Title: Pharmacokinetics, tolerability, and preliminary efficacy of paquinimod (ABR-215757), a new quinoline-3-carboxamide derivative: studies in lupus-prone mice and a multicenter, randomized, double-blind, placebo-controlled, repeat-dose, dose-ranging study in patients with systemic lupus erythematosus.
Journal: Arthritis and rheumatism 20120501
Title: Specific effect of immunomodulatory quinoline-3-carboxamide ABR-215757 in GM-CSF stimulated bone marrow cell cultures: block of initiation of proliferation of Gr-1+ cells.
Journal: International immunopharmacology 20110801
Title: The impact of a new immunomodulator oxo-quinoline-3-carboxamide on the progression of experimental lupus.
Journal: International immunopharmacology 20041101
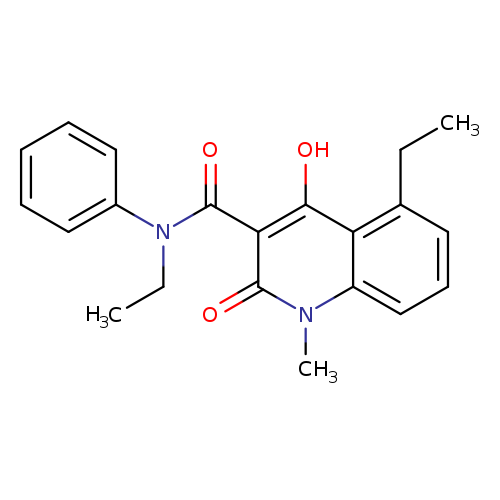
Title: Synthesis and biological evaluation of new 1,2-dihydro-4-hydroxy-2-oxo-3-quinolinecarboxamides for treatment of autoimmune disorders: structure-activity relationship.
Journal: Journal of medicinal chemistry 20040408
Title: Schelbergen RF, et al. Prophylactic treatment with S100A9 inhibitor paquinimod reduces pathology in experimental collagenase-induced osteoarthritis. Ann Rheum Dis. 2015 Dec;74(12):2254-8.
Title: Tahvili S, et al. Paquinimod prevents development of diabetes in the non-obese diabetic (NOD) mouse. PLoS One. 2018 May 9;13(5):e0196598.
Title: Qirui Guo, et al. Induction of alarmin S100A8/A9 mediates activation of aberrant neutrophils in the pathogenesis of COVID-19. Cell Host Microbe. 2021 Feb 10;29(2):222-235.e4.