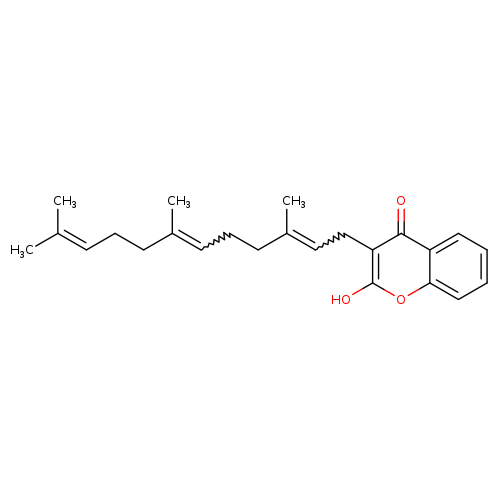
Title: Disruption of mitochondrial membrane potential by ferulenol and restoration by propolis extract: antiapoptotic role of propolis.
Journal: Acta biologica Hungarica 20091201
Title: Characterization of anti-coagulant properties of prenylated coumarin ferulenol.
Journal: Biochimica et biophysica acta 20071001
Title: Ferulenol specifically inhibits succinate ubiquinone reductase at the level of the ubiquinone cycle.
Journal: Biochemical and biophysical research communications 20070330
Title: Synthesis and structure-activity relationships of novel warfarin derivatives.
Journal: Bioorganic & medicinal chemistry 20070315
Title: Antimycobacterial coumarins from the sardinian giant fennel (Ferula communis).
Journal: Journal of natural products 20041201
Title: HPLC-DAD-MS identification of bioactive secondary metabolites from Ferula communis roots.
Journal: Fitoterapia 20040601
Title: Farnesyloxycoumarins, a new class of squalene-hopene cyclase inhibitors.
Journal: Bioorganic & medicinal chemistry letters 20040419
Title: Microtubule-interacting activity and cytotoxicity of the prenylated coumarin ferulenol.
Journal: Planta medica 20021201
Title: Acute toxicity of ferulenol, a 4-hydroxycoumarin isolated from Ferula communis L.
Journal: Veterinary and human toxicology 20020201
Title: Lahouel M, et al. Ferulenol specifically inhibits succinate ubiquinone reductase at the level of the ubiquinonecycle. Biochem Biophys Res Commun. 2007 Mar 30;355(1):252-7.
Title: Mamoci E, et al. Chemical composition and in vitro activity of plant extracts from Ferula communis and Dittrichia viscosa against postharvest fungi. Molecules. 2011 Mar 22;16(3):2609-25.
Title: DrissLamnaouer, et al. Ferulenol and ω-hydroxyferulenol, toxic coumarins from Ferula communis var. genuine. Volume 26, Issue 6, 1987, Pages 1613-1615.