Title: Role of Protein Kinase C in Endothelin Converting Enzyme-1 trafficking and shedding from endothelial cells.
Journal: Biochemical and biophysical research communications 20100723
Title: Diabetes-associated changes and role of N epsilon-(carboxymethyl)lysine in big ET-1-induced coronary vasoconstriction.
Journal: Peptides 20100201
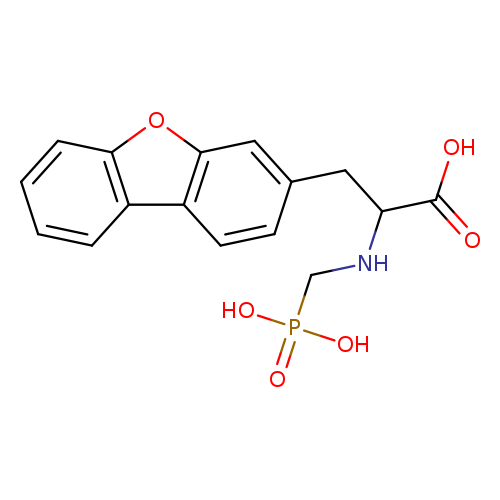
Title: Phosphinic tripeptides as dual angiotensin-converting enzyme C-domain and endothelin-converting enzyme-1 inhibitors.
Journal: Journal of medicinal chemistry 20100114
Title: Triple vasopeptidase inhibition normalizes blood pressure in conscious, unrestrained, and spontaneously hypertensive rats.
Journal: American journal of hypertension 20051201
Title: Endothelin-1 (1-31) is an intermediate in the production of endothelin-1 after big endothelin-1 administration in vivo.
Journal: Hypertension (Dallas, Tex. : 1979) 20050701
Title: Role of the endothelin system in secondary pulmonary hypertension related to air embolism: lessons learned from testing four classes of endothelin blockers in a rat model.
Journal: Journal of cardiovascular pharmacology 20041101
Title: Triple vasopeptidase inhibition of angiotensin-converting enzyme/neutral endopeptidase/endothelin-converting enzyme activities on the hemodynamic profile of chronically instrumented unrestrained conscious spontaneously hypertensive rats.
Journal: Journal of cardiovascular pharmacology 20041101
Title: [Physiological and pathophysiological roles of the endothelin converting enzymes].
Journal: Nihon rinsho. Japanese journal of clinical medicine 20040901
Title: Role of endothelin-converting enzyme, chymase and neutral endopeptidase in the processing of big ET-1, ET-1(1-21) and ET-1(1-31) in the trachea of allergic mice.
Journal: Clinical science (London, England : 1979) 20020801
Title: Effects of benazepril, an angiotensin-converting enzyme inhibitor, combined with CGS 35066, a selective endothelin-converting enzyme inhibitor, on arterial blood pressure in normotensive and spontaneously hypertensive rats.
Journal: Clinical science (London, England : 1979) 20020801
Title: Toward an optimal joint recognition of the S1' subsites of endothelin converting enzyme-1 (ECE-1), angiotensin converting enzyme (ACE), and neutral endopeptidase (NEP).
Journal: Journal of medicinal chemistry 20020328
Title: Attenuation of SAH-induced cerebral vasospasm by a selective ECE inhibitor.
Journal: Neuroreport 20020211
Title: Potent and selective non-peptidic inhibitors of endothelin-converting enzyme-1 with sustained duration of action.
Journal: Journal of medicinal chemistry 20000210