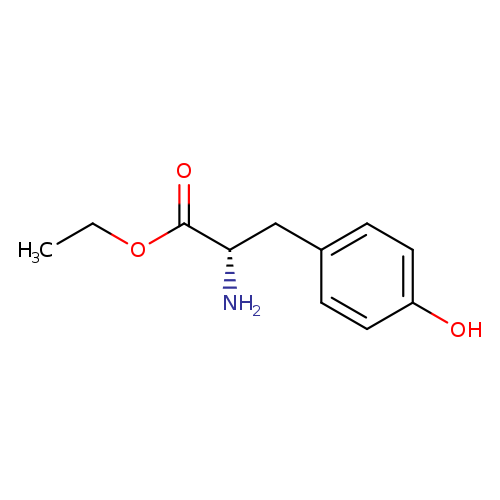
Title: Dimorphism of the prodrug L-tyrosine ethyl ester: pressure-temperature state diagram and crystal structure of phase II.
Journal: Journal of pharmaceutical sciences 20111101
Title: Synthesis and evaluation of amino acid-based radiotracer 99mTc-N4-AMT for breast cancer imaging.
Journal: Journal of biomedicine & biotechnology 20110101
Title: High level production of bioactive di- and tri-tyrosine peptides by protease-catalyzed reactions.
Journal: Journal of biotechnology 20101101
Title: Probing molecular motion by double-quantum (13C,13C) solid-state NMR spectroscopy: application to ubiquitin.
Journal: Journal of the American Chemical Society 20100113
Title: Singlet molecular oxygen [O2(1Deltag)]-mediated photodegradation of tyrosine derivatives in the presence of cationic and neutral micellar systems.
Journal: Amino acids 20080601
Title: Synthesis of gossypol atropisomers and derivatives and evaluation of their anti-proliferative and anti-oxidant activity.
Journal: Bioorganic & medicinal chemistry 20050701
Title: Transverse dephasing optimised NMR spectroscopy in solids: natural-abundance 13C correlation spectra.
Journal: Chemphyschem : a European journal of chemical physics and physical chemistry 20040621
Title: Fluorescence spectroscopic study of alpha-chymotrypsin relevant to the enantioselectivity for optical resolution of amino acid esters in organic solvents.
Journal: Biotechnology letters 20040401
Title: Light-driven tyrosine radical formation in a ruthenium-tyrosine complex attached to nanoparticle TiO2.
Journal: Inorganic chemistry 20021202
Title: Synthesis of 2-[18F]fluoro-L-tyrosine via regiospecific fluoro-de-stannylation.
Journal: Applied radiation and isotopes : including data, instrumentation and methods for use in agriculture, industry and medicine 20020801
Title: Approaches to the synthesis of some tyrosine-derived marine sponge metabolites: synthesis of verongamine and purealidin N.
Journal: The Journal of organic chemistry 20010504