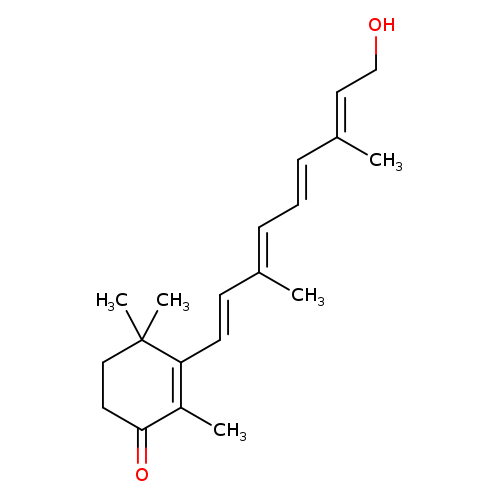
Title: Metabolism and regulation of gene expression by 4-oxoretinol versus all-trans retinoic acid in normal human mammary epithelial cells.
Journal: Journal of cellular physiology 20090901
Title: Metabolism and biological activities of topical 4-oxoretinoids in mouse skin.
Journal: The Journal of investigative dermatology 20080401
Title: LIF removal increases CRABPI and CRABPII transcripts in embryonic stem cells cultured in retinol or 4-oxoretinol.
Journal: Molecular and cellular endocrinology 20080102
Title: Beta-carotene and apocarotenals promote retinoid signaling in BEAS-2B human bronchioepithelial cells.
Journal: Archives of biochemistry and biophysics 20061101
Title: The structures of the unique sulfotransferase retinol dehydratase with product and inhibitors provide insight into enzyme mechanism and inhibition.
Journal: Protein science : a publication of the Protein Society 20050101
Title: Removal of LIF (leukemia inhibitory factor) results in increased vitamin A (retinol) metabolism to 4-oxoretinol in embryonic stem cells.
Journal: Proceedings of the National Academy of Sciences of the United States of America 19991109