2020-01-17 14:31:12
Yu Feng1 Xingxing Teng1 Jinhua Gu1 Bangwei Yu Yan Luo1 Lianbao Ye1,
Received: 27 March 2018 / Accepted: 4 December 2018 / Published online: 14 December 2018
© Springer Science+Business Media, LLC, part of Springer Nature 2018
Introduction
Finding the effective and specific anti-tumor drug has epoch-making significance since malignant tumor is a serious threat to human health. There are plenty of studies on the treatment of cancer by chemotherapeutic agents that can act directly on DNA or interfere with the synthesis of DNAs to inhibit the proliferation and metastasis of tumor cells (Johnson 2000; Lee et al. 2014; Bellacosa et al. 2013). Tumor-targeting therapies have significantly changed cancer treatments, which depend on specific vectors to kill the tumor cells so as to improve the efficacy and reduce the toxic side effects on normal cells (Kircheis et al. 2002; Hofmeister et al. 2008). In the previous research, our group designed and synthesized a new compound 1 (Fig. 1) bearing active skeleton 2,3,4,5- tetrahydro-1H-pyrido-[4,3-b]indole, which showed high antiproliferative activity and c-Met inhibitory potency (Ye et al. 2012). Additionally, molecular docking studies showed that the main binding mode of 2,3,4,5-tetrahydro- 1H-pyrido-[4,3-b]indole with c-Met kinase site was the hydrophobic region (Ye et al. 2016). In an ongoing effort to discover novel anti-tumor agents, we were going to design and synthesize novel 2,3,4,5-tetrahydro-1H-pyr- ido-[4,3-b]indoles using compound 1 as the leading compound based on combination principles.
Related references reported that the sulfonamide group was the pharmacophore of some anti-tumor drugs. Natural products such as coumarins, in which a sulfonamide group was introduced, have antiproliferative activity (Bhat et al. 2006; Luo et al. 2001; Farahi et al. 2015; Kovác et al. 2001; Thaisrivongs et al. 1994; Dang et al. 2010; Chandak et al., 2016; Desai et al. 2014). The sul- fonamide group has substituted for structurally similar amino sulfonic esters, and has shown well anti- proliferative activity in vivo and in vitro according to the biological isostere principles. Additionally, we expected that bearing an alkyl or aralkyl in position N-5 would both be absorbed by various tissues quickly and increase the antiproliferative activity. Our group reported that a series of small molecule inhibitors of c-Met, which are prepared by sulfonic acids and natural products, inhibited the pro- liferation of many tumor cell lines excellently (Ye et al. 2017). It is hoped that the introduction of sulfonyl, alkyl, or aralkyl (Fig. 2) can increase the antiproliferative activity of 2,3,4,5-tetrahydro-1H-pyrido-[4,3-b]indole. Hopefully, it was quickly and effectively absorbed by the creased polarity and increased the lipid solubility.
In this study, all compounds synthesized were evaluated for their antiproliferative activity in vitro against Hela, A549, HepG2, and MCF-7 cells. In addition, molecular docking study simulated the interaction between small molecule ligands and receptor macromolecules at the molecular level. Molecular dynamics (MD) experiments were performed to evaluate the binding stabilities between the compounds and their receptors.
Material and methods
Unless otherwise noted, all the chemicals were obtained from Aladdin or J&K Scientific Ltd., China. The solvents were purified and dried based on standard procedures and stored over 3A molecular sieves. The reactions progress was determined by thin layer chromatography (TLC) ana- lysis on silica gel F254 plate (Merck). Chromatography purification was run on silica gel (200–300 mesh) from Qingdao Ocean Chemical (Qingdao, Shandong, China). 1H NMR and 13C NMR spectra were recorded on a Bruker Digital NMR Spectrometer, rep, δ (ppm), J in Hz, using tetramethylsilane (TMS) as an internal standard and CDCl3 or DMSO-d6 as solvent. The chemical structures of the target compounds were determined by Electron Impact Mass Spectra (EI-MS) using a Waters ZQ400 instrument. RPMI-1640 culture medium and new-born calf serum were purchased from Gibco (Grand Island, NY), and methyl thiazolyl tetrazolium (MTT) was purchased from Amresco (Solon, OH).
Synthesis of compounds
Synthesis of 2
4-Bromophenylhydrazine hydrochloride (224 mg, 1 mmol) and N-tert-butoxycarbonyl-4-piperidone (298.5 mg, 1.5 mmol) were dissolved in 10 ml ethanol solution and satu- rated hydrochloric acid, and the mixture was stirred at 95 °C for 8 h. The solution was filtered; the filtered cake was rinsed with anhydrous ethanol and dried in vacuo to give 2 (151 mg, 60%) as a white solid.
Synthesis of 3
The compound 2 (251 mg, 1 mmol) and di-tert butyl dicarbonate (262 mg, 1.2 mmol) were dissolved in dichlor- omethane (8 ml). Then N, N-disopropylamine (268 mg, 2 mmol) was added slowly under stirring. The solution was poured into H2O, and extracted with dichloromethane, then dried over anhydrous sodium sulfate, filtered, and evapo- rated. The crude product was purified by column chroma- tography (dichloromethane/methanol, 23/1) to give 3 (299 mg, 88.9%) as a faint yellow solid.
Synthesis of 4a
The compound 3 (351 mg, 1 mmol), (Bu4N)2SO4 (50 wt%, solution in H2O, 0.4 ml), and 6 N NaOH solution (0.32 ml) were dissolved in toluene (8 ml) under an ice bath and stirred for 15 min. Benzenesulfonyl chloride (176 mg, 1 mmol) was added slowly. The mixture was vigorously stirred for 2.5 h at r.t. under nitrogen. The solution was poured into H2O and ethyl acetate was added to the sus- pension, dried over anhydrous sodium sulfate, filtered, and evaporated. The crude product was purified by column chromatography (petroleum ether/ethyl acetate, 10/4), to afford the compound (393 mg, 78%) as a yellow solid.
Synthesis of 4b–4d
Compounds were prepared in analogy to 4a.
Synthesis of 4e
To a solution of 3 (351 mg, 1 mmol) in N,N-Dimethylfor- mamide (DMF) (8 ml) at 0 °C under N2, sodium hydride (60% weight dispersion in mineral oil) was added, the mixture was stirred at 0 °C for 30 min. Methyl iodide (284 mg, 2 mmol) was added, and the reaction mixture was stirred overnight at ambient temperature, quenched by adding a saturated solution of ammonium chloride. The mixture was extracted three times with equivalent dichloromethane and the combined extracts dried over sodium sulfate. After concentration in vacuum, the residue was purified by column chromatography (petroleum ether/ethyl acetate, 10:4) to obtain the product (223 mg, 61%) as a yellow solid.
Synthesis of 4f–4g
Compounds were prepared in analogy to 4e.
Cell assay
The anti-proliferative activities of compounds 4a–4g were evaluated against Hela, A549, HepG2, and MCF-7 cell lines using the standard MTT assay in vitro. The cancer cell lines were cultured in minimum essential medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 100 μg ml−1 penicillin. Approximately 1.0 × 104 cells ml−1 with 200 μl of DMEM medium were plated onto each well of a 96-well plate and incubated in 5% CO2 at 37 °C for 24 h. The target products at indicated final concentrations (0–800 μM) or 0.1% Dimethyl sulfoxide (DMSO) were added to the medium for 24 and 48 h. Fresh MTT (10 μl) was added to each well and at a terminal concentration of 5 μg ml−1 and incubated with the cells at 37 °C in dark for 4 h. The formazan crystals were dissolved in 100 μl of DMSO in each well and shaken for 15 min to dissolve it completely. The absorbance at 492 nm was measured with a Spec- traMAX190 micro plate reader (Molecular Devices, USA). All target products were tested three times in each of the cell lines. The results expressed as IC50 (inhibitory concentra- tion 50%) were the averages of three determinations and were calculated by using the GraphPad Prism 5 software.
Molecular docking
Molecular docking was performed according to the related references (Pirali et al. 2010; Huang et al. 2013; Ye et al. 2015). To further elucidate the binding mode of compounds in the tyrosine kinase ligand binding region, the CDOKER program which connected with Accelrys Discovery Studio 2.5.5 was used to simulate. CDOKER can offer all the advantages of full ligand flexibility (including bonds, angels, and dihedrals), the CHARMm family of force field, the flexibility of CHARMm engine, and reasonable com- putation times, which has an important advantage of introducing the soft-core potentials. In this work, we maintained the root-mean-square deviation (RMSD) value of the optimum pose less than 1Å of the related crystal pose and optimized the crystal structure of 3EFJ with polar hydrogen atoms and CHARMm force field, but not water. We set the radius of the input site sphere as 10 Å from the center of the binding site, and each ligand generated 20 random conformations. The ligands’ other parameters were default values. The optimized ligands were docked into the corresponding proteins active binding site according to the protocol. The structure of the receptor was minimized to 10,000 cycles using the Powell method in DS2.5.5. The convergence criterion was identified as 0.001 kcal mol−1.
MD simulations
MD is a general simulation technique that is included in many molecular modeling packages such as CHARMm and AMBER. Applying MD to evaluate the binding stabilities between all the compounds and the 3EFJ due to the possible binding mode, which was predicted by molecular docking studies between a ligand and receptor, may be not reason- able or stable (Tian et al. 2014; Yuan et al. 2014; Hou et al. 2012; Yan et al., 2016; He et al. 2010). In this work, we used AMBER 10.0 for ligands and AMBER ff03 for pro- teins on the basis of molecular docking results. The Gaus- sian 0.3 program was used to calculate partial atomic charges of the ligand by using the HF/6-31G* basis set and the Antechamber module was used to fit the restricted electrostatic potential (RESP).The simulations were per- formed at a neutral pH and the hydrogen bonds were con- strained using SHAKE algorithm. The residue-based cut-off 10 Å was used for nonbonded interaction and the time step was set to 2 fs. The G-quadruplex complex and inner ions were initially fixed with force constants of 100 kcal mol−1. The system was heated from 0 to 300 K in 100 ps with solutes constrained at a weak harmonic constraint of 10 kcal mol−1 and then equilibrated for 100 ps after all the minimization steps contained 2000 cycles of steepest des- cent minimization, followed by 2000 cycles of conjugated gradient minimization. Finally, periodic boundary dynamics simulations of 8 ns were carried out in an NPT ensemble at 1 atm and 300 K in the production step. The output trajec- tory files were saved every 2 ps for subsequent analysis.
Results and discussion
Chemistry
The synthesis of the key intermediate of tert-butyl-8-bromo- 2, 3,4,5-tetrahydro-1H-pyrido-[4,3-b]indole carboxylate was achieved according to previous reported general procedure using commercially available 4-bromophenylhydrazine hydrochloride and N-tert-butoxycarbonyl-4-piperidone as starting materials based on Fischer Indolizations (Fig. 3). Different benzene sulfonyl groups were added to the N of 9- position of tert-butyl-8-bromo-2,3,4,5-tetra-hydro-1H-pyr- ido-[4,3-b]indole carboxylate to obtain tert-butyl-8-bromo- 5-(phenyl-sulfonyl)-3,4-dihydro-1H-pyrido-[4,3-b]indole-2- carboxylate (4a), tert-butyl-8-bromo-5-tosyl-3,4-dihydro- 1H-pyrido-[4,3-b]indole-2-carboxylate (4b), tert-butyl-8- bromo-5-[(4-chlorophenyl)sulfonyl]-3,4-dihydro-1H-pyrido- [4,3-b]indole-2-carboxylate (4c), tert-butyl-8-bromo-5-[(4- nitro-phenyl)sulfonyl]-3,4-dihydro-1H-pyrido-[4,3-b]indole- 2-carboxylate (4d) via nucleophilic substitution with yields of 63%, 61%, 58%, and 52%, respectively. This reaction provided good yields using sodium hydroxide as a catalyst and (Bu4N)2SO4 as a phase transfer catalyst and the reaction was completed within 10 h due to the hydrolysis of sulfonyl chloride when it was treated with water. Benzene sul- fochloride substituted by electron donating group would obtain high yield. Furthermore, the compounds tert-butyl-8- bromo-5-methyl-3,4-dihydro-1H-pyrido-[4,3-b]indole-2-car- boxylate(4e), tert-butyl-8-bromo-5-benzyl-3,4-dihydro-1H- pyrido-[4,3-b]indole-2-carboxylate (4f), and tert-butyl-8- bromo-5-phenethyl-3,4-dihydro-1H-pyrido-[4,3-b]indole-2- carbo-xylate (4g) were synthesized with yields of 69%, 63%, 61%, respectively. Sodium hydride was used as a strong base to make a compound alkylated by deprotonation and N, N-dimethylformamide was used as a solvent.
Evaluation of the biological activity
The antiproliferative activity of all the target compounds was evaluated against Hela, A549, HepG2, and MCF-7 cell lines using the MTT assay in vitro.The results were repre- sented by IC50 values as shown in Table 1. The IC50 values were obtained by at least three independent trials. All compounds showed moderate to excellent antiproliferative activity with IC50 values between 0 μM and 100 μM against cancer cells as illustrated in Fig. 4. The proliferations of Hela, A549, HepG2, and MCF-7 cell lines were inhibited in a dose-dependent manner, and the cytolytic activity was markedly inhibited at the same time. Moreover, there was no significant difference between the inhibitory effects of the all compounds on the growth of the tumor cells for 24 h and 48 h; thus, there was no significant influence on the antiproliferation effect of compounds on Hela cells, A549 cells, HepG2 cells, and MCF-7 cells with an extended treatment time after 24 h. The IC50 values of intermediate 3 inhibited against Hela, A549, HepG2, and MCF-7 cell lines were 52.75, 50.30, 60.31, 54.39 μM, respectively, which were higher than the new compounds that we expected. The compounds 4a–4d bearing sulfonyl, which was substituted by the electron donating group, showed moderate to sig- nificant antiproliferative activity, in which compound 4c was the best with the IC50 values of 13.71, 9.42, 15.06, 14.77 μM, and these results suggested that the introduction of sulfonyl group could increase the antiproliferative activity of 2,3,4,5-tetrahydro-1H-pyrido-[4,3-b]indole. Compounds 4e–4g bearing alkyl, phenyl, and arylated alkyl groups produced good antiproliferative activity and the IC50 value of 4g was lower than 30 μM. In general, the target compounds were more potent against A549 compared to the other three cell lines. In addition, DMSO alone did not show obvious inhibitory effect compared to untreated cells. These results revealed that this series of compounds possessed selectivity for A549 cancer cell lines, and had the makings of good drugs for lung cancer. Based on the molecular docking, MD simulations, and preliminary activity tests, we could initially confirm that the target compounds might well repay investigation. In conclusion, the compounds synthe- sized by our experimental scheme had a good inhibitory effect on cancer cells, and they could be used as the leading compounds for the development of new anticancer inhibi- tors, and this study was of great significance to the estab- lishment of the chemical library and further research studies.
Molecular docking
In pre-experiment, we carried out the docking experiment by using several pdb proteins including 3DKF, 3EFJ, 3F82, 3WGJ, and 3RTO. Only 3EFJ interacted with compound 2; compound 3 and derivatives achieved good binding effect owing to the stable indole fused ring structure, so we chose it for the docking experiment. The 3EFJ is the crystal structure of c-Met kinase in complex with ATP and its information is seen from http://www.rcsb.org/pdb/explore/ explore.do?structureId=3EFJ.
Molecular docking suggested that these compounds were bound to the active site of the protein 3EFJ (shown in Fig. 5). Docking experiments were performed to elucidate the binding model of the strongest compounds 4c with c-Met kinase, as shown in Fig. 6. Compound 4c was docked into the binding site of c-Met kinase using CDOCKER (dock ligands into an active site using CHARMm) program con- ducted through Discovery Studio 2.5.5. The docking experiments suggested that compound 4c would dock strongly into the ATP-binding site of c-Met. The binding energies of complexes between all compounds and 3EFJ are shown in Table 2. The strong interaction of 4c with c-Met was attributed to two H-bonds between two oxygen atoms on the sulfonyl group and THR1257 and GLN1256; in addition, there was also a H-bond between the chlorine atom and hydroxyl of THR1293. The π–π stacking inter- action between the indole ring and the benzene sulfonyl would make the binding more firm between small 4c and 3EFJ. Moreover, 4c showed highest activity owing to the π– π stacking interaction between the pyrrole ring and phenyl of indole and PHE1223, H-bond between carbonyl oxygen and GLN1258. Thus, it was illustrated that the compounds 4a–4d, bearing a benzene ring substituted by the electron donating group, showed higher binding energies and com- pounds 4e–4g, bearing arylated alkyl, produced the highest activity.
MD simulations
Molecular docking studies were first carried out to predict the plausible interactions between all compounds and 3EFJ. On the basis of the docking results, MD simulation may provide information on rearrangement and transition states. The crystal structures of 3EFJ complex with compounds 2, 3, and 4a–4g were used to evaluate the reliability of MD simulations. As a result, the MD models appeared to reach a stable state after 1 ns equilibration and the RMSD values converged below 2.5 Å, especially the binding state between 4c and 3EFJ was relatively stable and the trajec- tories were smooth (Fig. 7). The MD parameters we chose were appropriate for the MD simulations.
Most compounds gave stable RMSD curves during their simulations (shown in Fig. 8). The binding free energies ΔGpred were computed by means of MM-PBSA inside the Amber 10 program, where values more negative than −20 kcal mol−1 were selected to assess the inhibitory activity for 3EFJ. The predicted ΔGpred values of all compounds are listed in Table 3.
MM-PBSA estimation of binding free energy ΔGpred
For each system, the values were calculated using 100 snapshots recorded from the last with 1 ns trajectory at an interval of 10 ps by Molecular Mechanics Poisson– Boltzmann Surface Area method.
4-Chloro-3-methoxy-2-methylpyridineCatalog No.:AA007EDT CAS No.:107512-34-5 MDL No.:MFCD16610605 MF:C7H8ClNO MW:157.5975 |
6-Ethoxycarbonylindole-2-carboxylic acid ethyl esterCatalog No.:AA003PCD CAS No.:107516-75-6 MDL No.:MFCD03411566 MF:C14H15NO4 MW:261.2732 |
1-(chloromethyl)-2,6-dimethylnaphthaleneCatalog No.:AA01BRYR CAS No.:107517-28-2 MDL No.:MFCD24459529 MF:C13H13Cl MW:204.6953 |
COMUCatalog No.:AA0034JT CAS No.:1075198-30-9 MDL No.:MFCD11975052 MF:C12H19F6N4O4P++ MW:428.2678 |
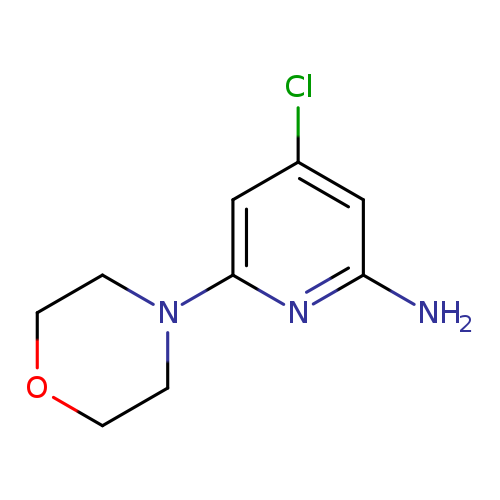
2-Amino-4-chloro-6-morpholinopyridineCatalog No.:AA00HAXI CAS No.:1075215-73-4 MDL No.:MFCD24624378 MF:C9H12ClN3O MW:213.6641 |
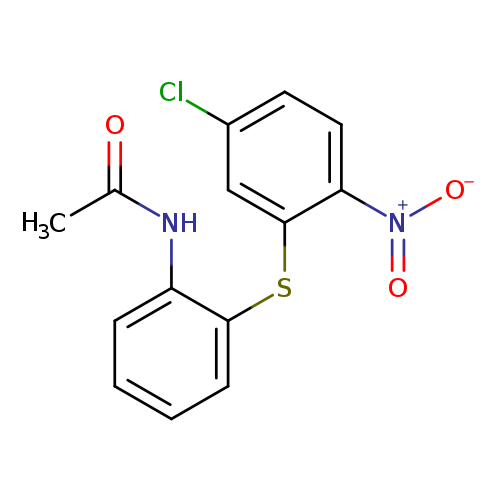
2-Acetamidophenyl 5-chloro-2-nitrophenyl sulfideCatalog No.:AA008SQW CAS No.:107522-19-0 MDL No.: MF:C14H11ClN2O3S MW:322.7667 |
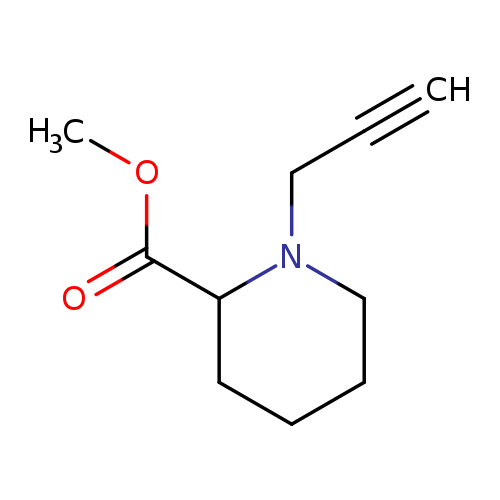
methyl 1-(prop-2-yn-1-yl)piperidine-2-carboxylateCatalog No.:AA01ABBF CAS No.:1075220-87-9 MDL No.:MFCD17977138 MF:C10H15NO2 MW:181.2316 |
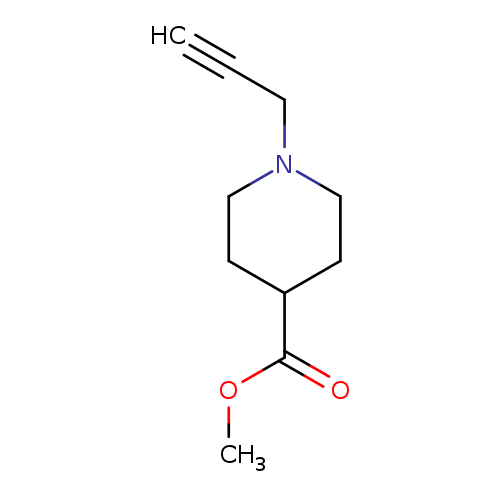
methyl 1-(prop-2-yn-1-yl)piperidine-4-carboxylateCatalog No.:AA01AFP5 CAS No.:1075221-06-5 MDL No.:MFCD14631441 MF:C10H15NO2 MW:181.2316 |
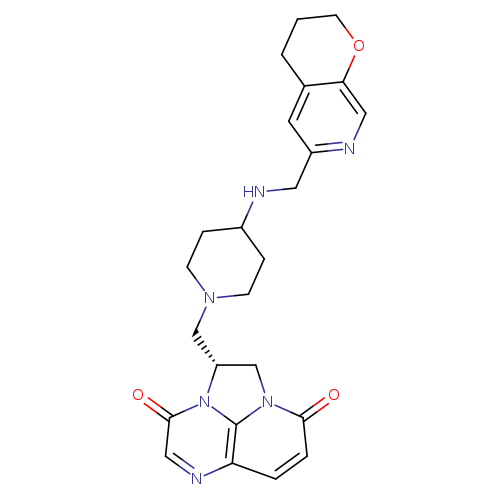
GepotidacinCatalog No.:AA01EOPM CAS No.:1075236-89-3 MDL No.:MFCD27987926 MF:C24H28N6O3 MW:448.5175 |
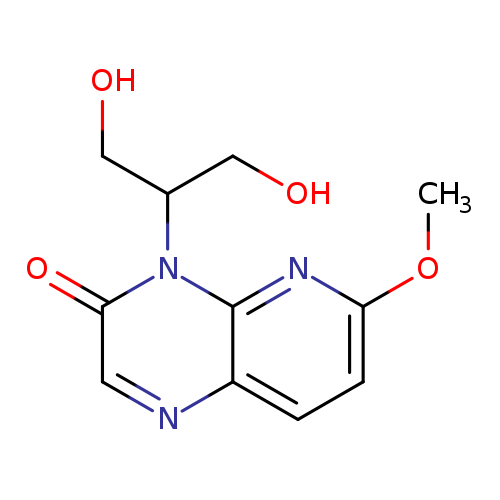
4-(1,3-Dihydroxypropan-2-yl)-6-methoxypyrido[2,3-b]pyrazin-3(4h)-oneCatalog No.:AA008TM6 CAS No.:1075237-97-6 MDL No.:MFCD28991959 MF:C11H13N3O4 MW:251.2386 |
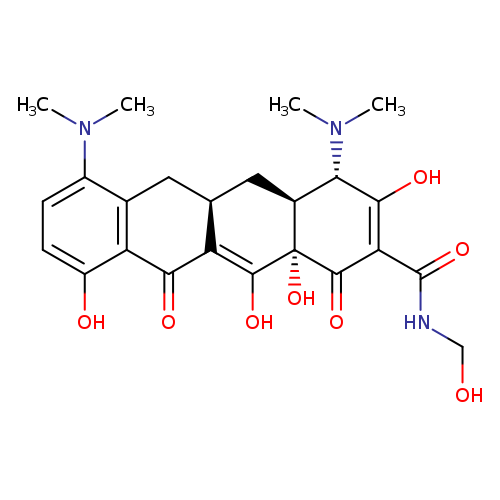
N-MethylolMinocyclineCatalog No.:AA01DZFZ CAS No.:1075240-33-3 MDL No.: MF:C24H29N3O8 MW:487.5024 |
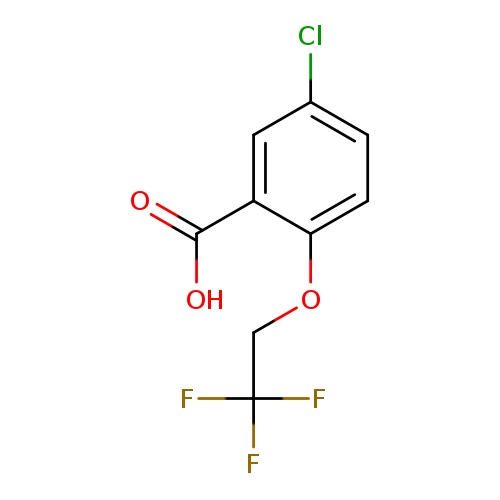
5-Chloro-2-(2,2,2-trifluoroethoxy)benzoic acidCatalog No.:AA00976F CAS No.:1075243-52-5 MDL No.:MFCD11180627 MF:C9H6ClF3O3 MW:254.5903 |

Ergoline-8β-carboxylic Acid Methyl Ester HydrochlorideCatalog No.:AA00961P CAS No.:1075250-77-9 MDL No.: MF: MW: |
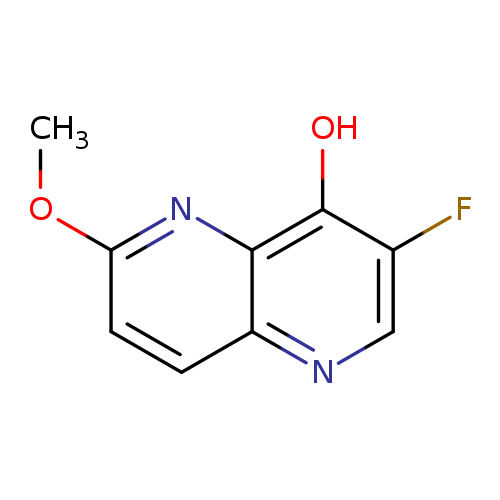
3-Fluoro-6-methoxy-1,5-naphthyridin-4-olCatalog No.:AA00941I CAS No.:1075259-77-6 MDL No.:MFCD19440497 MF:C9H7FN2O2 MW:194.1625 |
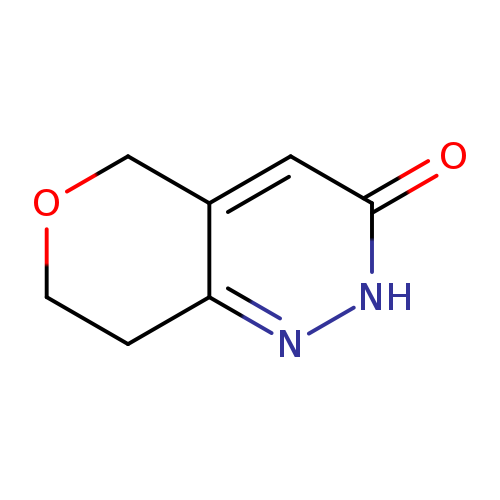
2H,3H,5H,7H,8H-Pyrano[4,3-c]pyridazin-3-oneCatalog No.:AA0093I7 CAS No.:1075260-60-4 MDL No.:MFCD16871374 MF:C7H8N2O2 MW:152.1506 |
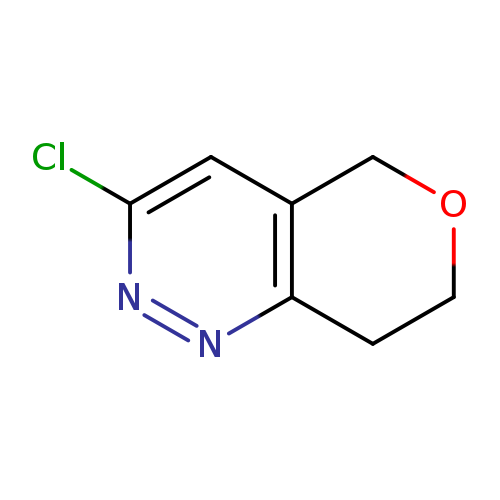
3-chloro-5H,7H,8H-pyrano[4,3-c]pyridazineCatalog No.:AA01C1OQ CAS No.:1075260-61-5 MDL No.:MFCD16871395 MF:C7H7ClN2O MW:170.5963 |
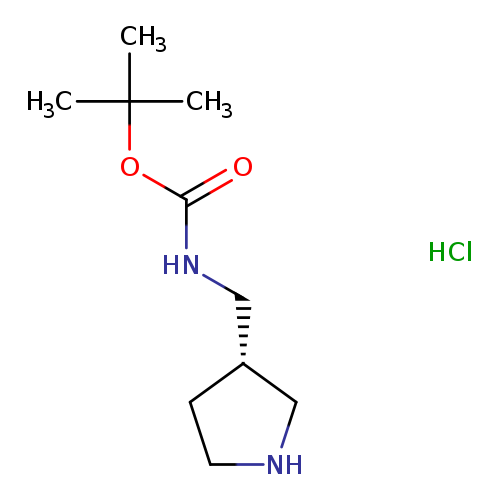
S-3-(Boc-aminomethyl)-pyrrolidine-hclCatalog No.:AA008Y9R CAS No.:1075260-66-0 MDL No.:MFCD11101394 MF:C10H21ClN2O2 MW:236.7389 |
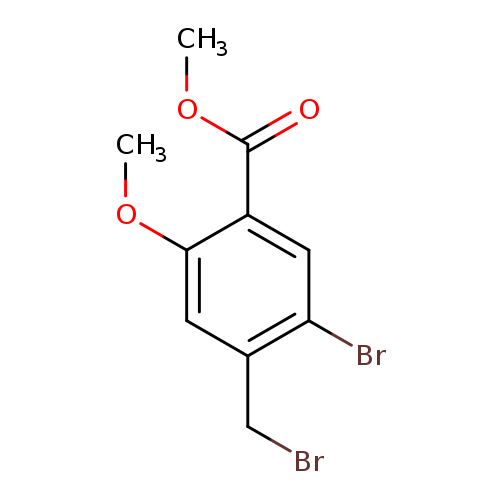
methyl 5-bromo-4-(bromomethyl)-2-methoxybenzoateCatalog No.:AA01BKCC CAS No.:1075281-18-3 MDL No.:MFCD25459843 MF:C10H10Br2O3 MW:337.9926 |
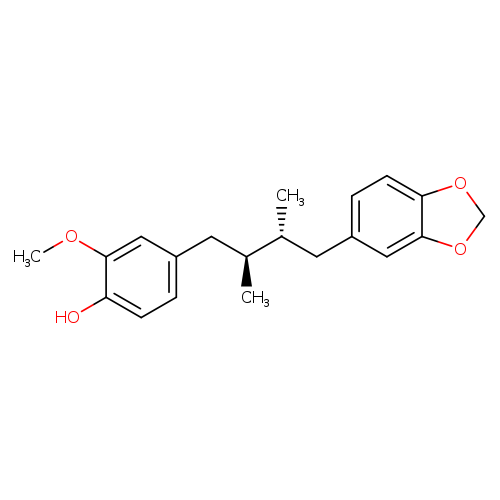
AnwulignanCatalog No.:AA008TJP CAS No.:107534-93-0 MDL No.:MFCD09953803 MF:C20H24O4 MW:328.4022 |
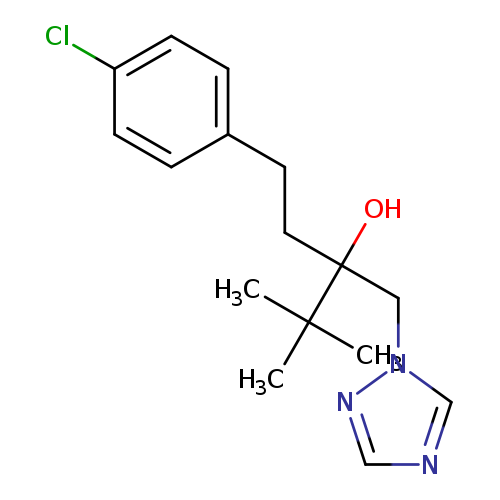
1-(4-chlorophenyl)-4,4-dimethyl-3-(1H-1,2,4-triazol-1-ylmethyl)pentan-3-olCatalog No.:AA0039NO CAS No.:107534-96-3 MDL No.:MFCD02674797 MF:C16H22ClN3O MW:307.8184 |
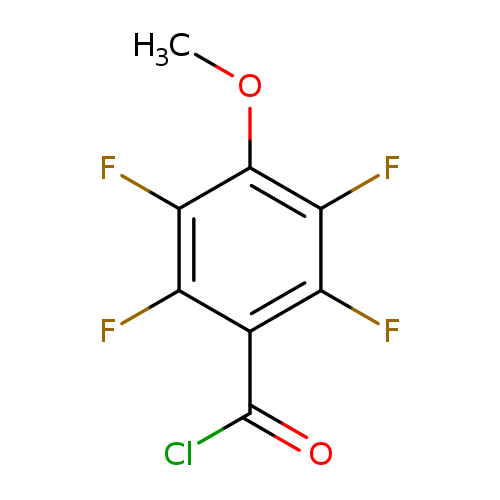
"2,3,5,6-tetrafluoro-4-methoxybenzoyl chloride"Catalog No.:AA01FP7Y CAS No.:107535-74-0 MDL No.:MFCD05819502 MF:C8H3ClF4O2 MW:242.5548 |
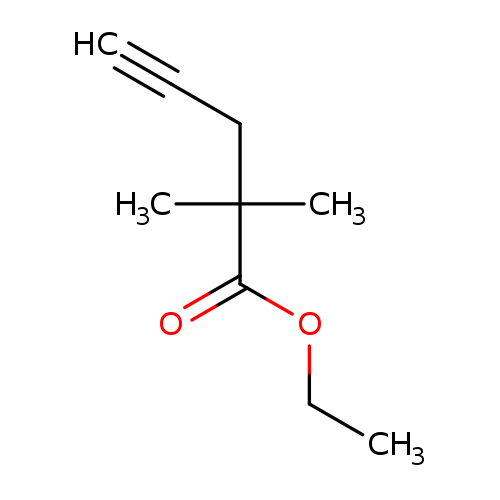
ETHYL 2,2-DIMETHYLPENT-4-YNOATECatalog No.:AA01DUVJ CAS No.:107540-02-3 MDL No.:MFCD22023966 MF:C9H14O2 MW:154.2063 |
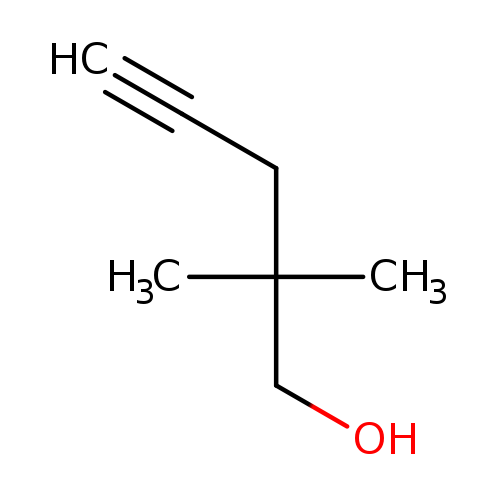
2,2-Dimethylpent-4-yn-1-olCatalog No.:AA00HAXP CAS No.:107540-03-4 MDL No.:MFCD19441316 MF:C7H12O MW:112.1696 |
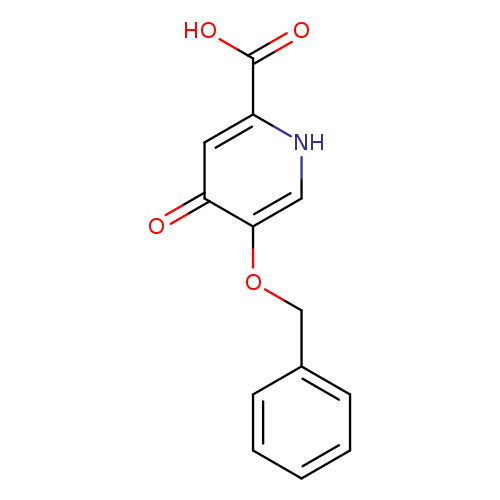
5-(Benzyloxy)-4-oxo-1,4-dihydropyridine-2-carboxylic acidCatalog No.:AA0092XX CAS No.:107550-30-1 MDL No.:MFCD18446980 MF:C13H11NO4 MW:245.2307 |
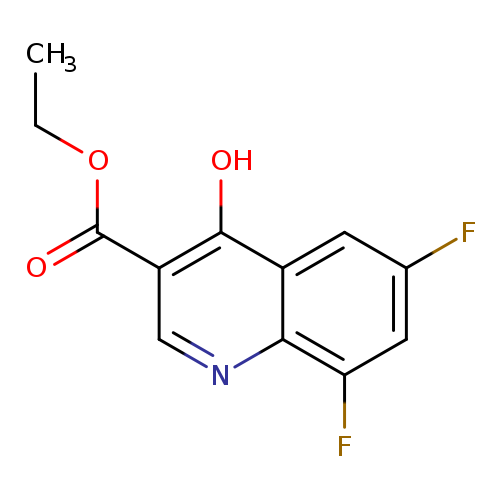
Ethyl 6,8-difluoro-4-hydroxyquinoline-3-carboxylateCatalog No.:AA003QAC CAS No.:107555-38-4 MDL No.:MFCD00173349 MF:C12H9F2NO3 MW:253.2016 |
Methyl 3-(4-nitrophenyl)benzoateCatalog No.:AA0084IR CAS No.:107558-26-9 MDL No.:MFCD20529487 MF:C14H11NO4 MW:257.2414 |
4-(4-Pyridylmethyl)-1(2h)-phthalazinoneCatalog No.:AA003K7G CAS No.:107558-48-5 MDL No.:MFCD00443664 MF:C14H11N3O MW:237.2566 |
4-Fluoro-2-methoxy-5-nitroanilineCatalog No.:AA0039KD CAS No.:1075705-01-9 MDL No.:MFCD23098941 MF:C7H7FN2O3 MW:186.1405 |
2-(3-ethylphenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolaneCatalog No.:AA00HAXS CAS No.:1075719-83-3 MDL No.:MFCD18734472 MF:C14H21BO2 MW:232.1263 |
2-(4-ethylphenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolaneCatalog No.:AA008SDG CAS No.:1075719-87-7 MDL No.:MFCD18643353 MF:C14H21BO2 MW:232.1263 |
2-(4-Chlorophenylthio)benzaldehydeCatalog No.:AA003ET4 CAS No.:107572-07-6 MDL No.:MFCD00051766 MF:C13H9ClOS MW:248.7280 |

AtracuriuM IMpurity VCatalog No.:AA0091SV CAS No.:1075726-86-1 MDL No.: MF:C25H34INO6 MW:571.4450 |
5-Boc-1-phenyl-1,4,6,7-tetrahydropyrazolo[4,3-c]pyridineCatalog No.:AA00923W CAS No.:1075729-08-6 MDL No.:MFCD12406149 MF:C17H21N3O2 MW:299.3675 |
1-Phenyl-4,5,6,7-tetrahydro-1H-pyrazolo[4,3-c]pyridine hydrochlorideCatalog No.:AA007EAV CAS No.:1075729-10-0 MDL No.:MFCD11867369 MF:C12H14ClN3 MW:235.7127 |
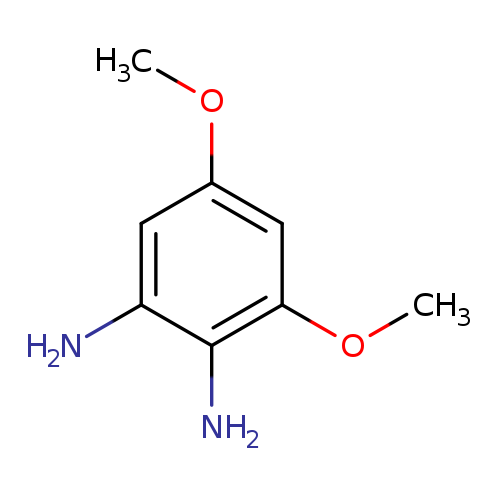
3,5-dimethoxybenzene-1,2-diamineCatalog No.:AA01A50K CAS No.:1075748-44-5 MDL No.:MFCD20702380 MF:C8H12N2O2 MW:168.1931 |
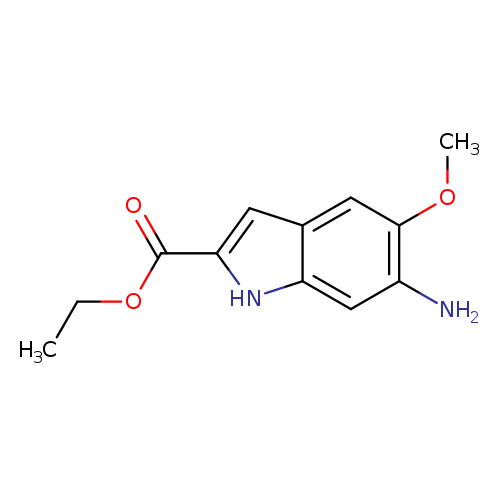
Ethyl 6-amino-5-methoxyindole-2-carboxylateCatalog No.:AA003QAE CAS No.:107575-60-0 MDL No.:MFCD05864806 MF:C12H14N2O3 MW:234.2512 |
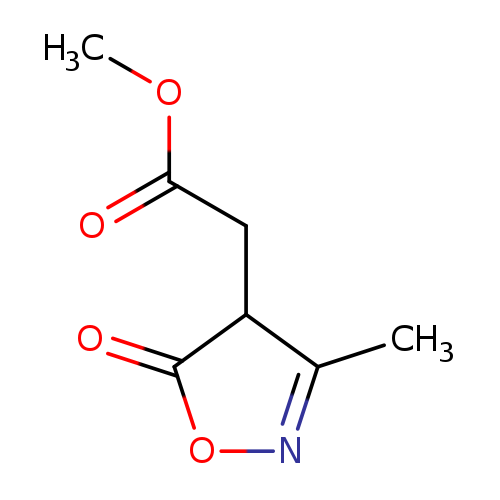
methyl 2-(3-methyl-5-oxo-4,5-dihydro-1,2-oxazol-4-yl)acetateCatalog No.:AA01CA8U CAS No.:107575-76-8 MDL No.:MFCD14653399 MF:C7H9NO4 MW:171.1507 |
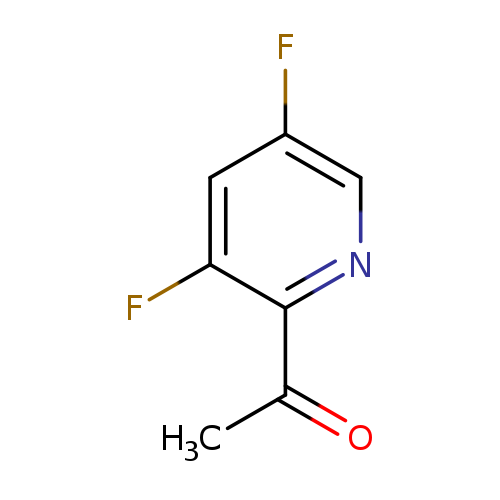
1-(3,5-Difluoro-2-pyridinyl)-ethanoneCatalog No.:AA00940F CAS No.:1075756-90-9 MDL No.:MFCD18711444 MF:C7H5F2NO MW:157.1175 |
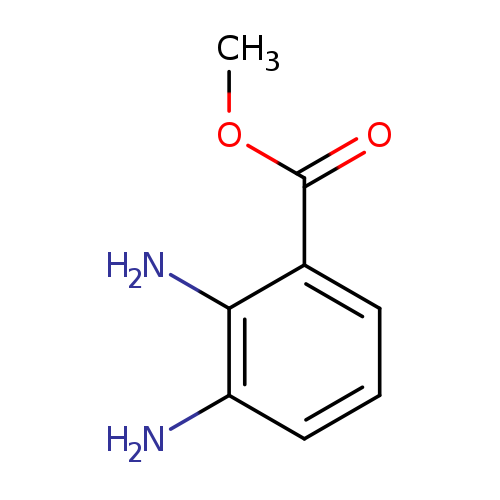
Methyl 2,3-diaminobenzoateCatalog No.:AA00352T CAS No.:107582-20-7 MDL No.:MFCD04038589 MF:C8H10N2O2 MW:166.1772 |
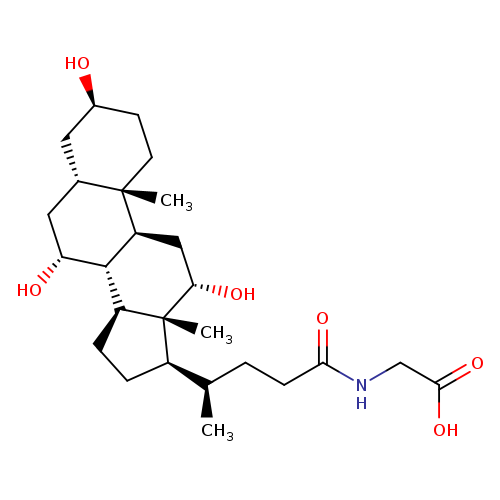
3β-GlycocholicAcidCatalog No.:AA008WAB CAS No.:107589-98-0 MDL No.: MF:C26H43NO6 MW:465.6227 |
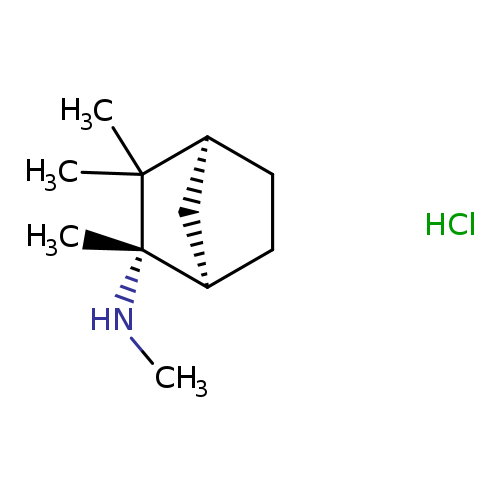
S-(+)-MecaMylaMine HydrochlorideCatalog No.:AA008W3N CAS No.:107596-30-5 MDL No.:MFCD19443650 MF:C11H22ClN MW:203.7521 |
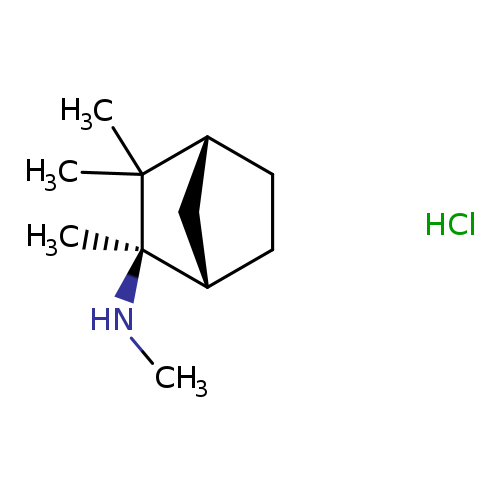
R-(-)-MecaMylaMine HydrochlorideCatalog No.:AA008VO9 CAS No.:107596-31-6 MDL No.:MFCD31562283 MF:C11H22ClN MW:203.7521 |
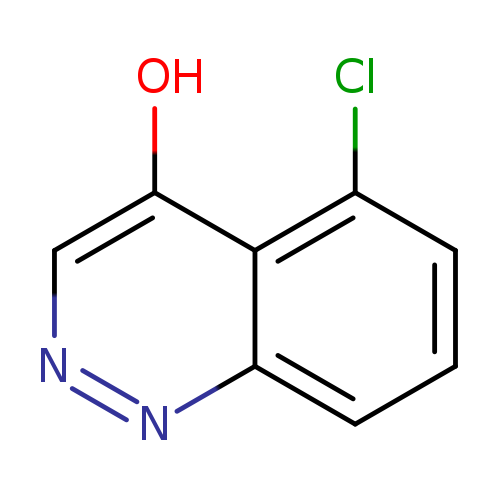
5-Chlorocinnolin-4-olCatalog No.:AA00M5GH CAS No.:1076-16-0 MDL No.:MFCD00956138 MF:C8H5ClN2O MW:180.5911 |
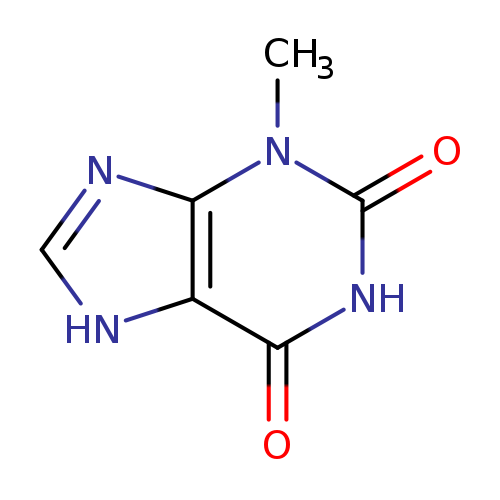
3-MethylxanthineCatalog No.:AA0033OW CAS No.:1076-22-8 MDL No.:MFCD00005580 MF:C6H6N4O2 MW:166.1374 |
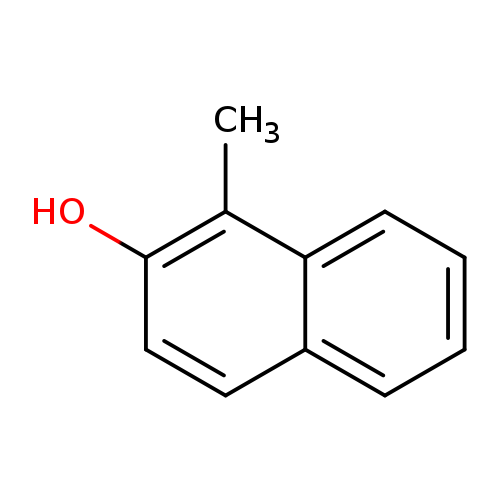
1-Methylnaphthalen-2-olCatalog No.:AA008SEO CAS No.:1076-26-2 MDL No.:MFCD00029266 MF:C11H10O MW:158.1965 |
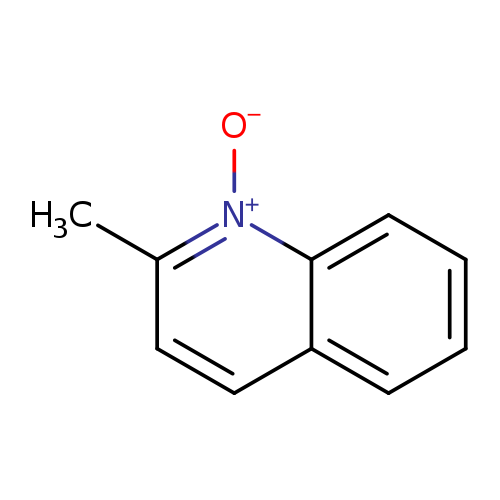
2-Methylquinoline 1-oxideCatalog No.:AA009QTO CAS No.:1076-28-4 MDL No.:MFCD01793857 MF:C10H9NO MW:159.1846 |
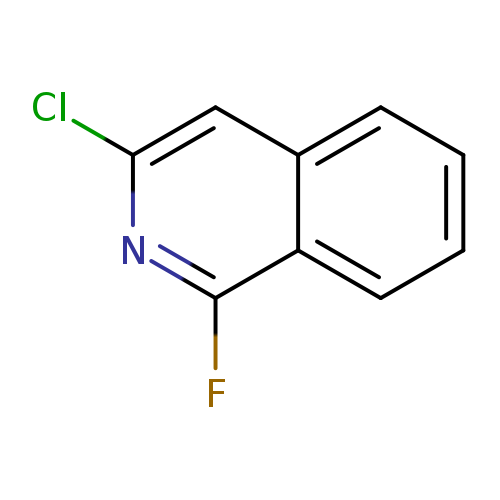
3-chloro-1-fluoroisoquinolineCatalog No.:AA01BIXN CAS No.:1076-37-5 MDL No.:MFCD28668858 MF:C9H5ClFN MW:181.5941 |
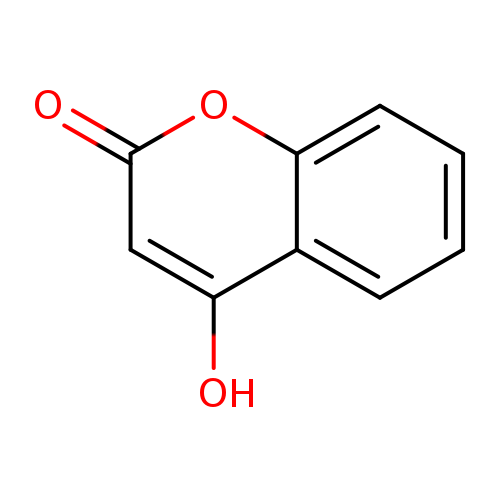
4-HydroxycoumarinCatalog No.:AA0033ZG CAS No.:1076-38-6 MDL No.:MFCD00006856 MF:C9H6O3 MW:162.1421 |
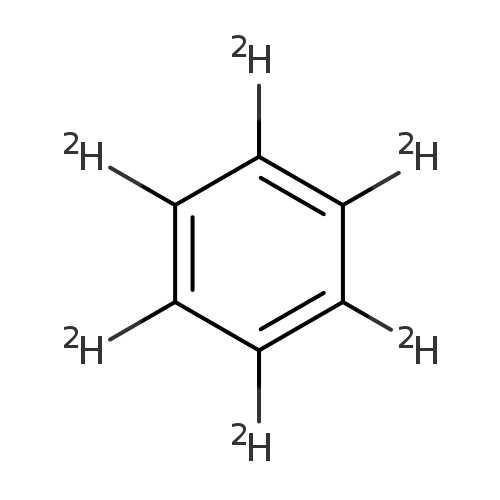
Benzene-d6Catalog No.:AA0034EW CAS No.:1076-43-3 MDL No.:MFCD00003010 MF:C6D6 MW:84.1488 |
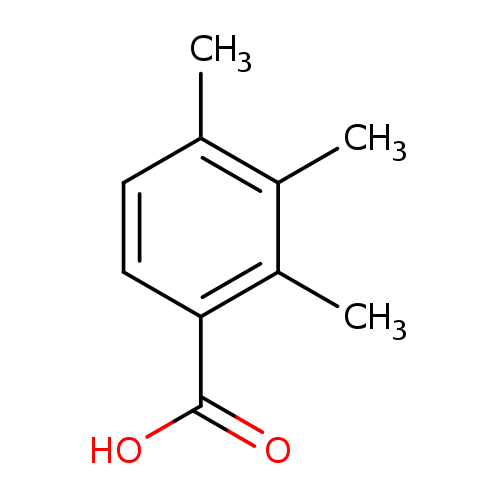
2,3,4-Trimethylbenzoic acidCatalog No.:AA0094OS CAS No.:1076-47-7 MDL No.:MFCD20638198 MF:C10H12O2 MW:164.2011 |
(S)-3-Amino-2-phenylpropanoic acidCatalog No.:AA00920Q CAS No.:1076-51-3 MDL No.:MFCD18782982 MF:C9H11NO2 MW:165.1891 |
Benzene,2-methoxy-4-methyl-1-(1-methylethyl)-Catalog No.:AA007VR8 CAS No.:1076-56-8 MDL No.:MFCD19441453 MF:C12H20O2 MW:196.2860 |
3-Phenyl-5-isoxazoloneCatalog No.:AA003JSR CAS No.:1076-59-1 MDL No.:MFCD00003158 MF:C9H7NO2 MW:161.1574 |
1-Phenyl-1H-pyrazol-4-olCatalog No.:AA009QD0 CAS No.:1076-60-4 MDL No.:MFCD00462161 MF:C9H8N2O MW:160.1726 |
Naphthalen-2-ylmethanethiolCatalog No.:AA007DTW CAS No.:1076-67-1 MDL No.:MFCD00155255 MF:C11H10S MW:174.2621 |
5-Methoxy-2-methylindoleCatalog No.:AA007VQQ CAS No.:1076-74-0 MDL No.:MFCD00005620 MF:C10H11NO MW:161.2004 |
Methyl 4-vinylbenzoateCatalog No.:AA003RTH CAS No.:1076-96-6 MDL No.:MFCD00017218 MF:C10H10O2 MW:162.1852 |
1,4-Cyclohexanedicarboxylic acidCatalog No.:AA0035YL CAS No.:1076-97-7 MDL No.:MFCD00001465 MF:C8H12O4 MW:172.1785 |
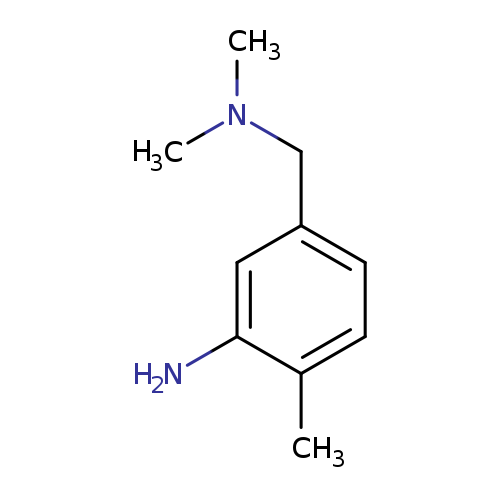
5-[(dimethylamino)methyl]-2-methylanilineCatalog No.:AA019U09 CAS No.:107600-25-9 MDL No.:MFCD10021452 MF:C10H16N2 MW:164.2474 |
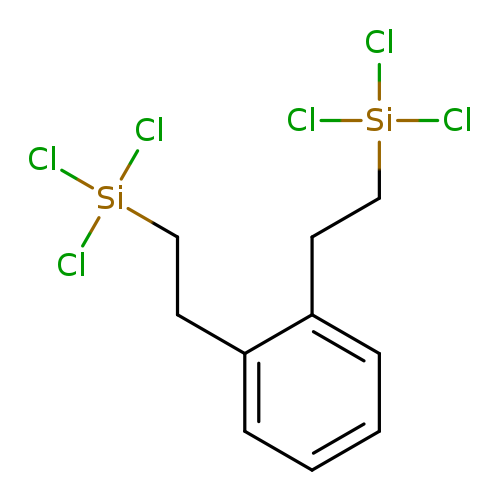
BIS(TRICHLOROSILYLETHYL)BENZENE,TECH-95Catalog No.:AA008ZPU CAS No.:107602-27-7 MDL No.:MFCD00054194 MF:C10H12Cl6Si2 MW:401.0913 |
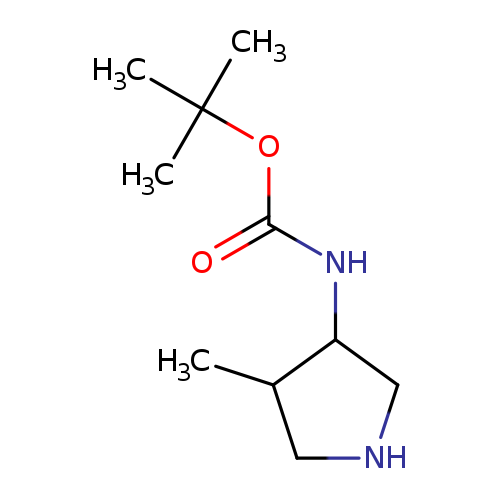
3-N-BOC-AMINO-4-METHYL-PYRROLIDINECatalog No.:AA008TSZ CAS No.:107610-92-4 MDL No.:MFCD08461138 MF:C10H20N2O2 MW:200.2780 |
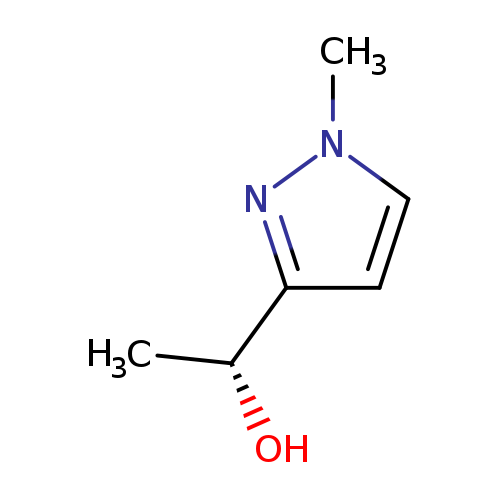
(1R)-1-(1-methyl-1H-pyrazol-3-yl)ethan-1-olCatalog No.:AA01C5JB CAS No.:1076195-50-0 MDL No.:MFCD21131937 MF:C6H10N2O MW:126.1564 |
2-(4-Aminophenoxy)-n,n,n-trimethylethanaminium bromide hydrobromideCatalog No.:AA0097C8 CAS No.:1076196-38-7 MDL No.:MFCD22580414 MF:C11H20Br2N2O MW:356.0973 |
Fmoc-3-amino-6-methyl-1-carboxymethyl-pyridin-2-oneCatalog No.:AA00HAXZ CAS No.:1076196-99-0 MDL No.:MFCD04974228 MF:C23H20N2O5 MW:404.4153 |
Fmoc-3-amino-2,2-dimethyl-propionic acidCatalog No.:AA00HAY0 CAS No.:1076197-00-6 MDL No.:MFCD09952626 MF:C20H21NO4 MW:339.3850 |
(R,S)-Boc-3-amino-2-(naphthalen-1-ylmethyl)-propionic acidCatalog No.:AA00HAY3 CAS No.:1076197-03-9 MDL No.:MFCD11226810 MF:C19H23NO4 MW:329.3902 |
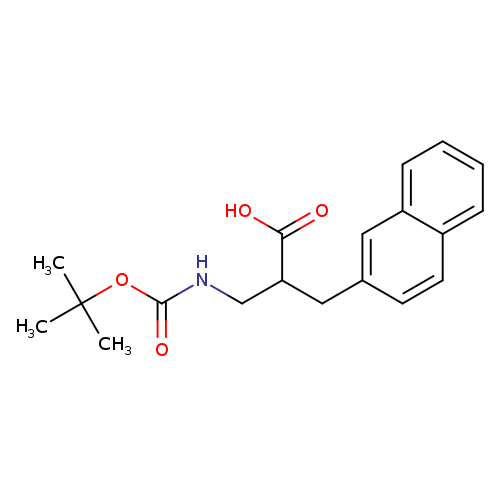
(R,S)-Boc-3-amino-2-(naphthalen-2-ylmethyl)-propionic acidCatalog No.:AA00HAY4 CAS No.:1076197-04-0 MDL No.:MFCD11226811 MF:C19H23NO4 MW:329.3902 |
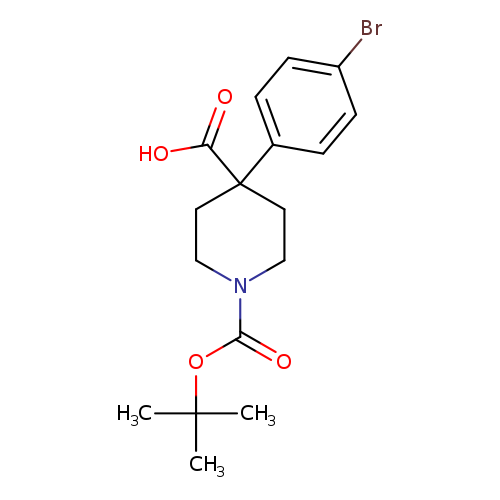
4-(4-Bromophenyl)-1-tert-butoxycarbonyl-piperidine-4-carboxylic acidCatalog No.:AA008U63 CAS No.:1076197-05-1 MDL No.:MFCD11226814 MF:C17H22BrNO4 MW:384.2649 |
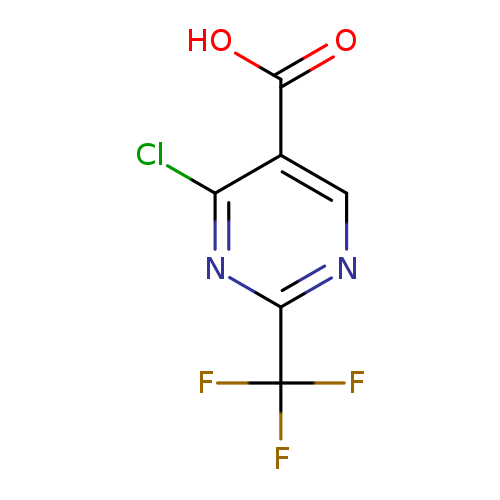
4-Chloro-2-(trifluoromethyl)pyrimidine-5-carboxylic acidCatalog No.:AA007W21 CAS No.:1076197-55-1 MDL No.:MFCD11558920 MF:C6H2ClF3N2O2 MW:226.5405 |
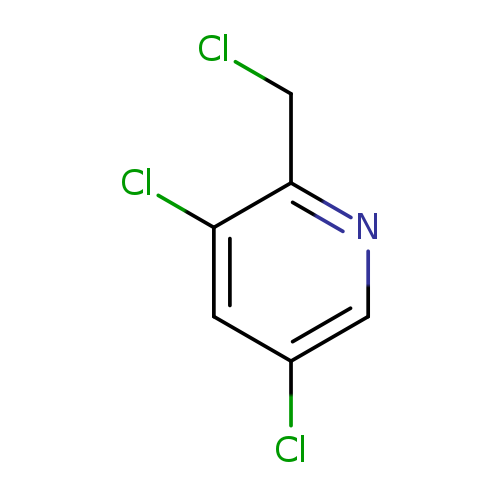
3,5-dichloro-pyridin-2-ylMethyl chlorideCatalog No.:AA0095AG CAS No.:1076197-56-2 MDL No.:MFCD11558921 MF:C6H4Cl3N MW:196.4617 |
2-Fluoro-5-methoxybenzyl chlorideCatalog No.:AA008ST8 CAS No.:1076197-70-0 MDL No.:MFCD09038458 MF:C8H8ClFO MW:174.5999 |
Methyl 4,5,6,7-tetrahydro-1H-indazole-5-carboxylateCatalog No.:AA00HAY8 CAS No.:1076197-88-0 MDL No.:MFCD11111582 MF:C9H12N2O2 MW:180.2038 |
6-Chloro-1-methyl-1H-pyrazolo[3,4-b]pyridin-3-amineCatalog No.:AA007W1Z CAS No.:1076197-93-7 MDL No.:MFCD11109857 MF:C7H7ClN4 MW:182.6103 |
2-(1-((3-Oxo-2-azaspiro[4.5]decan-2-yl)methyl)cyclohexyl)acetic acidCatalog No.:AA007VTA CAS No.:1076198-17-8 MDL No.:MFCD09952210 MF:C18H29NO3 MW:307.4278 |
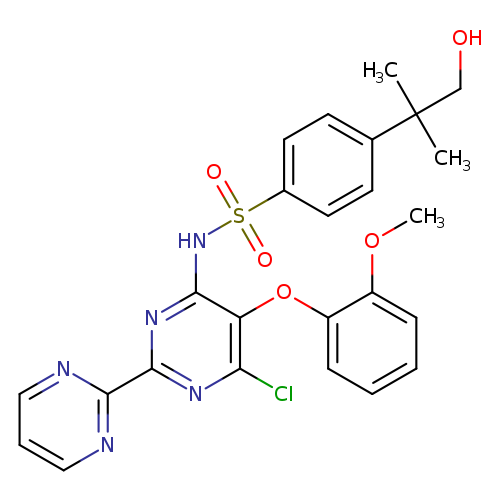
N-(6-Chloro-5-(2-methoxyphenoxy)-[2,2'-bipyrimidin]-4-yl)-4-(1-hydroxy-2-methylpropan-2-yl)benzenesulfonamideCatalog No.:AA008WT5 CAS No.:1076198-22-5 MDL No.:MFCD09952220 MF:C25H24ClN5O5S MW:542.0066 |
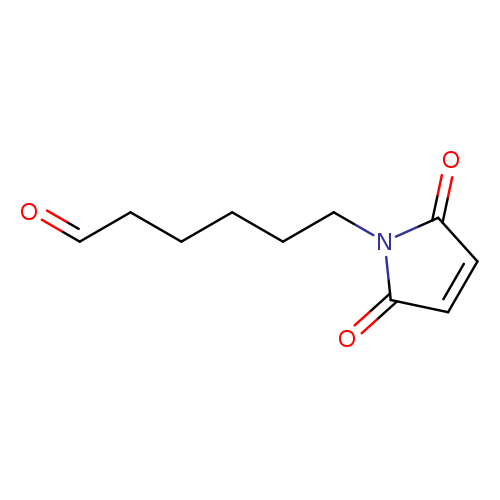
6-MALEIMIDO-1-HEXANALCatalog No.:AA0084ID CAS No.:1076198-37-2 MDL No.:MFCD09753612 MF:C10H13NO3 MW:195.2151 |
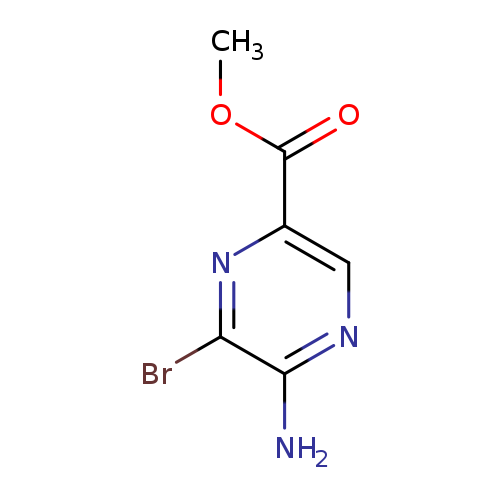
Methyl 5-amino-6-bromopyrazine-2-carboxylateCatalog No.:AA008TP0 CAS No.:1076198-49-6 MDL No.:MFCD09840911 MF:C6H6BrN3O2 MW:232.0347 |
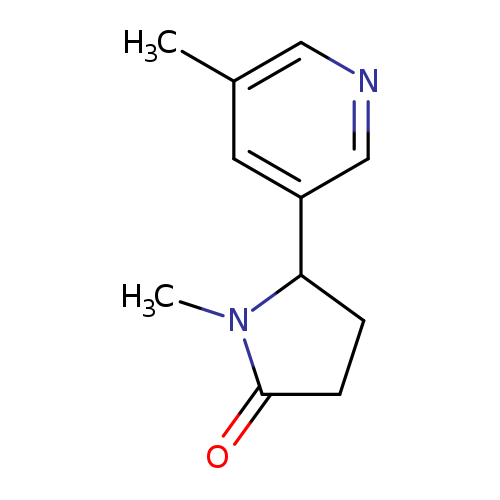
1-Methyl-5-(5-Methyl-3-pyridinyl)-2-pyrrolidinoneCatalog No.:AA008WWR CAS No.:1076198-50-9 MDL No.:MFCD09864793 MF:C11H14N2O MW:190.2417 |
Ondansetron Impurity BCatalog No.:AA008X27 CAS No.:1076198-52-1 MDL No.:MFCD25974002 MF:C37H38N6O2 MW:598.7366 |
2-[Methyl-1-(4-methoxyphenyl)methoxy]propyl-4'-nitrophenyl CarbonateCatalog No.:AA007VT6 CAS No.:1076198-54-3 MDL No.: MF:C19H21NO7 MW:375.3725 |
6-Methyl-3-pyridoyl-2-pyrrolidinoneCatalog No.:AA007E74 CAS No.:1076198-57-6 MDL No.:MFCD09841002 MF:C11H12N2O2 MW:204.2252 |
(4Z)-Mycophenolate Mofetil (EP Impurity C)Catalog No.:AA009611 CAS No.:1076198-64-5 MDL No.: MF:C23H31NO7 MW:433.4947 |
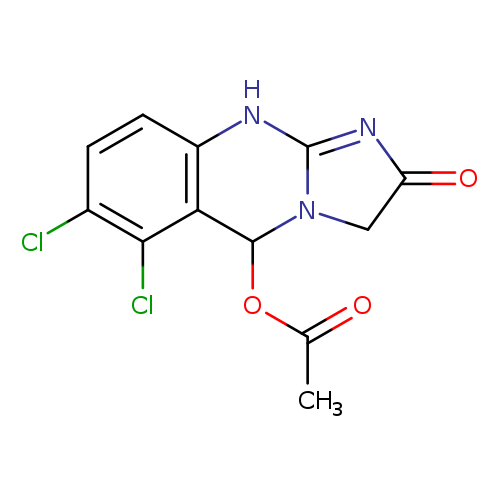
5-ACETOXY ANAGRELIDECatalog No.:AA008VUH CAS No.:1076198-71-4 MDL No.:MFCD09839816 MF:C12H9Cl2N3O3 MW:314.1242 |
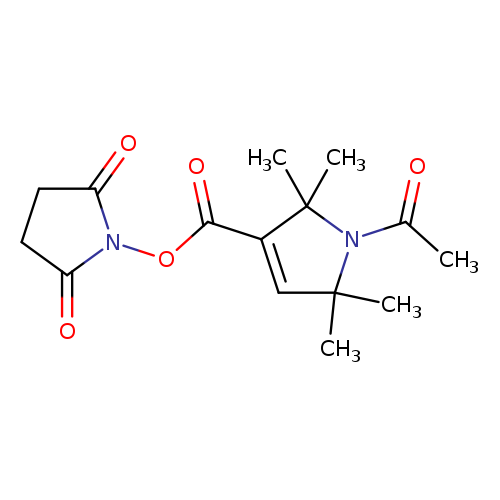
1-ACETYL-2,2,5,5-TETRAMETHYL-3-PYRROLINE-3-CARBOXYLIC ACID, N-HYDROXYSUCCINIMIDE ESTERCatalog No.:AA008WOK CAS No.:1076198-74-7 MDL No.:MFCD09839862 MF:C15H20N2O5 MW:308.3297 |
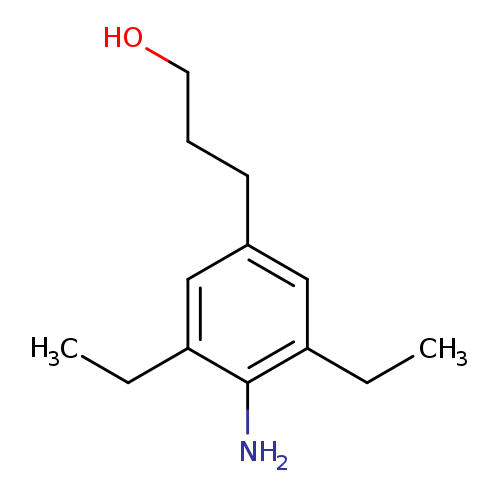
3-(4-AMINO-3,5-DIETHYLPHENYL)PROPAN-1-OLCatalog No.:AA008VOH CAS No.:1076198-78-1 MDL No.:MFCD09753563 MF:C13H21NO MW:207.3119 |
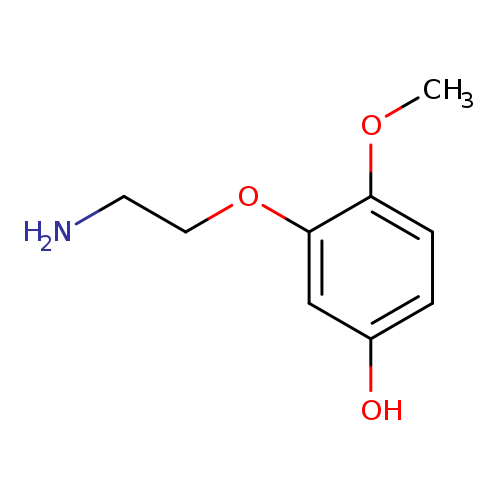
5- (2-AMINOETHOXY)-3-METHOXYPHENOLCatalog No.:AA008WBX CAS No.:1076198-81-6 MDL No.:MFCD09753596 MF:C9H13NO3 MW:183.2044 |
7-Aminosuccinylbenzo[a]pyreneCatalog No.:AA0084IB CAS No.:1076198-86-1 MDL No.:MFCD09842348 MF:C24H17NO3 MW:367.3967 |
Benzoxazolemethanesulfonamide-N-(6-methyl-hexanoate)Catalog No.:AA007E71 CAS No.:1076198-89-4 MDL No.:MFCD18382164 MF:C15H20N2O5S MW:340.3947 |
6-Benzyl-1,2,3,4-tetrahydro-6h-pyrrolo[3,4-b]pyridine-5,7-dioneCatalog No.:AA003N0G CAS No.:1076198-93-0 MDL No.:MFCD09907821 MF:C14H14N2O2 MW:242.2732 |
7-Benzyloxy-N-des{[2-(2-hydroxy)ethoxy]ethyl} QuetiapineCatalog No.:AA0084I8 CAS No.:1076198-97-4 MDL No.:MFCD09840033 MF:C24H23N3OS MW:401.5239 |
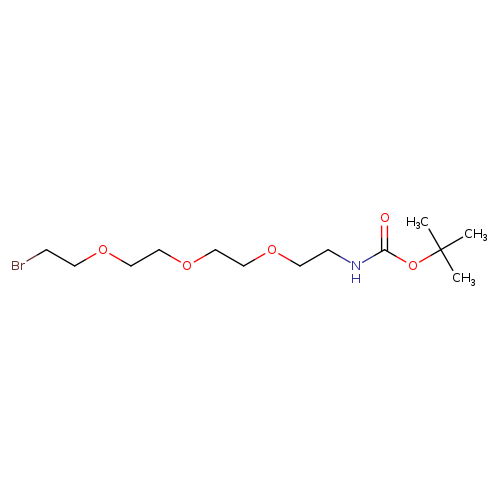
1-Boc-amino-3,6,9-trioxaundecanyl-11-bromideCatalog No.:AA007E6X CAS No.:1076199-21-7 MDL No.:MFCD09840081 MF:C13H26BrNO5 MW:356.2532 |
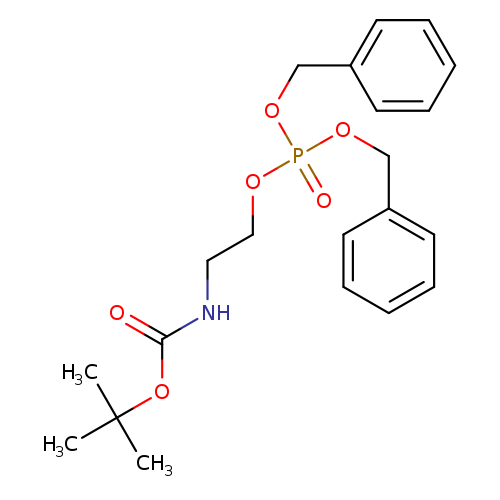
Boc-ethanolamine DibenzylphosphateCatalog No.:AA008W17 CAS No.:1076199-25-1 MDL No.:MFCD28138239 MF:C21H28NO6P MW:421.4239 |
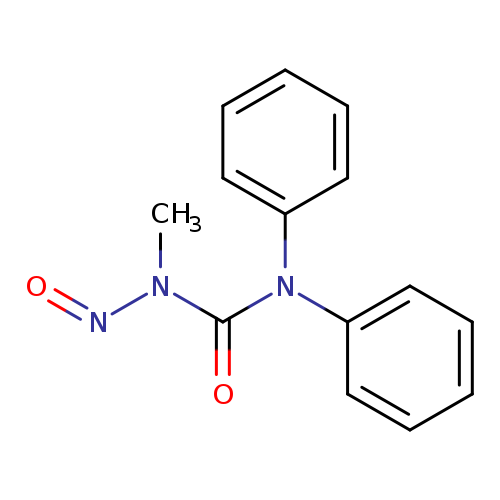
N-Nitroso akardite iiCatalog No.:AA008WBO CAS No.:1076199-26-2 MDL No.:MFCD09952259 MF:C14H13N3O2 MW:255.2719 |
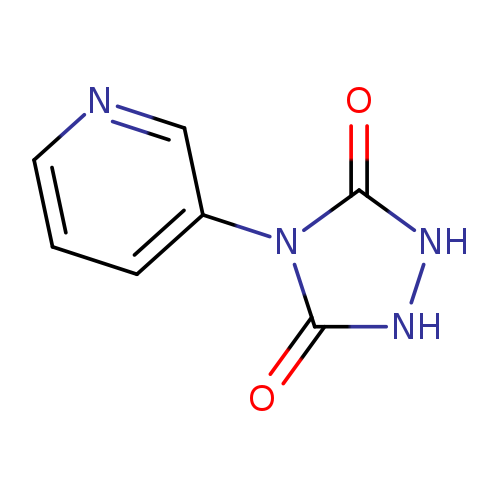
4-(3-Pyridyl)-1,2,4-triazolodone-3,5-dioneCatalog No.:AA0084I2 CAS No.:1076199-39-7 MDL No.:MFCD18382426 MF:C7H6N4O2 MW:178.1481 |
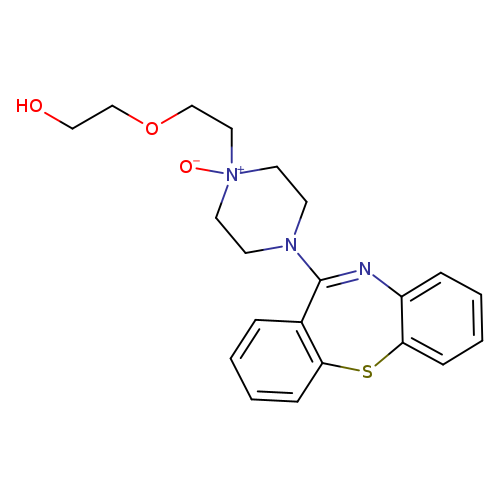
Quetiapine N-OxideCatalog No.:AA007VRX CAS No.:1076199-40-0 MDL No.:MFCD11977896 MF:C21H25N3O3S MW:399.5065 |
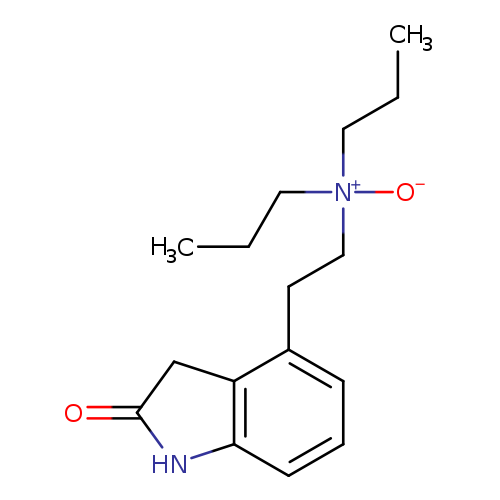
Ropinirole n-oxideCatalog No.:AA007VRW CAS No.:1076199-41-1 MDL No.:MFCD09841023 MF:C16H24N2O2 MW:276.3740 |
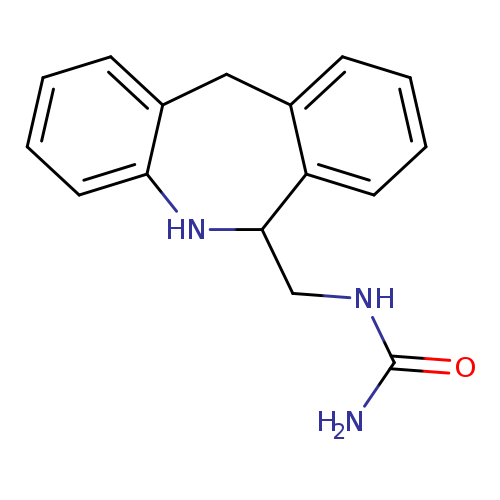
6-Ureidomethyl-5,6-dihydromorphanthridineCatalog No.:AA008WOU CAS No.:1076199-50-2 MDL No.:MFCD25973863 MF:C16H17N3O MW:267.3257 |
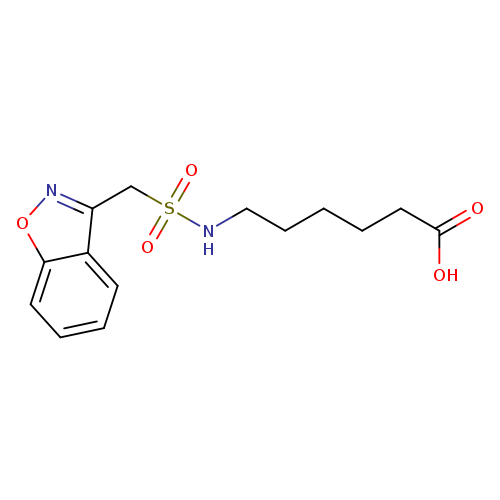
Zonisamide-N-(6-hexanoic Acid)Catalog No.:AA008W2K CAS No.:1076199-51-3 MDL No.:MFCD28898395 MF:C14H18N2O5S MW:326.3681 |
tert-Butyl 2-(pyridin-3-yl)pyrrolidine-1-carboxylateCatalog No.:AA008WIX CAS No.:1076199-53-5 MDL No.:MFCD18382197 MF:C14H20N2O2 MW:248.3208 |
1-tert-Butyl 4-[6-(5-Chloropyridin-2-yl)-7-oxo-6,7-dihydro-5H-pyrrolo[3,4-b]pyrazin-5-yl]piperazine-1,4-dicarboxylateCatalog No.:AA007E6V CAS No.:1076199-62-6 MDL No.:MFCD18382196 MF:C21H23ClN6O5 MW:474.8975 |
rac-3-tert-Butyl-5-(7-ethyl-2-benzofuranyl)-2-oxazolidinoneCatalog No.:AA008W70 CAS No.:1076199-68-2 MDL No.:MFCD07369206 MF:C17H21NO3 MW:287.3535 |
tert-Butyl 2-((4-(2-fluorophenyl)-6-methylthieno[2,3-d]pyrimidin-2-yl)amino)acetateCatalog No.:AA007VRP CAS No.:1076199-69-3 MDL No.:MFCD16293794 MF:C19H20FN3O2S MW:373.4444 |
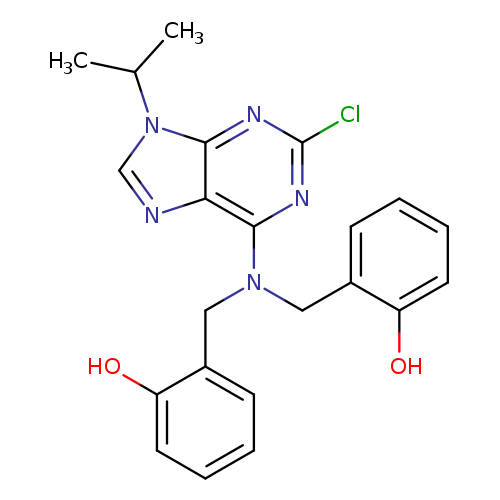
2-CHLORO-6-[N,N-DI(2-HYDROXYBENZYL)AMINO]-9-ISOPROPYLPURINECatalog No.:AA007E6T CAS No.:1076199-83-1 MDL No.:MFCD16293796 MF:C22H22ClN5O2 MW:423.8954 |
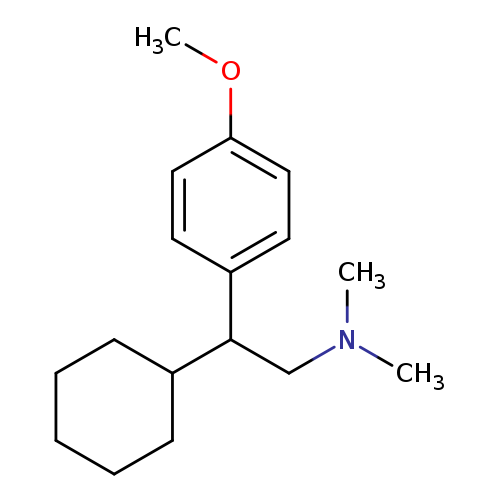
(2RS)-2-Cyclohexyl-2-(4-Methoxyphenyl)-N,NdiMethylethanaMineHydrochlorideCatalog No.:AA008W5I CAS No.:1076199-92-2 MDL No.:MFCD21396214 MF:C17H27NO MW:261.4024 |
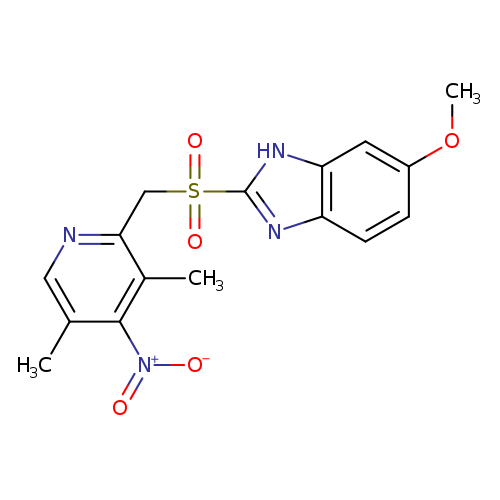
4-Desmethoxy-4-nitro Omeprazole SulfoneCatalog No.:AA008WNS CAS No.:1076199-93-3 MDL No.:MFCD09840356 MF:C16H16N4O5S MW:376.3870 |
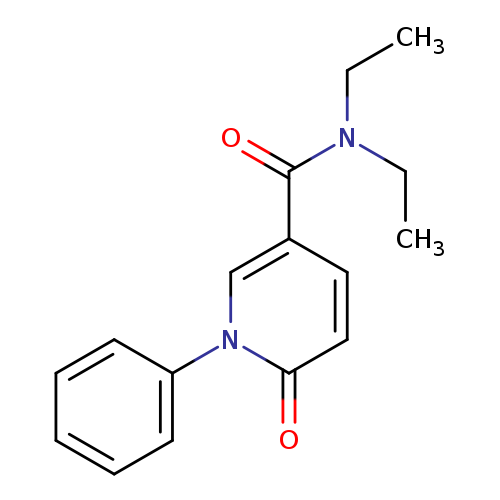
5-(N,N-Diethylcarboxamide)-1-phenylpyridin-2(1H)-oneCatalog No.:AA007E6R CAS No.:1076199-97-7 MDL No.:MFCD09840448 MF:C16H18N2O2 MW:270.3263 |
2,2'-[[[2-[(3-Hydroxypropyl)nitrilo]-9-(1-Methylethyl)-9H-purin-6-yl]iMino]bis(Methylene)]bisphenolCatalog No.:AA008WC3 CAS No.:1076200-04-8 MDL No.:MFCD28898388 MF:C25H30N6O3 MW:462.5441 |
ethyl 4-[(4-chlorophenyl)amino]-2-(methylsulfanyl)pyrimidine-5-carboxylateCatalog No.:AA00IYHQ CAS No.:107622-03-7 MDL No.:MFCD01444010 MF:C14H14ClN3O2S MW:323.7979 |
[2-(2-Phenylethyl)phenyl]aminehydrochlorideCatalog No.:AA01FO4W CAS No.:107622-07-1 MDL No.: MF:C14H16ClN MW:233.7365 |
4-PhenoxybenzylamineCatalog No.:AA0084HS CAS No.:107622-80-0 MDL No.:MFCD01310836 MF:C13H13NO MW:199.2484 |
N-(N-BOC-Piperidino)pyrazole-4-carboxaldehydeCatalog No.:AA00HAY9 CAS No.:1076224-00-4 MDL No.:MFCD19687734 MF:C14H21N3O3 MW:279.3348 |
4-((1R,2R)-2-Hydroxycyclohexyl)-2(trifluoromethyl)benzonitrileCatalog No.:AA008W94 CAS No.:1076225-27-8 MDL No.:MFCD17215970 MF:C14H14F3NO MW:269.2623 |
1-(Benzyloxy)-3-iodobenzeneCatalog No.:AA0032G4 CAS No.:107623-21-2 MDL No.:MFCD01318100 MF:C13H11IO MW:310.1303 |
(1S)-1-(4-Bromophenyl)-2-chloroethan-1-olCatalog No.:AA019QEA CAS No.:1076233-87-8 MDL No.:MFCD09863570 MF:C8H8BrClO MW:235.5055 |
4,4'-Diacetylbiphenyl diketoximeCatalog No.:AA01FEGP CAS No.:1076239-80-9 MDL No.:MFCD25977523 MF:C16H16N2O2 MW:268.3104 |
(2-phenoxyphenyl)methanamineCatalog No.:AA0084HR CAS No.:107624-14-6 MDL No.:MFCD07365247 MF:C13H13NO MW:199.2484 |
Methyl 9H-carbazole-9-carboxylateCatalog No.:AA01FE7O CAS No.:107624-52-2 MDL No.:MFCD05148718 MF:C14H11NO2 MW:225.2426 |
1-Methoxy-3-(4-nitrophenyl)benzeneCatalog No.:AA00948T CAS No.:107624-96-4 MDL No.:MFCD09909441 MF:C13H11NO3 MW:229.2313 |
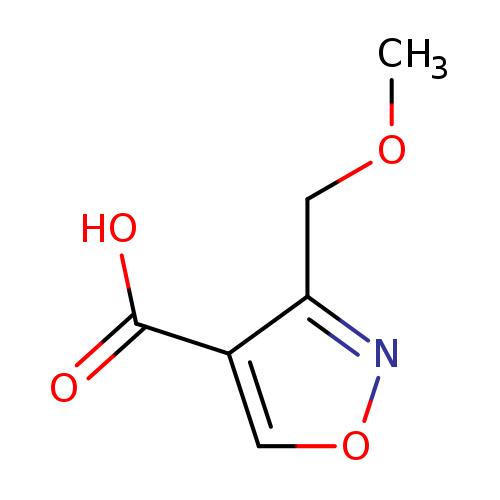
3-(methoxymethyl)-1,2-oxazole-4-carboxylic acidCatalog No.:AA01BAWJ CAS No.:1076245-90-3 MDL No.:MFCD20646109 MF:C6H7NO4 MW:157.1241 |
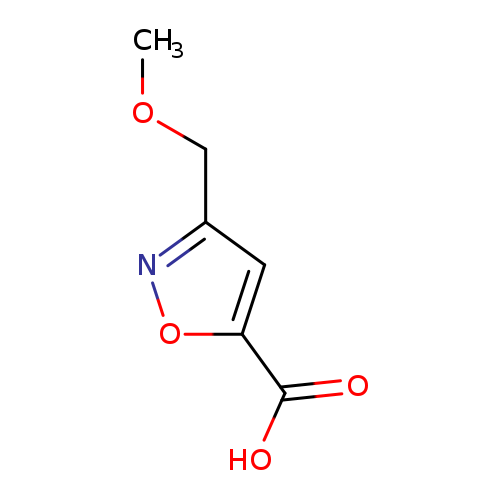
3-(METHOXYMETHYL)-1,2-OXAZOLE-5-CARBOXYLIC ACIDCatalog No.:AA01DUVL CAS No.:1076245-91-4 MDL No.:MFCD20646108 MF:C6H7NO4 MW:157.1241 |
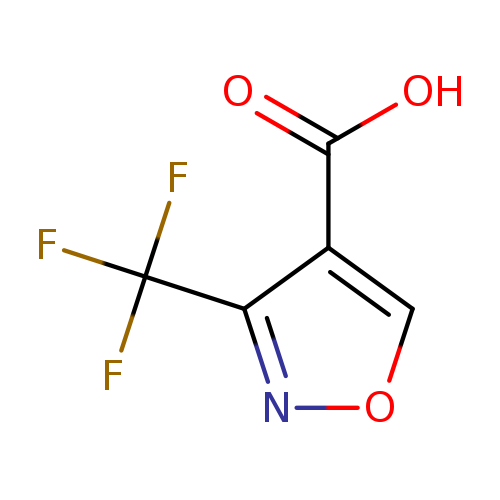
3-(Trifluoromethyl)isoxazole-4-carboxylic acidCatalog No.:AA008RZL CAS No.:1076245-98-1 MDL No.:MFCD11100076 MF:C5H2F3NO3 MW:181.0695 |
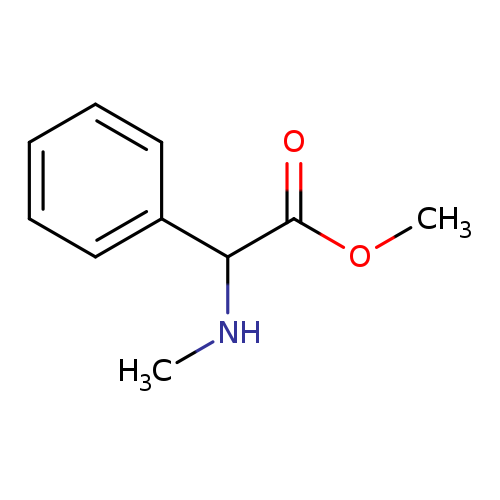
methyl 2-(methylamino)-2-phenylacetateCatalog No.:AA01AJMR CAS No.:107635-11-0 MDL No.:MFCD12148297 MF:C10H13NO2 MW:179.2157 |
Ethyl 2-(4-methoxy-3,5-dimethylphenyl)-2-oxoacetateCatalog No.:AA008VBN CAS No.:107642-57-9 MDL No.:MFCD06201703 MF:C13H16O4 MW:236.2637 |
2-([5-(4-Methoxyphenyl)-4-phenyl-4h-1,2,4-triazol-3-yl]sulfanyl)acetohydrazideCatalog No.:AA01A0N1 CAS No.:107645-46-5 MDL No.:MFCD01164022 MF:C17H17N5O2S MW:355.4142 |