2020-01-29 13:08:19
Yanling Ji, Xianghong He, Cheng Peng * and Wei Huang
1. Introduction
Indole scaffolds are the core of numerous natural alkaloids and pharmaceuticals exhibiting significant biological activities.1 Among these scaffolds, the spirooxindole, referred to as a “prominent scaffold”, plays an important role.2 C3-spirooxin- dole, for example, has good biological activity but is difficult to construct. Thus, numerous methods have been reported to construct spirooxindoles bearing a spirocyclic stereocenter at the C3 position.3 In recent years, many reviews have examined strategies to synthesize C3-spirooxindole.4 C2-spiropseudo- indoxyl is also widespread in natural products (Fig. 1).5 For instance, mitragynine pseudoindoxyl exhibits potent opioid agonistic activity in guinea pig ileum and mouse vas defer- ens,5a,b and ervaluteine can avoid multidrug resistance in vincristine-resistant KB cells.5e The enormous potential of C2- spiropseudoindoxyl has attracted many organic chemists to the study of its structural features and synthesis. While many methods have been described, few reviews have sought to bring them together and examine the progress in the field. The present review surveys methods to synthesize C2-spiro- pseudoindoxyl compounds and discusses the current challenges and future directions in this area.
The C2-spiropseudoindoxyl skeleton is generated from three types of substrates (Fig. 2): (i) nitrogen-containing phenylacetylene derivatives can undergo intramolecular cyclo- addition to synthesize C2-spiropseudoindoxyl; (ii) indole or indoline derivatives with a substituent at the C2 position can be coupled via cycloaddition with appropriate reaction part- ners; or (iii) 2-ylideneoxindole derivatives can react with other electron-rich substrates. The present review deals with all the three processes, which are covered in different sections based on the catalysis involved: metal catalysis, hypervalent iodine mediation, organic catalysis, or other catalytic mechanisms.
2. Metal-catalyzed synthesis
Due to their high efficiency, metal-catalytic reactions have become a research hotspot in chemical synthesis.6 Metal cata- lysis can synthesize many C2-spiropseudoindoxyl structures from simple substrates.
In 2011, Ramana and co-workers described a palladium- catalyzed intramolecular addition reaction of nitro- and alcohol units across alkyne 1, resulting in C2-spiropseudo- indoxyl derivatives 2. Optimal reaction conditions were 5 mol% Pd(CH3CN)2Cl2 as the catalyst in CH3CN at room temperature (Scheme 1a).7 With all the hexynols 1b and propargyl glycol derivatives 1c, C2-spiropseudoindoxyl derivatives were syn- thesized in good yields (78–84%). However, the cycloisomeriza- tion of 1a resulted in mixtures with a lower yield (32–38%). The proposed reaction mechanism explains the synthesis of C2-spiropseudoindoxyl derivatives from 5-exo-dig nitroalkyne via cycloisomerization, nucleophilic addition and reduction. As shown in Scheme 1b, a Pd-mediated cycle of 5-exo-dig nitroalkyne cyclization produces int. 1, which undergoes an internal N–O bond redox reaction to generate the intermediate metal carbene int. 2. Nitrogen addition to the metal carbene affords isatogen int. 3 and one molecule of PdCl2. Subsequent intramolecular addition of an OH group leads to int. 4, and subsequent reduction of the N–O bond produces the C2-spiro- pseudoindoxyl derivatives 2.
The same research group subsequently subjected similar substrates 3 to the cycloisomerization–cycloaddition cascade, generating the tricyclic core of C2-spiropseudoindoxyl deriva- tives 4.8 As shown in Scheme 2, the optimal reaction con- ditions proved to be 10 mol% AuCl(PPh3) as the catalyst, 20 mol% AgSbF6 as the additive, and reaction temperature of 0 °C for 4 h. The reaction was tolerant of substituents on the alkene and aromatic rings and of protecting groups on the nitrogen. The presence of substituents such as MeO– and Me–on the aryl ring produced the best results. However, the pres- ence of N–Ms, N–Ts, or –Cl groups on the aromatic ring reduced the yield of the desired product (13–26%) because these groups rendered the reaction less selective, and com- pounds 4′ or 4″ were also generated as by-products. The researchers proposed the intermediate int. 5 as shown in Scheme 2.
Subsequently, Wang and coworkers used the o-alkynylnitro- benzenes 5 as substrates to directly synthesize N,N′-ketal spiro- pseudoindoxyls 6 for the first time (Scheme 3).9 Optimal reac- tion conditions were tmphen (3,4,7,8-tetramethyl-1,10-phenan- throline) (40 mol%) as the ligand, Pd(OAc)2 (20 mol%) as the catalyst, Mo(CO)6 (1 equiv.) as the reductant, and CF3CH2OH as the solvent at 70 °C for 8 h. The reaction was tolerant of a wide range of o-alkynylnitrobenzenes (X = Ts) and functional groups. Notably, substituents at C-1 or C-4 on the benzene ring reduced the yield (27–45%), probably because of steric hin- drance. The reaction worked well when the methyl- phenylsulfonyl group (X = Ts) was changed to a substituted benzenesulfonyl group or to a thiophen-2-ylsulfonyl, cyclo- propyl or methyl sulfonyl group. This is a simple and efficient method to synthesize a wide range of C2-spiropseudoindoxyls, several of which show bactericidal activity.
In 2015, Verniest’s group reported a similar reaction in which Au(III) catalyzed the cycloisomerization of o-nitrophenyl-
propiolamides 7, affording novel C2-spiropseudoindoxyls 8 in high yields (Scheme 4).10 The optimal reaction conditions were toluene as the solvent, 5 mol% Au(III) as the catalyst, and room temperature for 0.5 h. The resulting polycyclic spiropseudo- indoxyl int. 9 was converted to single-diastereomer spiropyrryl- oxys 8 in 91–99% yields under hydrogen at atmospheric pressure in the presence of 10% Pd/C. This reaction tolerated various substituents that exerted different electronic effects on the substrates. To elucidate the mechanism of this reaction, the group performed preliminary density functional theory (DFT) calculations. The amide oxygen facilitates ring opening in int. 6 via formation of the remarkable oxirane structure int. 7, which is easily converted to isatogen int. 8. DFT calcu- lations suggest that, contrary to the conventional assumptions, the transformation does not involve gold carbene (int. 7′). Instead, isatogen int. 8 undergoes [3 + 2] intramolecular cyclo- addition, and hydrogen reduction affords spiropseudoindoxyls. This process can allow reduction of benzylic ketones under certain conditions.
Tu’s team described a C2-selective cyclization of gold-cata- lyzed alkynyl oxime 9 with an electron-withdrawing protecting group to synthesize a useful C2-spiropseudoindoxyl structure (Scheme 5).11 The optimal reaction conditions were Au(PPh3) Cl/AgOTf (5 mol%) as the catalyst and CH2Cl2 as the solution at room temperature in 0.5 h, giving a useful spiro-indolenine derivative 10. The reaction tolerated indole substrates with propyl (Pr), isopropyl (i-Pr), or Cy (cyclohexyl) on the alkyne chain and with N-protecting groups, giving the desired products in good to excellent yields (51–98%). The researchers proposed a mechanism in Scheme 5b. Isotopic labeling experiments con- firmed that H2O participates in this 5-exo-dig cyclization. Treating compound 9 with Au(PPh3)Cl/AgOTf (5 mol%) in ultra- dry CH2Cl2 saturated with H 18O afforded product 18O-labeled 10, which was confirmed by electrospray ionization-based mass spectrometry and by high-resolution mass spectrometry.
In 2015, Pal’s group proposed an unprecedented Cu-cata- lyzed cascade reaction of cyclopenta[b]indoles 11 to construct C2-spiropseudoindoxyls 12 (Scheme 6).12 By screening the experimental conditions, the optimal reaction conditions were found to be 10 mol% of CuI as the catalyst in a solution mixture of DMF and H2O (7 : 3) at 120 °C for 1.5–4 h with the assistance of air. This reaction tolerates cyclopentanediol 11 containing alkyl, aryl and heteroaryl sulfonamide groups and offers the desired product in good to acceptable yields (35–72%). It is worth noting that when the reaction was per- formed under nitrogen protection, the reaction time was increased to 8 hours, which indicated the air played an impor- tant role in the reaction. The reaction rate is higher in the aqueous DMF solution than in pure DMF solution potentially because the presence of water greatly enhances the ability to absorb and retain higher volumes of oxygen through the DMF aqueous solution. However, due to the low solubility of the aqueous solution, the presence of a larger amount of water does not further improve the reaction time or yield. The pro- posed reaction mechanism according to the reaction phenom- enon in the experimental screening process is shown in Scheme 6b.
In 2016, Li’s group synthesized spiropseudoindoxyl 14 via copper-catalyzed spirocyclization of 1-H-indole-2-carboxamide 13 bearing an N-phenyl ring (Scheme 7).13 The optimal reac- tion conditions were 5 mol% Cu(OTf)2 and 3.0 equivalents of tert-butylhydroperoxide (TBHP) in dichloroethane (DCE) at 60 °C for 4 h. The reaction tolerated diverse substituents on the substrates, including different N-alkyl substituents (R1) on the amide group, different N-aryl substituents (R2), and chlor- ine or fluorine substituents on the indole moiety (R3). Products were obtained in good yields (63–71%) in the pres- ence of various electron-donating groups (R2) at the para-posi- tion of the N-phenyl ring. However, product yields were lower when electron-withdrawing groups weakened the nucleophili- city of the N-phenyl ring. Indole-2-carboxamide with tetra- hydroquinoline on the amide was easily cyclized under standard reaction conditions to afford the highly strained polycyclic spiroindole in 45% yield. These results led to two possible mechanistic pathways (Scheme 7b). Regardless of the mecha- nism, the process transforms the readily available starting materials rapidly and efficiently into C2-spiro-indolyl groups under mild conditions. These C2-spiro-indolyl groups are ubi- quitous structural units in indole alkaloids yet are generally difficult to prepare.
In 2014, Li’s group demonstrated Rh(III)-catalyzed hydrogen- ation coupling of N-sulfonyl 2-aminobenzaldehyde 15 with olefins 16 (Scheme 8).14 The optimal reaction conditions were [Cp*RhCl2]2 (2.5 mol%) as the catalyst, Ag2CO3 (1 equiv.) as the base, and DCE as the solvent at 120 °C under argon for 18 h. This reaction tolerated various electron-donating or electron- withdrawing substitutions on the phenyl group, providing the expected product in 47–86% yield. The researchers proposed two possible intermediates (Scheme 8).
3. Hypervalent iodine-mediated annulation reaction
Hypervalent iodine reagents are easy to handle, environmen- tally friendly, “green” nonmetallic oxidants that have under- gone explosive development in oxidative annulation reactions.
Iodine reagent-mediated oxidative cyclization is widely used to synthesize various heterocyclic compounds, such as aziridine, cyclopropane, oxetane, azetidine, and dihydrofuran.15 Recent studies have shown that iodine reagents can also be used to mediate the synthesis of C2-spiropseudoindoxyl.
Fan and coworkers investigated the synthesis of a C2-spiro- pseudoindoxyl compound 19 from an acyclic precursor 18, iodobenzene diacetate, and tetrabutylammonium iodide via iodophenyltriene(III)-mediated tandem carbon–hydrogen bond oxidation (Scheme 9).16 The reaction proceeded smoothly at room temperature in DMF as the solvent and the combination of PhI(OAc)2 (2.0 equiv.) and Bu4NI (2.0 equiv.) as the oxidant. The desired products were obtained in high yields (72–90%). This reaction tolerated electron-donating and electron-with- drawing substituents at the para-positions of the benzene ring in R1. When the cyclohexane-1,3-dione group was replaced by the 5,5-dimethylcyclohexane-1,3-dione group, the corres- ponding products also formed in good yields (79–81%). Remarkably, no desired product formed when an Ms, benzoyl, or acetyl protecting group was used, indicating that the Ts protecting group in substrate 18 is essential for formation of the oxa-aza spirobicyclic product. The researchers proposed a mechanism (Scheme 9b). This reaction was the first to use a hypervalent iodine reagent to synthesize C2- spiropseudoindoxyl.
In 2015, Du’s team used the same hypervalent iodine reagent (PIDA)-mediated cascade cyclization to achieve the unique trans-aminocarboxylation of diarylalkyl and oxoamino- carboxylates 20, resulting in C2-spiropseudoindoxyl 22 (Scheme 10).17 First, the Lewis acid BF3·Et2O was added to a solution of the substrate 20 in DCE at room temperature. The mixture was stirred at 80 °C overnight to synthesize 6-endo intermediate 21. Subsequently, 2.1 equivalents of PIDA were gradually added to generate the final product 22. The reaction tolerated a variety of substrates with electron-donating or elec- tron-withdrawing groups at the 4-position of the phenyl ring (R1, R2). The researchers proposed a plausible mechanism (Scheme 10b) in which the acid moiety in 20 is activated by BF3·Et2O and converted to the ylide intermediate int. 11, which undergoes tautomerization/aromatization to obtain intermediate int. 12. This compound undergoes intra- molecular 1,4-addition to afford 6-endo-dig intermediate 21. Oxidation of 21 by PIDA, accompanied by the loss of one acetic acid, produces intermediate int. 13, which undergoes intra- molecular cyclization to yield intermediate int. 14, and one molecule of iodobenzene and acetate anions. The acetate anion promotes protonation of the intermediate int. 14 to produce intermediate int. 15 with two additional rings. Following activation by BF3·Et2O, PIDA oxidizes int. 15 while losing an acetate anion, producing intermediate int. 16. Nucleophilic attack by the acetate anion at the most electrophi- lic carbon in intermediate int. 16 generates intermediate int. 17 and liberates one iodobenzene and one acetate anion. The released acetate anion promotes the ring opening of the lactone moiety in int. 17. Subsequent 1,2-addition of the nucleophilic benzoate to the imine moiety affords the final C2- spiropseudoindoxyl product 22.
The same research group later used hypervalent iodine reagents in single-oxidant cascade cyclization to oxidize the diarylacetylene compounds 23a and form a series of novel C2- spiropseudoindoxyl compounds 24a (Scheme 11).18 Optimal reaction conditions were PhI(OCOCF3)2 as the oxidizing agent, DCM as the solvent, and temperature between −20 and 0 °C. The reaction was unaffected by various electron-deficient and electron-rich substituents on the phenyl ring of the aniline or benzamide moiety, with the desired spiro-compounds gener- ated in good yields (51–88%). Product yield was, however, sub- stantially reduced when the substrate exerted a spatial effect. Replacing the OMe group with an aryl or alkyl group led to the desired products in moderate yields (49–69%). In fact, repla- cing NH–OMe in the substrate by OH gave a satisfactory yield (57–92%). Under similar conditions, substrates 23b could be transformed to obtain the desired products 24b in good yields. The research group proposed two reaction mechanisms (Scheme 11b). In path a, the intermediate int. 18 is obtained by reacting substrate 23 with 2.0 equivalents of the oxidant PhI(OCOCF3)2, which also releases one molecule of trifluoroacetic acid. Subsequent intramolecular C–O bond formation and release of one molecule of iodobenzene and a trifluoroacetate anion generate the cationic intermediate int. 19. The energeti- cally favorable combination of the negatively charged trifluoro- acetate and the positively charged carbon center in int. 19 provide intermediate int. 20. Oxidation of the tosylamide moiety provides intermediate int. 21 and another molecule of trifluoroacetic acid. Subsequent intramolecular indolization generates intermediate int. 22, whose most electrophilic carbon is again attacked by the nucleophilic trifluoroacetate anion, affording the intermediate int. 23, which is converted to the iminium int. 24. Release of the trifluoroacetate anion forms intermediate int. 24, which undergoes ring opening to form iminium int. 25, which in turn rapidly cyclizes to provide the desired spirocyclic compound 24.
In the alternative mechanistic path b, activation of the triple bond by PhI(OCOCF3)2 produces electrophilic intermedi- ate int. 26, which reacts with nucleophilic benzamide to gene- rate intermediate int. 27. Release of one molecule of iodoben- zene from int. 27 produces the same intermediate int. 20. PhI(OCOCF3)2 again activates the intramolecular double bond to form the intermediate int. 28, which generates intermediate int. 29 via an electrophilic reaction. Intermediate int. 29 is con- verted into oxonium int. 22, accompanied by the loss of one molecule of iodobenzene. The intermediate int. 22 is con- verted into the final product 24 via a process similar to that in path a. This work was the first report of the complex spirocycli- zation of alkyne substrates under metal-free conditions. This unprecedented cascade reaction includes not only two sequen- tial C–N/C–O bond formations, but also the insertion of carbo- nyl oxygen.
The same research group later synthesized a novel class of C2-spiropseudoindoxyls 26 using the same hypervalent iodine reagent oxidative-cascade annulation reaction of 2-sulfon- amido-N-phenylpropiolamide derivatives 25 (Scheme 12).19 The optimal reaction conditions were 2.2 equivalents of PhI(OCOCF3)2 as the sole oxidant at 0 °C in DCE. These opti- mized conditions were used to generate a series of C2-spiro- pseudoindoxyl compounds in moderate to good yields (43–90%). This cascade oxidation with hypervalent iodine reagents broadly tolerated electrons on the benzene ring of 2-aminoben- zyl 26 (R1 and R2). The substituent on the nitrogen atom (R3) was tosyl, methanesulfonyl, phenylsulfonyl, or p-chlorobenze nesulfonyl, and the substituent at R4 was ethyl or n-butyl. Based on control experiments, the researchers proposed two rational mechanisms for PIFA-mediated tandem oxidation (Scheme 12b). In both cases, spirocyclization involves iodine(III)- mediated cascade formation of C(sp2)–C(sp) and C(sp2)–N bonds, with the transfer of a carbonyl group from the hypervalent iodine reagent.
4. Organic catalysis
Organocatalysis stands out because of its various activation modes and the structural simplicity of most organic catalysts, and it has become an important synthetic route to many drug molecules and natural products.20 In recent years, chemists have gradually used organic catalysis to synthesize C2-spiro- pseudoindoxyl derivatives with high enantioselectivities.
In 2014, Glorius and coworkers first reported the N-heterocyclic carbene-catalyzed (NHC) reaction of α,β-unsaturated aldehydes 27 with the ring of aza-aurones 28 (Scheme 13).21 This synthetic method provides an alternative route to useful enantiomerically enriched substituted C2-spiro- pseudoindoxyl 29. Optimal conditions for the reaction were NHC Cat. B (10 mol%) as the catalyst, DBU (150 mol%) as the base, THF as the solvent, and temperature of 50 °C. The reac- tion tolerated diverse substituents bearing an electron-rich or electron-deficient group on the phenyl ring of the R1 and R2 groups, delivering products 29 in high yields and with excel- lent stereoselectivities (3 : 1 to >20 : 1 dr and 90–95% ee). The reaction worked well with substrates containing substituents on the indolin-3-one (R3), alkyl-substituted enals, and aza- aurones with a cinnamyl substituent. The researchers pro- posed a plausible mechanism for this catalytic cycle (Scheme 13b). First, the precatalyst salt is deprotonated to produce the NHC organic catalyst, which then adds to the enal 27 to generate the NHC-homoenolate. The intermediate under- goes Michael addition from the back face, and then a carbon– carbon bond forms with aza-aurone 28, followed by tautomeri- zation to produce an acyl group. Acyl azolium was subjected to C-acylation to produce the final product 29 and regenerates the NHC organocatalyst. This pathway is a unique, NHC-cata- lyzed, formal [3 + 2] annulation for obtaining the C2-spiro- pseudoindoxyl derivatives of high optical purity, which are difficult to synthesize by conventional strategies.
Similar to the work of Glorius, Xu’s group reported for the first time that hydrogen bond network-catalyzed o-hydroxy aro- matic aldimines 30 and (Z)-1-acetyl-2-benzylideneindoline-3- one 31 undergo asymmetric [3 + 2] cyclization directly to give 2′-pyrrolidinyl-spirooxoxime 32 (Scheme 14).22 The optimal reaction conditions were 10 mol% Cat. C as the catalyst and DCE as the solvent at room temperature. The reaction tolerated substituents bearing an electron-withdrawing or electrondonating group at the ortho-, meta-, or para-positions on the phenyl rings (R2). A wide range of target products 32 were obtained in high to excellent yields (86–82%) and enantio- selectivities (86–95% ee). The reaction also tolerated substi- tuted o-hydroxy aromatic aldimines, and products were obtained in good yields (83–84%) and stereoselectivities (87–90% ee). In contrast, aza-aurones possessing a furan or piperonyl aldehyde substituent gave the desired products with low enantioselectivities (77–80% ee). Control experiments using pure (Z)- or (E)-aza-aurone revealed that under optimal reaction conditions, the product 32 formed with a similar yield and stereoselectivity. The researchers proposed a transition state involving hydrogen bonding and spatial interactions that can account for the observed stereoconfiguration of 32.
In 2016, Han’s group used a chiral secondary amine catalyst cat. D (20 mol%) to catalyze an inverse-electron-demand oxa- DA reaction involving [4 + 2] cycloaddition of aldehyde 33 and (Z)-2-mercaptopurine 34, generating the product 35. In the presence of 2-iodobenzoic acid (IBX), hemiacetal 35 underwent efficient α-hydroxylation, acetalization and oxidation in mixed DMSO–ethyl acetate solvent at 80 °C, affording two diastereo- isomers with the C2-spiropseudoindoxyl skeleton structure (36 and 36′) with excellent enantioselectivity (97% ee) (Scheme 15).23 Optimal reaction conditions turned out to be the mixture CH2Cl2/H2O as the solvent and 3-nitrobenzoic acid (20 mol%) as the additive. This method allows asymmetric syn- thesis of indole-based natural products or drug-like scaffolds bearing C2-spiroquaternary stereocenters.
You’s group developed an organocatalytic enantioselective reaction to construct the chiral indoline skeletons 39a or 39b with a continuous spiro-quaternary carbon center and a tertiary carbon center using indole derived benzamides 37a or 37b (Scheme 16).24 The reaction employed 1,3-dichloro-5,5- diphenylhydantoin 38 as the halogen source and Cat. E as the bifunctional catalyst in MeCN at −30 °C. Substrates 37a bearing the electron withdrawing or electron donating groups on the phenyl rings (R1 and R2) were all well tolerated and pro- vided corresponding products in good to high yields (77–90%) and with excellent enantioselectivities (84–96% ee). In addition, substituents bearing an electron withdrawing or electron donating group at the meta- or para-positions on the phenyl rings (R3) also were well tolerated (70–87% yields, 91–95% ee). However, when tBu is used instead of phenyl, the reduced enantioselectivity was observed (76% yield, 80 ee). The presence of Boc as the protecting group also gave the desired product in an acceptable result (74% yield and 88% ee). For substrates 37b, the corresponding products 39b can also be acquired in good to high yields (75–81%) with excellent enantioselectivities (75–81% yield, 90–96% ee). The authors proposed a possible transition state model to explain the observed enantioselectivities. In this model, the catalyst mainly has two functions. On the one hand, the 2,3-naphthyri- dine nitrogen in the catalyst forms a hydrogen bond with the benzamide in the substrate to increase the nucleophilicity of the amide group; on the other hand, the tertiary amine nitro- gen moiety acts as a Lewis base to activate the chloronium species to provide a chiral environment to induce high enantioselectivity.
In 2018, Liu’s group described a cyclization between 2-alkynyl-3,3-difluoro-3H-indoles 40 and the dinucleophilic reagent 2-aminobenzothiazole 41, obtaining C2-spiropseudo- indoxyl compounds 42 (Scheme 17).25 Optimal reaction con- ditions were TBD (50 mol%) as the catalyst and EtOAc as the solvent at 25 °C. This organocatalysis tolerated electron-donat- ing and electron-withdrawing substituents at the ortho-, meta-, and para-positions of the benzene ring in 2-alkynyl-3,3- difluoro-3H-indoles, and various substituents exerting different electronic effects on the 2-aminobenzothiazole sub- strate. The reaction produced the desired difluorinated C2- spiropseudoindoxyl compound in 61–88% yields with com- plete regioselectivity within 5 h. Substitution at the indole rings allowed smooth conversion to the corresponding pro- ducts with yields of 10–80%. The yield was only 10% with a substrate that had a chlorine group at the 4-position, perhaps an unfavorable spatial factor. On the basis of experimental observations and previous reports, the group envisioned two reaction mechanisms for this novel transformation (Scheme 17b). The reaction selectively afforded structurally diverse gem-difluorinated C2-spiro-indolines in moderate to excellent yields. This protocol features high regioselectivity, good functional group tolerance, broad substrate scope, facile scalability, and mild reaction conditions.
Magnesium MethoxideCatalog No.:AA003RC6 CAS No.:109-88-6 MDL No.:MFCD00053671 MF:C2H6MgO2 MW:86.3728 |
DiethylamineCatalog No.:AA003867 CAS No.:109-89-7 MDL No.:MFCD00009032 MF:C4H11N MW:73.1368 |
Ethyl formateCatalog No.:AA0034TE CAS No.:109-94-4 MDL No.:MFCD00003294 MF:C3H6O2 MW:74.0785 |
3-PyrrolineCatalog No.:AA0033PG CAS No.:109-96-6 MDL No.:MFCD00005213 MF:C4H7N MW:69.1051 |
PyrroleCatalog No.:AA0035H5 CAS No.:109-97-7 MDL No.:MFCD00005216 MF:C4H5N MW:67.0892 |
2-PyrazolineCatalog No.:AA003HU8 CAS No.:109-98-8 MDL No.:MFCD00059715 MF:C3H6N2 MW:70.0931 |
TetrahydrofuranCatalog No.:AA0035LF CAS No.:109-99-9 MDL No.:MFCD00005356 MF:C4H8O MW:72.1057 |
5,12-NaphthacenequinoneCatalog No.:AA003M5U CAS No.:1090-13-7 MDL No.:MFCD00003701 MF:C18H10O2 MW:258.2708 |
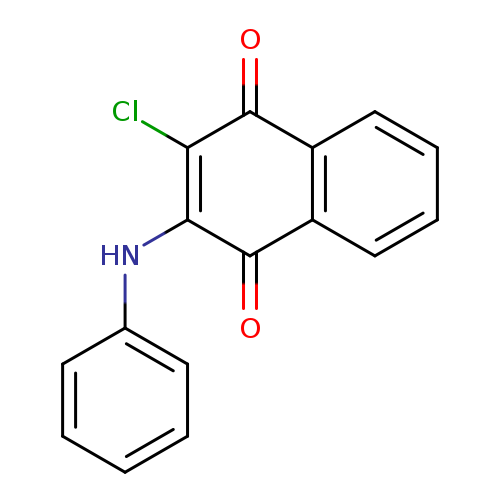
2-Chloro-3-(phenylamino)naphthalene-1,4-dioneCatalog No.:AA007BGM CAS No.:1090-16-0 MDL No.:MFCD00010250 MF:C16H10ClNO2 MW:283.7091 |
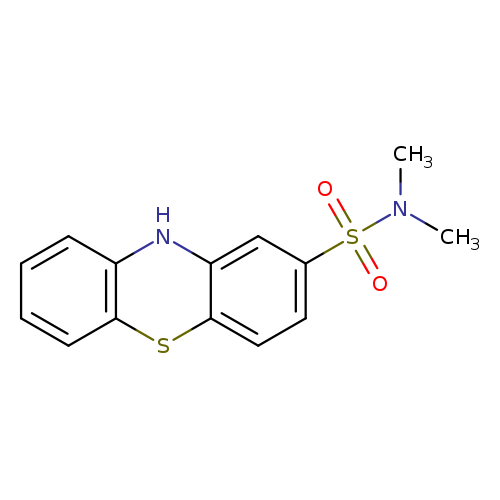
N,N-Dimethyl-10H-phenothiazine-2-sulfonamideCatalog No.:AA008SJY CAS No.:1090-78-4 MDL No.:MFCD00129995 MF:C14H14N2O2S2 MW:306.4032 |
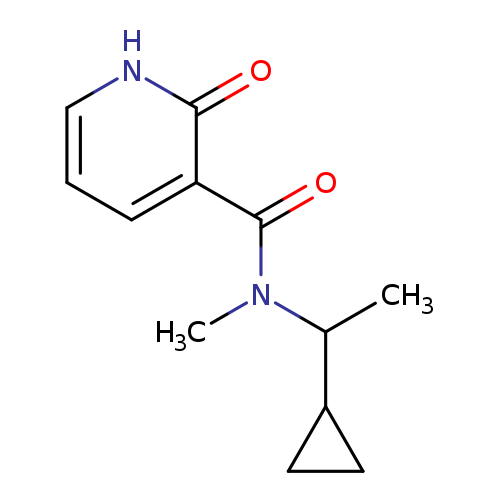
N-(1-cyclopropylethyl)-N-methyl-2-oxo-1,2-dihydropyridine-3-carboxamideCatalog No.:AA01EIDK CAS No.:1090002-20-2 MDL No.:MFCD17480431 MF:C12H16N2O2 MW:220.2676 |
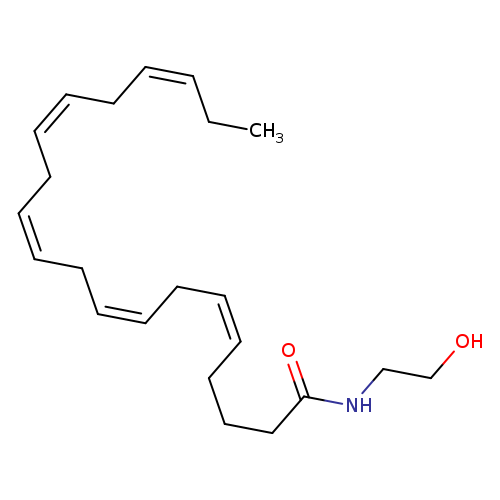
Eicosapentaenoyl EthanolamideCatalog No.:AA008SP0 CAS No.:109001-03-8 MDL No.:MFCD22416528 MF:C22H35NO2 MW:345.5188 |
N-(2-nitrophenyl)pyrrolidine-1-carboxamideCatalog No.:AA019UHZ CAS No.:1090012-11-5 MDL No.:MFCD11728278 MF:C11H13N3O3 MW:235.2392 |
4-(2,3-dihydro-1H-indole-1-carbonyl)-1-[3-(methylsulfanyl)phenyl]pyrrolidin-2-oneCatalog No.:AA01BA62 CAS No.:1090029-95-0 MDL No.:MFCD11723525 MF:C20H20N2O2S MW:352.4500 |
10,15-Dihydro-5H-diindolo[3,2-aCatalog No.:AA009TOF CAS No.:109005-10-9 MDL No.:MFCD21609440 MF:C24H15N3 MW:345.3960 |
glutarylcarnitineCatalog No.:AA009OUA CAS No.:109006-11-3 MDL No.: MF:C11H19NO6 MW:261.2717 |
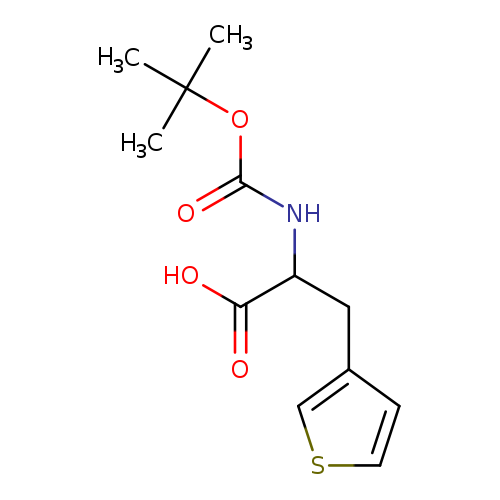
BOC-DL-3-THIENYLALANINECatalog No.:AA009LMZ CAS No.:109007-59-2 MDL No.:MFCD09264320 MF:C12H17NO4S MW:271.3327 |
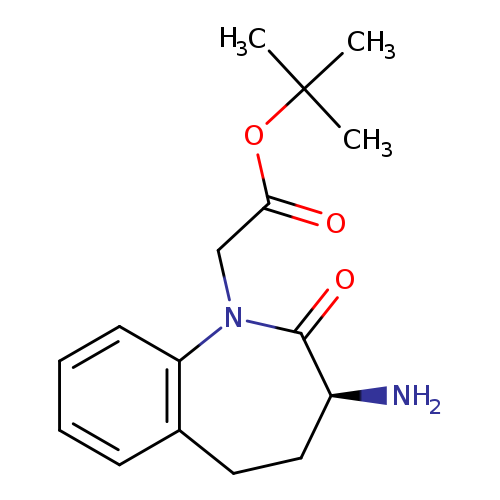
(S)-t-Butyl 2-(3-amino-2-oxo-2,3,4,5-tetrahydro-1H-benzo[b]azepin-1-yl)acetateCatalog No.:AA003UG6 CAS No.:109010-60-8 MDL No.:MFCD03426719 MF:C16H22N2O3 MW:290.3575 |
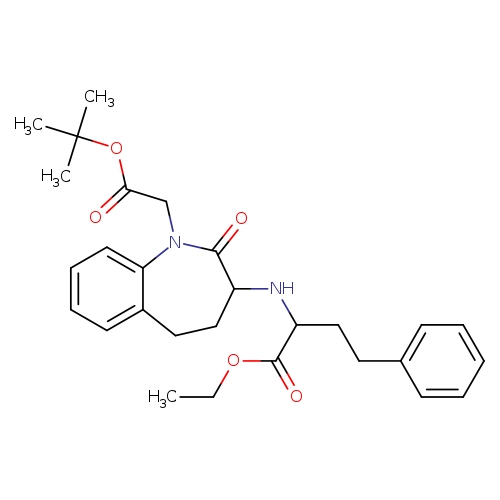
1H-1-Benzazepine-1-acetic acid,3-[[(1S)-1-(ethoxycarbonyl)-3-phenylpropyl]amino]-2,3,4,5-tetrahydro-2-oxo-, 1,1-dimethylethyl ester, (3S)-Catalog No.:AA01CBWH CAS No.:109010-61-9 MDL No.:MFCD31729380 MF:C28H36N2O5 MW:480.5958 |
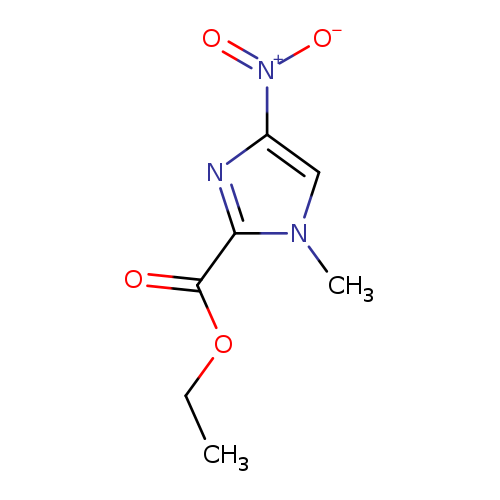
Ethyl 1-methyl-4-nitroimidazole-2-carboxylateCatalog No.:AA007BGO CAS No.:109012-23-9 MDL No.:MFCD03789110 MF:C7H9N3O4 MW:199.1641 |
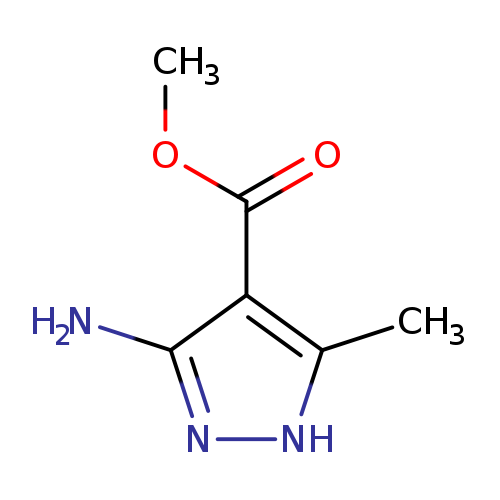
Methyl 5-amino-3-methyl-1h-pyrazole-4-carboxylateCatalog No.:AA002MOH CAS No.:109012-96-6 MDL No.:MFCD09953592 MF:C6H9N3O2 MW:155.1546 |
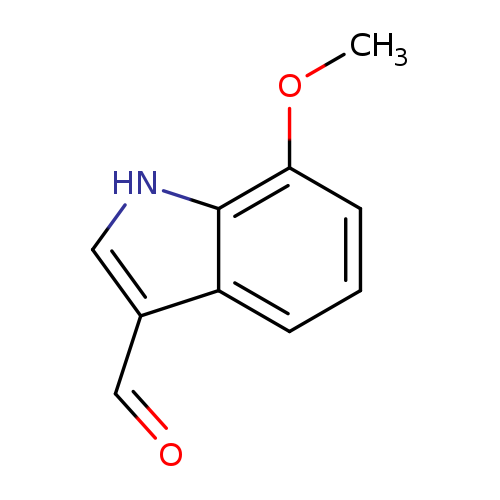
7-Methoxy-3-indolecarboxaldehydeCatalog No.:AA007BGE CAS No.:109021-59-2 MDL No.:MFCD02667609 MF:C10H9NO2 MW:175.1840 |
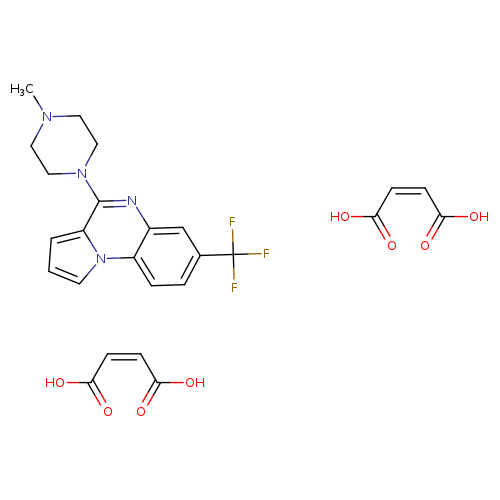
CGS 12066B DIMALEATECatalog No.:AA008SD7 CAS No.:109028-10-6 MDL No.:MFCD00055048 MF:C25H25F3N4O8 MW:566.4832 |
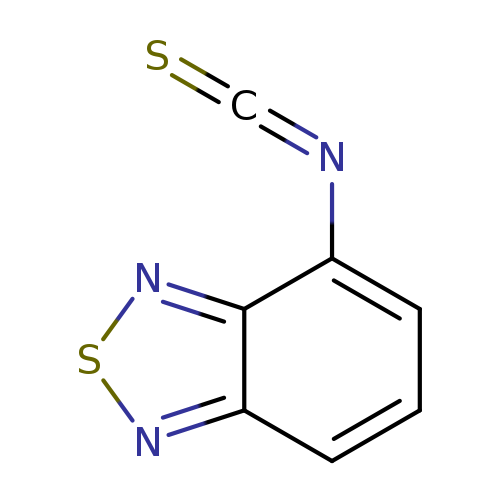
2,1,3-Benzothiadiazol-4-yl isothiocyanateCatalog No.:AA0082U9 CAS No.:109029-21-2 MDL No.:MFCD02681991 MF:C7H3N3S2 MW:193.2488 |
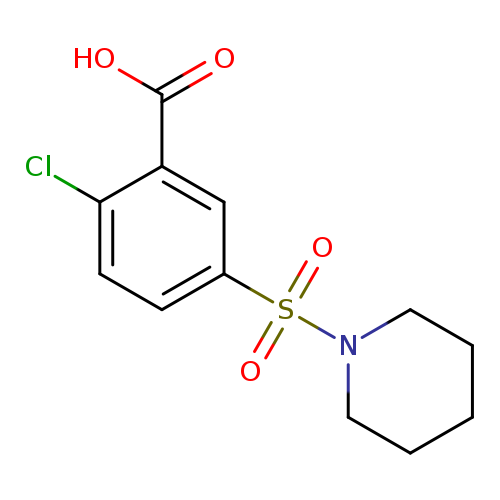
2-Chloro-5-(piperidin-1-ylsulfonyl)benzoic acidCatalog No.:AA0082U8 CAS No.:109029-95-0 MDL No.:MFCD00625735 MF:C12H14ClNO4S MW:303.7619 |
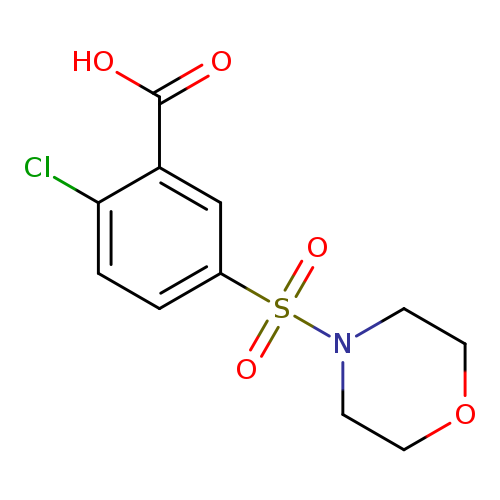
2-Chloro-5-(morpholinosulfonyl)benzoic acidCatalog No.:AA008TVC CAS No.:109029-96-1 MDL No.:MFCD00492872 MF:C11H12ClNO5S MW:305.7347 |
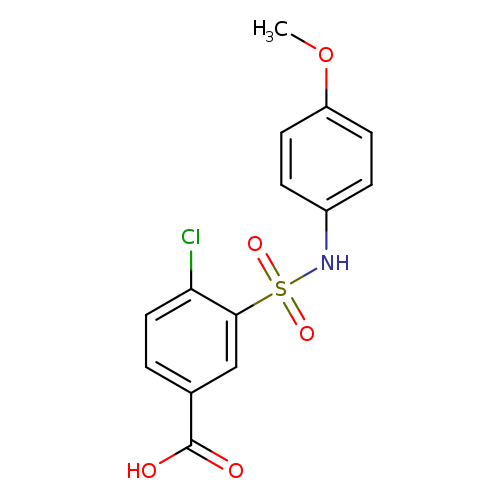
4-chloro-3-[(4-methoxyphenyl)sulfamoyl]benzoic acidCatalog No.:AA019GGF CAS No.:109029-99-4 MDL No.:MFCD02695610 MF:C14H12ClNO5S MW:341.7668 |
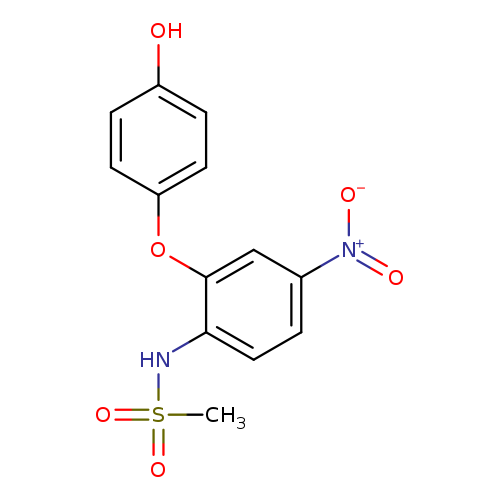
4'-Hydroxy NimesulideCatalog No.:AA007TOW CAS No.:109032-22-6 MDL No.:MFCD08063679 MF:C13H12N2O6S MW:324.3092 |
2,3-Dichloro-N-ethylbenzamideCatalog No.:AA00HB7B CAS No.:1090344-11-8 MDL No.:MFCD10646088 MF:C9H9Cl2NO MW:218.0799 |
1-(o-Chloro-α-phenylbenzyl)piperazineCatalog No.:AA008VME CAS No.:109036-15-9 MDL No.:MFCD05186073 MF:C17H19ClN2 MW:286.7992 |
Ethanone, 1-(4-acetyl-1-piperazinyl)-2-(4-nitrophenyl)-Catalog No.:AA01DH8A CAS No.:1090361-16-2 MDL No.:MFCD10664873 MF:C14H17N3O4 MW:291.3025 |

Triphenylsulfonium chloride solutionCatalog No.:AA003V3F CAS No.:109037-76-5 MDL No.:MFCD01939421 MF: MW: |
1-(2-Fluorophenyl)cyclopentane-1-carboxamideCatalog No.:AA0082RD CAS No.:1090385-94-6 MDL No.:MFCD10669587 MF:C12H14FNO MW:207.2441 |
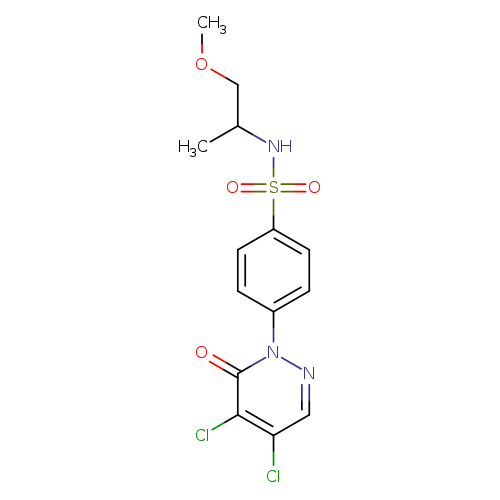
4-(4,5-dichloro-6-oxo-1,6-dihydropyridazin-1-yl)-N-(1-methoxypropan-2-yl)benzene-1-sulfonamideCatalog No.:AA01BTAB CAS No.:1090393-42-2 MDL No.:MFCD10667175 MF:C14H15Cl2N3O4S MW:392.2576 |
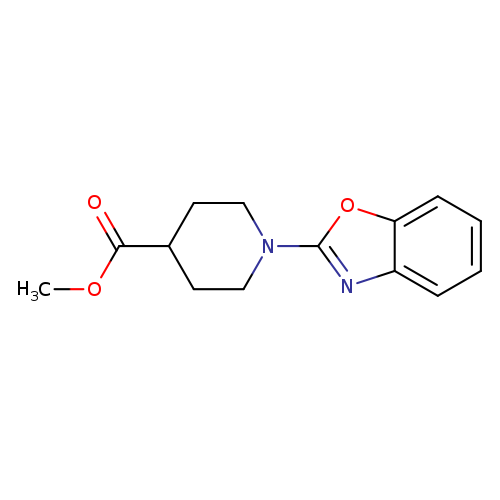
methyl 1-(1,3-benzoxazol-2-yl)piperidine-4-carboxylateCatalog No.:AA019SAM CAS No.:1090401-36-7 MDL No.:MFCD10649788 MF:C14H16N2O3 MW:260.2884 |
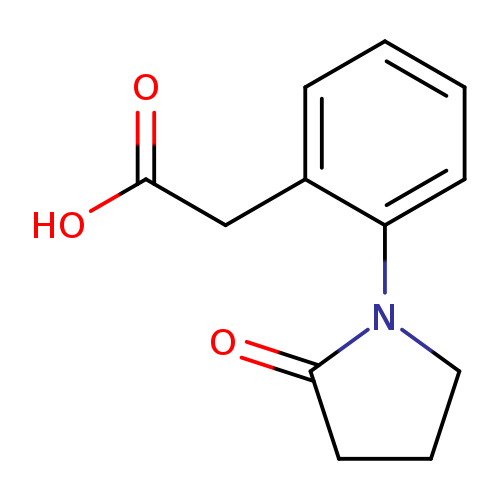
2-(2-(2-oxopyrrolidin-1-yl)phenyl)acetic acidCatalog No.:AA01BHRV CAS No.:109049-91-4 MDL No.:MFCD16788684 MF:C12H13NO3 MW:219.2365 |
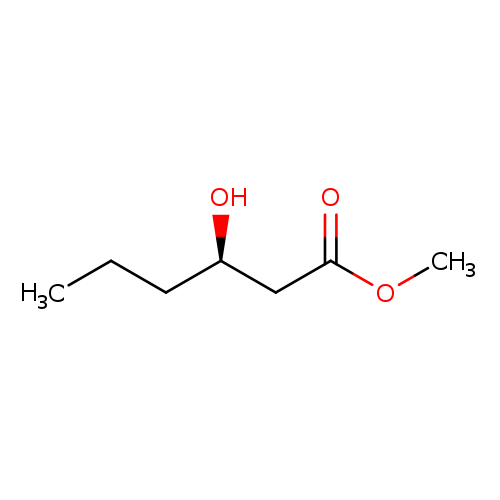
Hexanoic acid, 3-hydroxy-, methyl ester, (3R)-Catalog No.:AA0082R7 CAS No.:109053-86-3 MDL No.:MFCD30336670 MF:C7H14O3 MW:146.1843 |
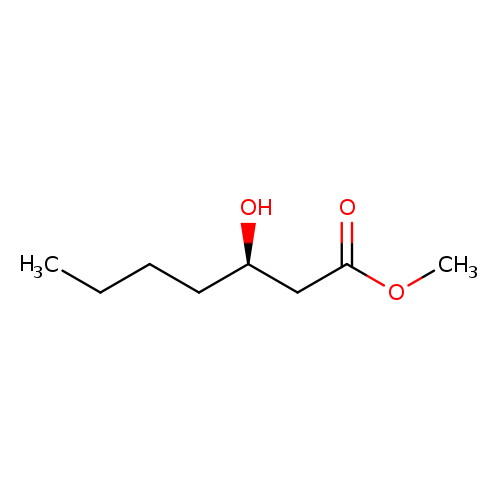
methyl (3R)-3-hydroxyheptanoateCatalog No.:AA00IXJ4 CAS No.:109053-87-4 MDL No.:MFCD28990655 MF:C8H16O3 MW:160.2108 |
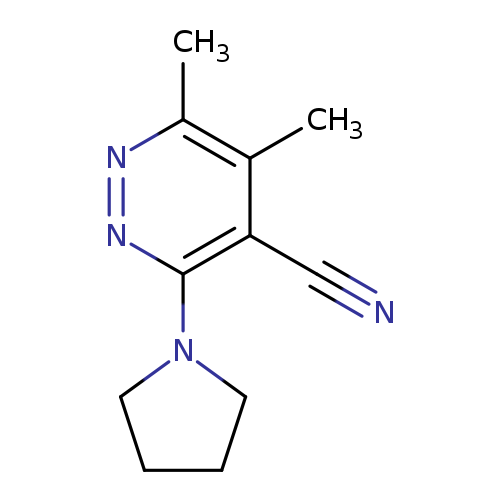
5,6-dimethyl-3-(pyrrolidin-1-yl)pyridazine-4-carbonitrileCatalog No.:AA01AI12 CAS No.:1090535-67-3 MDL No.:MFCD18838864 MF:C11H14N4 MW:202.2557 |
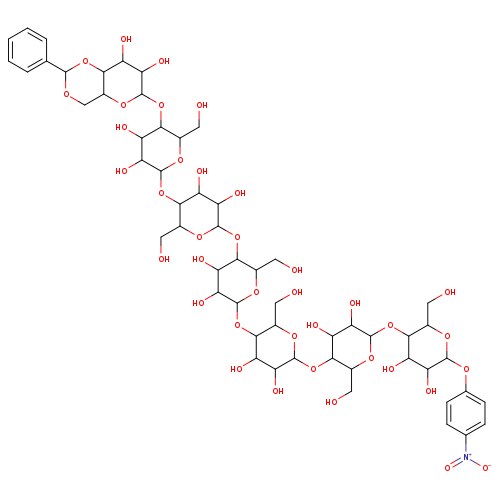
4-Nitrophenyl 4,6-benzylidene-a-D-maltoheptaosideCatalog No.:AA009TJ9 CAS No.:109055-07-4 MDL No.:MFCD28064285 MF:C55H79NO38 MW:1362.1997 |
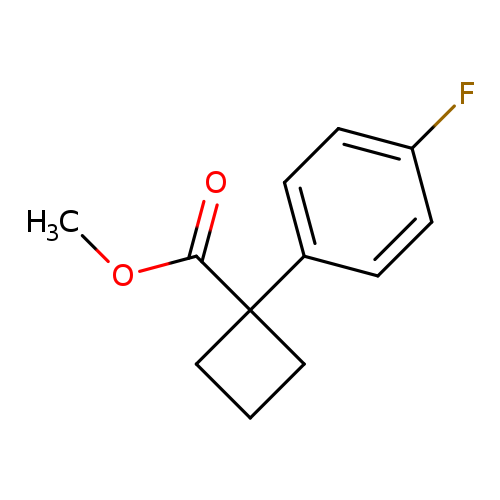
Methyl 1-(4-fluorophenyl)cyclobutane-1-carboxylateCatalog No.:AA0093YZ CAS No.:1090553-79-9 MDL No.:MFCD11810428 MF:C12H13FO2 MW:208.2288 |

Gly-Phe-β-naphthylamideTrifluoroaceticAcidSaltCatalog No.:AA01E3PB CAS No.:109057-30-9 MDL No.: MF: MW: |
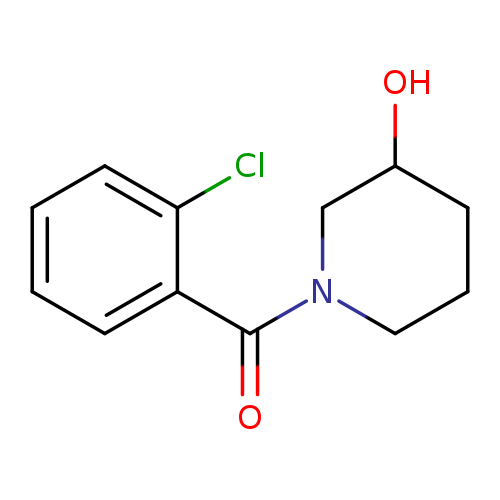
1-(2-chlorobenzoyl)piperidin-3-olCatalog No.:AA01AHJ6 CAS No.:1090598-03-0 MDL No.:MFCD11738633 MF:C12H14ClNO2 MW:239.6981 |
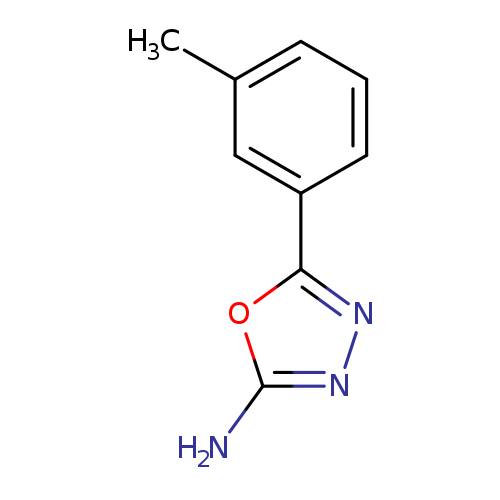
5-(3-Methylphenyl)-1,3,4-oxadiazol-2-amineCatalog No.:AA007BFX CAS No.:109060-64-2 MDL No.:MFCD00469195 MF:C9H9N3O MW:175.1873 |

5-(3-Bromophenyl)-1,3,4-oxadiazol-2-amineCatalog No.:AA007BFW CAS No.:109060-66-4 MDL No.:MFCD04627325 MF:C8H6BrN3O MW:240.0567 |
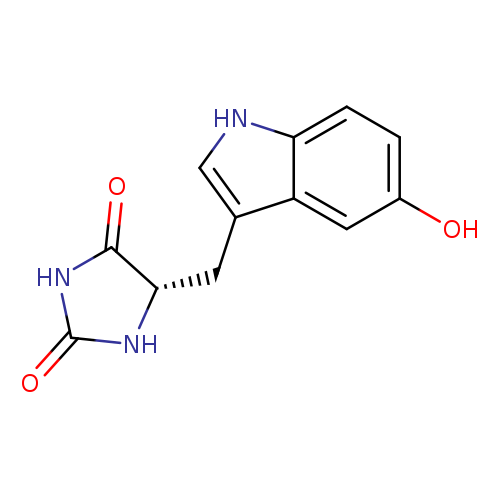
2,4-Imidazolidinedione, 5-[(5-hydroxy-1H-indol-3-yl)methyl]-, (S)-Catalog No.:AA007TLI CAS No.:109063-48-1 MDL No.:MFCD31666555 MF:C12H11N3O3 MW:245.2340 |
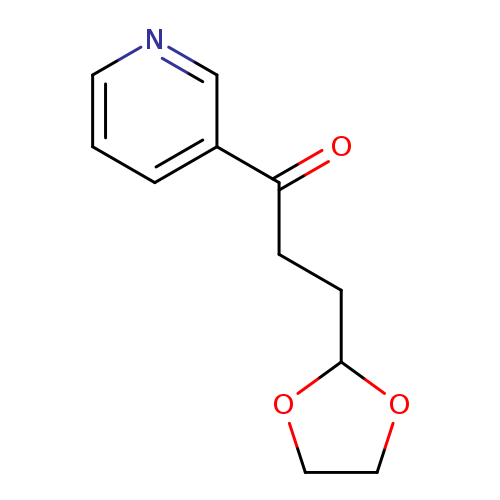
1-Propanone,3-(1,3-dioxolan-2-yl)-1-(3-pyridinyl)-Catalog No.:AA007BFV CAS No.:109065-57-8 MDL No.:MFCD01320448 MF:C11H13NO3 MW:207.2258 |
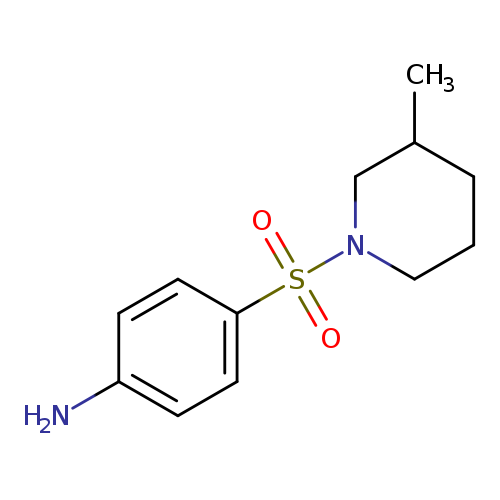
4-[(3-Methylpiperidin-1-yl)sulfonyl]anilineCatalog No.:AA008U5U CAS No.:109069-00-3 MDL No.:MFCD02746182 MF:C12H18N2O2S MW:254.3485 |
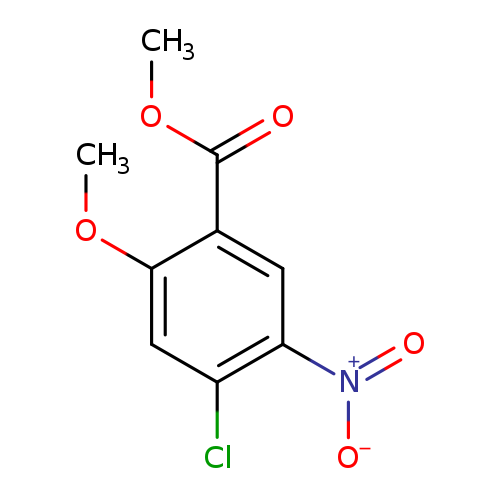
4-Chloro-2-methoxy-5-nitro-benzoic acid methyl esterCatalog No.:AA0082QX CAS No.:109069-75-2 MDL No.:MFCD10574787 MF:C9H8ClNO5 MW:245.6165 |
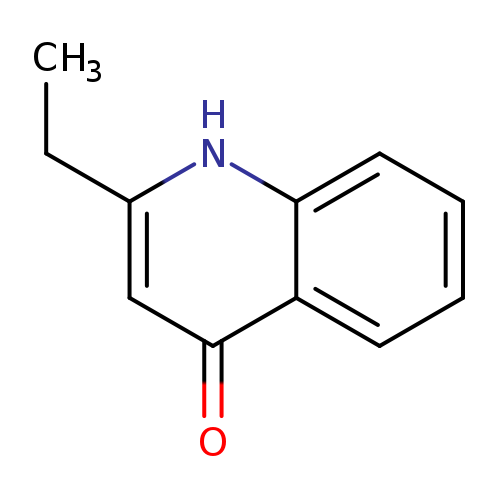
2-ethyl-1,4-dihydroquinolin-4-oneCatalog No.:AA01A227 CAS No.:109072-25-5 MDL No.:MFCD12114553 MF:C11H11NO MW:173.2111 |
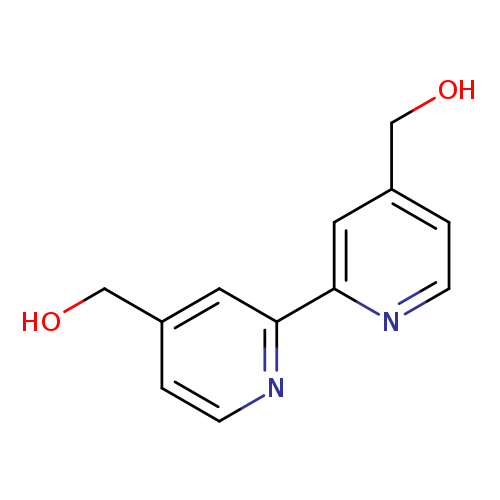
4,4'-Bis(hydroxymethyl)-2,2'-bipyridineCatalog No.:AA007TLF CAS No.:109073-77-0 MDL No.:MFCD06202808 MF:C12H12N2O2 MW:216.2359 |
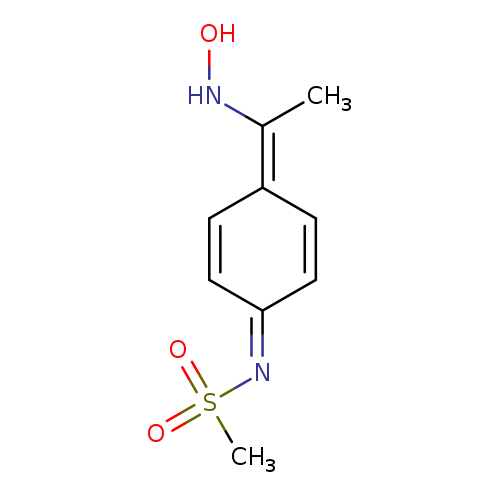
N-(4-[1-(Hydroxyamino)ethylidene]cyclohexa-2,5-dien-1-ylidene)methanesulfonamideCatalog No.:AA01BVDB CAS No.:1090738-49-0 MDL No.:MFCD11817507 MF:C9H12N2O3S MW:228.2682 |
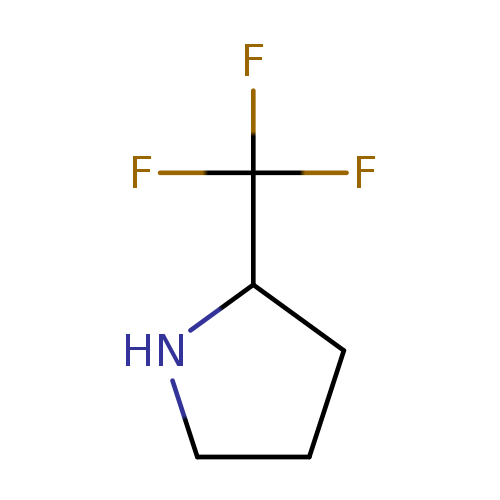
(R)-(-)-2-(Trifluoromethyl)pyrrolidineCatalog No.:AA008S5O CAS No.:109074-67-1 MDL No.:MFCD02663405 MF:C5H8F3N MW:139.1189 |
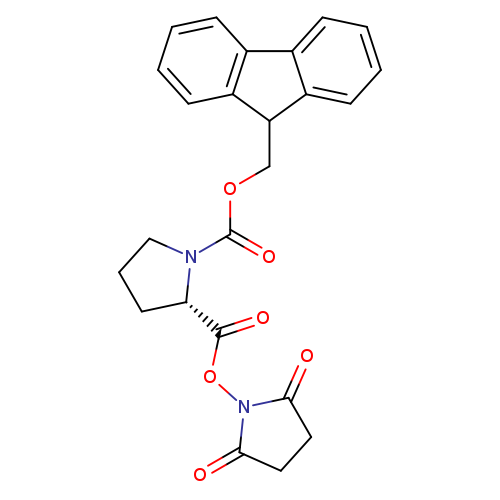
Fmoc-pro-osuCatalog No.:AA008S6D CAS No.:109074-94-4 MDL No.:MFCD00065673 MF:C24H22N2O6 MW:434.4413 |
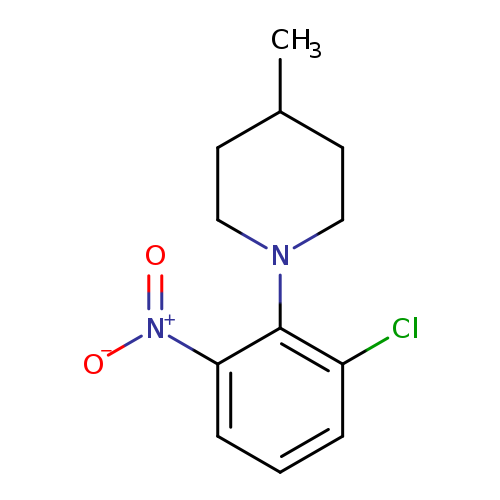
Piperidine, 1-(2-chloro-6-nitrophenyl)-4-methyl-Catalog No.:AA00IMMV CAS No.:1090783-85-9 MDL No.:MFCD11765018 MF:C12H15ClN2O2 MW:254.7127 |
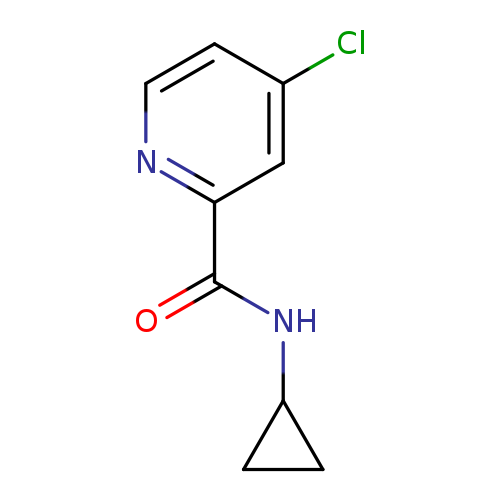
N-Cyclopropyl 4-chloropicolinamideCatalog No.:AA007BFP CAS No.:1090815-16-9 MDL No.:MFCD10663418 MF:C9H9ClN2O MW:196.6336 |
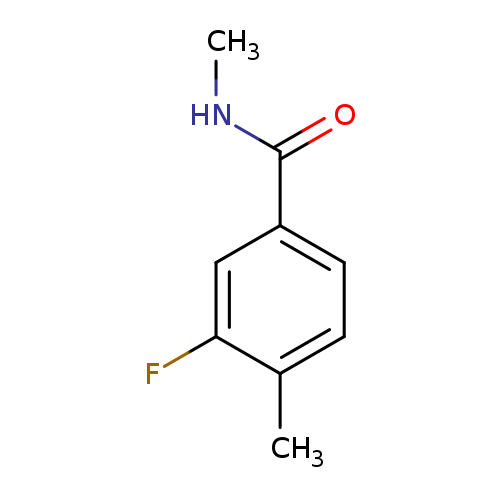
3-fluoro-N,4-dimethylbenzamideCatalog No.:AA01ELNA CAS No.:1090829-74-5 MDL No.:MFCD11767632 MF:C9H10FNO MW:167.1802 |
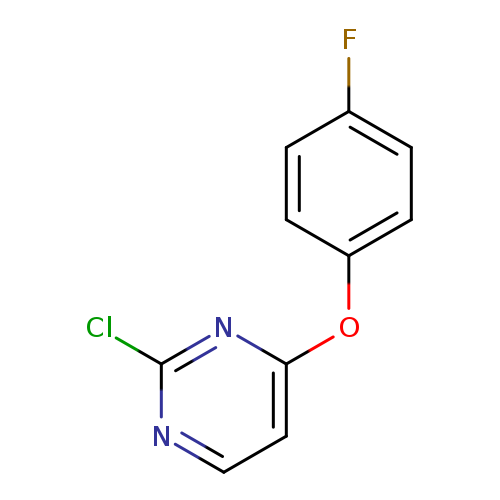
2-chloro-4-(4-fluorophenoxy)pyrimidineCatalog No.:AA01AAJ4 CAS No.:1090835-72-5 MDL No.:MFCD11812088 MF:C10H6ClFN2O MW:224.6188 |
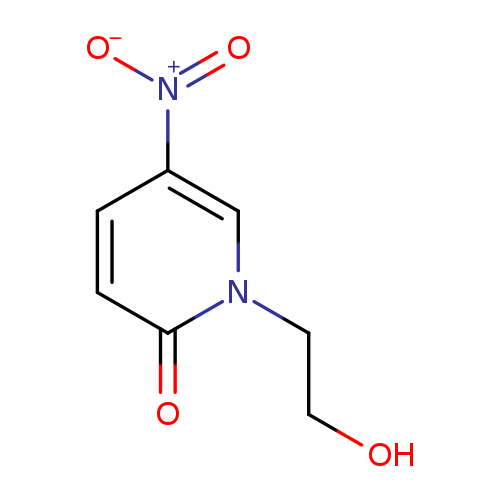
1-(2-hydroxyethyl)-5-nitro-1,2-dihydropyridin-2-oneCatalog No.:AA01C4XD CAS No.:1090858-69-7 MDL No.:MFCD11823874 MF:C7H8N2O4 MW:184.1494 |
2-(3-Trifluoromethyl-phenyl)-pyrrolidineCatalog No.:AA0082QS CAS No.:109086-17-1 MDL No.:MFCD02663523 MF:C11H12F3N MW:215.2149 |

POTASSIUM DIPHENYLBIS(PYRAZOL-1-YL)BORATECatalog No.:AA008V1T CAS No.:109088-11-1 MDL No.:MFCD02684554 MF:C18H16BKN4 MW:338.2557 |
2-Oxo-1,2,3,4-tetrahydroquinoline-6-sulfonamideCatalog No.:AA019KF2 CAS No.:1090885-01-0 MDL No.:MFCD11745347 MF:C9H10N2O3S MW:226.2523 |
3-(2-Methoxy-5-methylphenyl)-3-phenylpropanoic acidCatalog No.:AA0082QP CAS No.:109089-77-2 MDL No.:MFCD01098026 MF:C17H18O3 MW:270.3230 |
N-Cyclopropylthiophene-3-carboxamideCatalog No.:AA00HB7M CAS No.:1090897-27-0 MDL No.:MFCD10674150 MF:C8H9NOS MW:167.2282 |
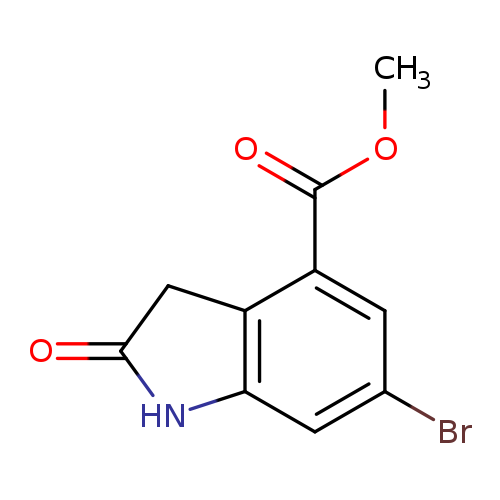
methyl 6-bromo-2-oxo-1,3-dihydroindole-4-carboxylateCatalog No.:AA00HB7N CAS No.:1090903-69-7 MDL No.:MFCD26744232 MF:C10H8BrNO3 MW:270.0794 |
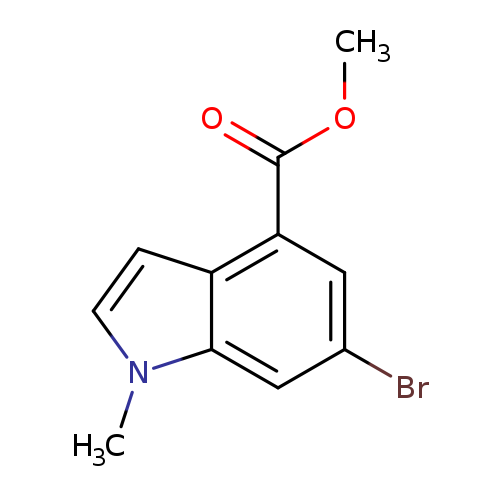
Methyl 6-bromo-1-methyl-1h-indole-4-carboxylateCatalog No.:AA00HB7O CAS No.:1090903-89-1 MDL No.:MFCD28641837 MF:C11H10BrNO2 MW:268.1066 |
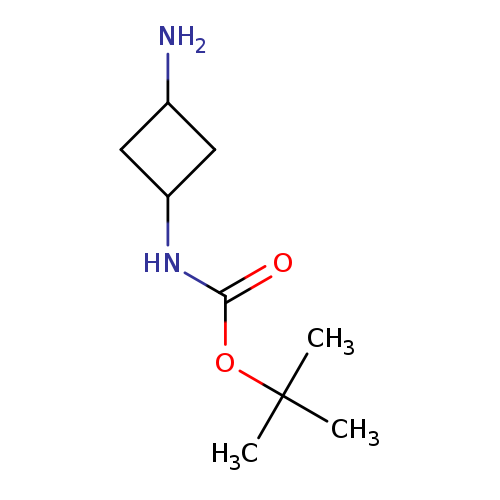
tert-Butyl 3-aminocyclobutylcarbamateCatalog No.:AA007TLD CAS No.:1090904-48-5 MDL No.:MFCD12755997 MF:C9H18N2O2 MW:186.2514 |
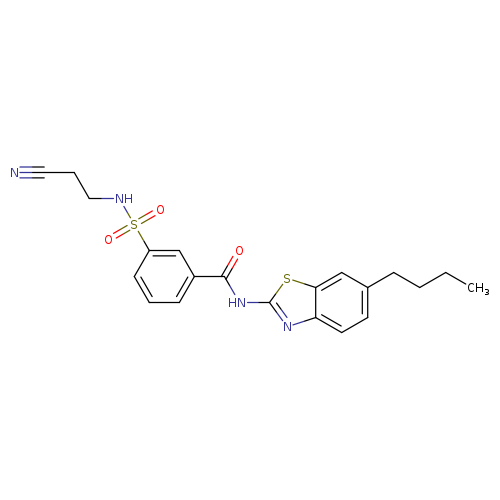
N-(6-Butyl-1,3-benzothiazol-2-yl)-3-[(2-cyanoethyl)sulfamoyl]benzamideCatalog No.:AA01DUVX CAS No.:1090911-74-2 MDL No.:MFCD11807900 MF:C21H22N4O3S2 MW:442.5544 |
2-chloro-6-(4-fluorophenoxy)pyridineCatalog No.:AA01AN6E CAS No.:1090916-41-8 MDL No.:MFCD11812089 MF:C11H7ClFNO MW:223.6308 |
2-(3-Ethyl-1h-1,2,4-triazol-5-yl)anilineCatalog No.:AA01E967 CAS No.:1090921-40-6 MDL No.:MFCD13704511 MF:C10H12N4 MW:188.2291 |
1-(2-fluorophenyl)-3-(prop-2-yn-1-yl)ureaCatalog No.:AA019WNR CAS No.:1090922-05-6 MDL No.:MFCD29763482 MF:C10H9FN2O MW:192.1897 |
4-[(Thiophen-3-yl)carbonyl]morpholineCatalog No.:AA00HB7Q CAS No.:1090955-28-4 MDL No.:MFCD11742082 MF:C9H11NO2S MW:197.2541 |
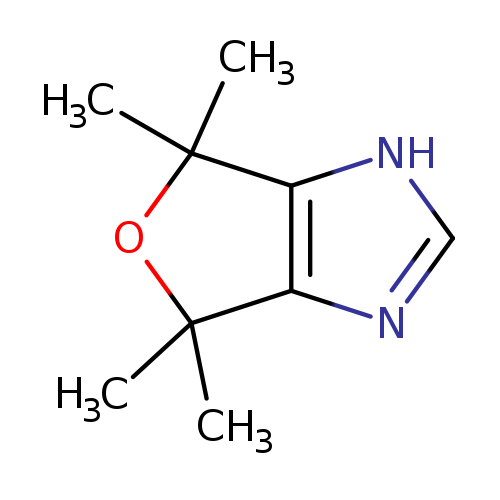
4,4,6,6-tetramethyl-4,6-dihydro-1H-furo[3,4-d]imidazoleCatalog No.:AA00J2IY CAS No.:109096-87-9 MDL No.:MFCD19982754 MF:C9H14N2O MW:166.2203 |
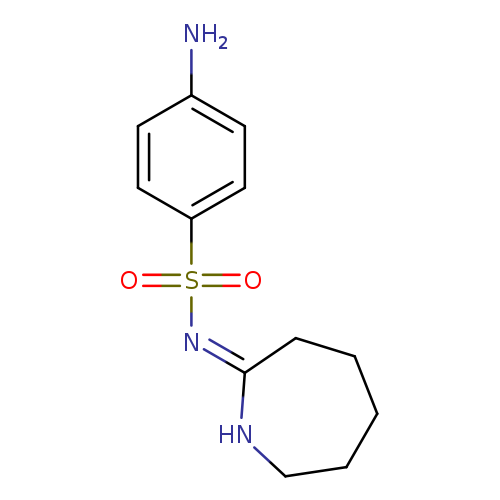
4-amino-N-[(2E)-azepan-2-ylidene]benzenesulfonamideCatalog No.:AA0197KF CAS No.:109097-77-0 MDL No.:MFCD03414804 MF:C12H17N3O2S MW:267.3473 |
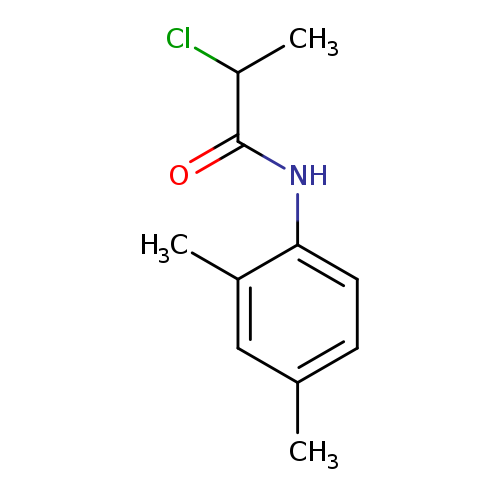
2-Chloro-n-(2,4-dimethylphenyl)propanamideCatalog No.:AA009M2A CAS No.:109099-55-0 MDL No.:MFCD09403766 MF:C11H14ClNO MW:211.6880 |
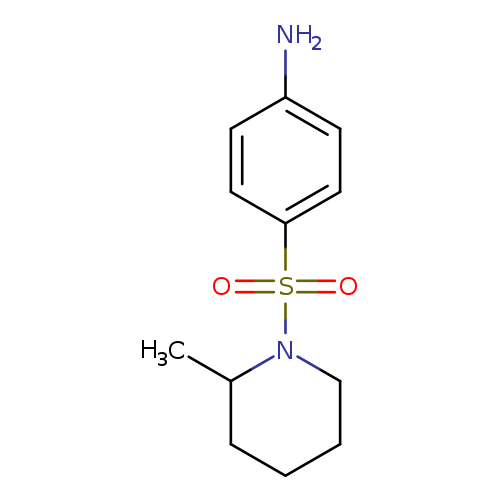
4-[(2-Methylpiperidin-1-yl)sulfonyl]anilineCatalog No.:AA008V2O CAS No.:109099-69-6 MDL No.:MFCD01055485 MF:C12H18N2O2S MW:254.3485 |
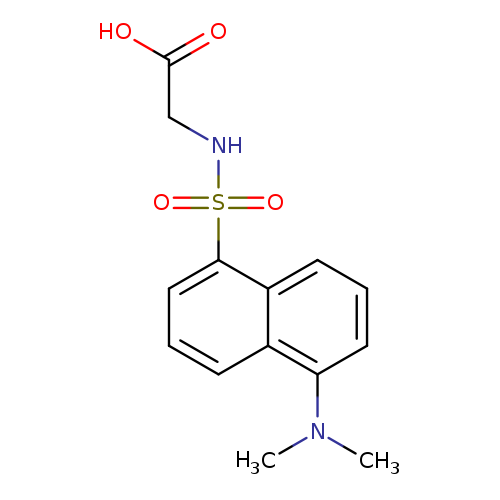
DansylglycineCatalog No.:AA003P5R CAS No.:1091-85-6 MDL No.:MFCD00037734 MF:C14H16N2O4S MW:308.3528 |
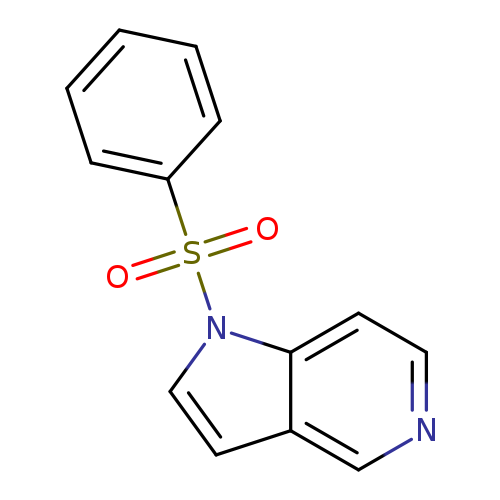
1-(Phenylsulfonyl)-1h-pyrrolo[3,2-c]pyridineCatalog No.:AA007BFI CAS No.:109113-39-5 MDL No.:MFCD11848711 MF:C13H10N2O2S MW:258.2957 |
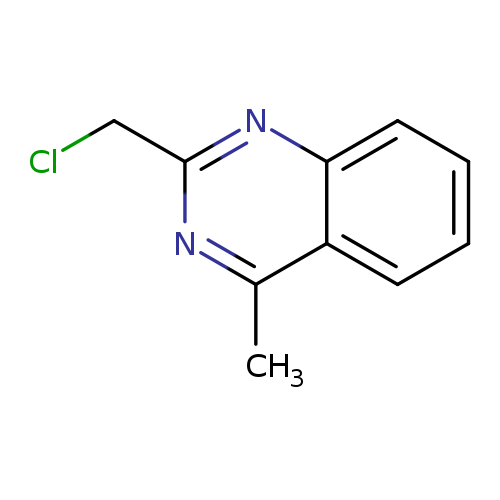
2-(Chloromethyl)-4-methylquinazolineCatalog No.:AA0039N8 CAS No.:109113-72-6 MDL No.:MFCD09807547 MF:C10H9ClN2 MW:192.6449 |
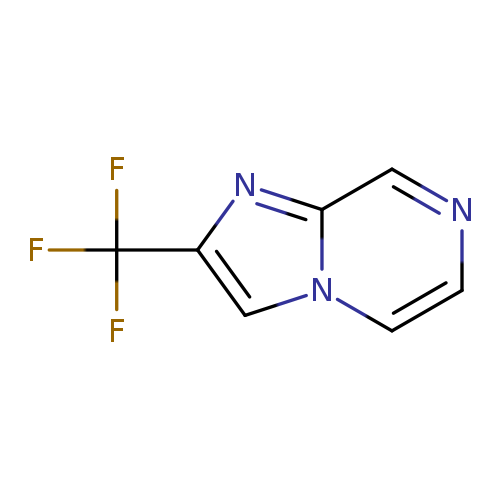
2-(Trifluoromethyl)imidazo[1,2-a]pyrazineCatalog No.:AA0082QK CAS No.:109113-96-4 MDL No.:MFCD05248878 MF:C7H4F3N3 MW:187.1220 |
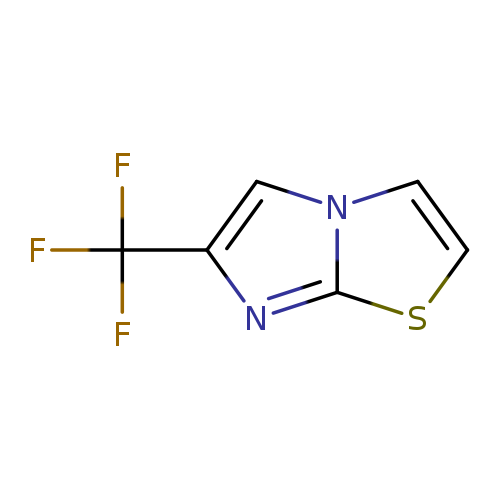
6-(Trifluoromethyl)imidazo[2,1-b]thiazoleCatalog No.:AA007BFH CAS No.:109113-98-6 MDL No.:MFCD12828656 MF:C6H3F3N2S MW:192.1616 |
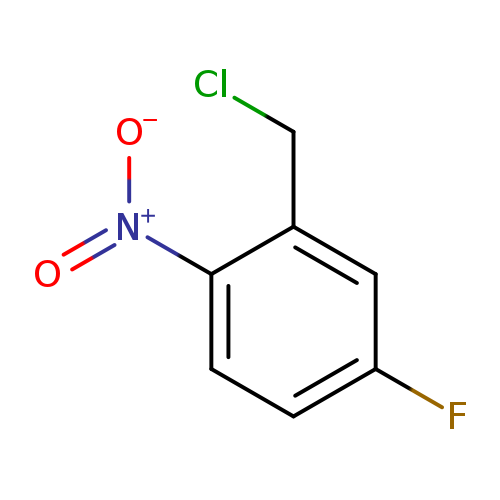
2-(Chloromethyl)-4-fluoro-1-nitrobenzeneCatalog No.:AA01BZGN CAS No.:109115-05-1 MDL No.:MFCD13185628 MF:C7H5ClFNO2 MW:189.5715 |
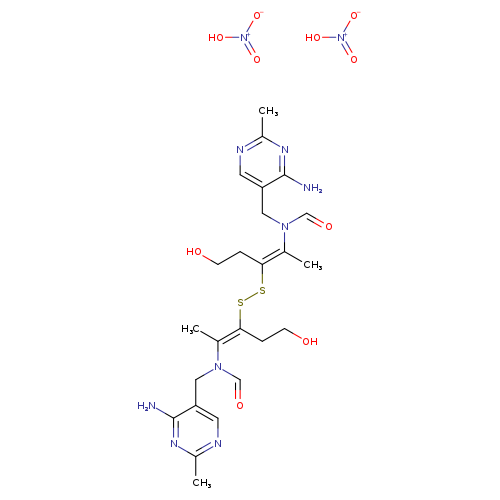
THIAMINE DISULFIDE NITRATECatalog No.:AA008WHU CAS No.:109125-52-2 MDL No.:MFCD00060189 MF:C24H36N10O10S2 MW:688.7336 |
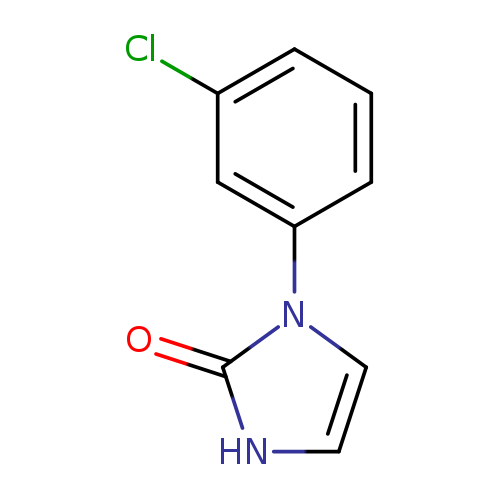
1-(3-Chlorophenyl)-1H-imidazol-2(3H)-oneCatalog No.:AA01C59B CAS No.:109130-25-8 MDL No.:MFCD12965845 MF:C9H7ClN2O MW:194.6177 |
3-(N-Boc-Amino)benzotrifluorideCatalog No.:AA0082QD CAS No.:109134-07-8 MDL No.:MFCD11040633 MF:C12H14F3NO2 MW:261.2403 |
2-(3-Methoxyphenyl)propan-2-amineCatalog No.:AA007TL2 CAS No.:109138-28-5 MDL No.:MFCD09864419 MF:C10H15NO MW:165.2322 |
S-trifluoromethyl-p-fluorophenylsulfoximineCatalog No.:AA008WEY CAS No.:109139-20-0 MDL No.:MFCD16660921 MF:C7H5F4NOS MW:227.1793 |
5-Methoxychroman-3-oneCatalog No.:AA007TKY CAS No.:109140-20-7 MDL No.:MFCD12828271 MF:C10H10O3 MW:178.1846 |
N-Hydroxy NorfloxacinCatalog No.:AA007TKX CAS No.:109142-49-6 MDL No.:MFCD01685693 MF:C16H18FN3O4 MW:335.3302 |
TERT-BUTYLISOPROPYLDIMETHOXYSILANECatalog No.:AA007TKQ CAS No.:109144-59-4 MDL No.:MFCD09909985 MF:C9H22O2Si MW:190.3553 |
7-Nitro-2H-imidazo[4,5-d]pyridineCatalog No.:AA0037W5 CAS No.:109151-82-8 MDL No.:MFCD11846334 MF:C6H4N4O2 MW:164.1216 |
Methyl cyclopentanecarbimidate hydrochlorideCatalog No.:AA007B96 CAS No.:109152-86-5 MDL No.:MFCD25955361 MF:C7H14ClNO MW:163.6452 |
2-(Diethylamino)ethyl [1,1'-bi(cyclohexan)]-1'-ene-1-carboxylateCatalog No.:AA01CBA9 CAS No.:109158-77-2 MDL No.: MF:C19H33NO2 MW:307.4708 |
N7-benzylpyrimido[4,5-d]pyrimidine-2,4,7-triamineCatalog No.:AA01BTGO CAS No.:109160-46-5 MDL No.:MFCD30539857 MF:C13H13N7 MW:267.2892 |
(4S,5R)-4,5-Diphenyl-1,2,3-oxathiazolidine-2,2-dioxide-3-carboxylic acid t-butyl esterCatalog No.:AA008V8E CAS No.:1091606-63-1 MDL No.:MFCD17018791 MF:C19H21NO5S MW:375.4387 |
(4S,5R)-4-Methyl-5-phenyl-1,2,3-oxathiazolidine-2,2-dioxide-3-carboxylic acid t-butyl esterCatalog No.:AA008VGO CAS No.:1091606-65-3 MDL No.:MFCD17018783 MF:C14H19NO5S MW:313.3694 |
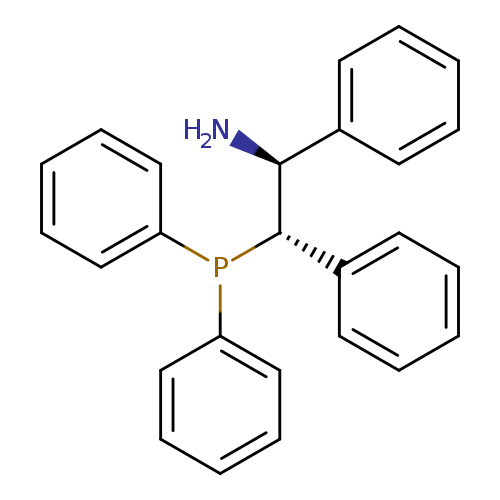
(1S,2S)-2-(Diphenylphosphino)-1,2-diphenylethanamineCatalog No.:AA003BG8 CAS No.:1091606-67-5 MDL No.:MFCD11045444 MF:C26H24NP MW:381.4492 |
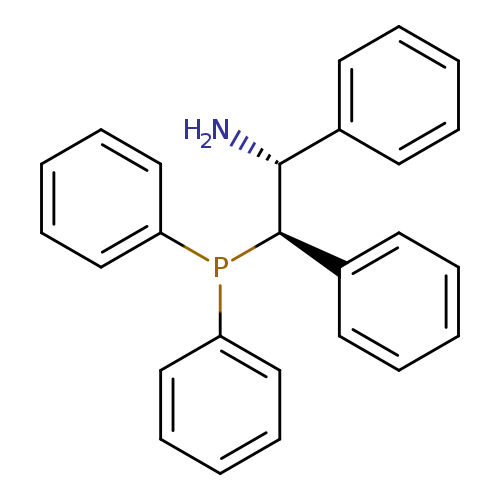
(1R,2R)-2-(Diphenylphosphino)-1,2-diphenylethylamineCatalog No.:AA008V1M CAS No.:1091606-68-6 MDL No.:MFCD17013982 MF:C26H24NP MW:381.4492 |
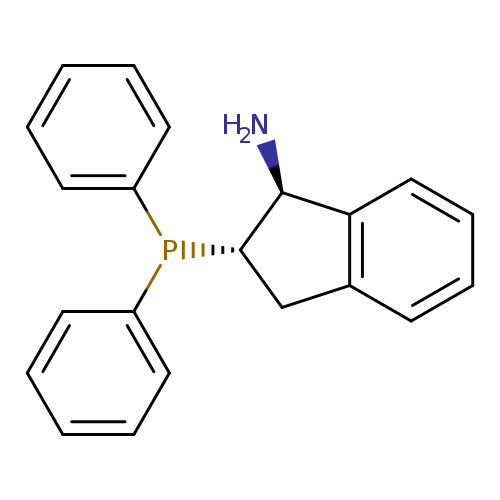
(1S,2S)-2-(Diphenylphosphino)-2,3-dihydro-1H-inden-1-amineCatalog No.:AA003BG9 CAS No.:1091606-69-7 MDL No.:MFCD17013984 MF:C21H20NP MW:317.3640 |
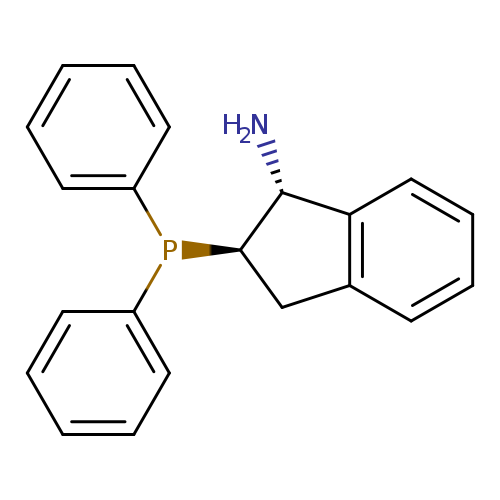
(1R,2R)-2-(Diphenylphosphino)-2,3-dihydro-1h-inden-1-amineCatalog No.:AA003BDM CAS No.:1091606-70-0 MDL No.:MFCD17013983 MF:C21H20NP MW:317.3640 |
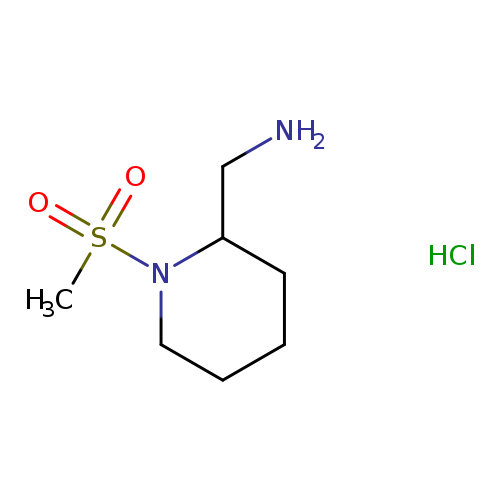
(1-methanesulfonylpiperidin-2-yl)methanamine hydrochlorideCatalog No.:AA01BAP1 CAS No.:1091613-74-9 MDL No.:MFCD28397724 MF:C7H17ClN2O2S MW:228.7401 |
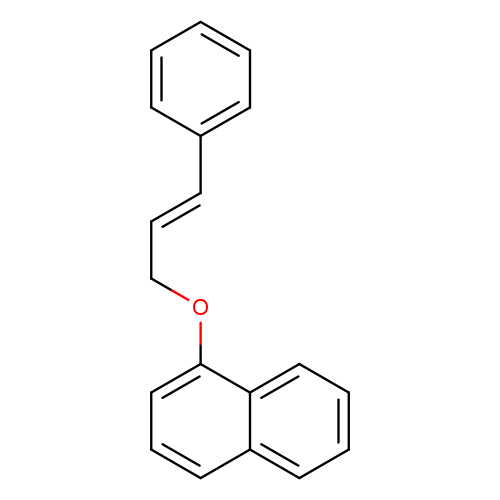
1-[[(2E)-3-Phenyl-2-propen-1-yl]oxy]naphthaleneCatalog No.:AA008WMG CAS No.:1091626-77-5 MDL No.:MFCD29039419 MF:C19H16O MW:260.3297 |
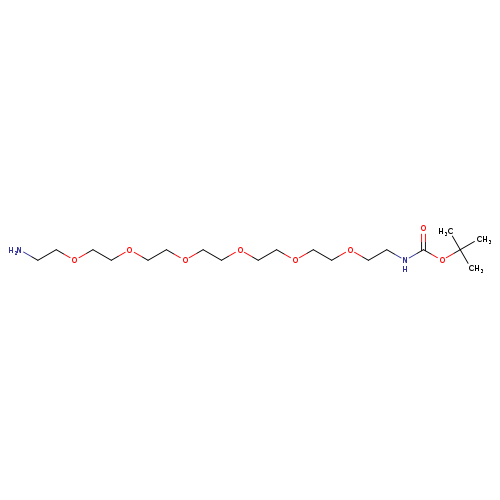
t-Boc-N-amido-PEG6-AmineCatalog No.:AA008UXN CAS No.:1091627-77-8 MDL No.:MFCD16619391 MF:C19H40N2O8 MW:424.5295 |
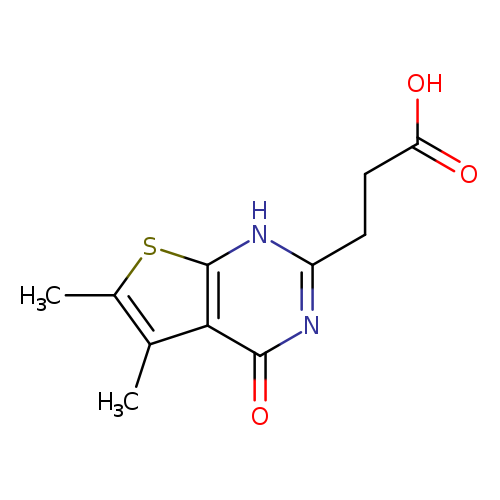
3-(5,6-Dimethyl-4-oxo-1h,4h-thieno[2,3-d]pyrimidin-2-yl)propanoic acidCatalog No.:AA019XGN CAS No.:109164-46-7 MDL No.:MFCD13196184 MF:C11H12N2O3S MW:252.2896 |
2-Bromo-3-methylbenzaldehydeCatalog No.:AA0096WT CAS No.:109179-31-9 MDL No.:MFCD18392002 MF:C8H7BrO MW:199.0446 |
N-[1-(4-Bromophenyl)ethyl]methanesulfonamideCatalog No.:AA00947R CAS No.:1091796-50-7 MDL No.:MFCD13891254 MF:C9H12BrNO2S MW:278.1661 |
21-Acetyloxy DeschloroMoMetasone Furoate 9,11-EpoxideCatalog No.:AA008WZI CAS No.:109183-56-4 MDL No.:MFCD28143241 MF:C29H32O8 MW:508.5596 |
Boc-L-cyclohexylglycineCatalog No.:AA0034H9 CAS No.:109183-71-3 MDL No.:MFCD02684452 MF:C13H23NO4 MW:257.3260 |
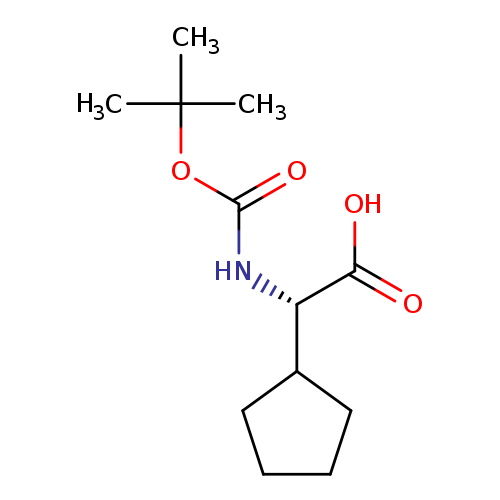
Boc-L-cyclopentylglycineCatalog No.:AA008R76 CAS No.:109183-72-4 MDL No.:MFCD00671385 MF:C12H21NO4 MW:243.2994 |
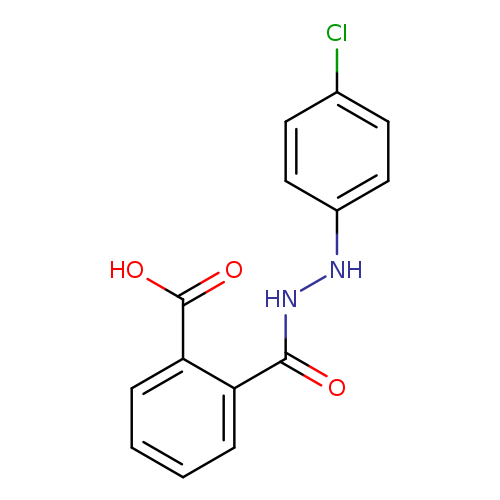
2-[N'-(4-chlorophenyl)hydrazinecarbonyl]benzoic acidCatalog No.:AA00IQ7A CAS No.:109187-07-7 MDL No.:MFCD00793721 MF:C14H11ClN2O3 MW:290.7017 |
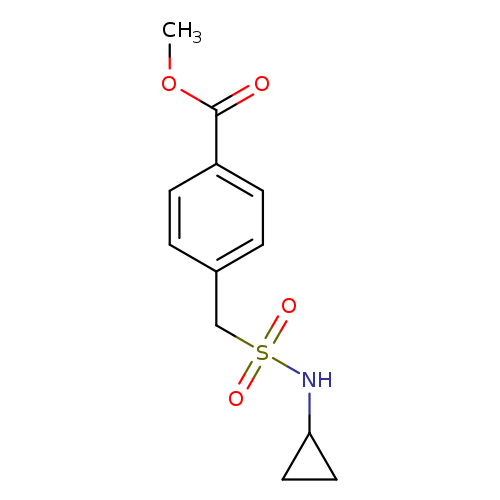
Methyl 4-[(cyclopropylsulfamoyl)methyl]benzoateCatalog No.:AA01EH6M CAS No.:1091876-96-8 MDL No.:MFCD13894909 MF:C12H15NO4S MW:269.3168 |
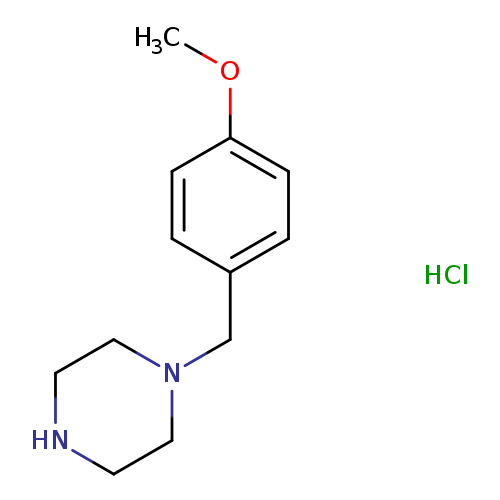
1-(4-METHOXYBENZYL)PIPERAZINEHYDROCHLORIDECatalog No.:AA009TKG CAS No.:109188-09-2 MDL No.:MFCD05656277 MF:C12H19ClN2O MW:242.7451 |
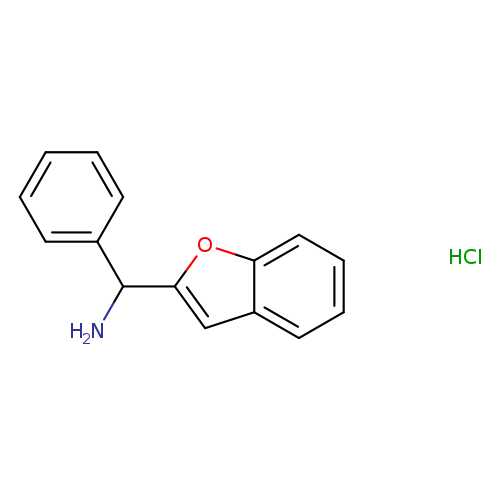
1-benzofuran-2-yl(phenyl)methanamine hydrochlorideCatalog No.:AA019PJM CAS No.:109194-13-0 MDL No.:MFCD08245282 MF:C15H14ClNO MW:259.7308 |
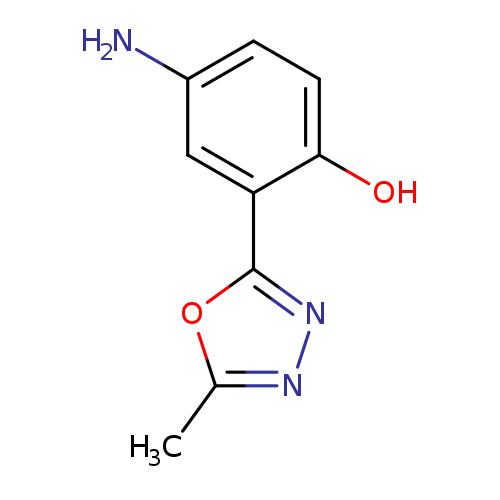
4-Amino-2-(5-methyl-1,3,4-oxadiazol-2-yl)phenolCatalog No.:AA008V39 CAS No.:1091990-90-7 MDL No.:MFCD12198473 MF:C9H9N3O2 MW:191.1867 |
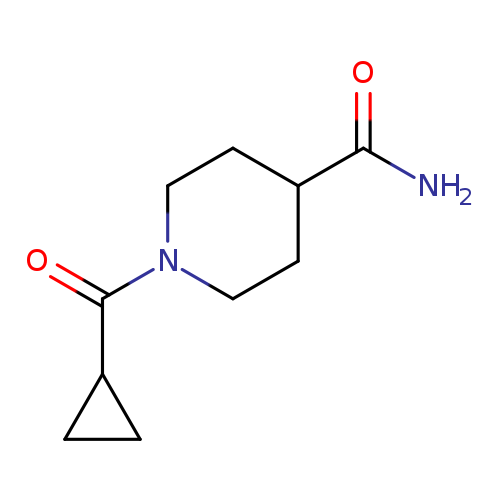
1-(Cyclopropanecarbonyl)piperidine-4-carboxamideCatalog No.:AA01FODN CAS No.:1092023-78-3 MDL No.: MF:C10H16N2O2 MW:196.2462 |
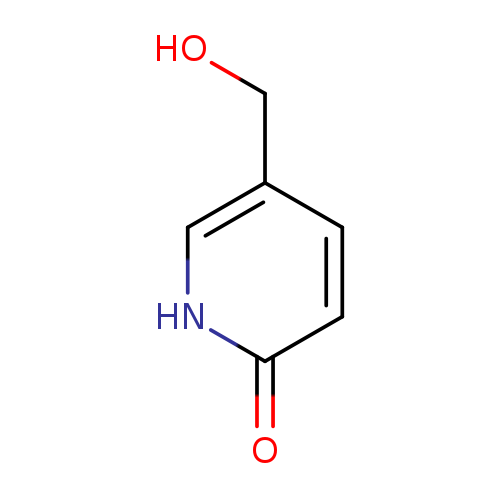
5-(Hydroxymethyl)pyridin-2(1h)-oneCatalog No.:AA007TK9 CAS No.:109205-68-7 MDL No.:MFCD09839753 MF:C6H7NO2 MW:125.1253 |