2020-01-25 15:53:09
Priyanka Reddy, Myrna A. Deseo,Vilnis Ezernieks, Kathryn Guthridge, German Spangenberg and Simone Rochfort
1.Introduction Perennial ryegrass (Lolium perenne L.), is used for forage in temperate regions throughout the world including Northern Europe, the Pacific North West of USA, Japan, South-Eastern Australia and New Zealand [1-5]. It is the most utilized pasture grass on dairy farms in Australia and has a high economic importance [6]. Perennial ryegrass is commonly infected with the fungus Epichloefestucae var. lolii (also termed LpTG-1) that provides the grass with both abiotic and biotic stress tolerances. Since the 1980s it has been known that this association leads to the production of the indole diterpene lolitrem B that causes ryegrass staggers disease [7].
Lolitrem B was found to be the most abundant and potent of the indole diterpenes produced by LpTG-1 in symbiotic association with perennial ryegrass [8,9]. For forage grass improvement programs, the selection of novel endophytes that produce indole diterpenes that are safe for animals while maintaining the agronomic benefits of having an endophyte, such as insect resistance, is essential. Targeted screening assays for toxic indole diterpene alkaloids and bioprotective compounds are required to enable better selection of novel endophytes. However, it is a challenge to study these compounds effectively as they are very difficult to isolate and there have been limited studies on the synthesis of the indole diterpenes [10]. Many of the lolitrems and their derivatives have been isolated and the structures elucidated [9,11-19]; however, the biological activity remains unknown for many of them.
Also, several intermediate compounds along the indole diterpene pathway are not commercially available and the lolitrems in particular show little or no expression in culture but are highly expressed in planta. Thus, isolation from perennial ryegrass has been the key source of these compounds [20,21]. The availability of these compounds is essential to undertake further mechanistic studies on their toxicity and mode of action. The first isolation of lolitrem B was carried out by Gallagher et al., which led to the purification of small quantities of lolitrem B using bioactivity-guided isolation and purification steps over several months [7,17] yielding 15% recovery of lolitrem B.
Since the concentration of these alkaloids are generally higher in ryegrass seeds than the ryegrass plant itself [17,22], Miles et al. were able to carry out large scale purification of ryegrass seeds resulting in a 34% yield of lolitrem B using several complex partition and chromatographic steps over a few weeks [17]. Grancher et al. also carried out large scale isolation and purification using a novel counter current chromatography method and multiple steps, resulting in a 32% yield of lolitrem B [23]. In this study, a rapid purification approach was developed to isolate a series of indole diterpenes from a crude hexane extract as required. A crude extract was the preferred starting material of the indole diterpenes, as compounds in natural product extracts are known to increase long-term compound stability [24].
Lolitrem B has been reported to be unstable in acidic conditions [15], but the long-term stability of lolitrem B and its intermediate compounds were not known. Here, we were able to develop an isolation scheme that led to the purification of lolitrem B, lolitrem E [17], paspaline [25] and terpendole B [26] in a short period of time. Compound identity was confirmed by mass spectrometry and NMR spectroscopy in comparison with database or published literature. In this study, it was found that lolitrem B readily degraded to the compound lolitriol [16], which was previously reported to be a biosynthetic intermediate of lolitrem B in the indole diterpene pathway [27]. We also found that lolitrem B can readily degrade depending on the solvent and storage conditions. The isolation scheme developed in this study could easily be repeated to obtain greater quantities of compounds and at the same time could be adapted for the isolation of other indole diterpenes. 2.Results A preliminary study was carried out using a combination of solvents for extraction (hexane, acetone and methanol (MeOH)) to determine the optimum solvent system that can be used for the selective extraction of the lipophilic alkaloids, lolitrem B and its biosynthetic intermediate compounds (lolitrem E, lolitriol, paspaline and terpendole B).
The extracts were qualitatively analyzed by liquid chromatography-mass spectrometry (LCMS) in triplicate. Since the more polar alkaloids such as peramine and ergovaline are also present in perennial ryegrass-endophyte associations [28], these compounds were included in the preliminary assessment of the LCMS profiles to determine the selectivity of the solvent(s) for the lipophilic alkaloids. The exact mass of the molecular ion of lolitrem B, lolitrem E, paspaline, peramine and ergovaline (Table 1) was used to assess the presence of the metabolites from the total ion chromatograms (TIC) of the extracts using extracted ion technique. The peak area of the target metabolites in the extracted ion chromatogram (EIC) was integrated and the responses were compared (Figure 1). In the sequential extraction with acetone-MeOH (solvent system A), all target metabolites were extracted in both acetone and MeOH at varying proportions.
The sequential extraction with hexane-acetone-MeOH (solvent system B) indicated the extractability of most of lolitrem B, lolitrem E and paspaline in hexane and acetone, but acetone was also able to extract ergovaline and peramine, The sequential extractionwith hexane-MeOH (solvent system C) showed that thebulk of lolitrem B and paspaline were extracted in hexane [17] and lolitrem E was extracted in both hexane and MeOH. Peramine and ergovaline were selectively extracted in MeOH and thus, solvent system C would be the best choice to carry out the large-scale extraction, whereby the bulk of ielitrem B and tts intermediates would be in the hexaneextract, and the other alkaloids (if of interest) could be extracted subsequently with MeOH. The large-scale extraction was carried out using a sample-to-solvent ratio of 1:10 (w/v); i.e., 50 kg of seeds with 2 x 500 L of hexane. The preliminary study showed that a second extraction of hexane was necessary as it contributed to 30% of the total lolitrem B.
The concentrated crude hexane extract was subjected to fractionation steps (Figure 2) to obtain lolitrem B and its intermediate compounds. The use of the Reveleris flash chromatography system allowed efficient use of solvent and time for the initial chromatographic separation of the crude extract. This step could be repeated several times to obtain enriched fractions for the purification of targeted indole diterpenes.
2.1.Lolitrem B and Lolitrem E Lolitrem B fraction was obtained by preparative High performance liquid chromatography (HPLC) separation of Fraction B from the flash chromatography fractionation (Figure 2) and LCMS and 1H NMR spectroscopic analysis (in deuterated chloroform) confirmed the presence of lolitrem B in this fraction (Fr B43-B45), which was allowed to crystallize out of solution to obtain 98% purity by 1H NMR spectroscopy and mass spectrometry (Figures S1 and S2).
The three-step isolation and purification method resulted in a 25% yield of lolitrem B (1.5 mg/kg of seed) from endophyte-infected perennial ryegrass seed containing 6 mg/kg lolitrem B. Repeated fractionation by flash chromatography and preparative HPLC to obtain greater amounts of lolitrem B was carried out and 1H NMR analysis indicated the stability of lolitrem B up to this isolation step. Lolitrem B (1) was purified from the preparative HPLC fraction by crystallization and after analysis by 1H NMR spectroscopy, it was found that lolitrem B in its pure form spontaneously degraded while in deuterated chloroform solvent. This was interesting new information because the degree of instability of lolitrem B had not been reported before.
Consequently, 1H NMR analysis was carried out using deuterated chloroform that was passed through potassium carbonate to neutralize the acid. Lolitrem B was isolated numerous times using the scheme outlined in Figure 2 and 1H NMR spectra were acquired for all fractions. The chemical shift data of the isolated lolitrem B (1) was found to be in agreement with what had been reported in the literature [15] (Table S1). Consequently, to minimize the risk of degradation of lolitrem B from exposure to acid present in deuterated chloroform, further NMR spectral analysis was carried out using benzene-d6 as solvent (Figure S3). Lolitrem E (2) was obtained from the fraction eluting after lolitrem B by preparative HPLC and was also purified by crystallization [17]. 1H NMR spectroscopy and mass spectrometry (Figures S4 and S5) of the isolated lolitrem E (2) indicated 95% purity. The method resulted in 0.2 mg lolitrem E/kg endophyte-infected perennial ryegrass seed. Lolitrem E (2) structurally differs from lolitrem B (1) from ring I of the latter wherein the ring had opened at the C-42 position (Figure 3). Lolitrem E has been isolated previously from endophyte-infected perennial ryegrass [17].
2.2.Paspaline and Terpendole B The fraction containing paspaline and terpendole B from flash chromatography (Fraction A) was subjected to preparative HPLC that separated paspaline [25] from its analogue, terpendole B (Figure 2). LCMS analysis indicated that the paspaline fraction (Fr A22-A30) contained other components and crystallization resulted in the impurities precipitating out, leaving the supernatant relatively pure with paspaline. 1H NMR spectroscopy and mass spectrometry (Figures S6 and S7) of the isolated paspaline (3) indicated 98% purity. The 1H and 13C NMR data are summarized in Table S2 and are in agreement with what had been reported in the literature [25,29]. The preparative HPLC fraction Fr A31-A34 was further subjected to semi-preparative HPLC that yielded terpendole B (4), which was confirmed by 1H and 13C NMR analysis (Table S2). 1H NMR spectroscopy and mass spectrometry (Figures S8 and S9) of terpendole B (4) indicated 95% purity. Terpendole B was previously isolated by Huang et al [26]. The isolation method resulted in 0.5 mg and 0.2 mg of paspaline (3) and terpendole B (4) (Figure 3) respectively, per kg of endophyte-infected perennial ryegrass seed.
2.3.Lolitrem B Degradation Lolitrem B rapidly converted to lolitriol (5) upon dissolution of lolitrem B in DMSO with sonication. LCMS and NMR spectral analysis (Figures S10 and S11) confirmed the conversion of lolitrem B to lolitriol. Lolitriol has been previously reported with NMR data in 2:1 deuterated chloroform: DMSO [15]. In the present study, NMR spectral data of lolitriol was obtained in deuterated chloroform, as summarized in Table S1. Lolitrem B in deuterated chloroform was found to spontaneously degrade. NMR spectral analysis confirmed the changes in the chemical structure of what lolitrem B should have been. Since deuterated chloroform can become acidic over time, it is possible that the presence of acid caused the degradation.
3.Discussion The procedure described here resulted in the concurrent isolation of lolitrem B (1), lolitrem E (2), paspaline (3) and terpendole B (4). The endophyte-infected perennial ryegrass seeds extracted contained 6 mg lolitrem B/kg seed and yielded 1.5 mg/kg of lolitrem B (25% yield). The reported quantities of lolitrem B in ryegrass seed can vary significantly [30]. Thus, extraction and isolation methods described in this study can be applied to seeds containing higher levels of lolitrem B (11-13 mg/kg) and its biosynthetic intermediates, to allow greater yields to be isolated. Although methods described by Miles et al. [17] and Grancher et al. [23] have been shown to achieve better yields (32-34% yield), both methods used ryegrass seeds with high lolitrem B content (11-13 mg/kg) and a significant number of complex isolation steps and procedures for the targeted purification of lolitrem B only [17,23].
The simplified method described herein also allowed the stability of the major neurotoxin lolitrem B to be investigated. In deuterated chloroform lolitrem B was found to easily degrade, most probably due to the acidic condition of the solvent. Chloroform in the presence of O2 and light turns into phosgene (COCl?) and HCl [31]. Typically, alkaloids containing secondary amines have been reported to react with phosgene, incorporating a carbonyl group (C=O) to the molecule [31]. NMR spectral analysis indicated the presence of an additional carbonyl carbon signal in lolitrem B that had degraded in deuterated chloroform and the reaction of lolitrem B with phosgene would probably explain the formation of the breakdown product. Previous reports propose that acid catalyzed hydrolysis of 85% pure lolitrem B results in complete conversion to lolitriol (5) [16]. In the present study, it was found that 98% pure lolitrem B could rapidly convert to lolitriol (5) upon dissolution of lolitrem B in DMSO with sonication.
LCMS and NMR analysis revealed complete conversion of lolitrem B to lolitriol. Lolitriol (5) was reported as a naturally occurring constituent of endophyte-infected ryegrass [16] and LCMS analysis of the hexane extract of ryegrass seed used in this study confirmed its presence in the extract. Given that lolitrem B can easily degrade while in solution, it is also possible that lolitriol could be an artefact and not an intermediate in the biosynthetic pathway [20], depending on the process used for the extraction and isolation of lolitrem B. The observations in this study thus brings in doubt whether lolitriol is naturally occurring or a breakdown product of lolitrem B as a result of the extraction and isolation procedure. However, there is a brief mention of lolitriol being reported to be produced in very low levels from cultures of the fungus LpTG-1 [16]. Further observations during our study confirmed the instability of lolitrem B whereby it degraded while stored in acetonitrile at4 0 C after 4 weeks and this was verified by LCMS analysis. However, lolitrem B was stable when stored in 80% methanol at -20 OC for over 24 months.
Paspaline (3) a key intermediate in the biosynthesis of lolitrem B, is likely to be the first stable indole diterpene intermediate and core molecule that is enzymatically altered for the generation of the indole diterpene chemical diversity in various species [21]. Paspaline is not commercially available, although its total synthesis was recently published [10]. It has also been purified and crystallized from other species such as Penicillium paxilli and Claviceps paspali [25,29]. Terpendole B (4) is structurally similar to paspaline (Figure 3) and has been purified from Albophoma yamanshiensis; however its biological activity has never been reported in mammals. The amounts and purity of the compounds isolated are sufficient for in vitro and small mouse in vivo studies, as well as LCMS analytical standards. Also, the proposed isolation method is time efficient, as only a maximum of four steps are required for the purification of one compound along the indole diterpene pathway. The method will also allow purification of other intermediates within the indole diterpene pathway that are not commercially available.
TeludipineCatalog No.:AA0083AI CAS No.:108687-08-7 MDL No.:MFCD09837740 MF:C28H38N2O6 MW:498.6111 |
GUANIDINE-D5 DEUTERIOCHLORIDECatalog No.:AA008S1D CAS No.:108694-93-5 MDL No.:MFCD00144417 MF:CD5N3 MW:64.1013 |
Boc-n-me-arg(tos)-ohCatalog No.:AA007C0S CAS No.:108695-16-5 MDL No.:MFCD00134858 MF:C19H30N4O6S MW:442.5297 |
13,16,19-Docosatrienoicacid, methyl ester, (13Z,16Z,19Z)-Catalog No.:AA00837M CAS No.:108698-01-7 MDL No.:MFCD00058485 MF:C23H40O2 MW:348.5625 |
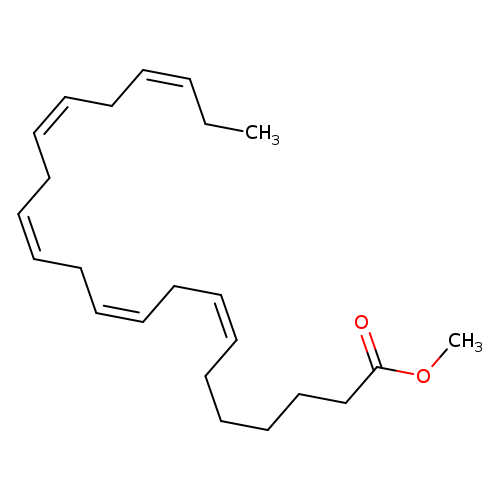
CIS-7,10,13,16,19-DOCOSAPENTAENOIC ACID METHYL ESTERCatalog No.:AA008RI6 CAS No.:108698-02-8 MDL No.:MFCD00674894 MF:C23H36O2 MW:344.5307 |
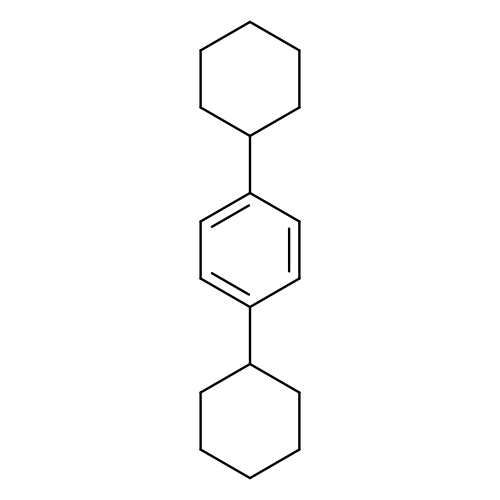
1,4-DicyclohexylbenzeneCatalog No.:AA003DLF CAS No.:1087-02-1 MDL No.:MFCD00001452 MF:C18H26 MW:242.3990 |
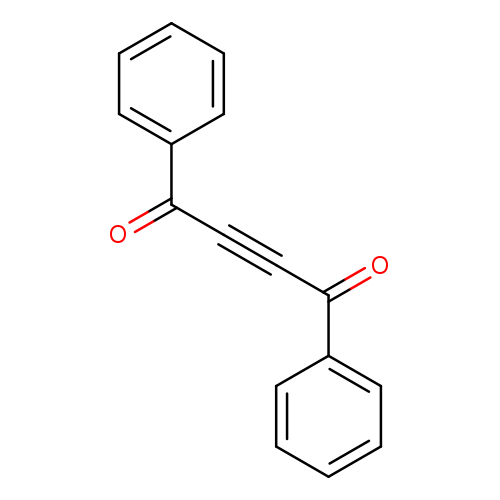
1,4-Diphenyl-2-butyne-1,4-dioneCatalog No.:AA007U1X CAS No.:1087-09-8 MDL No.:MFCD00033371 MF:C16H10O2 MW:234.2494 |
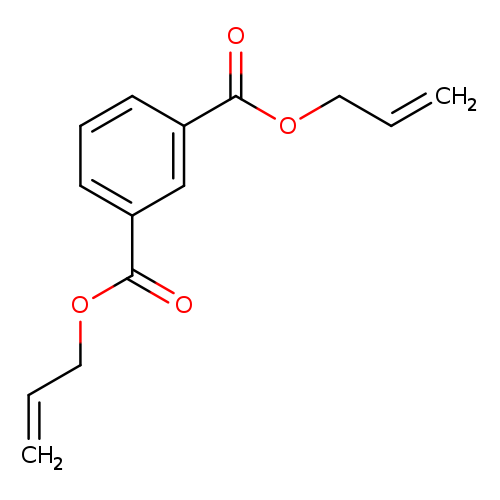
Diallyl isophthalateCatalog No.:AA0034MF CAS No.:1087-21-4 MDL No.:MFCD00048174 MF:C14H14O4 MW:246.2586 |
Hexyl 3,4,5-trihydroxybenzoateCatalog No.:AA007C0D CAS No.:1087-26-9 MDL No.:MFCD00051936 MF:C13H18O5 MW:254.2790 |
2-phenyl-N'-[(1E)-(pyridin-3-yl)methylidene]acetohydrazideCatalog No.:AA00IWR4 CAS No.:1087-40-7 MDL No.:MFCD00617305 MF:C14H13N3O MW:239.2725 |
Diethyl 2-benzoylmalonateCatalog No.:AA003O0C CAS No.:1087-97-4 MDL No.:MFCD00059381 MF:C14H16O5 MW:264.2738 |
QUINOLINIUM CHLOROCHROMATECatalog No.:AA008WRM CAS No.:108703-35-1 MDL No.:MFCD00077687 MF:C9H7ClCrNO3------- MW:264.6059 |
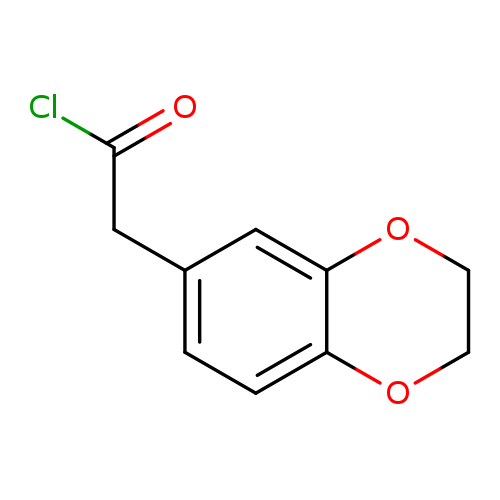
2-(2,3-dihydrobenzo[b][1,4]dioxin-6-yl)acetyl chlorideCatalog No.:AA0188WK CAS No.:108704-93-4 MDL No.:MFCD07400599 MF:C10H9ClO3 MW:212.6297 |
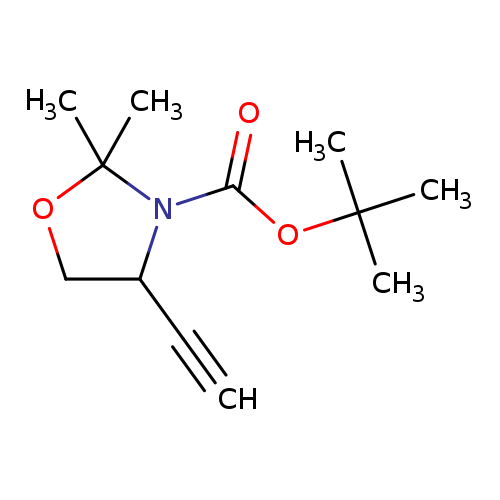
tert-Butyl 4-ethynyl-2,2-dimethyloxazolidine-3-carboxylateCatalog No.:AA00HB5C CAS No.:1087043-97-7 MDL No.:MFCD16620634 MF:C12H19NO3 MW:225.2842 |
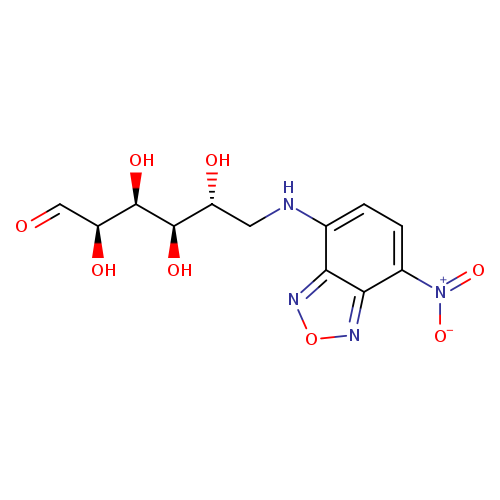
D-Glucose,6-deoxy-6-[(7-nitro-2,1,3-benzoxadiazol-4-yl)amino]-Catalog No.:AA00837E CAS No.:108708-22-1 MDL No.: MF:C12H14N4O8 MW:342.2616 |
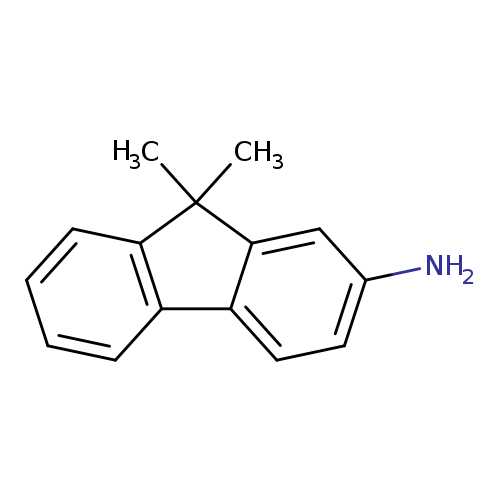
2-Amino-9,9-dimethylfluoreneCatalog No.:AA00HB5D CAS No.:108714-73-4 MDL No.:MFCD09953790 MF:C15H15N MW:209.2863 |
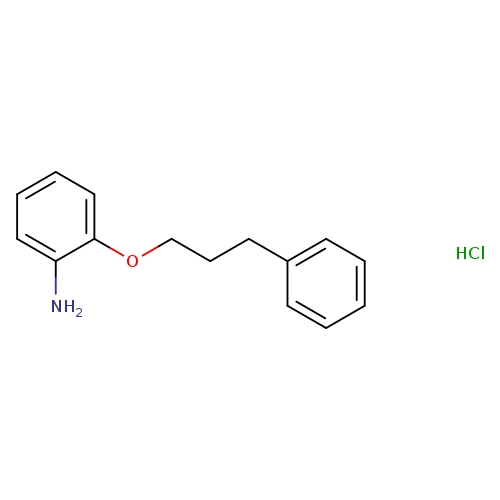
[2-(3-Phenylpropoxy)phenyl]amine hydrochlorideCatalog No.:AA008379 CAS No.:108715-56-6 MDL No.:MFCD08559357 MF:C15H18ClNO MW:263.7625 |
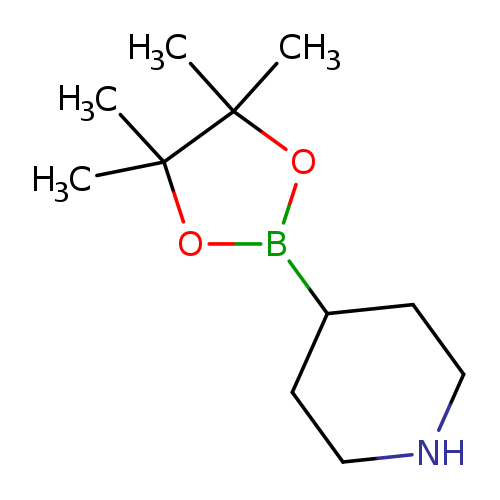
Piperidine-4-boronic acid pinacol esterCatalog No.:AA008SG2 CAS No.:1087160-40-4 MDL No.:MFCD11506079 MF:C11H22BNO2 MW:211.1089 |
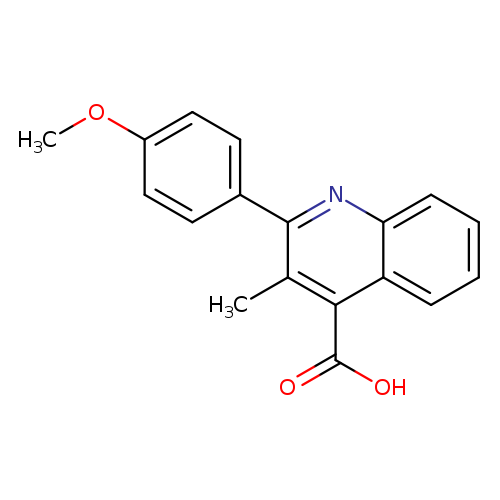
2-(4-Methoxyphenyl)-3-methylquinoline-4-carboxylic acidCatalog No.:AA00HB5E CAS No.:108717-24-4 MDL No.:MFCD01114914 MF:C18H15NO3 MW:293.3166 |
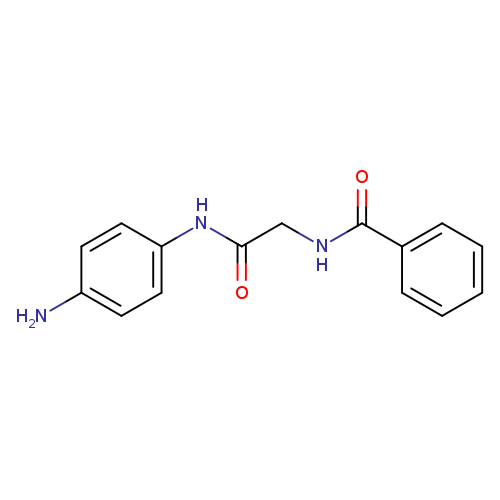
N-(2-((4-Aminophenyl)amino)-2-oxoethyl)benzamideCatalog No.:AA008UZW CAS No.:108717-59-5 MDL No.:MFCD00172388 MF:C15H15N3O2 MW:269.2985 |
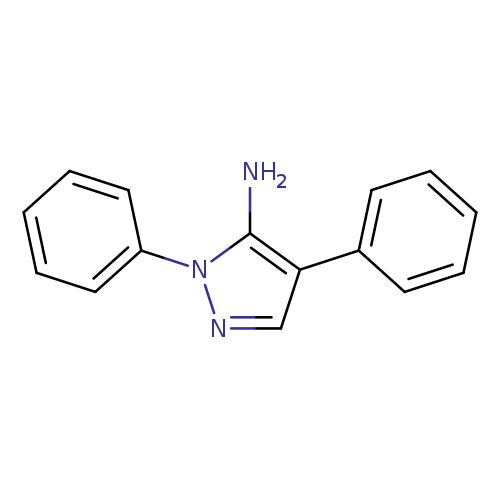
1,4-Diphenyl-1h-pyrazol-5-amineCatalog No.:AA008YT5 CAS No.:108719-40-0 MDL No.:MFCD00140147 MF:C15H13N3 MW:235.2838 |
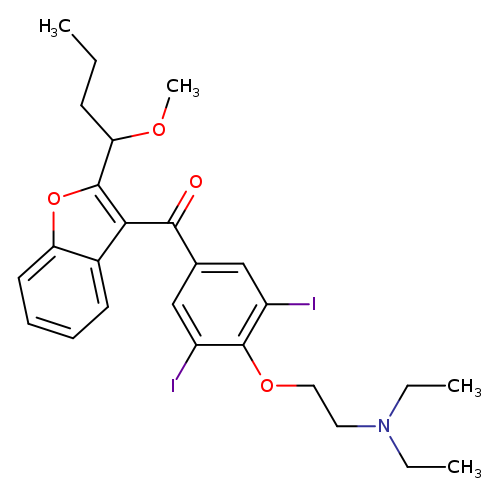
(4-(2-(Diethylamino)ethoxy)-3,5-diiodophenyl)(2-(1-methoxybutyl)benzofuran-3-yl)methanoneCatalog No.:AA008VZC CAS No.:1087223-70-8 MDL No.: MF:C26H31I2NO4 MW:675.3376 |
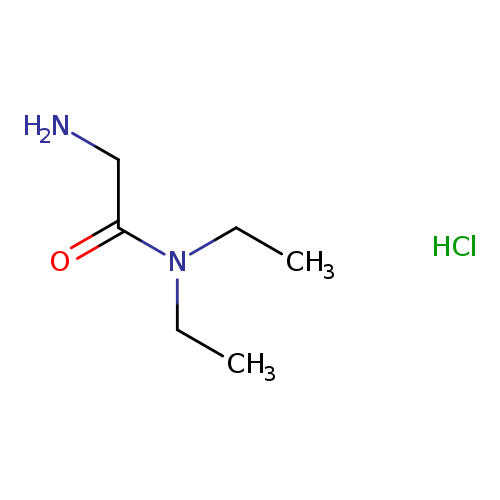
2-Amino-N,N-diethylacetamide hydrochlorideCatalog No.:AA003GGT CAS No.:108723-79-1 MDL No.:MFCD07366745 MF:C6H15ClN2O MW:166.6491 |
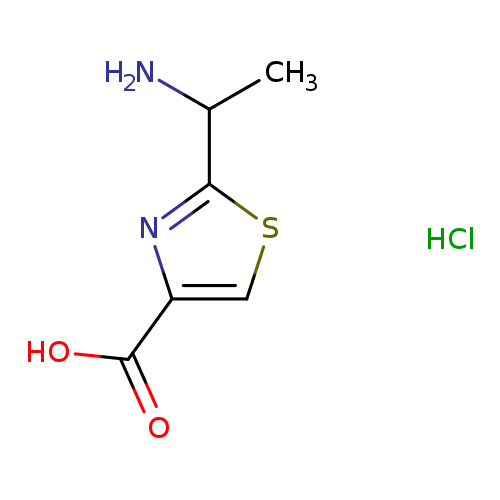
2-(1-aminoethyl)-1,3-thiazole-4-carboxylic acid hydrochlorideCatalog No.:AA01B2MN CAS No.:1087345-72-9 MDL No.:MFCD21090470 MF:C6H9ClN2O2S MW:208.6659 |
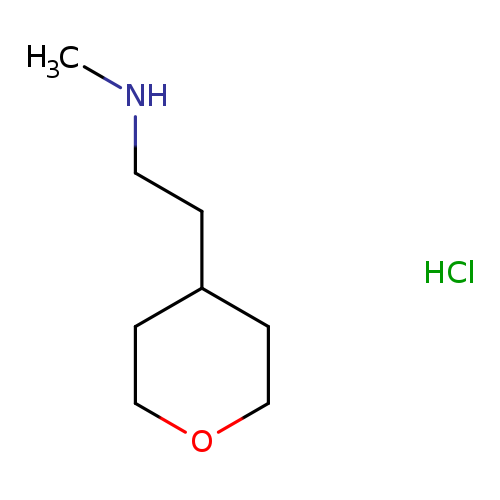
N-Methyl-2-(tetrahydro-2H-pyran-4-yl)ethanamine hydrochlorideCatalog No.:AA00J16B CAS No.:1087351-66-3 MDL No.:MFCD09864283 MF:C8H18ClNO MW:179.6876 |
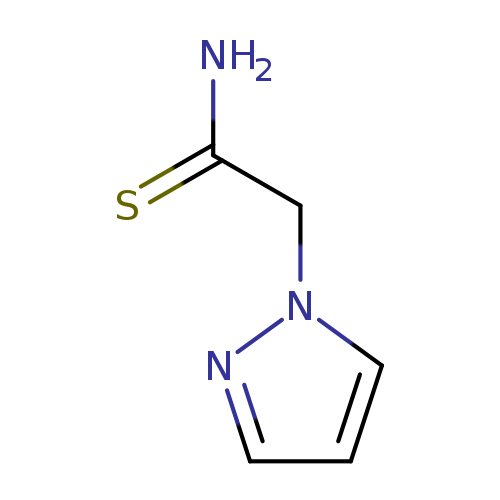
2-(1H-pyrazol-1-yl)ethanethioamideCatalog No.:AA00VUIA CAS No.:1087353-55-6 MDL No.:MFCD09933278 MF:C5H7N3S MW:141.1942 |
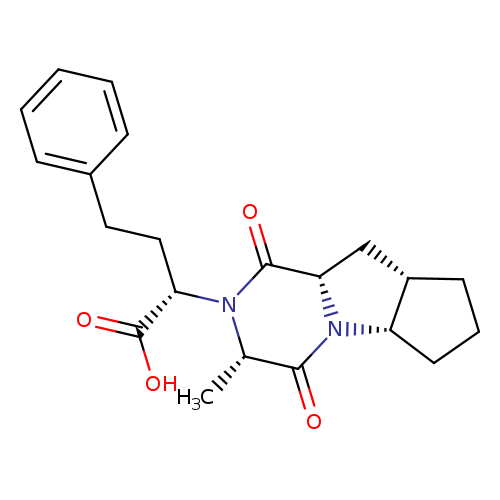
Ramiprilat DiketopiperazineCatalog No.:AA008X2T CAS No.:108736-10-3 MDL No.:MFCD09841234 MF:C21H26N2O4 MW:370.4421 |
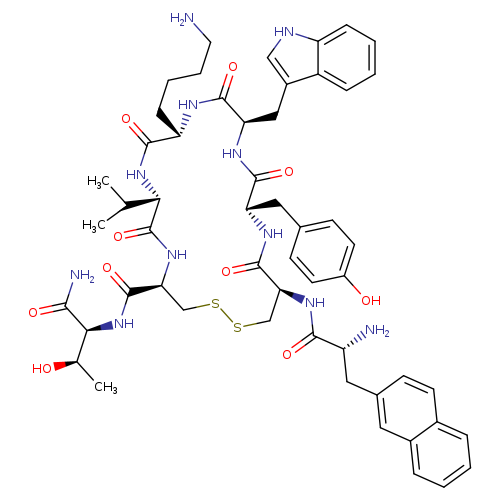
L-Threoninamide, 3-(2-naphthalenyl)-D-alanyl-L-cysteinyl-L-tyrosyl-D-tryptophyl-L-lysyl-L-valyl-L-cysteinyl-, cyclic (2→7)-disulfideCatalog No.:AA0039II CAS No.:108736-35-2 MDL No.:MFCD03424071 MF:C54H69N11O10S2 MW:1096.3234 |
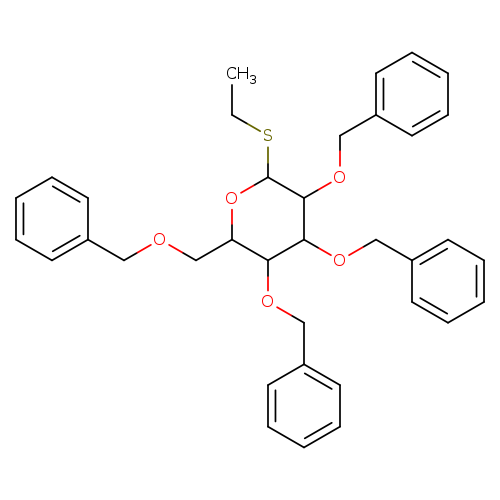
Ethyl 2,3,4,6-tetra-o-benzyl-beta-d-thioglucopyranosideCatalog No.:AA008W0L CAS No.:108739-67-9 MDL No.:MFCD13182858 MF:C36H40O5S MW:584.7648 |
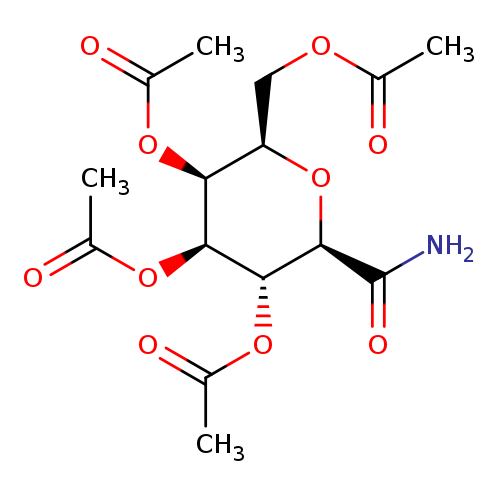
2,3,4,6-Tetra-O-acetyl-b-D-galactopyranosyl formamideCatalog No.:AA007BVS CAS No.:108739-88-4 MDL No.:MFCD08703924 MF:C15H21NO10 MW:375.3279 |
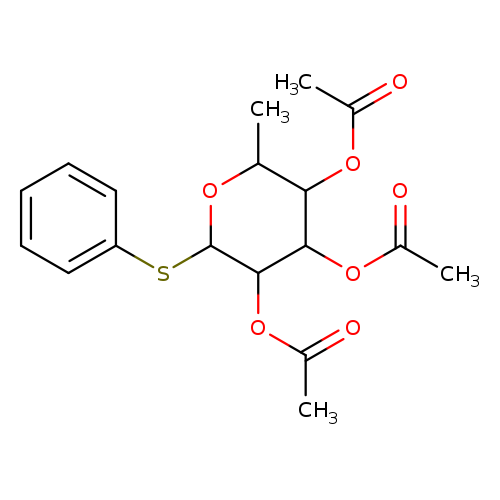
Phenyl 2,3,4-Tri-O-acetyl-1-thio-α-L-rhamnopyranosideCatalog No.:AA008VN8 CAS No.:108740-74-5 MDL No.:MFCD09841185 MF:C18H22O7S MW:382.4281 |
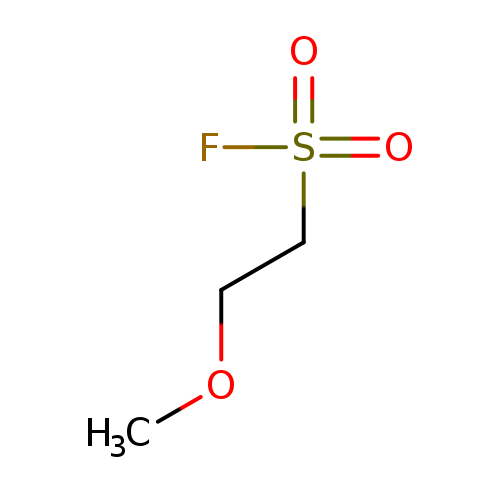
2-methoxyethane-1-sulfonyl fluorideCatalog No.:AA01A8ZD CAS No.:1087410-86-3 MDL No.:MFCD18917239 MF:C3H7FO3S MW:142.1493 |
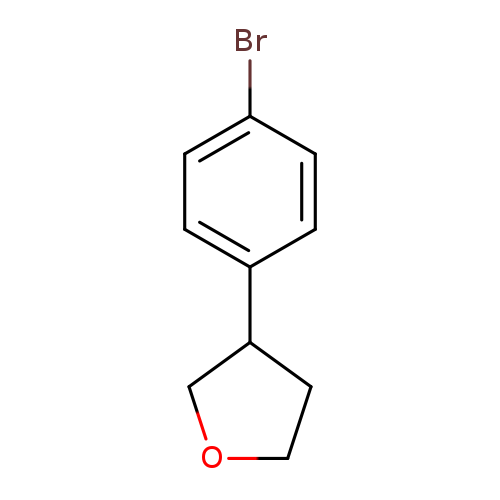
3-(4-Bromophenyl)tetrahydrofuranCatalog No.:AA00HB5H CAS No.:1087415-99-3 MDL No.:MFCD21099831 MF:C10H11BrO MW:227.0977 |
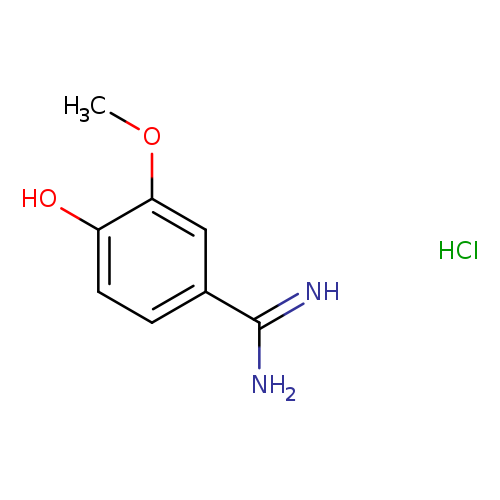
4-Hydroxy-3-methoxybenzimidamide hydrochlorideCatalog No.:AA00J07J CAS No.:108748-73-8 MDL No.:MFCD16090015 MF:C8H11ClN2O2 MW:202.6381 |
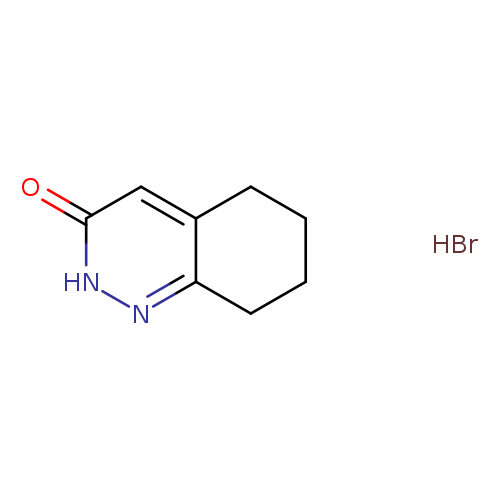
5,6,7,8-Tetrahydrocinnolin-3(2H)-one hydrobromideCatalog No.:AA008X8Q CAS No.:108749-03-7 MDL No.:MFCD06809755 MF:C8H11BrN2O MW:231.0897 |
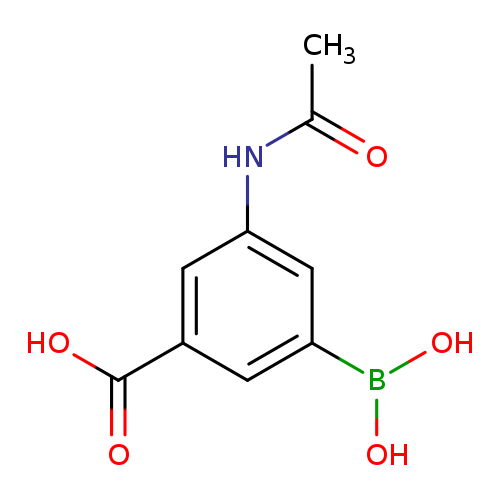
3-Acetamido-5-boronobenzoic acidCatalog No.:AA007TXC CAS No.:108749-15-1 MDL No.:MFCD08436066 MF:C9H10BNO5 MW:222.9904 |
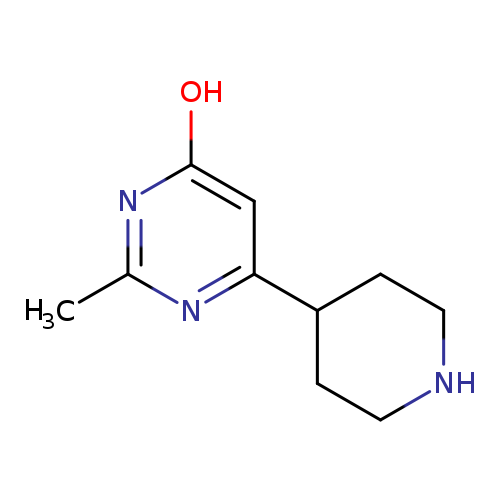
2-Methyl-6-(piperidin-4-yl)pyrimidin-4-olCatalog No.:AA008VFI CAS No.:1087527-83-0 MDL No.:MFCD11054029 MF:C10H15N3O MW:193.2456 |
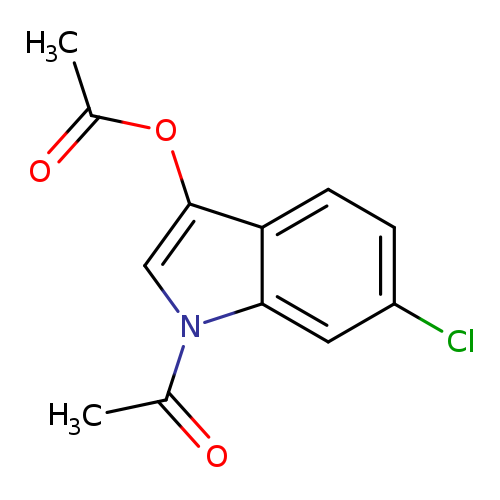
6-Chloroindoxyl-1,3-diacetateCatalog No.:AA008ROH CAS No.:108761-33-7 MDL No.:MFCD00269702 MF:C12H10ClNO3 MW:251.6657 |
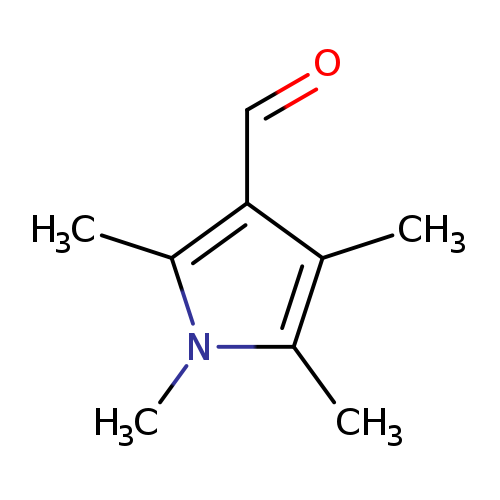
1,2,4,5-Tetramethyl-1h-pyrrole-3-carbaldehydeCatalog No.:AA008VEK CAS No.:1087610-71-6 MDL No.:MFCD13176469 MF:C9H13NO MW:151.2056 |
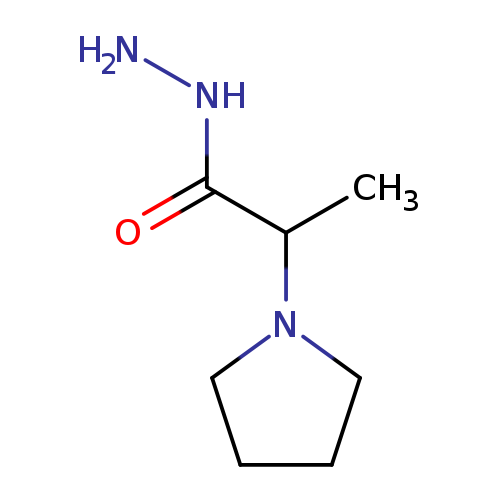
2-(Pyrrolidin-1-yl)propanehydrazideCatalog No.:AA008VF7 CAS No.:1087614-09-2 MDL No.:MFCD13961272 MF:C7H15N3O MW:157.2135 |
4-(4-Morpholinyl)butanohydrazideCatalog No.:AA008VDH CAS No.:1087616-69-0 MDL No.:MFCD11868666 MF:C8H17N3O2 MW:187.2395 |
methyl 3-[(E)-2-(dimethylamino)ethenyl]-4-nitrobenzoateCatalog No.:AA00IRCU CAS No.:108763-41-3 MDL No.:MFCD06496141 MF:C12H14N2O4 MW:250.2506 |
2-Amino-2-(1h-indol-5-yl)acetic acidCatalog No.:AA007BVM CAS No.:108763-43-5 MDL No.:MFCD06656882 MF:C10H10N2O2 MW:190.1986 |
1-butyl-5-(5-fluoro-2-hydroxybenzoyl)-2-oxo-1,2-dihydropyridine-3-carbonitrileCatalog No.:AA01AAE4 CAS No.:1087634-93-2 MDL No.:MFCD10649405 MF:C17H15FN2O3 MW:314.3110 |
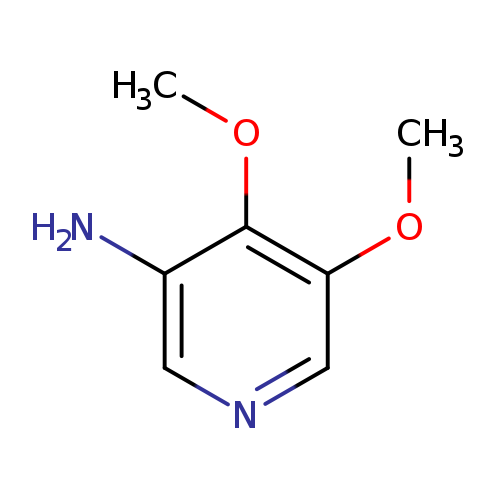
4,5-Dimethoxypyridin-3-amineCatalog No.:AA0090Q1 CAS No.:1087659-17-3 MDL No.:MFCD11052856 MF:C7H10N2O2 MW:154.1665 |
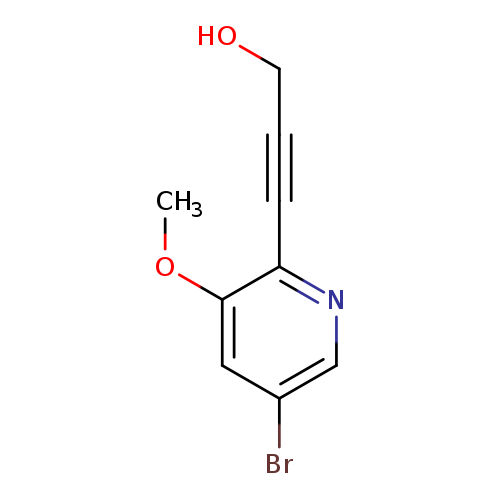
3-(5-Bromo-3-methoxypyridin-2-yl)prop-2-yn-1-olCatalog No.:AA00945J CAS No.:1087659-22-0 MDL No.:MFCD11857630 MF:C9H8BrNO2 MW:242.0693 |
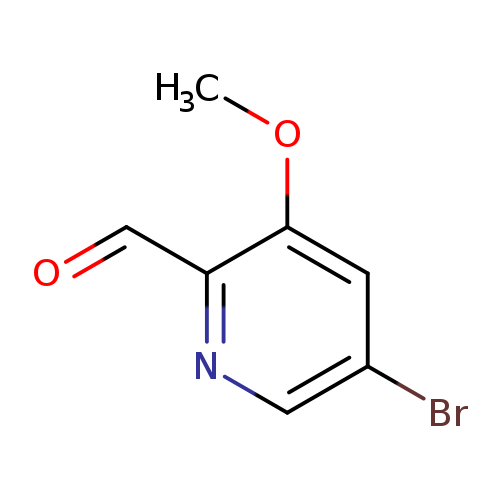
5-Bromo-3-methoxypicolinaldehydeCatalog No.:AA00945W CAS No.:1087659-24-2 MDL No.:MFCD11857632 MF:C7H6BrNO2 MW:216.0320 |
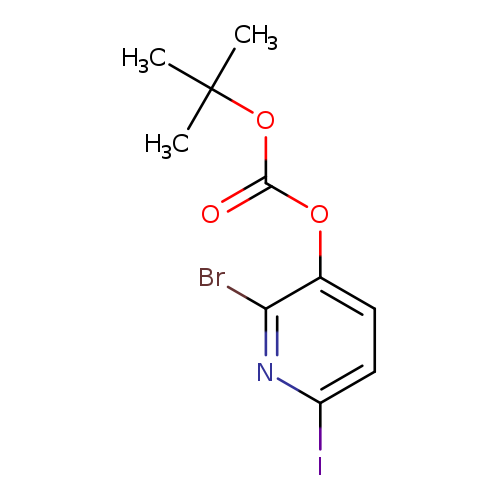
2-Bromo-6-iodopyridin-3-yl tert-butyl carbonateCatalog No.:AA0094DW CAS No.:1087659-26-4 MDL No.:MFCD11857635 MF:C10H11BrINO3 MW:400.0077 |
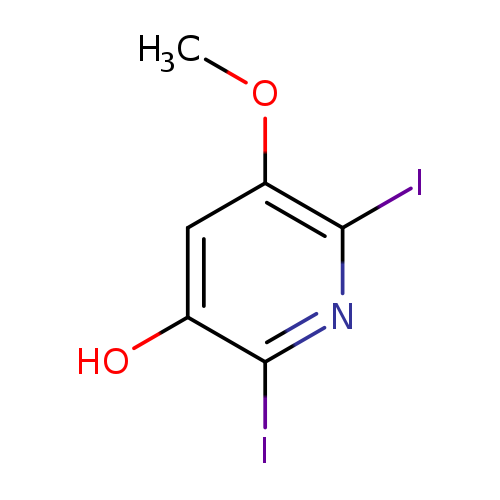
2,6-Diiodo-5-methoxypyridin-3-olCatalog No.:AA0090I1 CAS No.:1087659-27-5 MDL No.:MFCD11857637 MF:C6H5I2NO2 MW:376.9183 |
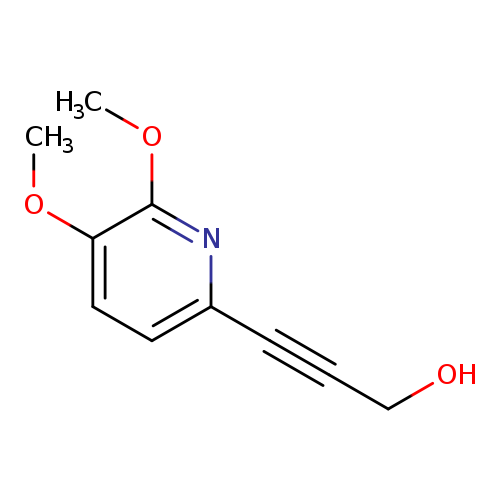
3-(5,6-Dimethoxypyridin-2-yl)prop-2-yn-1-olCatalog No.:AA0090T5 CAS No.:1087659-28-6 MDL No.:MFCD11857639 MF:C10H11NO3 MW:193.1992 |
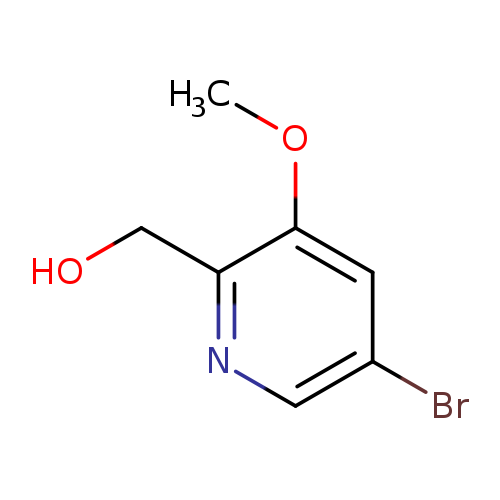
(5-Bromo-3-methoxypyridin-2-yl)methanolCatalog No.:AA009LZ6 CAS No.:1087659-32-2 MDL No.:MFCD11857646 MF:C7H8BrNO2 MW:218.0479 |
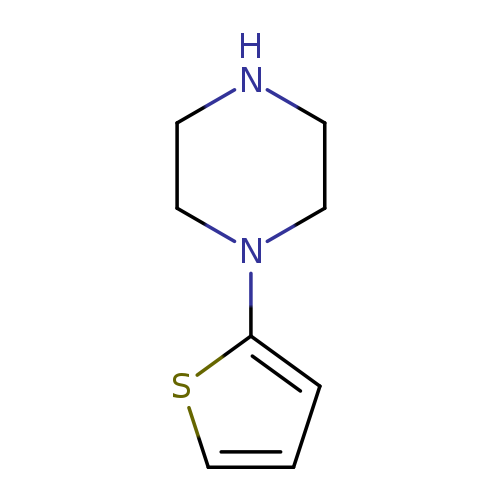
1-(Thiophen-2-yl)piperazineCatalog No.:AA0091ZO CAS No.:108768-19-0 MDL No.:MFCD11872772 MF:C8H12N2S MW:168.2593 |
1-(2,6-Dimethoxyphenyl)ethanamine hydrochlorideCatalog No.:AA00969Y CAS No.:1087707-43-4 MDL No.:MFCD30478930 MF:C10H16ClNO2 MW:217.6925 |
4-(2H-1,2,3-Triazol-2-yl)aniline hydrochlorideCatalog No.:AA008TLL CAS No.:1087712-11-5 MDL No.:MFCD11505547 MF:C8H9ClN4 MW:196.6369 |
2-(1H-1,2,4-Triazol-5-yl)acetic acid hydrochlorideCatalog No.:AA00HB5R CAS No.:1087714-25-7 MDL No.:MFCD19689158 MF:C4H6ClN3O2 MW:163.5623 |
ethyl 4-methyl-2-(phenylamino)-1,3-thiazole-5-carboxylate hydrochlorideCatalog No.:AA019MMB CAS No.:1087715-00-1 MDL No.:MFCD11099442 MF:C13H15ClN2O2S MW:298.7884 |
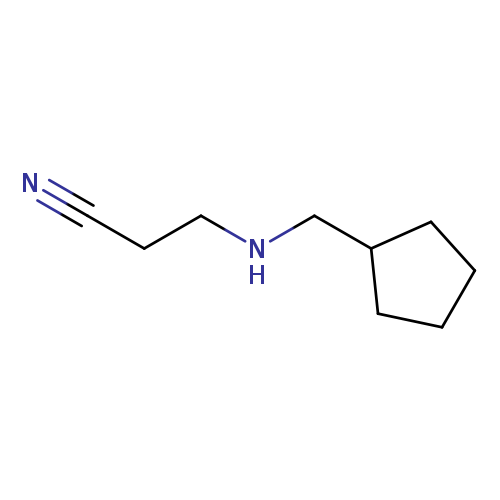
3-[(Cyclopentylmethyl)amino]propanenitrileCatalog No.:AA01AAM7 CAS No.:1087717-80-3 MDL No.:MFCD12166311 MF:C9H16N2 MW:152.2367 |
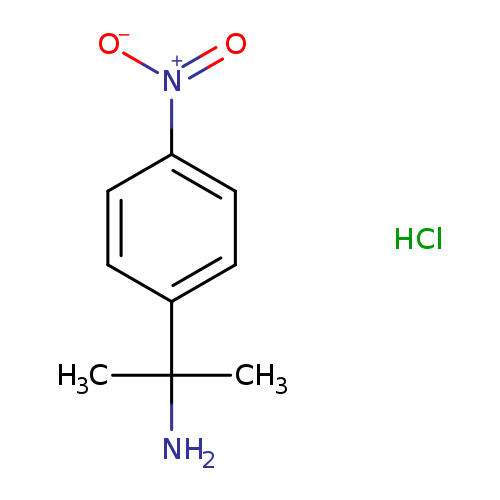
2-(4-Nitrophenyl)propan-2-amine hydrochlorideCatalog No.:AA019YHV CAS No.:1087719-23-0 MDL No.:MFCD08689977 MF:C9H13ClN2O2 MW:216.6647 |
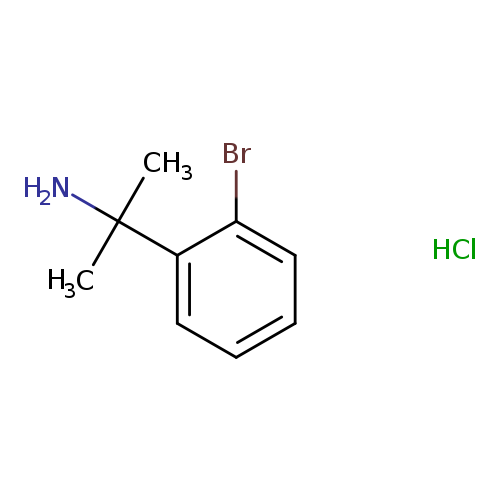
2-(2-Bromophenyl)propan-2-amine HClCatalog No.:AA00968F CAS No.:1087723-47-4 MDL No.:MFCD11870089 MF:C9H13BrClN MW:250.5632 |
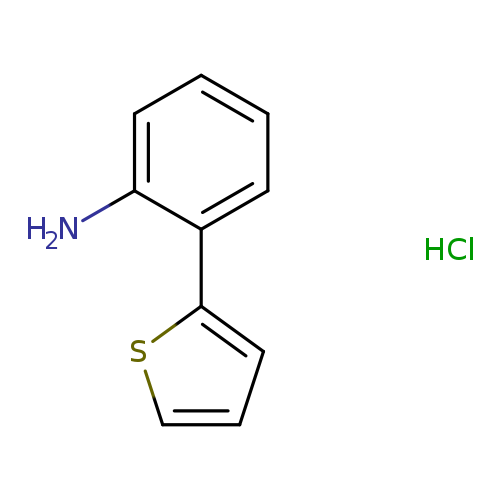
2-(2-Aminophenyl)thiophene hydrochlorideCatalog No.:AA008U7J CAS No.:1087723-62-3 MDL No.:MFCD07365346 MF:C10H10ClNS MW:211.7111 |
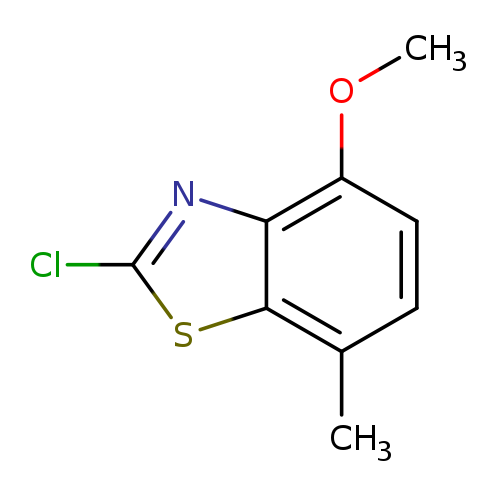
Benzothiazole, 2-chloro-4-methoxy-7-methyl- (9CI)Catalog No.:AA0091IJ CAS No.:108773-00-8 MDL No.:MFCD04971832 MF:C9H8ClNOS MW:213.6839 |
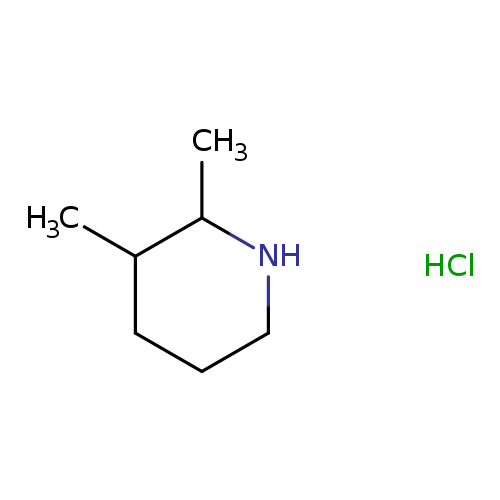
2,3-Dimethylpiperidine hydrochlorideCatalog No.:AA01AKQX CAS No.:1087730-30-0 MDL No.:MFCD19160661 MF:C7H16ClN MW:149.6616 |
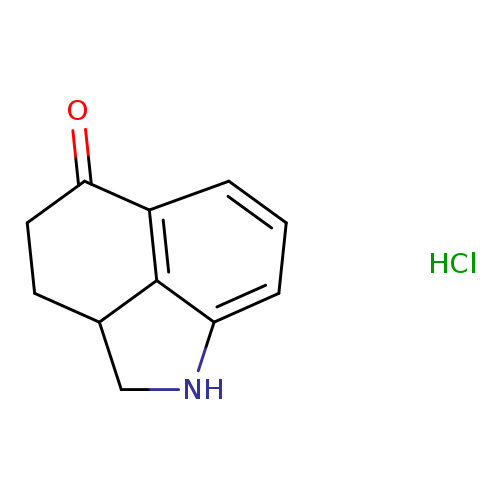
2-azatricyclo[6.3.1.0,4,12]dodeca-1(11),8(12),9-trien-7-one hydrochlorideCatalog No.:AA01ACEP CAS No.:1087737-80-1 MDL No.:MFCD26407990 MF:C11H12ClNO MW:209.6721 |
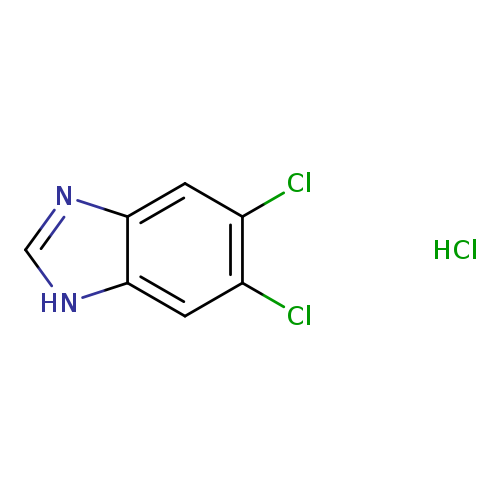
5,6-Dichlorobenzimidazole hydrochlorideCatalog No.:AA008WIN CAS No.:1087737-96-9 MDL No.:MFCD16810292 MF:C7H5Cl3N2 MW:223.4870 |
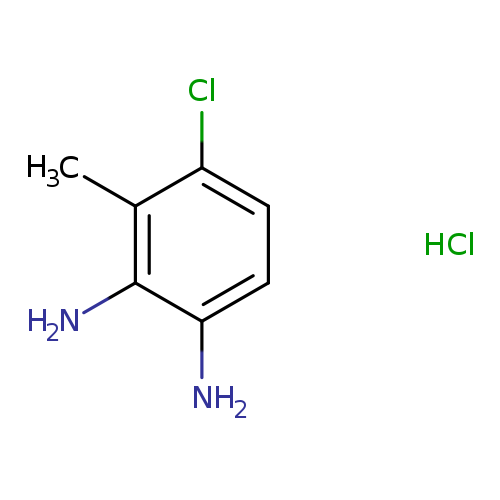
4-Chloro-3-methylbenzene-1,2-diamine hydrochlorideCatalog No.:AA00836S CAS No.:1087743-89-2 MDL No.:MFCD23135721 MF:C7H10Cl2N2 MW:193.0737 |
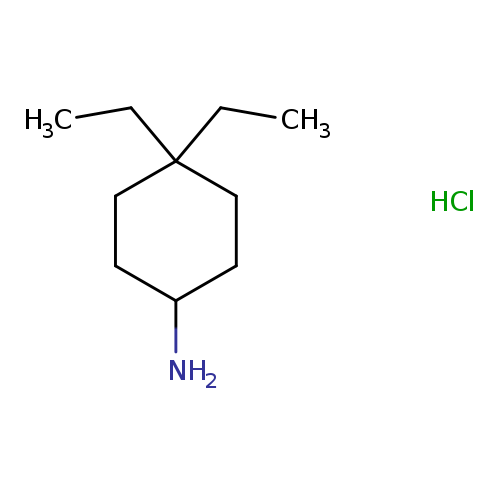
4,4-Diethylcyclohexan-1-amine hydrochlorideCatalog No.:AA01BCY4 CAS No.:1087747-58-7 MDL No.:MFCD28012373 MF:C10H22ClN MW:191.7414 |
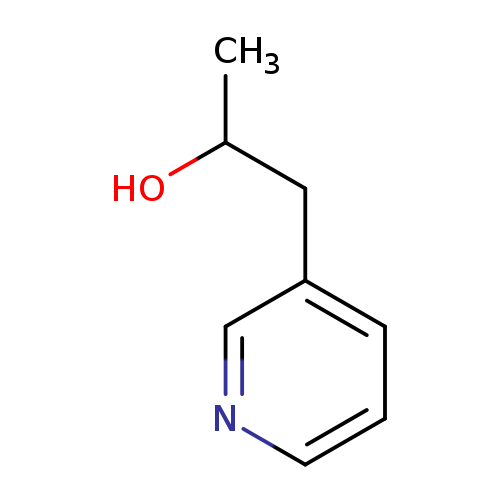
1-(pyridin-3-yl)propan-2-olCatalog No.:AA01A4LY CAS No.:1087751-29-8 MDL No.:MFCD16786236 MF:C8H11NO MW:137.1790 |
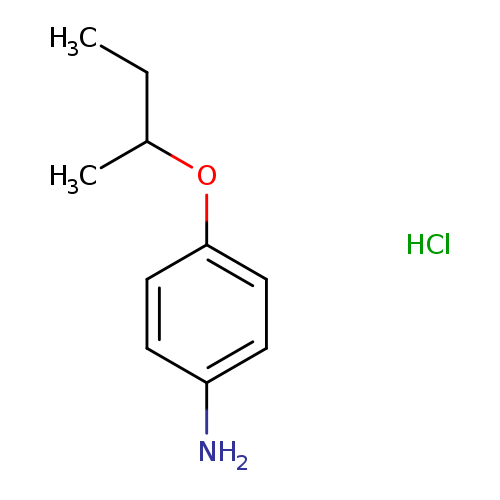
(4-sec-butoxyphenyl)amine hydrochlorideCatalog No.:AA00J1NS CAS No.:1087751-49-2 MDL No.:MFCD00025317 MF:C10H16ClNO MW:201.6931 |
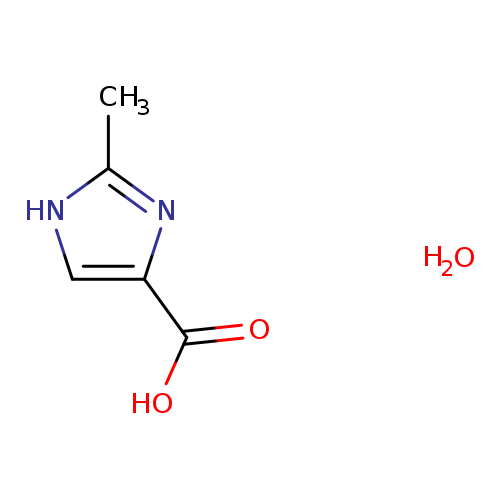
2-methyl-1H-imidazole-4-carboxylic acid hydrateCatalog No.:AA00J1I2 CAS No.:1087768-73-7 MDL No.:MFCD13193967 MF:C5H8N2O3 MW:144.1286 |
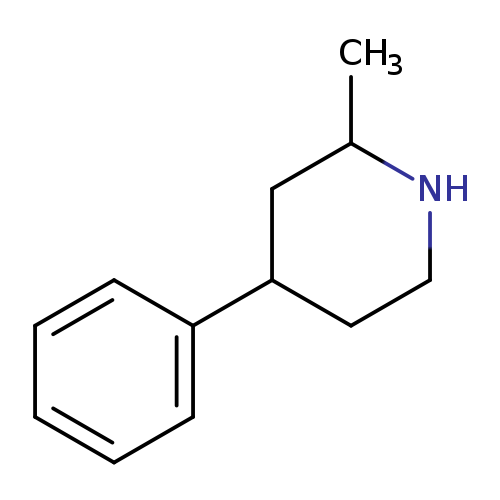
2-methyl-4-phenylpiperidineCatalog No.:AA01B7UD CAS No.:108777-57-7 MDL No.:MFCD20669851 MF:C12H17N MW:175.2701 |
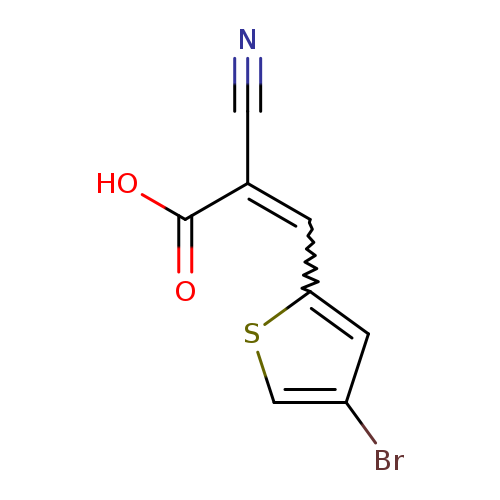
3-(4-Bromothiophen-2-yl)-2-cyanoacrylic acidCatalog No.:AA00IWWO CAS No.:1087780-82-2 MDL No.:MFCD17392921 MF:C8H4BrNO2S MW:258.0919 |
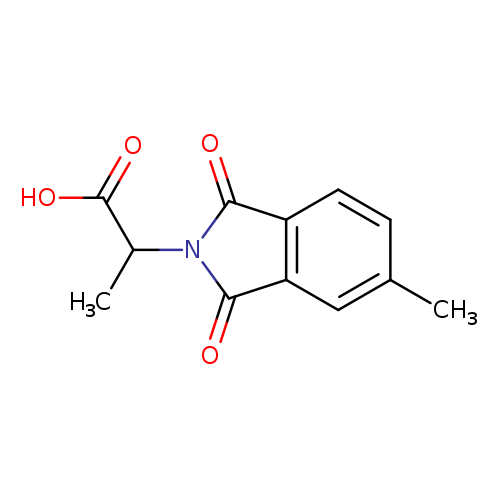
2-(5-methyl-1,3-dioxo-2,3-dihydro-1H-isoindol-2-yl)propanoic acidCatalog No.:AA019P6B CAS No.:1087784-06-2 MDL No.:MFCD11099376 MF:C12H11NO4 MW:233.2200 |
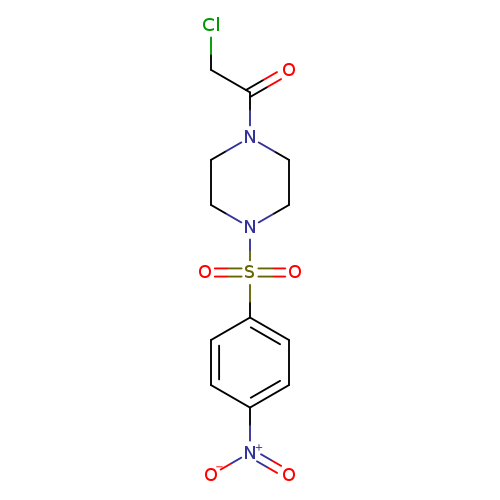
1-(Chloroacetyl)-4-[(4-nitrophenyl)sulfonyl]piperazineCatalog No.:AA019P85 CAS No.:1087784-07-3 MDL No.:MFCD11099379 MF:C12H14ClN3O5S MW:347.7747 |
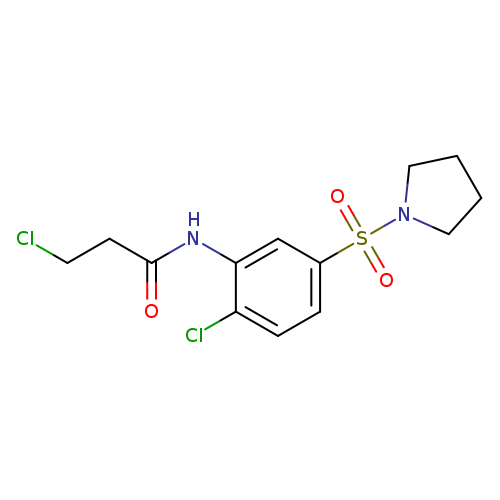
3-chloro-N-[2-chloro-5-(pyrrolidine-1-sulfonyl)phenyl]propanamideCatalog No.:AA019K4G CAS No.:1087784-08-4 MDL No.:MFCD02215971 MF:C13H16Cl2N2O3S MW:351.2487 |
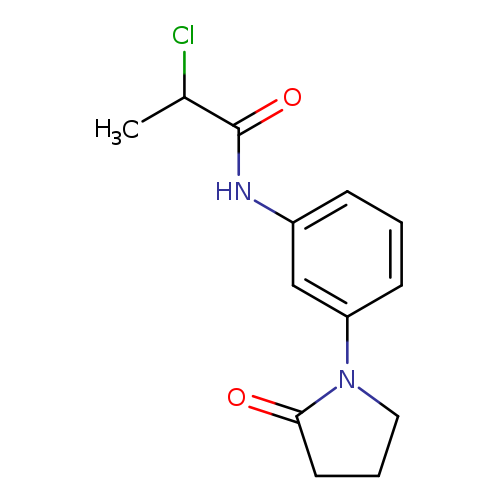
2-chloro-N-[3-(2-oxopyrrolidin-1-yl)phenyl]propanamideCatalog No.:AA019KOA CAS No.:1087784-11-9 MDL No.:MFCD11505362 MF:C13H15ClN2O2 MW:266.7234 |
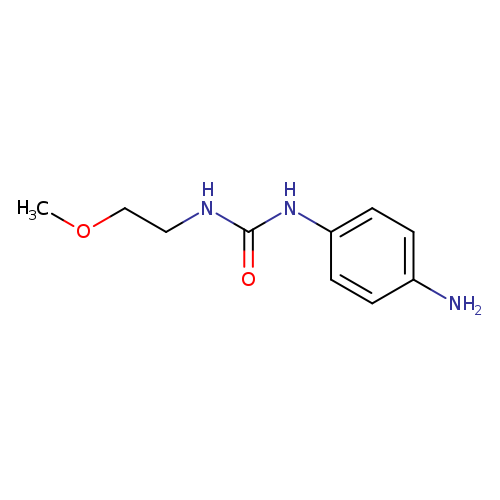
1-(4-aminophenyl)-3-(2-methoxyethyl)ureaCatalog No.:AA019KTY CAS No.:1087784-17-5 MDL No.:MFCD11505367 MF:C10H15N3O2 MW:209.2450 |
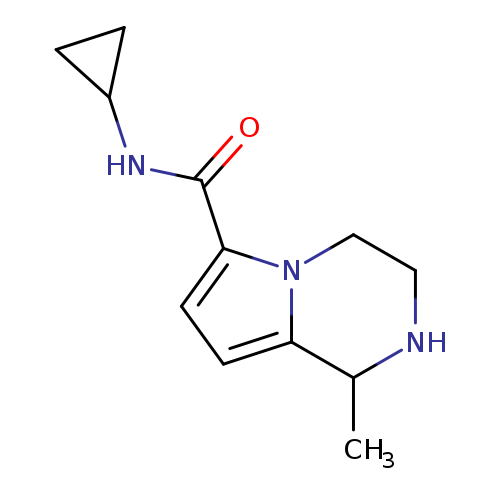
N-cyclopropyl-1-methyl-1H,2H,3H,4H-pyrrolo[1,2-a]pyrazine-6-carboxamideCatalog No.:AA019LIA CAS No.:1087784-19-7 MDL No.:MFCD11099400 MF:C12H17N3O MW:219.2829 |
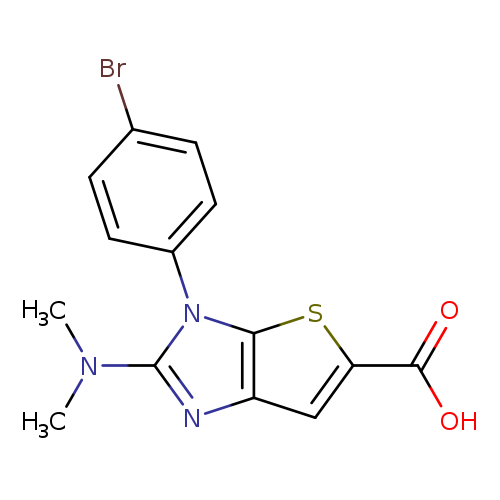
3-(4-bromophenyl)-2-(dimethylamino)-3H-thieno[2,3-d]imidazole-5-carboxylic acidCatalog No.:AA019LV6 CAS No.:1087784-20-0 MDL No.:MFCD11505370 MF:C14H12BrN3O2S MW:366.2330 |
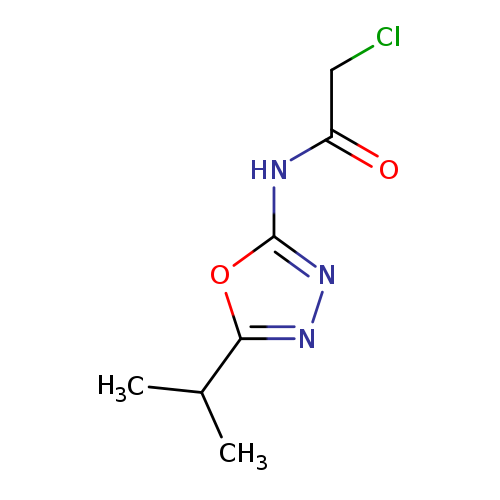
2-chloro-N-[5-(propan-2-yl)-1,3,4-oxadiazol-2-yl]acetamideCatalog No.:AA019MAS CAS No.:1087784-21-1 MDL No.:MFCD11099408 MF:C7H10ClN3O2 MW:203.6262 |
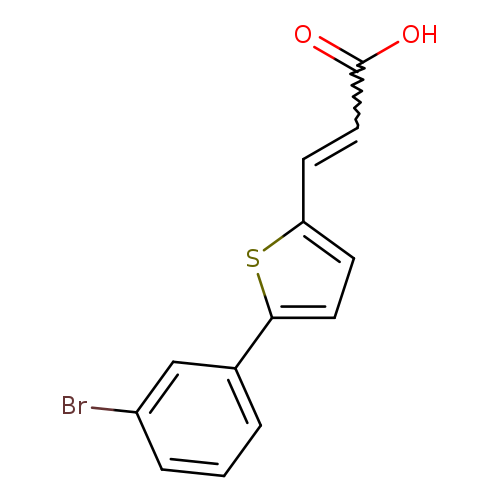
3-[5-(3-bromophenyl)thiophen-2-yl]prop-2-enoic acidCatalog No.:AA019MLG CAS No.:1087784-22-2 MDL No.:MFCD11099423 MF:C13H9BrO2S MW:309.1784 |
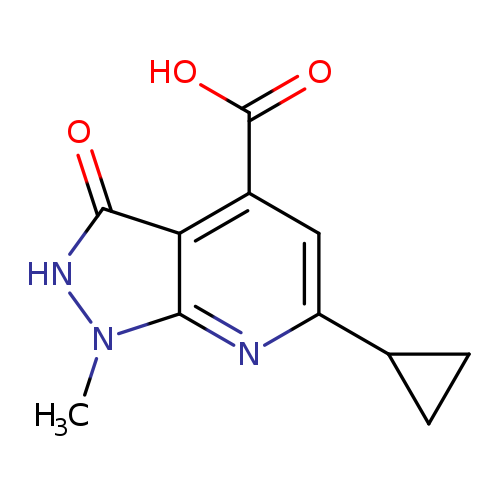
6-Cyclopropyl-1-methyl-3-oxo-2,3-dihydro-1H-pyrazolo[3,4-b]pyridine-4-carboxylic acidCatalog No.:AA019MLW CAS No.:1087784-23-3 MDL No.:MFCD11099431 MF:C11H11N3O3 MW:233.2233 |
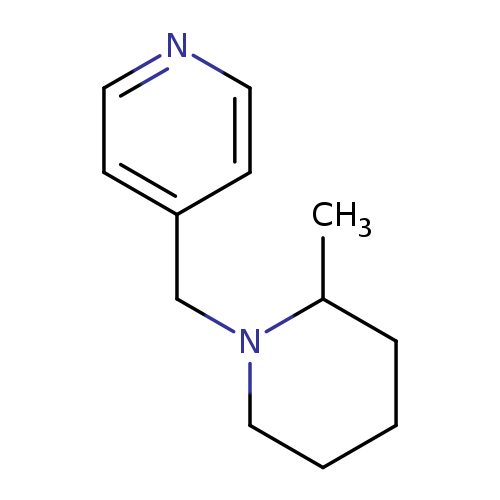
4-[(2-methylpiperidin-1-yl)methyl]pyridineCatalog No.:AA019MML CAS No.:1087784-25-5 MDL No.:MFCD11099444 MF:C12H18N2 MW:190.2847 |
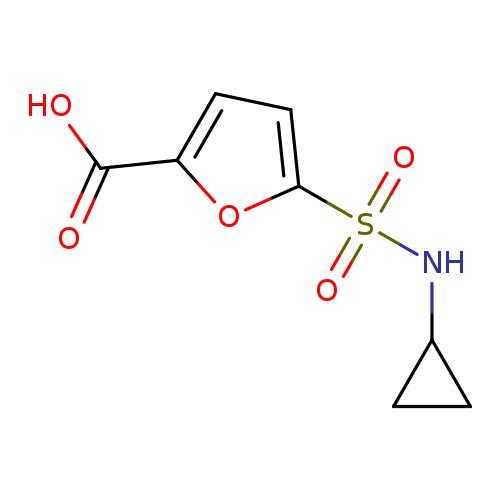
5-(cyclopropylsulfamoyl)furan-2-carboxylic acidCatalog No.:AA019MNE CAS No.:1087784-27-7 MDL No.:MFCD10694673 MF:C8H9NO5S MW:231.2258 |
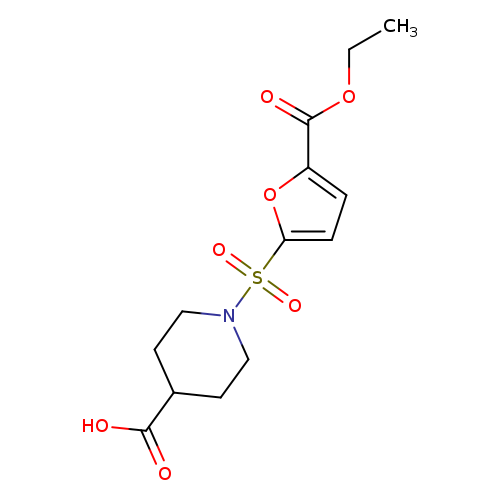
1-{[5-(ethoxycarbonyl)furan-2-yl]sulfonyl}piperidine-4-carboxylic acidCatalog No.:AA019MNM CAS No.:1087784-28-8 MDL No.:MFCD11099467 MF:C13H17NO7S MW:331.3416 |
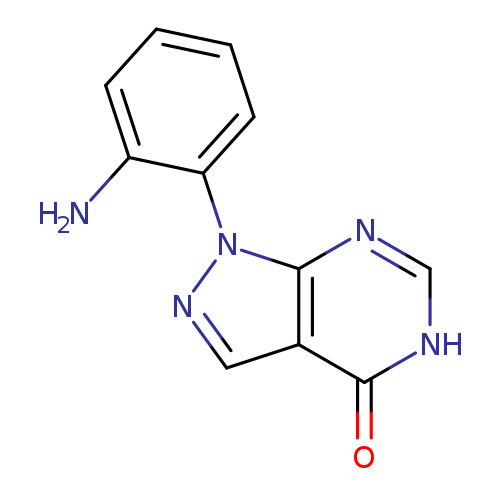
1-(2-aminophenyl)-1H,4H,5H-pyrazolo[3,4-d]pyrimidin-4-oneCatalog No.:AA019MOB CAS No.:1087784-31-3 MDL No.:MFCD11099478 MF:C11H9N5O MW:227.2221 |
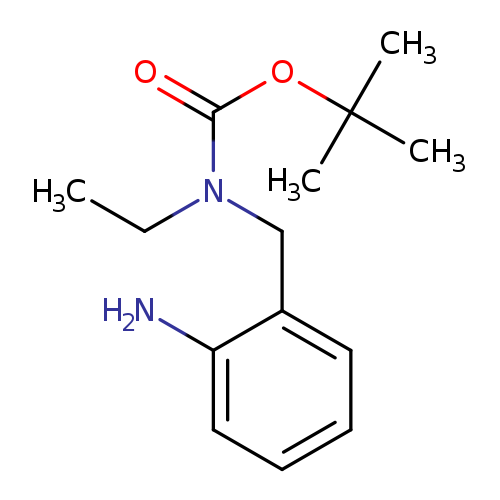
tert-butyl N-[(2-aminophenyl)methyl]-N-ethylcarbamateCatalog No.:AA019MOZ CAS No.:1087784-32-4 MDL No.:MFCD11099489 MF:C14H22N2O2 MW:250.3367 |
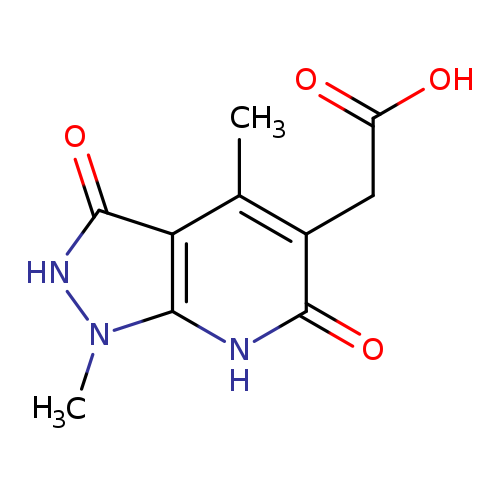
2-{1,4-dimethyl-3,6-dioxo-1H,2H,3H,6H,7H-pyrazolo[3,4-b]pyridin-5-yl}acetic acidCatalog No.:AA019MPG CAS No.:1087784-33-5 MDL No.:MFCD11099496 MF:C10H11N3O4 MW:237.2120 |
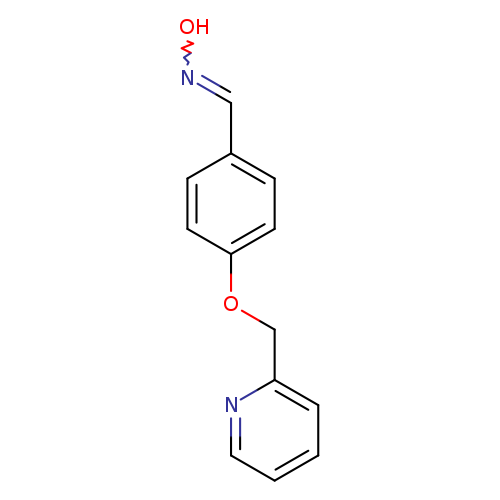
N-{[4-(pyridin-2-ylmethoxy)phenyl]methylidene}hydroxylamineCatalog No.:AA019MQZ CAS No.:1087784-36-8 MDL No.:MFCD11099519 MF:C13H12N2O2 MW:228.2466 |
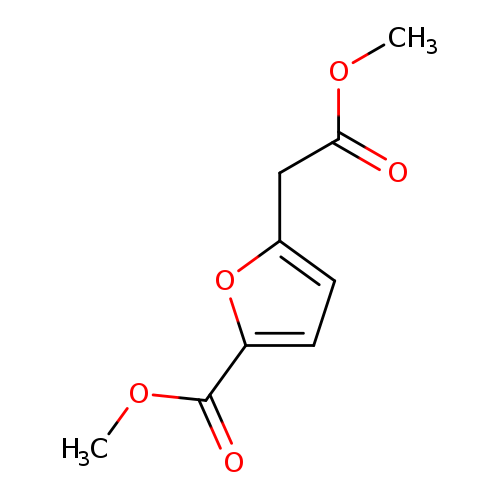
methyl 5-(2-methoxy-2-oxoethyl)furan-2-carboxylateCatalog No.:AA019MRG CAS No.:1087784-37-9 MDL No.:MFCD11099528 MF:C9H10O5 MW:198.1727 |
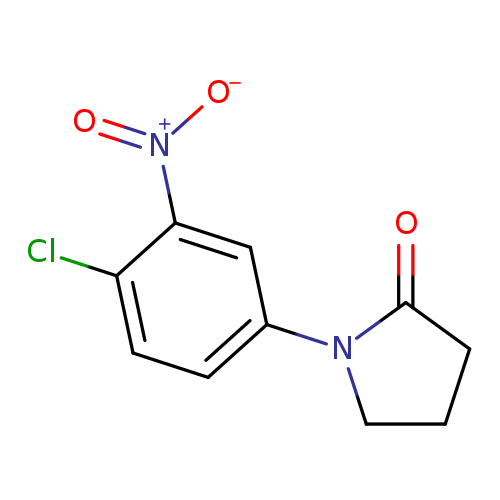
1-(4-Chloro-3-nitrophenyl)pyrrolidin-2-oneCatalog No.:AA019MRZ CAS No.:1087784-40-4 MDL No.:MFCD11099539 MF:C10H9ClN2O3 MW:240.6431 |
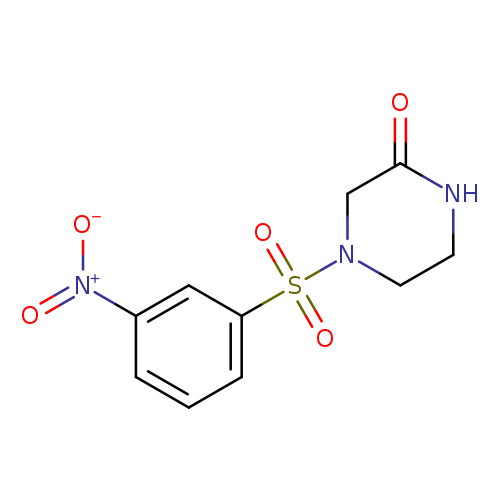
4-(3-nitrobenzenesulfonyl)piperazin-2-oneCatalog No.:AA019MTA CAS No.:1087784-42-6 MDL No.:MFCD11099556 MF:C10H11N3O5S MW:285.2764 |
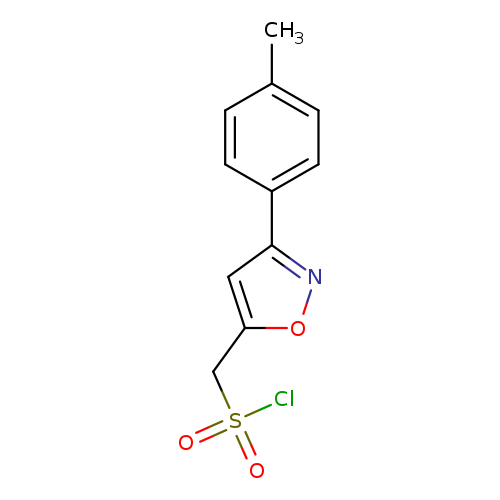
[3-(4-methylphenyl)-1,2-oxazol-5-yl]methanesulfonyl chlorideCatalog No.:AA019MTL CAS No.:1087784-43-7 MDL No.:MFCD11099561 MF:C11H10ClNO3S MW:271.7200 |
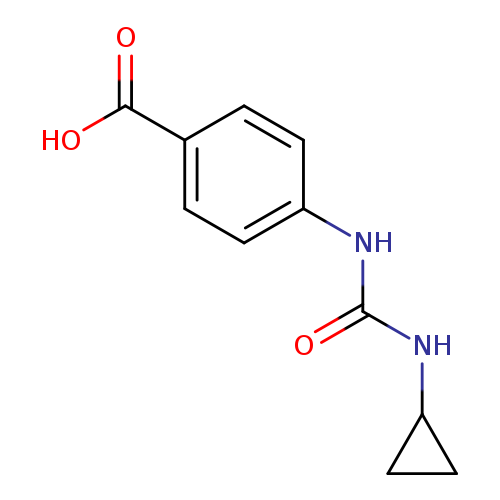
4-([(Cyclopropylamino)carbonyl]amino)benzoic acidCatalog No.:AA019MUS CAS No.:1087784-47-1 MDL No.:MFCD11099579 MF:C11H12N2O3 MW:220.2246 |
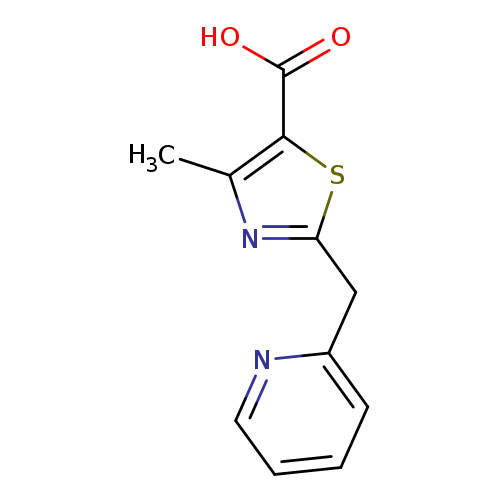
4-methyl-2-(pyridin-2-ylmethyl)-1,3-thiazole-5-carboxylic acidCatalog No.:AA019MWC CAS No.:1087784-51-7 MDL No.:MFCD11099601 MF:C11H10N2O2S MW:234.2743 |
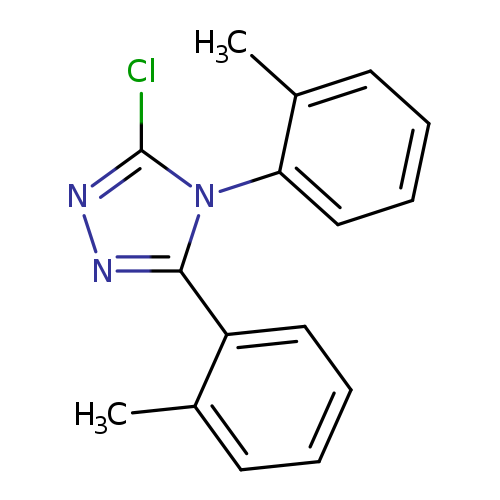
3-chloro-4,5-bis(2-methylphenyl)-4H-1,2,4-triazoleCatalog No.:AA019MWF CAS No.:1087784-52-8 MDL No.:MFCD11099604 MF:C16H14ClN3 MW:283.7555 |
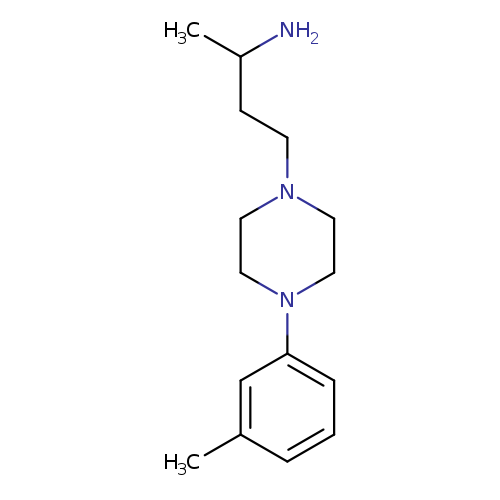
1-Methyl-3-[4-(3-methylphenyl)piperazin-1-yl]propylamineCatalog No.:AA019MWS CAS No.:1087784-53-9 MDL No.:MFCD11099611 MF:C15H25N3 MW:247.3791 |
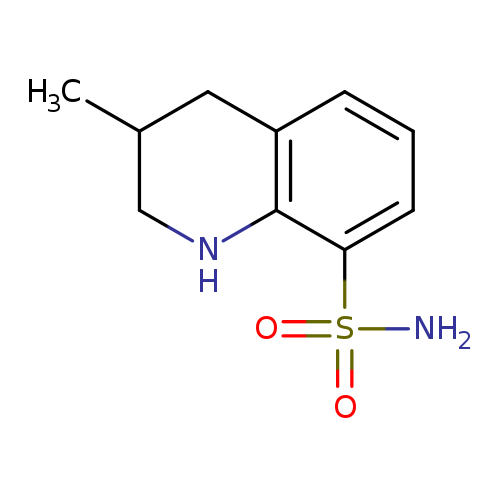
3-methyl-1,2,3,4-tetrahydroquinoline-8-sulfonamideCatalog No.:AA019MX7 CAS No.:1087784-54-0 MDL No.:MFCD11099620 MF:C10H14N2O2S MW:226.2954 |
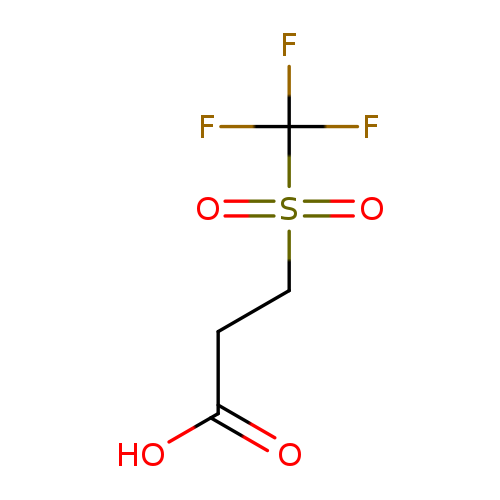
3-[(Trifluoromethyl)sulfonyl]propanoic acidCatalog No.:AA019MXT CAS No.:1087784-56-2 MDL No.:MFCD11099632 MF:C4H5F3O4S MW:206.1403 |
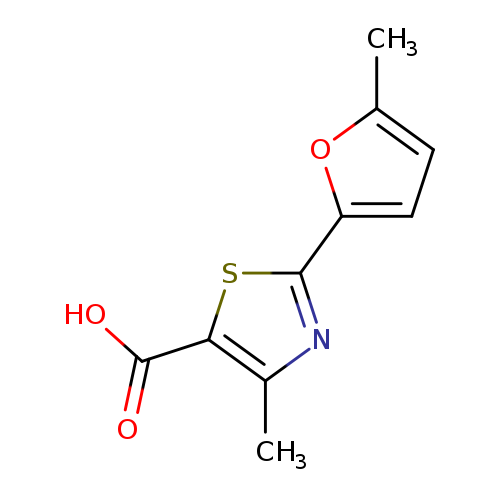
4-methyl-2-(5-methylfuran-2-yl)-1,3-thiazole-5-carboxylic acidCatalog No.:AA019MZL CAS No.:1087784-62-0 MDL No.:MFCD11505389 MF:C10H9NO3S MW:223.2484 |
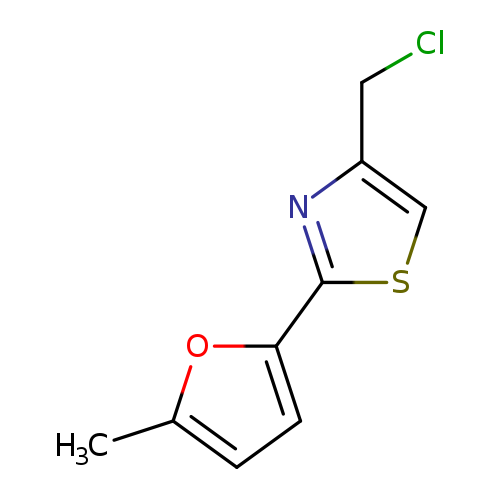
4-(chloromethyl)-2-(5-methylfuran-2-yl)-1,3-thiazoleCatalog No.:AA019MZP CAS No.:1087784-63-1 MDL No.:MFCD11505392 MF:C9H8ClNOS MW:213.6839 |
5-chloro-1,3-benzothiazol-4-amineCatalog No.:AA019N0X CAS No.:1087784-64-2 MDL No.:MFCD11505410 MF:C7H5ClN2S MW:184.6460 |
2-chloro-N-[3-cyano-1-(oxolan-2-ylmethyl)-4,5,6,7-tetrahydro-1H-indol-2-yl]acetamideCatalog No.:AA019N1U CAS No.:1087784-66-4 MDL No.:MFCD11505428 MF:C16H20ClN3O2 MW:321.8019 |
5-amino-N-(4-bromophenyl)-2-fluorobenzene-1-sulfonamideCatalog No.:AA019N37 CAS No.:1087784-69-7 MDL No.:MFCD11505452 MF:C12H10BrFN2O2S MW:345.1874 |
5-(piperazine-1-sulfonyl)furan-2-carboxamideCatalog No.:AA019N3T CAS No.:1087784-70-0 MDL No.:MFCD11505464 MF:C9H13N3O4S MW:259.2822 |
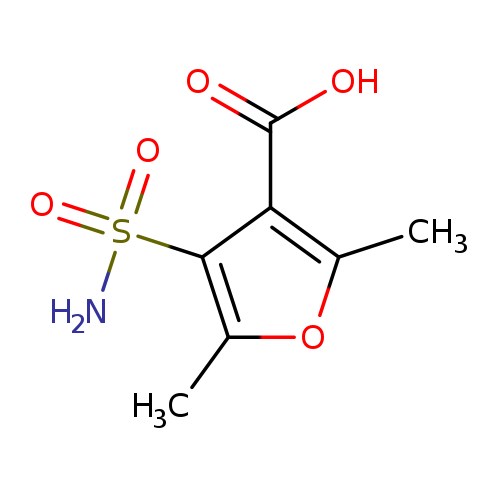
2,5-dimethyl-4-sulfamoylfuran-3-carboxylic acidCatalog No.:AA019N3V CAS No.:1087784-71-1 MDL No.:MFCD11505466 MF:C7H9NO5S MW:219.2151 |
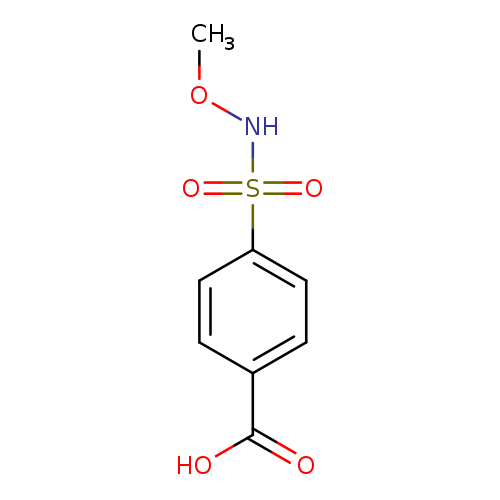
4-(methoxysulfamoyl)benzoic acidCatalog No.:AA019N3Y CAS No.:1087784-72-2 MDL No.:MFCD11505469 MF:C8H9NO5S MW:231.2258 |
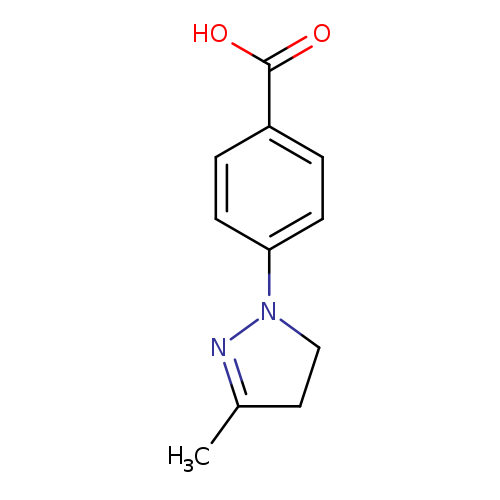
4-(3-Methyl-4,5-dihydro-1h-pyrazol-1-yl)benzoic acidCatalog No.:AA019N7S CAS No.:1087784-74-4 MDL No.:MFCD11505520 MF:C11H12N2O2 MW:204.2252 |
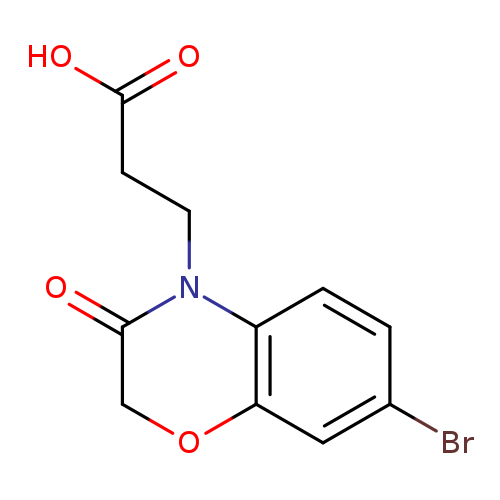
3-(7-Bromo-3-oxo-2H-benzo[b][1,4]oxazin-4(3H)-yl)propanoic acidCatalog No.:AA019N7X CAS No.:1087784-75-5 MDL No.:MFCD11505521 MF:C11H10BrNO4 MW:300.1054 |
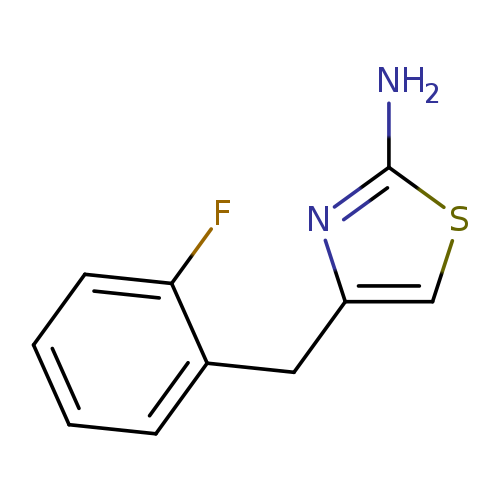
4-[(2-fluorophenyl)methyl]-1,3-thiazol-2-amineCatalog No.:AA019N95 CAS No.:1087784-76-6 MDL No.:MFCD11505537 MF:C10H9FN2S MW:208.2553 |
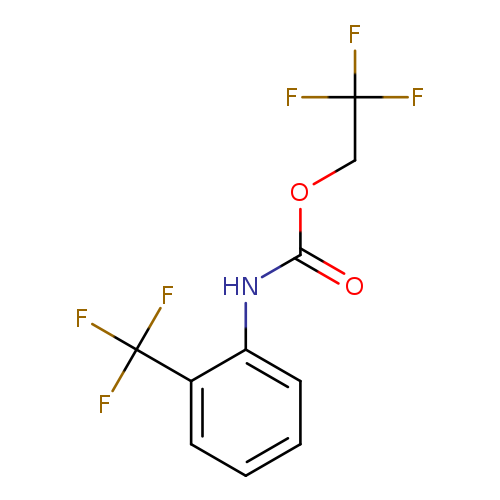
2,2,2-trifluoroethyl N-[2-(trifluoromethyl)phenyl]carbamateCatalog No.:AA01A8AK CAS No.:1087788-47-3 MDL No.:MFCD11099769 MF:C10H7F6NO2 MW:287.1585 |
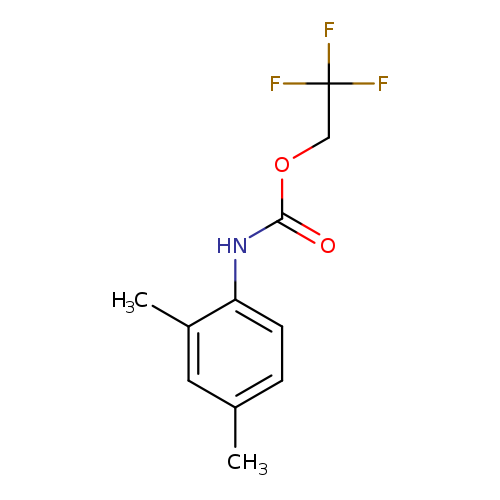
2,2,2-trifluoroethyl N-(2,4-dimethylphenyl)carbamateCatalog No.:AA019QPE CAS No.:1087788-52-0 MDL No.:MFCD11099778 MF:C11H12F3NO2 MW:247.2137 |
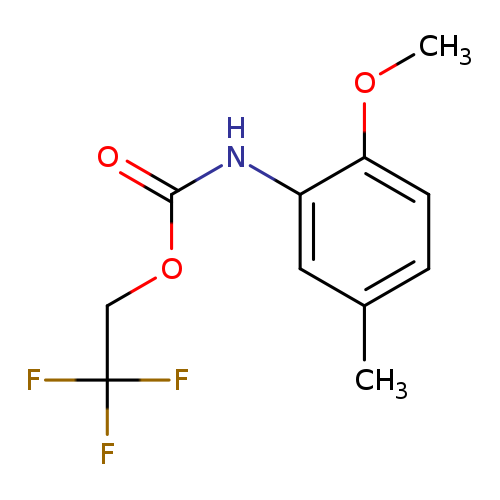
2,2,2-trifluoroethyl N-(2-methoxy-5-methylphenyl)carbamateCatalog No.:AA019QPG CAS No.:1087788-53-1 MDL No.:MFCD11099780 MF:C11H12F3NO3 MW:263.2131 |
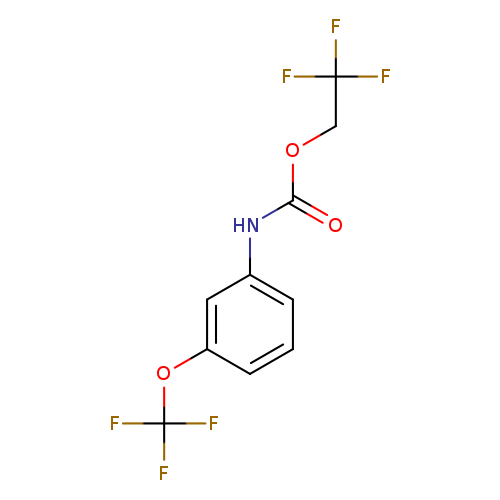
2,2,2-Trifluoroethyl 3-(trifluoromethoxy)phenylcarbamateCatalog No.:AA019QPI CAS No.:1087788-54-2 MDL No.:MFCD11099782 MF:C10H7F6NO3 MW:303.1579 |
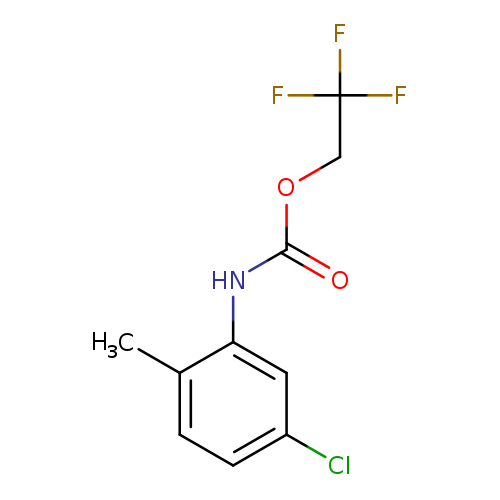
2,2,2-Trifluoroethyl 5-chloro-2-methylphenylcarbamateCatalog No.:AA019QPM CAS No.:1087788-56-4 MDL No.:MFCD11099786 MF:C10H9ClF3NO2 MW:267.6322 |
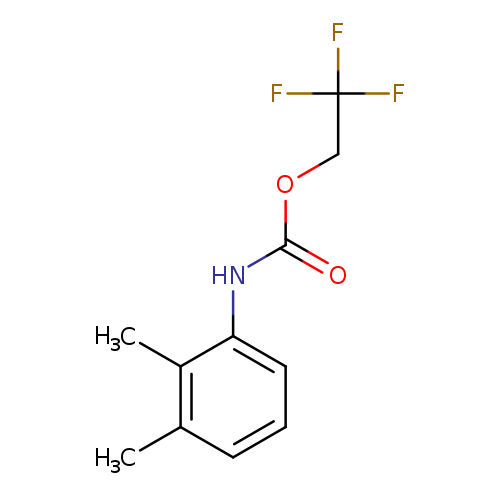
2,2,2-Trifluoroethyl 2,3-dimethylphenylcarbamateCatalog No.:AA019QPO CAS No.:1087788-57-5 MDL No.:MFCD11099788 MF:C11H12F3NO2 MW:247.2137 |
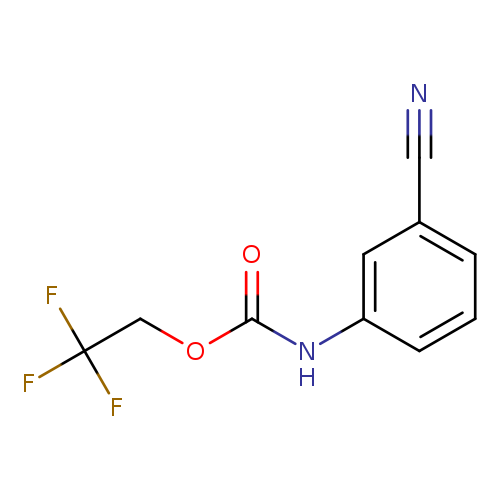
2,2,2-trifluoroethyl N-(3-cyanophenyl)carbamateCatalog No.:AA019U7L CAS No.:1087788-60-0 MDL No.:MFCD11099795 MF:C10H7F3N2O2 MW:244.1700 |
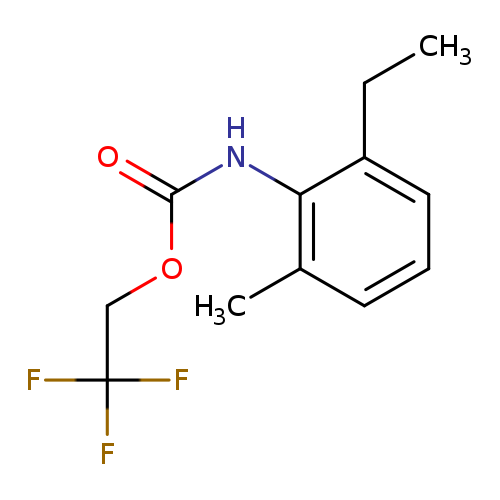
2,2,2-Trifluoroethyl 2-ethyl-6-methylphenylcarbamateCatalog No.:AA019QPY CAS No.:1087788-62-2 MDL No.:MFCD11099799 MF:C12H14F3NO2 MW:261.2403 |
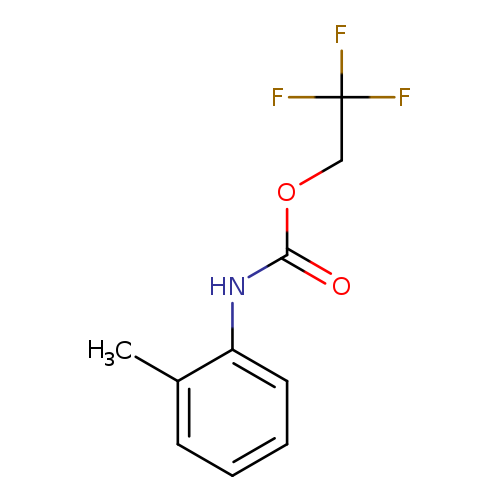
2,2,2-trifluoroethyl N-(2-methylphenyl)carbamateCatalog No.:AA019QQ2 CAS No.:1087788-64-4 MDL No.:MFCD01013358 MF:C10H10F3NO2 MW:233.1871 |
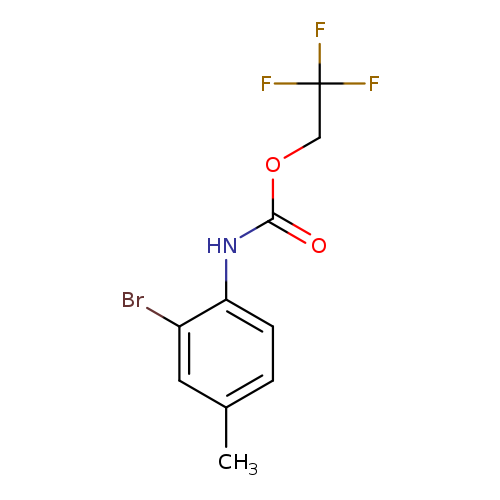
2,2,2-trifluoroethyl N-(2-bromo-4-methylphenyl)carbamateCatalog No.:AA019QQ7 CAS No.:1087788-66-6 MDL No.:MFCD11099807 MF:C10H9BrF3NO2 MW:312.0832 |
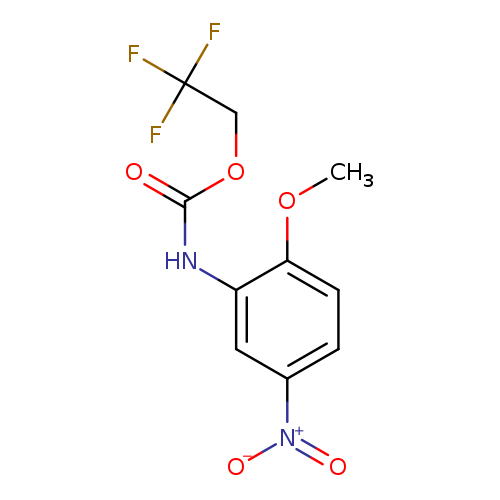
2,2,2-trifluoroethyl N-(2-methoxy-5-nitrophenyl)carbamateCatalog No.:AA019QQG CAS No.:1087788-70-2 MDL No.:MFCD11099815 MF:C10H9F3N2O5 MW:294.1841 |