Title: Intranasally administered neuropeptide S (NPS) exerts anxiolytic effects following internalization into NPS receptor-expressing neurons.
Journal: Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 20120501
Title: [tBu-D-Gly5]NPS, a pure and potent antagonist of the neuropeptide S receptor: in vitro and in vivo studies.
Journal: Peptides 20120401
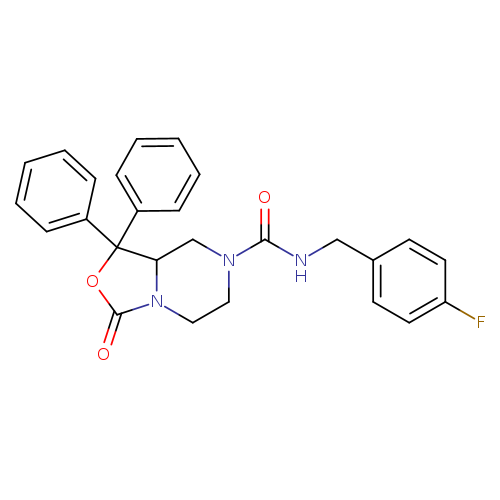
Title: Synthesis and separation of the enantiomers of the neuropeptide S receptor antagonist (9R/S)-3-oxo-1,1-diphenyl-tetrahydro-oxazolo[3,4-a]pyrazine-7-carboxylic acid 4-fluoro-benzylamide (SHA 68).
Journal: Journal of medicinal chemistry 20110428
Title: Neuropeptide S facilitates cue-induced relapse to cocaine seeking through activation of the hypothalamic hypocretin system.
Journal: Proceedings of the National Academy of Sciences of the United States of America 20101109
Title: Further studies on the pharmacological profile of the neuropeptide S receptor antagonist SHA 68.
Journal: Peptides 20100501
Title: Importance of extracellular loop one of the neuropeptide S receptor for biogenesis and function.
Journal: Peptides 20100101
Title: Identifying structural features on 1,1-diphenyl-hexahydro-oxazolo[3,4-a]pyrazin-3-ones critical for Neuropeptide S antagonist activity.
Journal: Bioorganic & medicinal chemistry letters 20080715
Title: Synthesis and pharmacological in vitro and in vivo profile of 3-oxo-1,1-diphenyl-tetrahydro-oxazolo[3,4-a]pyrazine-7-carboxylic acid 4-fluoro-benzylamide (SHA 68), a selective antagonist of the neuropeptide S receptor.
Journal: The Journal of pharmacology and experimental therapeutics 20080601
Title: Okamura N, et al. Synthesis and pharmacological in vitro and in vivo profile of 3-oxo-1,1-diphenyl-tetrahydro-oxazolo,4-apyrazine-7-carboxylic acid 4-fluoro-benzylamide (SHA 68), a selective antagonist of the neuropeptide S receptor.J Pharmacol Exp Ther. 2008 Jun;325(3):893-901.
Title: Ensho T, et al. Neuropeptide S increases motor activity and thermogenesis in the rat through sympathetic activation.Neuropeptides. 2017 Oct;65:21-27.