Title: 5-Modified-2'-dU and 2'-dC as mutagenic anti HIV-1 proliferation agents: synthesis and activity.
Journal: Journal of medicinal chemistry 20100225
Title: 3D-QSAR studies on antitubercular thymidine monophosphate kinase inhibitors based on different alignment methods.
Journal: Bioorganic & medicinal chemistry letters 20060215
Title: Synthesis and in vitro anti-mycobacterial activity of 5-substituted pyrimidine nucleosides.
Journal: Bioorganic & medicinal chemistry 20051215
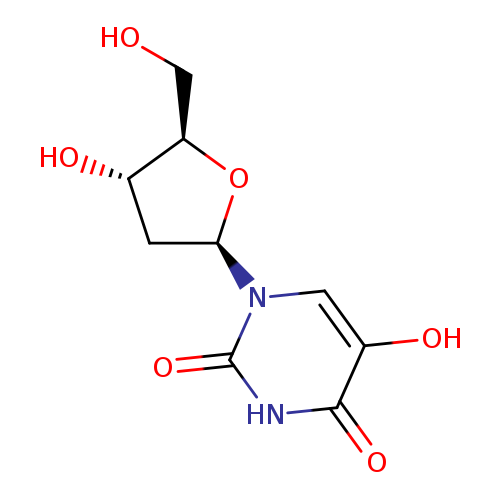
Title: Oxidation of 5-hydroxypyrimidine nucleosides to 5-hydroxyhydantoin and its alpha-hydroxy-ketone isomer.
Journal: Chemical research in toxicology 20050801
Title: Oxidation of 5-hydroxy-2'-deoxyuridine into isodialuric acid, dialuric acid, and hydantoin products.
Journal: Journal of the American Chemical Society 20040602
Title: Comparative study of purine and pyrimidine nucleoside analogues acting on the thymidylate kinases of Mycobacterium tuberculosis and of humans.
Journal: Chembiochem : a European journal of chemical biology 20030804
Title: Design of Mycobacterium tuberculosis thymidine monophosphate kinase inhibitors.
Journal: Nucleosides, nucleotides & nucleic acids 20030101
Title: New substrates for old enzymes. 5-Hydroxy-2'-deoxycytidine and 5-hydroxy-2'-deoxyuridine are substrates for Escherichia coli endonuclease III and formamidopyrimidine DNA N-glycosylase, while 5-hydroxy-2'-deoxyuridine is a substrate for uracil DNA N-glycosylase.
Journal: The Journal of biological chemistry 19940722
Title: Comparison of susceptibilities of varicella-zoster virus and herpes simplex viruses to nucleoside analogs.
Journal: Antimicrobial agents and chemotherapy 19860301
Title: 5-O-Alkylated derivatives of 5-hydroxy-2'-deoxyuridine as potential antiviral agents. Anti-herpes activity of 5-propynyloxy-2'-deoxyuridine.
Journal: Journal of medicinal chemistry 19780201
Title: Nucleosides. 3. Studies on 5-methylamino-2'-deoxyuridine as a specific antiherpes agent.
Journal: Journal of medicinal chemistry 19660501
Title: Purmal AA, et al. Major oxidative products of cytosine, 5-hydroxycytosine and 5-hydroxyuracil, exhibit sequence context-dependent mispairing in vitro. Nucleic Acids Res. 1994 Jan 11;22(1):72-8.