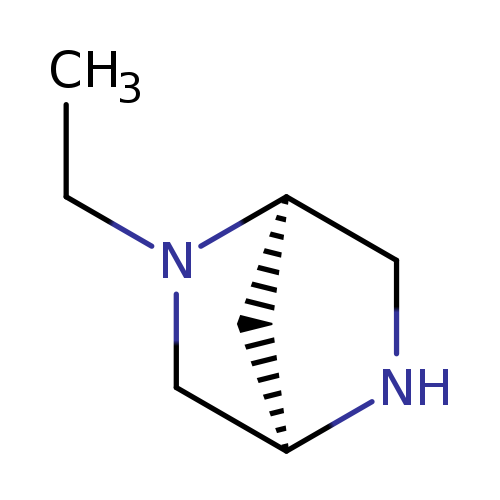
(1R,4R)-2-Ethyl-2,5-diaza-bicyclo[2.2.1]heptaneCatalog No.:AA00HAX2 CAS No.:1073556-32-7 MDL No.:MFCD19237471 MF:C7H14N2 MW:126.1995 |
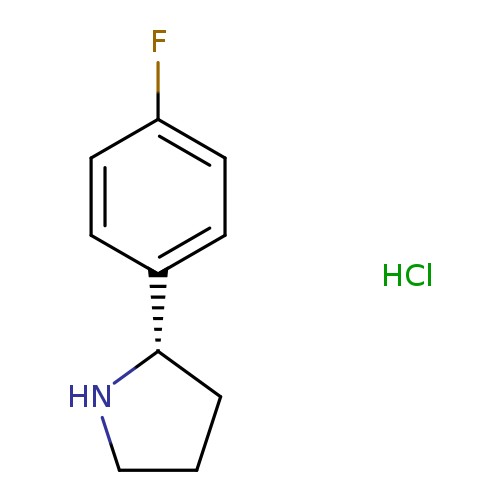
(S)-2-(4-Fluorophenyl)pyrrolidine hydrochlorideCatalog No.:AA00HAX3 CAS No.:1073556-40-7 MDL No.:MFCD08751463 MF:C10H13ClFN MW:201.6683 |
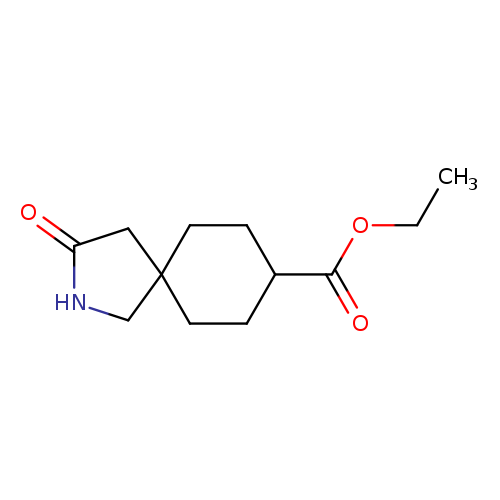
Ethyl 3-oxo-2-azaspiro[4.5]decane-8-carboxylateCatalog No.:AA009982 CAS No.:1073559-59-7 MDL No.:MFCD20488669 MF:C12H19NO3 MW:225.2842 |
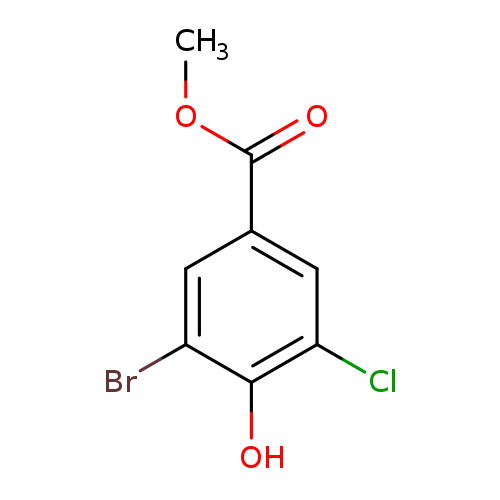
methyl 3-bromo-5-chloro-4-hydroxybenzoateCatalog No.:AA01BQZ4 CAS No.:107356-01-4 MDL No.:MFCD25955178 MF:C8H6BrClO3 MW:265.4884 |
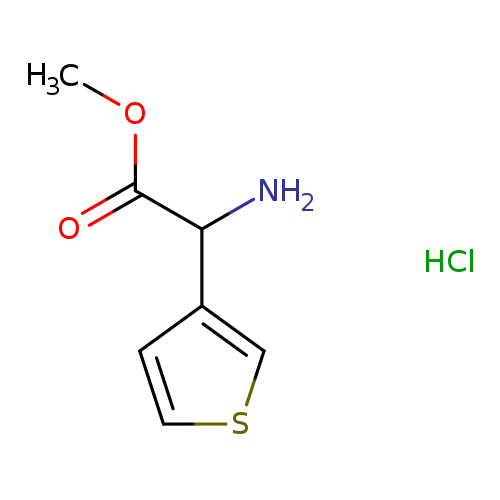
methyl 2-amino-2-(thiophen-3-yl)acetate hydrochlorideCatalog No.:AA01ACN3 CAS No.:107357-02-8 MDL No.:MFCD26936076 MF:C7H10ClNO2S MW:207.6778 |
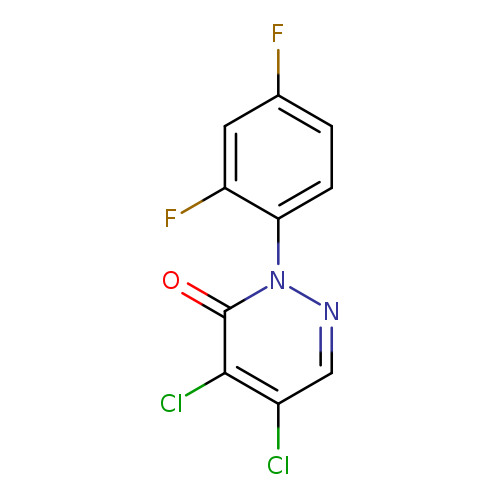
4,5-dichloro-2-(2,4-difluorophenyl)-2,3-dihydropyridazin-3-oneCatalog No.:AA019U8C CAS No.:107360-91-8 MDL No.:MFCD09051393 MF:C10H4Cl2F2N2O MW:277.0544 |
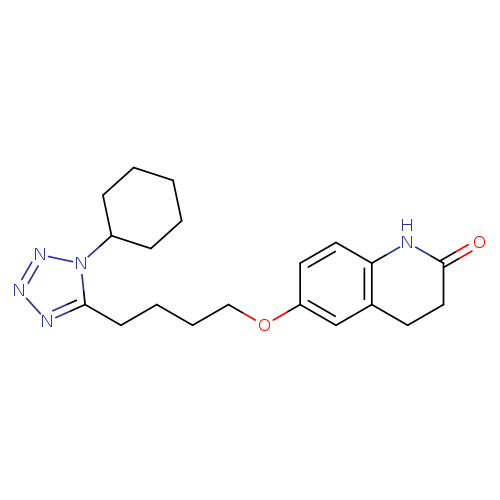
Cilostazol-d11Catalog No.:AA008VYE CAS No.:1073608-02-2 MDL No.:MFCD28138296 MF:C20H27N5O2 MW:369.4607 |
3,4-DehydroCilostazol-d11Catalog No.:AA01DZFU CAS No.:1073608-13-5 MDL No.:MFCD09840314 MF:C20H14D11N5O2 MW:378.5126 |
EnazadremCatalog No.:AA008SEV CAS No.:107361-33-1 MDL No.:MFCD00866625 MF:C18H25N3O MW:299.4106 |
Cy-09Catalog No.:AA01DEX8 CAS No.:1073612-91-5 MDL No.:MFCD31619349 MF:C19H12F3NO3S2 MW:423.4287 |
3-(1H-Pyrrol-1-yl)benzo[b]thiophene-2-carbohydrazideCatalog No.:AA0084N1 CAS No.:107363-01-9 MDL No.:MFCD06200944 MF:C13H11N3OS MW:257.3109 |
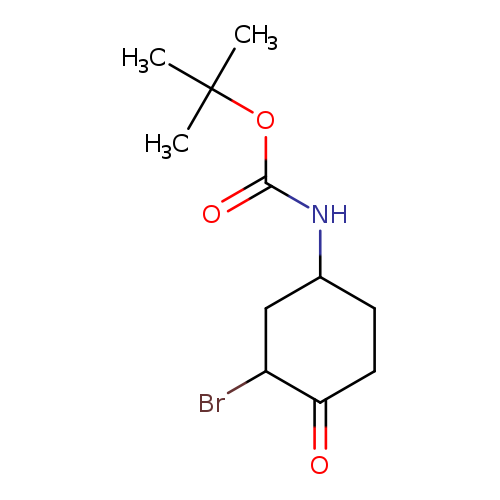
(3-Bromo-4-oxo-cyclohexyl)-carbamic acid tert-butyl esterCatalog No.:AA0093HS CAS No.:1073632-93-5 MDL No.:MFCD23115355 MF:C11H18BrNO3 MW:292.1695 |
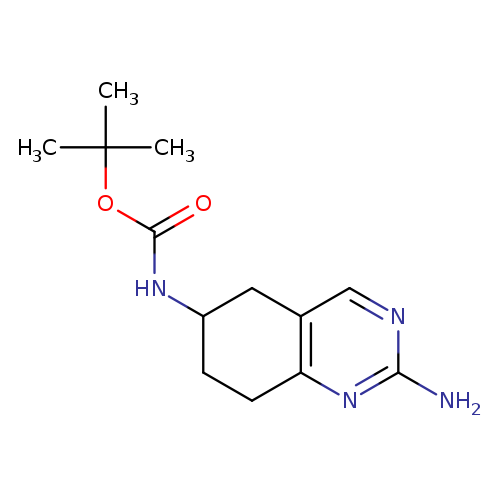
(2-Amino-5,6,7,8-tetrahydro-quinazolin-6-yl)-carbamic acid tert-butyl esterCatalog No.:AA0093I0 CAS No.:1073633-86-9 MDL No.:MFCD24843107 MF:C13H20N4O2 MW:264.3235 |
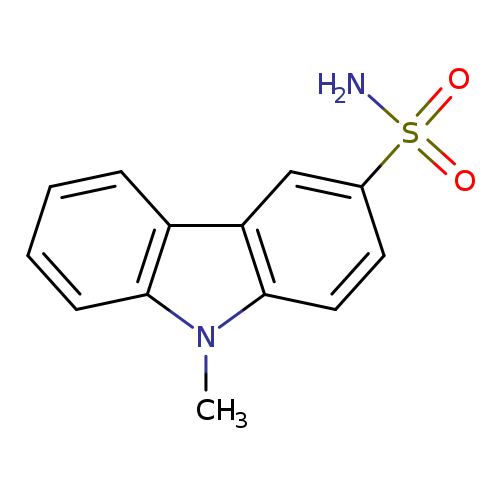
9-methyl-9H-carbazole-3-sulfonamideCatalog No.:AA01C3UK CAS No.:1073653-45-8 MDL No.:MFCD29762920 MF:C13H12N2O2S MW:260.3116 |
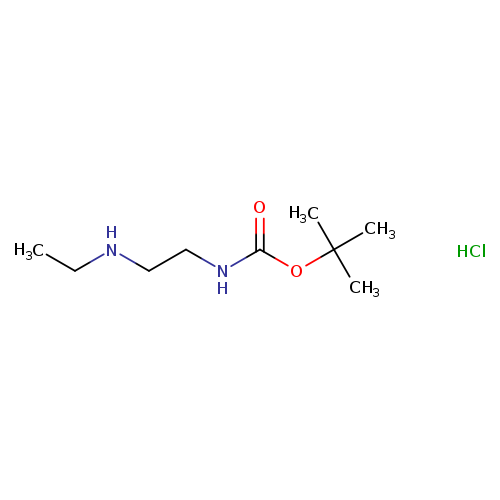
Boc-eda-et hclCatalog No.:AA008UY3 CAS No.:1073659-87-6 MDL No.:MFCD06796899 MF:C9H21ClN2O2 MW:224.7282 |
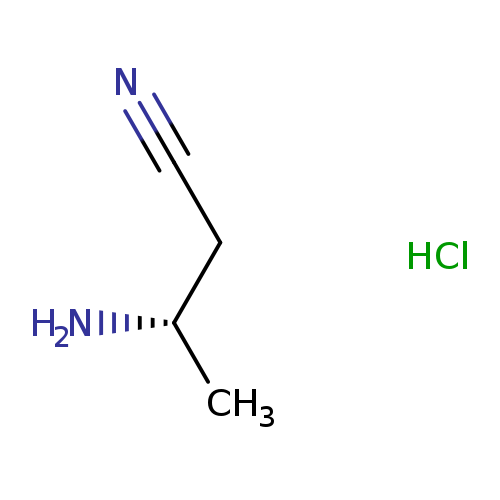
(S)-3-Aminobutanenitrile hydrochlorideCatalog No.:AA003A91 CAS No.:1073666-54-2 MDL No.:MFCD18651598 MF:C4H9ClN2 MW:120.5807 |
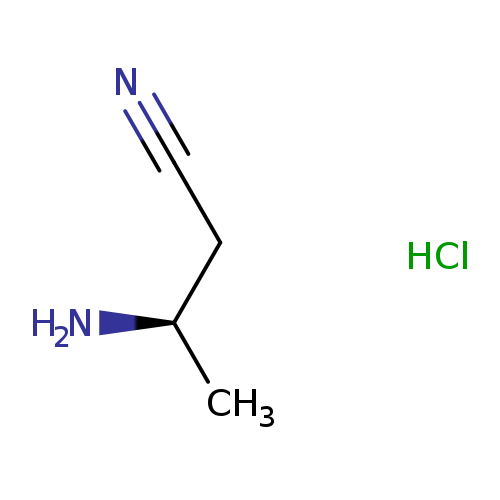
(R)-3-Aminobutanenitrile hclCatalog No.:AA009695 CAS No.:1073666-55-3 MDL No.:MFCD18651597 MF:C4H9ClN2 MW:120.5807 |
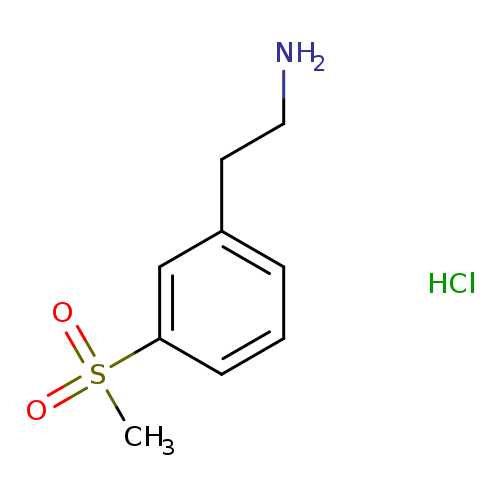
2-[3-(Methylsulfonyl)phenyl]ethylamine HydrochlorideCatalog No.:AA00HAX6 CAS No.:1073666-57-5 MDL No.:MFCD27986815 MF:C9H14ClNO2S MW:235.7310 |
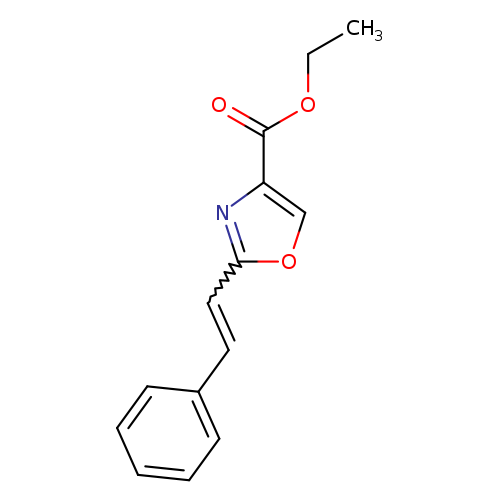
ethyl 2-(2-phenylethenyl)-1,3-oxazole-4-carboxylateCatalog No.:AA01ACPX CAS No.:107367-69-1 MDL No.:MFCD00793709 MF:C14H13NO3 MW:243.2579 |
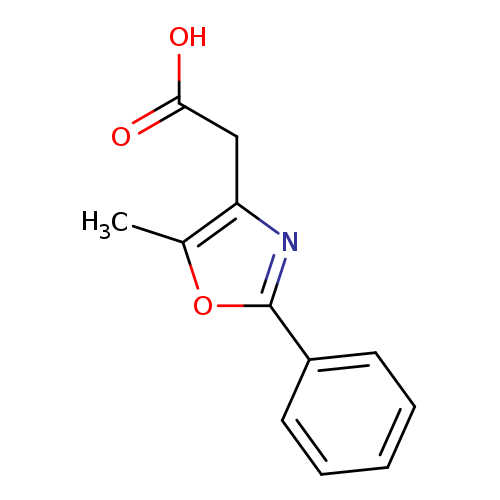
2-(5-Methyl-2-phenyl-1,3-oxazol-4-yl)acetic acidCatalog No.:AA007EKE CAS No.:107367-98-6 MDL No.:MFCD00100005 MF:C12H11NO3 MW:217.2206 |
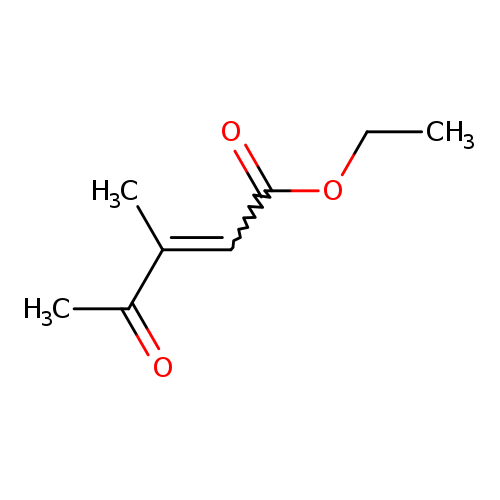
2-Pentenoic acid, 3-methyl-4-oxo-, ethyl ester, (Z)-Catalog No.:AA01B9LI CAS No.:107368-27-4 MDL No.:MFCD28383942 MF:C8H12O3 MW:156.1791 |
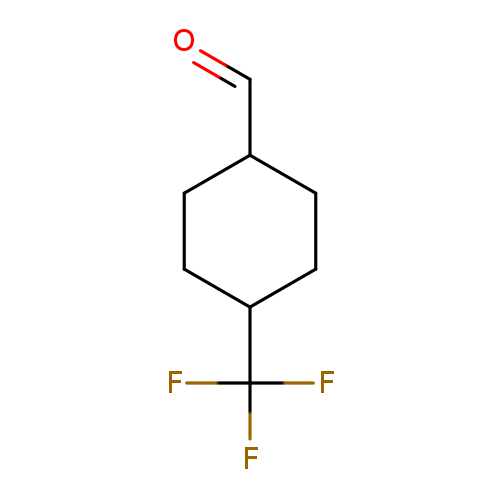
4-(trifluoromethyl)cyclohexane-1-carbaldehyde, Mixture of diastereomersCatalog No.:AA01ACIO CAS No.:1073722-40-3 MDL No.:MFCD21321762 MF:C8H11F3O MW:180.1675 |
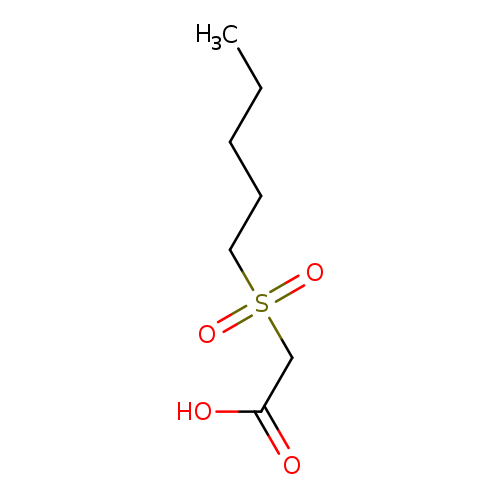
2-(pentane-1-sulfonyl)acetic acidCatalog No.:AA01AA6W CAS No.:107375-91-7 MDL No.:MFCD09940130 MF:C7H14O4S MW:194.2487 |
(R)-PropafenoneCatalog No.:AA008W4Q CAS No.:107381-31-7 MDL No.:MFCD00869669 MF:C21H27NO3 MW:341.4440 |
1-Propanone,1-[2-[(2S)-2-hydroxy-3-(propylamino)propoxy]phenyl]-3-phenyl-Catalog No.:AA0084MV CAS No.:107381-32-8 MDL No.:MFCD00869668 MF:C21H27NO3 MW:341.4440 |
1H-Pyrrolo[3,2-c]pyridine-6-carboxylic acid, ethyl esterCatalog No.:AA0095VV CAS No.:107384-68-9 MDL No.:MFCD20527767 MF:C11H11NO2 MW:189.2105 |