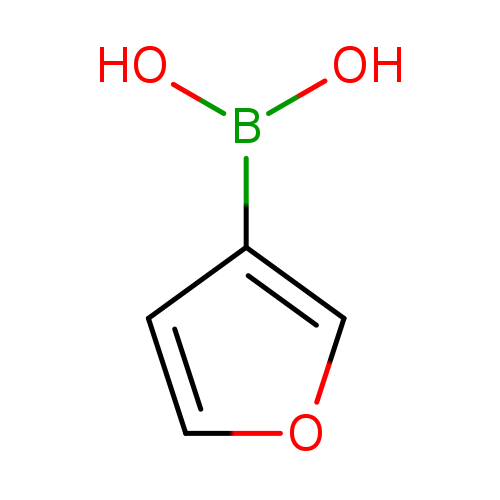
[1]BioorganicandMedicinalChemistryLetters,2006,vol.16,#19,p.4987-4993
[2]MedicinalChemistryResearch,2002,vol.11,#2,p.87-101
[3]JournalofOrganicChemistry,2013,vol.78,#13,p.6427-6439
[1]Heterocycles,2010,vol.80,#1,p.313-328
[1]OrganicProcessResearchandDevelopment,2017,vol.21,#11,p.1859-1863
[1]JournaloftheAmericanChemicalSociety,2015,vol.137,#3,p.1330-1340
[1]JournalofOrganicChemistry,1988,vol.53,p.2052-2055
[2]Patent:US2011/306589,2011,A1.Locationinpatent:Page/Pagecolumn43
[3]Patent:WO2011/154327,2011,A1.Locationinpatent:Page/Pagecolumn112;113
[4]Patent:WO2008/36272,2008,A1.Locationinpatent:Page/Pagecolumn47
[1]JournalofHeterocyclicChemistry,1990,vol.27,p.2165-2173
[1]TetrahedronLetters,2005,vol.46,p.1779-1782
[1]TetrahedronLetters,2005,vol.46,p.3053-3056
[1]Steroids,2006,vol.71,p.585-590
Title: Synthesis of 8-aryl-substituted coumarins based on ring-closing metathesis and Suzuki-Miyaura coupling: synthesis of a furyl coumarin natural product from Galipea panamensis.
Journal: The Journal of organic chemistry 20120302