2020-02-17 22:20:38
Nermin S. Ahmed
1| INTRODUCTION
The nitric oxide/cGMP pathway is an essential pathway in many normal physiological functions; disruption of this pathway plays a role in the pathophysiology of several diseases. Nitric oxide (NO) binds to sol- uble guanylyl cyclase (sGC) an action that triggers (sGC)-cGMP signal- ing pathway. NO is synthesized by the oxidation of L-arginine, nitric oxide synthase (NOS) catalyzes the oxidation process in the presence of NADPH and O2 as substrates. NO activates sGC, sGC converts GTP to cGMP. The formed cGMP activates cGMP-dependent protein kinase (PKG, cGK); such kinases activate a cascade of proteins result- ing in numerous physiological effects. Therefore, NO-sGC-cGMP signaling pathway plays essential role in physiological processes like growth, cell viability, smooth muscle relaxation, neurotransmission, inflammation, and gene transcription. cGMP are hydrolyzed to GMP (inactive form) via cGMP specific PDE enzymes (PDE5, PDE6, and PDE9), which break its phosphodiester bond. PDE inhibitors block the action of PDE and thus elevate the levels of cGMP (Denninger & Marletta, 1999; Moncada, Palmer, & Higgs, 1991; Murad, 2006).
The synthesis of sildenafil (1), the first commercially available PDE5 inhibitor originally studied as antianginal agent, was a break- through in the treatment of erectile dysfunction (ED). Sildenafil discovery encouraged researchers to investigate novel clinical applications of PDE5 inhibitors. Although many PDE5 inhibitors were synthesized, sildenafil (1), tadalafil (2), and vardenafil (3) were the focus of scientific studies. Since sildenafil (1) was discovered, PDE5 inhibitors are perceived as the first line of therapy for ED. New PDE5 inhibitors were introduced to the market with clinical applications beyond male erectile dysfunction (MED). Sildenafil (1), tadalafil (2), vardenafil (3), lodenafil (4), and mirodenafil (5) are applied in the treat- ment of asthma, chronic obstructive pulmonary disease (COPD), pul- monary arterial hypertension (PAH), cardiac failure, autoimmune diseases, and ED (Maurice et al., 2014).
Tadalafil inhibits both PDE5 and PDE11 enzymes; PDE11 enzyme is abundant in skeletal muscle. Inhibition of PDE11 with tadalafil leads to the common side effects, namely, back and muscle pain (myalgia) (Makhlouf, Kshirsagar, & Niederberger, 2006). It was found that the catalytic site of PDE11 resembles that of PDE5, how- ever, there is no available crystal structure for PDE11 and no ade- quate knowledge about its physiological role in human body. This lack of data restricts our understanding and limits our conception to how this PDE isoform works.
2| ROUTES OF SYNTHESIS ADOPTED IN PREPARATION OF TADALAFIL AND ITS ANALOGUES
The huge success of tadalafil and its analogues have encouraged tremendous research that focuses on developing synthetic routes to these tetrahydro-β-carboline derivatives. A straightforward synthetic scheme was initially adopted for the preparation of Tadalafil (2). This scheme is based on the work of Saxena et al. using four main starting blocks, namely, D-tryptophan methyl ester, commercially available piperonal, chloroacetyl chloride, and methylamine (Saxena, Jain, & Anand, 1973).
Pictet–Spengler (P–S) reaction is used to construct chiral tetrahydro-β-carbolines moieties. The P–S reaction of D-tryptophan methyl ester with piperonal in acidic medium is the fundamental step in the synthesis of tadalafil (2). Daugan et al. describe a process for the synthesis of tadalafil (2), D-tryptophan methyl ester reacts with a piperonal in methylene chloride in the presence of trifluoroacetic acid as a catalyst, and reaction is stirred for 5 days at 4 ○C. The reaction gives a mixture of cis- and trans-tetrahydro-β-carboline derivatives (cis-/trans- 60:40). Reaction of the pure cis-isomer with chloroacetyl chloride in trichloromethane in basic medium (sodium bicarbonate or triethylamine in dichloromethane) form the N-chloroacetyl tetrahydro-β-carboline derivatives (62%), which then reacts with methylamine in methanol at 50 ○C for 16 hr to furnish tadalafil (2) (70%) (Scheme 1) (Daugan et al., 2003b).
In 2004, two concise methods of synthesis were developed. A 2-day synthesis procedure was adopted instead of the 5-days synthe- sis adopted by Icos. In this route, piperonal and D-tryptophan methyl ester HCl react to produce an imine intermediate. The intermediate reacts with Fmoc–sarcosyl chloride to yield an acyl chloride derivative. Upon using Fmoc-sarcoyl chloride the cis-diastereomer undergoes smooth and rapid cyclization to tadalafil in the appropriate basic medium (Scheme 2) (Revell, Srinivasan, & Ganesan, 2004).
On an attempt to lower the cost of the reaction, chloroacetyl chloride was used as the acylating agent instead of the expensive Fmoc–sarcosyl chloride. The reaction of the imine intermediate with chloroacetyl chloride yielded an acyl chloride derivative (78%), a higher yield when compared to reaction with sarcosyl chloride (62%). Cyclization of the chloroacyl derivative using methylamine in metha- nol for 16 hr yielded tadalafil in 92% (Scheme 3) (Revell et al., 2004).
On attempt to improve stereoselectivity, Xiao et al. studied the stereoselectivity of P–S reaction under various conditions. They con- ducted the reaction using ester HCl to avoid the use of trifloroacetic acid (TFA); reactions were conducted in various solvents. This study concluded that in the absence of any catalyst, the reaction was slower and of a poor yield with no stereoselectivity. Furthermore, the use of an acid catalyst improved the yield, the reaction rate, and the stereoselectivity. Using benzoic acid gave the best results with high selectivity (cis-; trans- 92:8). Results showed that isopropanol, butanol, pentanol, nitromethane, acetonitrile, 1,2-dichloroethane and 1,1-dimethoxyethane were suitable solvents, those solvents improved both yields and stereoselectivities. Methanol, DMSO (dimethyl sulfox- ide), and DMF (dimethyl formamide) provided only moderate yields and lower stereoselectivities. The best stereoselectivity was noticed with solvents that can precipitate the cis-isomer while the trans- isomer remains in the supernatant this stereoselectivity suggests that in certain solvents (e.g., acetonitrile or nitromethane) equilibrium develops between cis- and trans-tadalafil–(6S,12aR)-6-(1,3-benzo- dioxol-5-yl)-2-methyl-2,3,6,7,12,12a-hexahydropyrazino[10,20:1,6] pyrido[3,4-b]indole-1,4- dione HCl epimers. The major driving force of this transformation was the large difference is solubility between the cis- and trans-isomers. It is noteworthy that this stereoselectivity was observed only when the D-tryptophane methyl ester HCl was reacted with piperonal.
However, it could not be achieved using other ester salts or other aromatic aldehydes. To further extend the THBC HCl salt to the tetracyclic skeleton of tadalafil, the product of the P–S reaction was reacted with 1.5 equiv. of chloroacetyl chloride in dichloromethane at 0 ○C, in a basic medium to form chloroacyl deriva- tive (92%). This was followed by an overnight reaction with 5 equiv. methylamine in DMF at 25 ○C to furnish tadalafil (95%). Epimerization of tadalafil during cyclization is noticed if the reaction was carried out in DMSO/i –PrOH under basic conditions (DBU: 1,8-Diazabicyclo [5.4.0]undec-7-ene) and refluxed at high temperature for 5 hr. 6a epi- tadalafil –(6S,12aR)-6-(1,3-benzodioxol-5-yl)-2-methyl-2,3,6,7,12,12a- hexahydropyrazino[10,20:1,6] pyrido[3,4-b]indole-1,4- dione was obtained from tadalafil (98%) (Scheme 4) (Shi, Liu, Xu, & Xu, 2008).
In 2008, Anumula et al. developed an alternative pathway for the synthesis of tadalafil avoiding the use of toxic chloroacetyl chloride and expensive solvents. The protocol also circumvented the need for column chromatography meeting the standards of International Con- ference on Harmonization (ICH). This method adopts P–S reaction to produce the tetrayhydro-β-carboline skeleton, the tetrayhydro- β-carboline HCl salt is subjected to amidation conditions with sarco- sine ethyl ester hydrochloride in presence of DCC (N,N0-dicyclohexyl carbodiimide)/HOBt (N-hydroxybenzotriazole). Pure tadalafil is obtained (55%) (Scheme 5) (Anumula et al., 2008).
Tadalafil was also prepared from N-Boc-D-tryptophan. The N-protected tryptophan was treated with ethyl chloroformate to gener- ate its mixed anhydride which reacted in situ with sarcosine ester to yield an intermediate (a) in 50% yield. The reaction of the anhydride intermediate with piperonal using TFA as a catalyst and toluene as a solvent at high temperature (110 ○C) gave a trans-(S,R)-tadalafil prod- uct with 70% yield, while at a lower temperature (45 ○C) cis-(R,R)- tadalafil in 50% yield was observed via an intermediate formation.
Maleic acidCatalog No.:AA0038WQ CAS No.:110-16-7 MDL No.:MFCD00063177 MF:C4H4O4 MW:116.0722 |
Fumaric acidCatalog No.:AA0034VQ CAS No.:110-17-8 MDL No.:MFCD00002700 MF:C4H4O4 MW:116.0722 |
N,N,N',N'-TetramethylethylenediamineCatalog No.:AA00362F CAS No.:110-18-9 MDL No.:MFCD00008335 MF:C6H16N2 MW:116.2046 |
Isobutyl acetateCatalog No.:AA003QZQ CAS No.:110-19-0 MDL No.:MFCD00008932 MF:C6H12O2 MW:116.1583 |
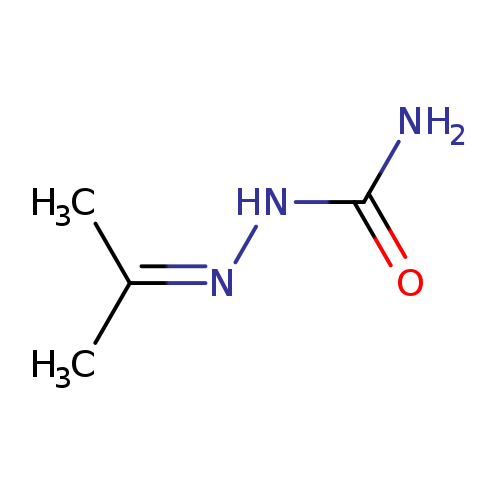
2-(Propan-2-ylidene)hydrazinecarboxamideCatalog No.:AA003NKI CAS No.:110-20-3 MDL No.:MFCD00014785 MF:C4H9N3O MW:115.1338 |
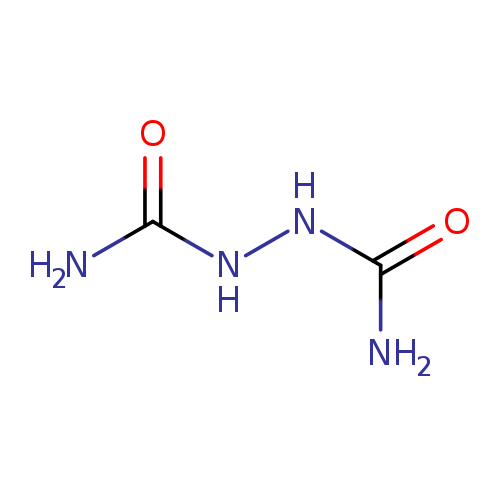
BiureaCatalog No.:AA003SG2 CAS No.:110-21-4 MDL No.:MFCD00025398 MF:C2H6N4O2 MW:118.0946 |
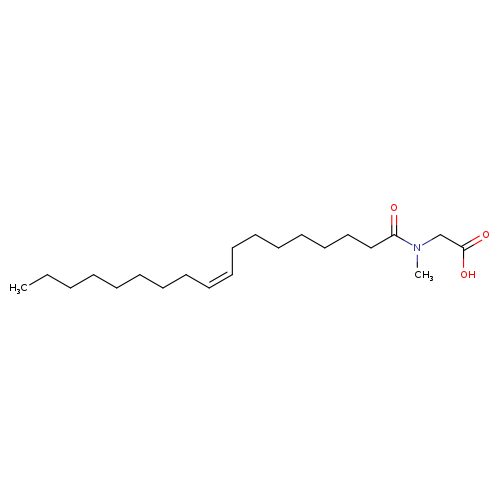
2-(N-Methyloleamido)acetic acidCatalog No.:AA003T6W CAS No.:110-25-8 MDL No.:MFCD03936142 MF:C21H39NO3 MW:353.5393 |
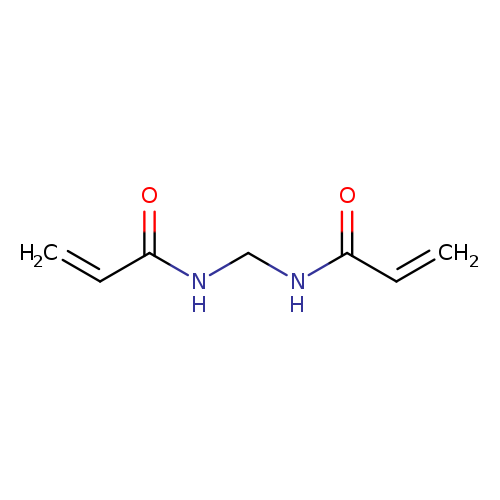
N,N'-MethylenebisacrylamideCatalog No.:AA00358W CAS No.:110-26-9 MDL No.:MFCD00008625 MF:C7H10N2O2 MW:154.1665 |
Tetradecanoic acid, 1-methylethyl esterCatalog No.:AA00HBKR CAS No.:110-27-0 MDL No.:MFCD00008982 MF:C17H34O2 MW:270.4507 |
N,N'-Ethylenebis(stearamide)Catalog No.:AA003SJD CAS No.:110-30-5 MDL No.:MFCD00059224 MF:C38H76N2O2 MW:593.0222 |
N,N'-1,2-Ethanediylbis-9-octadecenamideCatalog No.:AA007V7P CAS No.:110-31-6 MDL No.:MFCD00216615 MF:C38H72N2O2 MW:588.9905 |
Butyl myristateCatalog No.:AA003S43 CAS No.:110-36-1 MDL No.:MFCD00015077 MF:C18H36O2 MW:284.4772 |
Octyl butyrateCatalog No.:AA003TE8 CAS No.:110-39-4 MDL No.:MFCD00048939 MF:C12H24O2 MW:200.3178 |
Undecanal, 2-methyl-Catalog No.:AA007V1N CAS No.:110-41-8 MDL No.:MFCD00006990 MF:C12H24O MW:184.3184 |
Methyl decanoateCatalog No.:AA003RX8 CAS No.:110-42-9 MDL No.:MFCD00009580 MF:C11H22O2 MW:186.2912 |
2-HeptanoneCatalog No.:AA003398 CAS No.:110-43-0 MDL No.:MFCD00233829 MF:C7H14O MW:114.1855 |
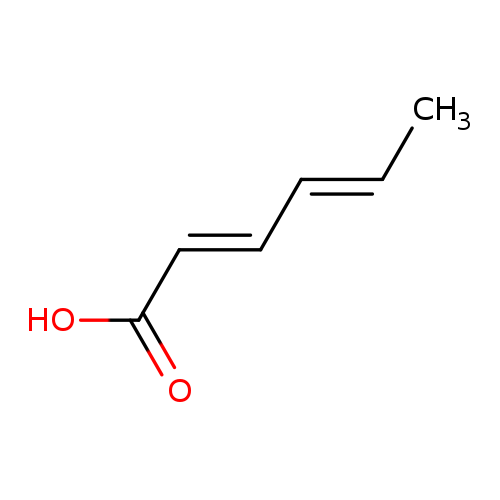
Sorbic acidCatalog No.:AA003UBX CAS No.:110-44-1 MDL No.:MFCD00002703 MF:C6H8O2 MW:112.1265 |
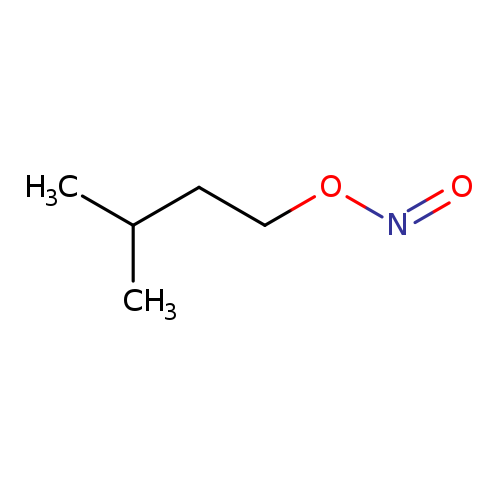
Isoamyl nitriteCatalog No.:AA00385V CAS No.:110-46-3 MDL No.:MFCD00002057 MF:C5H11NO2 MW:117.1463 |
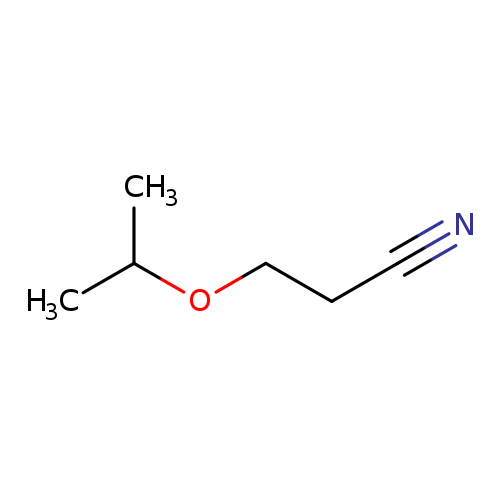
BETA-ISOPROPOXYPROPIONITRILECatalog No.:AA008R2L CAS No.:110-47-4 MDL No.:MFCD00019866 MF:C6H11NO MW:113.1576 |
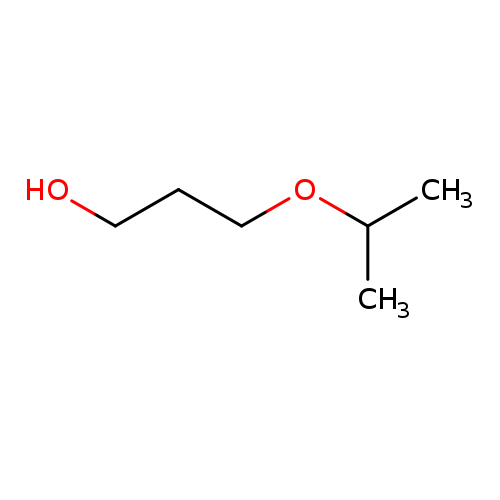
IsopropoxypropanolCatalog No.:AA008V5Z CAS No.:110-48-5 MDL No.:MFCD01696843 MF:C6H14O2 MW:118.1742 |
2-Methoxyethyl AcetateCatalog No.:AA003HHB CAS No.:110-49-6 MDL No.:MFCD00008719 MF:C5H10O3 MW:118.1311 |
Pyridin-1-ium-1-yltrihydroborateCatalog No.:AA003OG4 CAS No.:110-51-0 MDL No.:MFCD00012435 MF:C5H8BN MW:92.9347 |
1,4-DibromobutaneCatalog No.:AA0032JY CAS No.:110-52-1 MDL No.:MFCD00000261 MF:C4H8Br2 MW:215.9143 |
1-BromopentaneCatalog No.:AA00HBKS CAS No.:110-53-2 MDL No.:MFCD00000267 MF:C5H11Br MW:151.0448 |
HexaneCatalog No.:AA0034X5 CAS No.:110-54-3 MDL No.:MFCD00009520 MF:C6H14 MW:86.1754 |
1,4-DichlorobutaneCatalog No.:AA0032K2 CAS No.:110-56-5 MDL No.:MFCD00001011 MF:C4H8Cl2 MW:127.0123 |
1,4-Dichloro-trans-2-buteneCatalog No.:AA009NR0 CAS No.:110-57-6 MDL No.:MFCD00000988 MF:C4H6Cl2 MW:124.9964 |
AmylamineCatalog No.:AA003NTA CAS No.:110-58-7 MDL No.:MFCD00008236 MF:C5H13N MW:87.1634 |
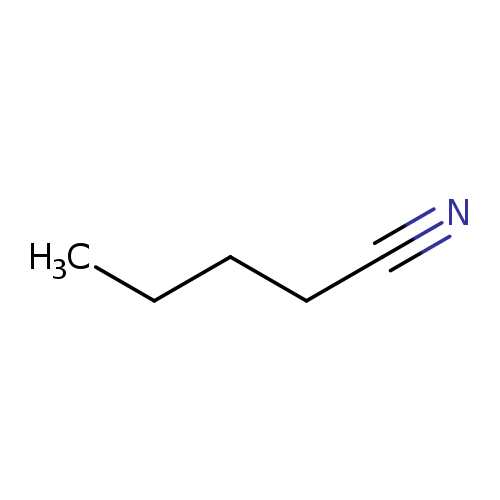
ValeronitrileCatalog No.:AA0038WJ CAS No.:110-59-8 MDL No.:MFCD04113629 MF:C5H9N MW:83.1317 |
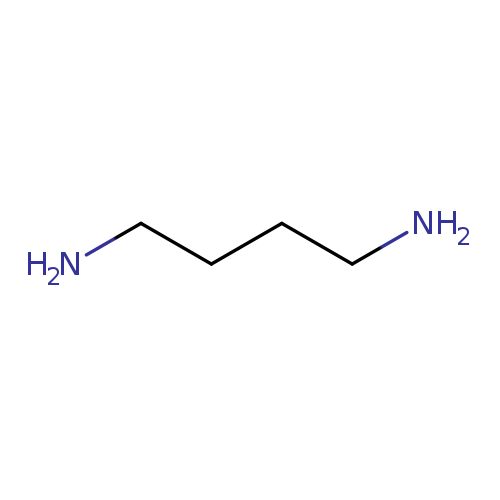
1,4-DiaminobutaneCatalog No.:AA003DKW CAS No.:110-60-1 MDL No.:MFCD00008235 MF:C4H12N2 MW:88.1515 |
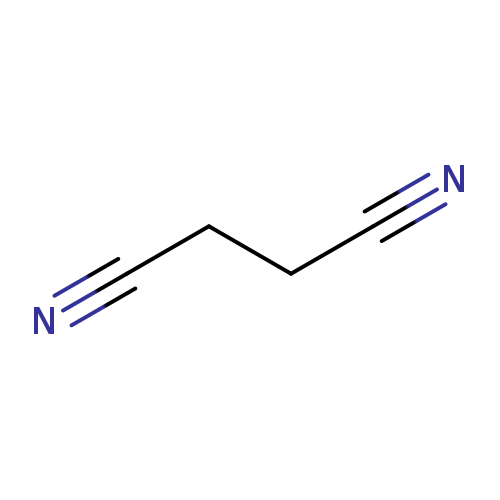
SuccinonitrileCatalog No.:AA003UDR CAS No.:110-61-2 MDL No.:MFCD00001949 MF:C4H4N2 MW:80.0880 |
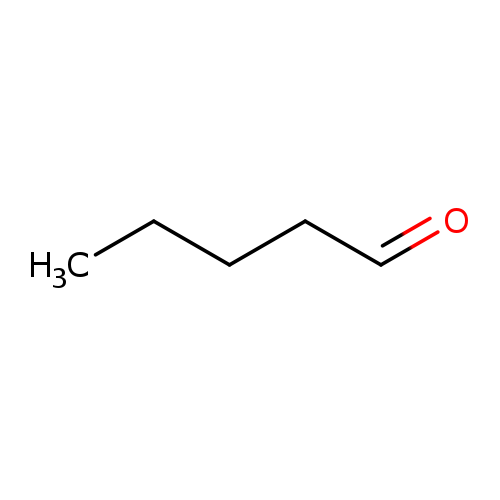
ValeraldehydeCatalog No.:AA003V7E CAS No.:110-62-3 MDL No.:MFCD00007026 MF:C5H10O MW:86.1323 |
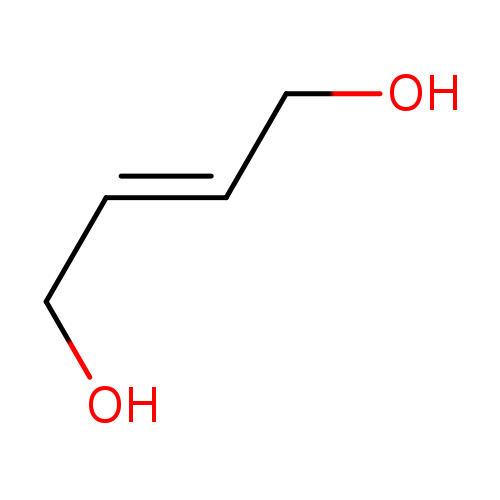
2-Butene-1,4-diol, cis/trans mixtureCatalog No.:AA00334A CAS No.:110-64-5 MDL No.:MFCD00002924 MF:C4H8O2 MW:88.1051 |
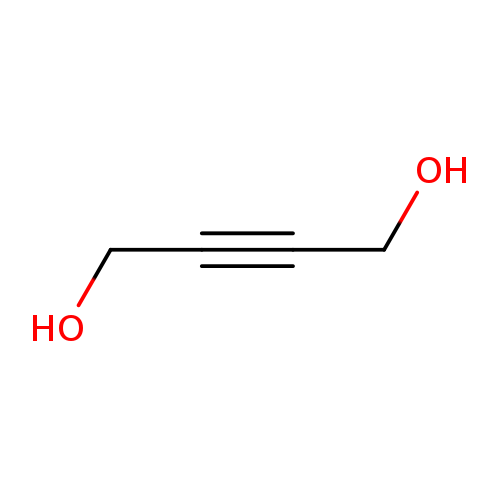
2-Butyne-1,4-diolCatalog No.:AA003GR7 CAS No.:110-65-6 MDL No.:MFCD00002915 MF:C4H6O2 MW:86.0892 |
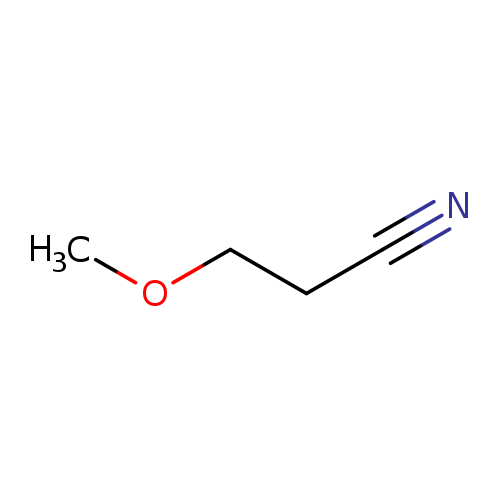
3-MethoxypropanenitrileCatalog No.:AA003JL9 CAS No.:110-67-8 MDL No.:MFCD00001958 MF:C4H7NO MW:85.1045 |
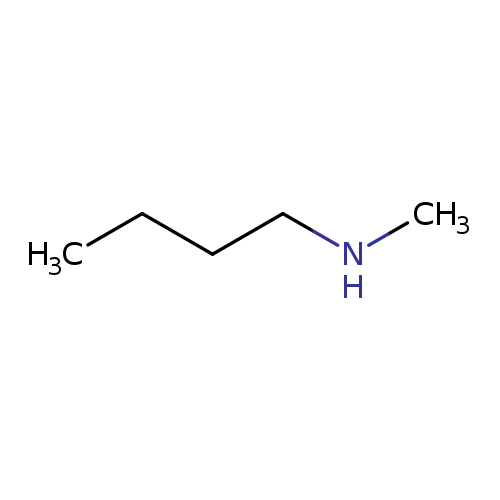
N-METHYLBUTYLAMINECatalog No.:AA003T4F CAS No.:110-68-9 MDL No.:MFCD00009426 MF:C5H13N MW:87.1634 |
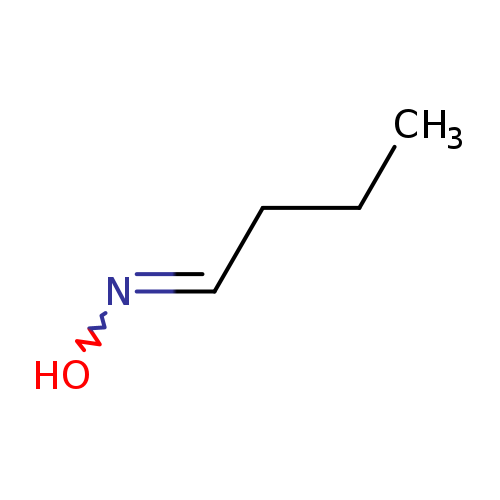
Butyraldehyde oximeCatalog No.:AA003OJP CAS No.:110-69-0 MDL No.:MFCD00013943 MF:C4H9NO MW:87.1204 |
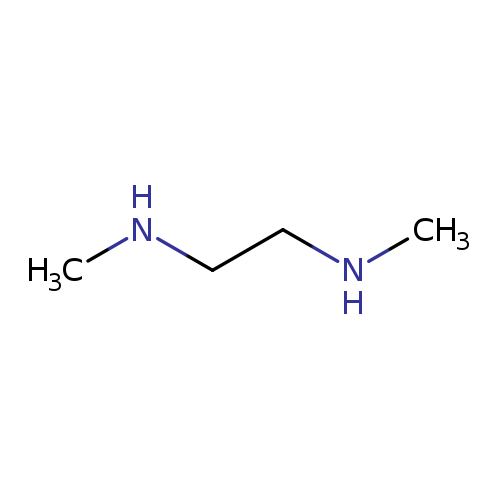
N,N'-Dimethylethane-1,2-diamineCatalog No.:AA0032HM CAS No.:110-70-3 MDL No.:MFCD00008290 MF:C4H12N2 MW:88.1515 |
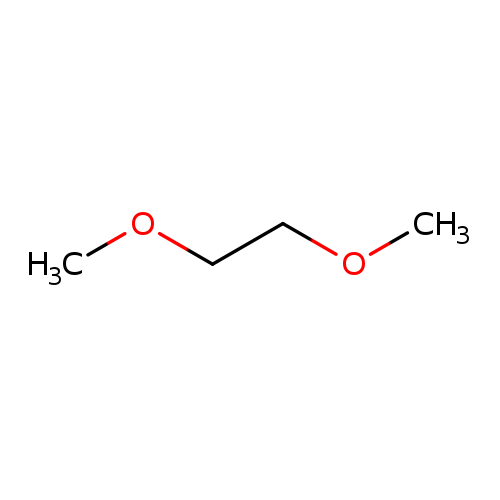
1,2-DimethoxyethaneCatalog No.:AA0032I3 CAS No.:110-71-4 MDL No.:MFCD00008502 MF:C4H10O2 MW:90.1210 |
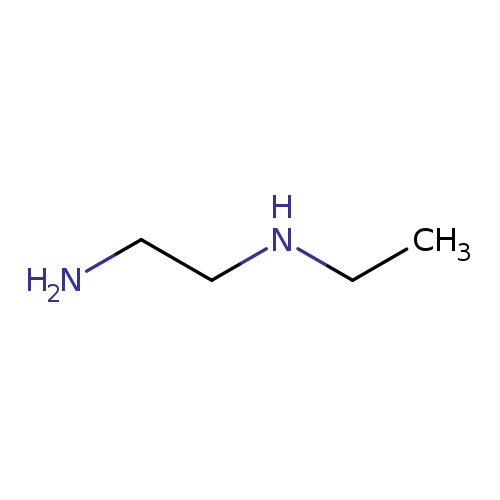
N-EthylethylenediamineCatalog No.:AA003SXH CAS No.:110-72-5 MDL No.:MFCD00008166 MF:C4H12N2 MW:88.1515 |
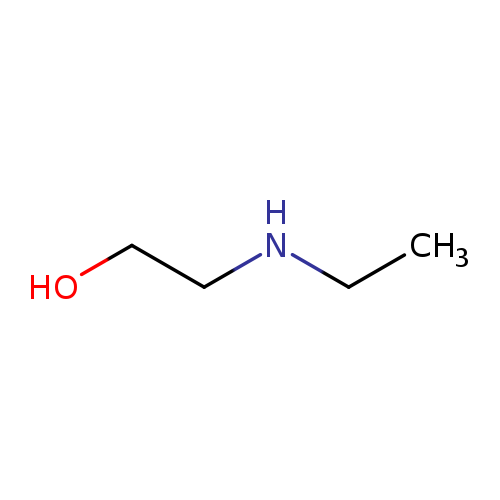
2-(Ethylamino)ethanolCatalog No.:AA0032ST CAS No.:110-73-6 MDL No.:MFCD00002841 MF:C4H11NO MW:89.1362 |
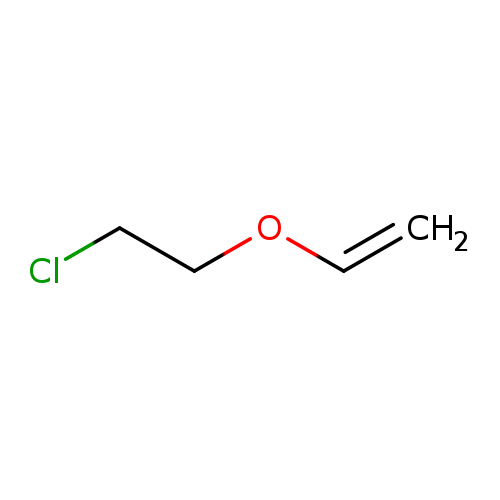
Ethene,(2-chloroethoxy)-Catalog No.:AA0083A8 CAS No.:110-75-8 MDL No.:MFCD00000973 MF:C4H7ClO MW:106.5508 |
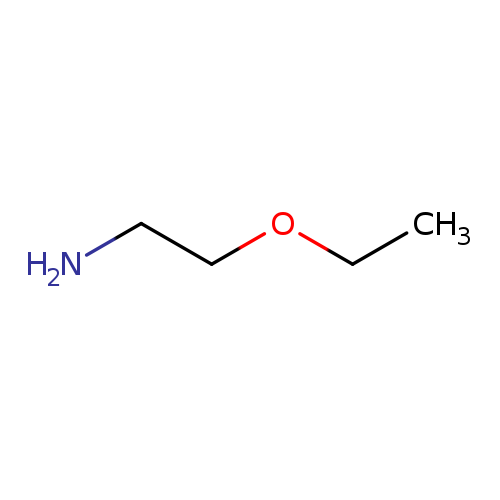
2-EthoxyethylamineCatalog No.:AA00337R CAS No.:110-76-9 MDL No.:MFCD00025604 MF:C4H11NO MW:89.1362 |
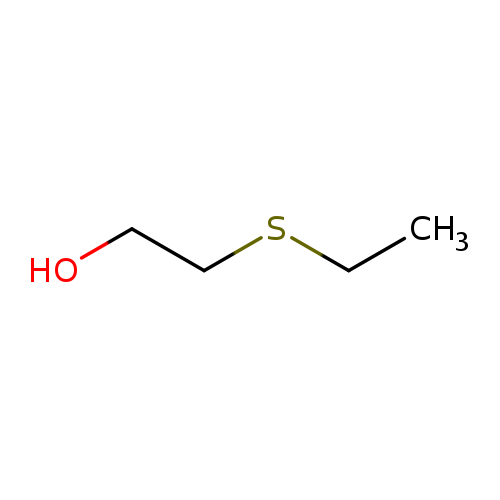
Ethyl 2-Hydroxyethyl SulfideCatalog No.:AA003Q1J CAS No.:110-77-0 MDL No.:MFCD00002909 MF:C4H10OS MW:106.1866 |
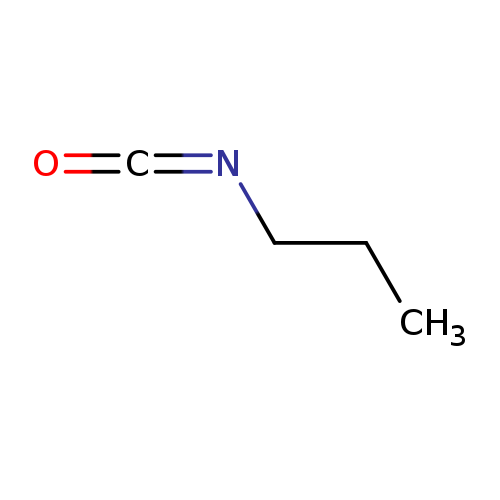
Propyl isocyanateCatalog No.:AA0035GA CAS No.:110-78-1 MDL No.:MFCD00002045 MF:C4H7NO MW:85.1045 |
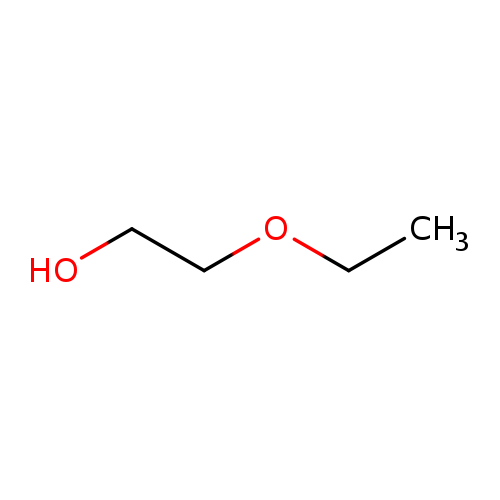
2-EthoxyethanolCatalog No.:AA00327N CAS No.:110-80-5 MDL No.:MFCD00002869 MF:C4H10O2 MW:90.1210 |
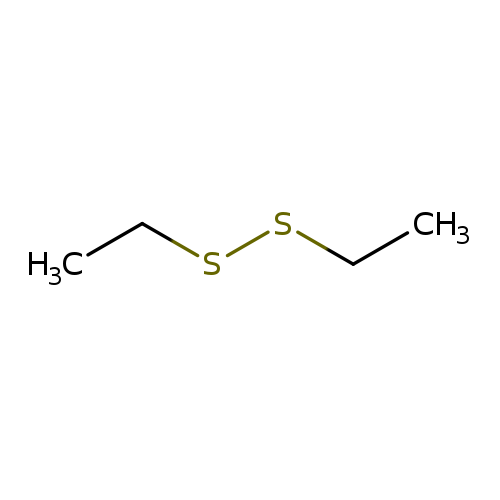
Diethyl DisulfideCatalog No.:AA0034NH CAS No.:110-81-6 MDL No.:MFCD00009266 MF:C4H10S2 MW:122.2522 |
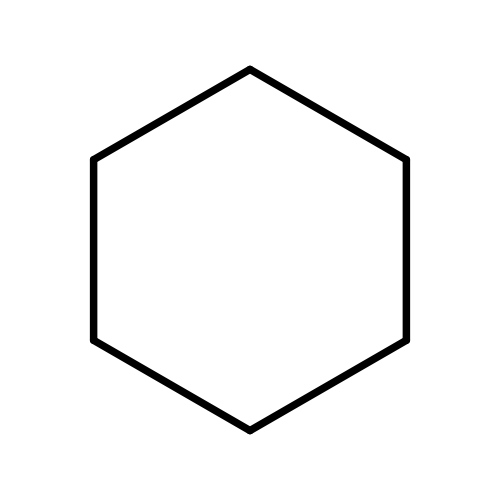
CyclohexaneCatalog No.:AA003648 CAS No.:110-82-7 MDL No.:MFCD00003814 MF:C6H12 MW:84.1595 |
CyclohexeneCatalog No.:AA0034KU CAS No.:110-83-8 MDL No.:MFCD00001539 MF:C6H10 MW:82.1436 |
PiperazineCatalog No.:AA003TPQ CAS No.:110-85-0 MDL No.:MFCD00005953 MF:C4H10N2 MW:86.1356 |
PyridineCatalog No.:AA0035R3 CAS No.:110-86-1 MDL No.:MFCD00011732 MF:C5H5N MW:79.0999 |
3,4-Dihydro-2H-pyranCatalog No.:AA0035YK CAS No.:110-87-2 MDL No.:MFCD00006558 MF:C5H8O MW:84.1164 |
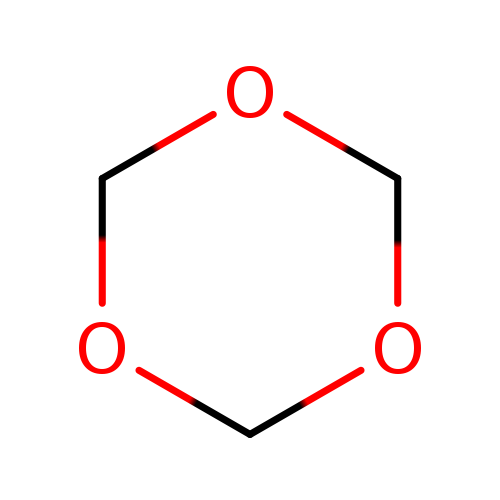
1,3,5-TrioxaneCatalog No.:AA0035JK CAS No.:110-88-3 MDL No.:MFCD00006563 MF:C3H6O3 MW:90.0779 |
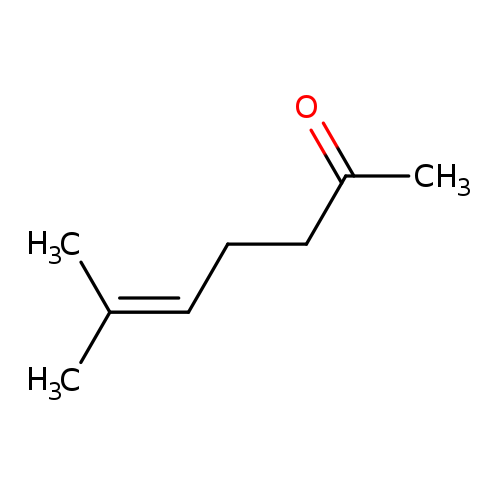
6-Methylhept-5-en-2-oneCatalog No.:AA003N7Q CAS No.:110-93-0 MDL No.:MFCD00008905 MF:C8H14O MW:126.1962 |
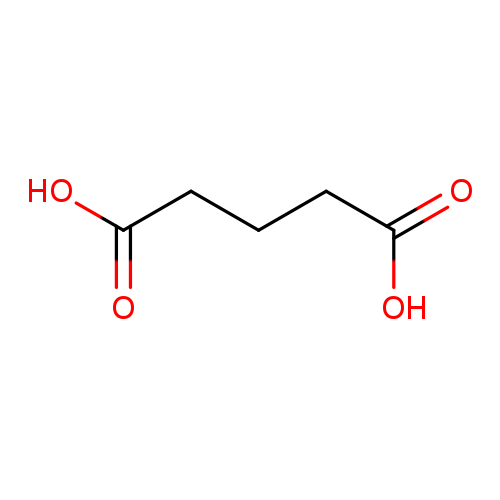
Glutaric acidCatalog No.:AA003QP0 CAS No.:110-94-1 MDL No.:MFCD00004410 MF:C5H8O4 MW:132.1146 |
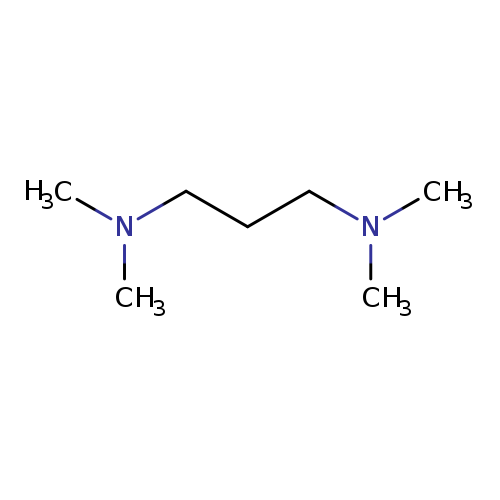
N,N,N,N-Tetramethyl-1,3-propanediamineCatalog No.:AA003SDU CAS No.:110-95-2 MDL No.:MFCD00008337 MF:C7H18N2 MW:130.2312 |
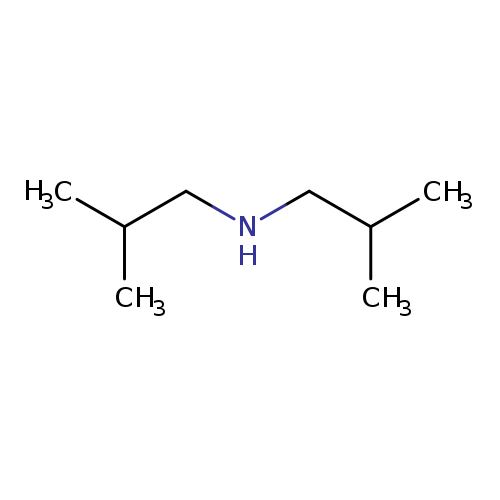
DiisobutylamineCatalog No.:AA0034NZ CAS No.:110-96-3 MDL No.:MFCD00008930 MF:C8H19N MW:129.2432 |
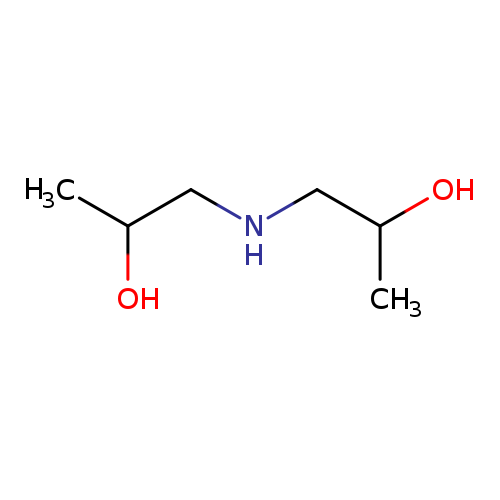
DiisopropanolamineCatalog No.:AA007TU1 CAS No.:110-97-4 MDL No.:MFCD00004531 MF:C6H15NO2 MW:133.1888 |
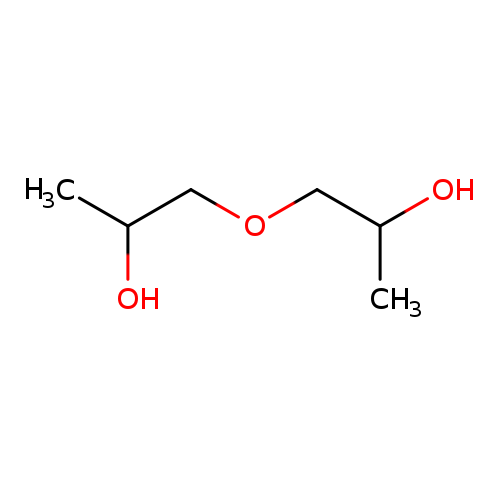
1,1-Oxydi-2-PropanolCatalog No.:AA003AWQ CAS No.:110-98-5 MDL No.:MFCD00004538 MF:C6H14O3 MW:134.1736 |
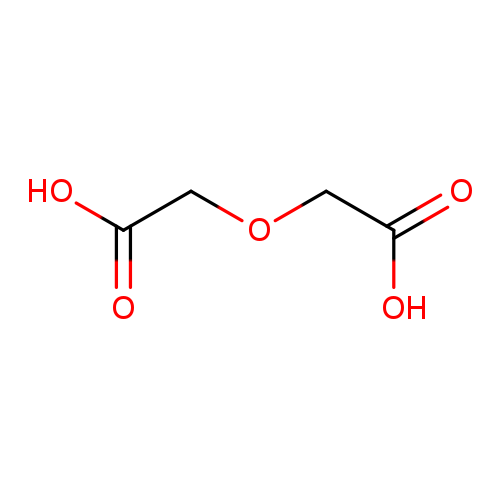
Diglycolic acidCatalog No.:AA003F9Q CAS No.:110-99-6 MDL No.:MFCD00004309 MF:C4H6O5 MW:134.0874 |
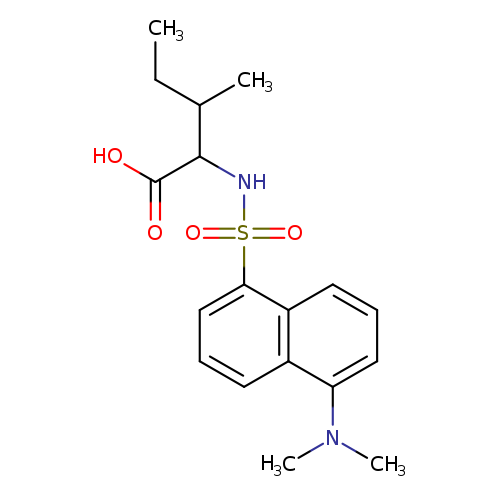
DANSYL-L-ISOLEUCINECatalog No.:AA003P5S CAS No.:1100-21-6 MDL No.:MFCD00069525 MF:C18H24N2O4S MW:364.4592 |
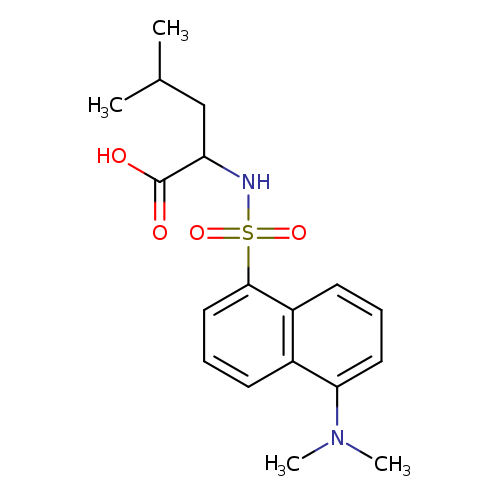
5-Dimethylaminonaphthalene-1-sulfonyl-l-leucineCatalog No.:AA003P5T CAS No.:1100-22-7 MDL No.:MFCD00069532 MF:C18H24N2O4S MW:364.4592 |
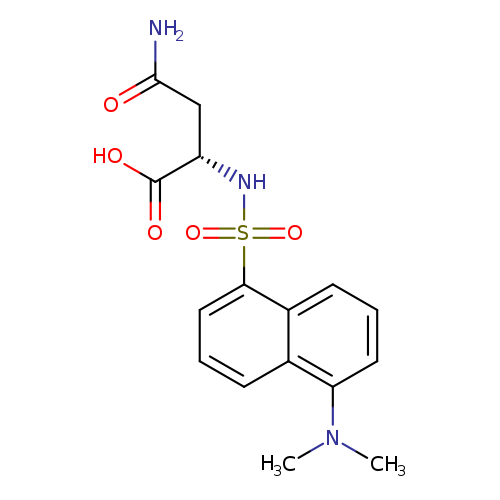
DANSYL-L -ASPARAGINECatalog No.:AA008S1U CAS No.:1100-23-8 MDL No.:MFCD00037731 MF:C16H19N3O5S MW:365.4042 |
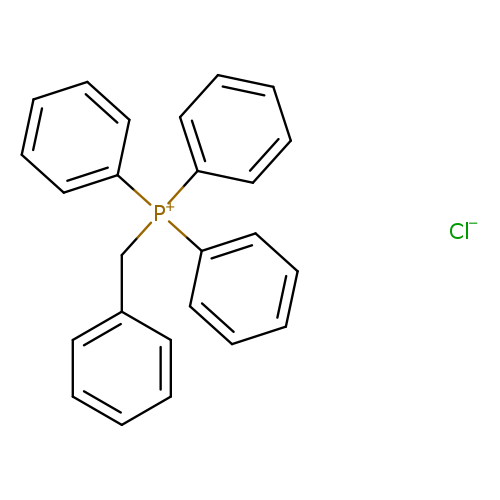
Benzyltriphenylphosphonium chlorideCatalog No.:AA003O2P CAS No.:1100-88-5 MDL No.:MFCD00011913 MF:C25H22ClP MW:388.8689 |
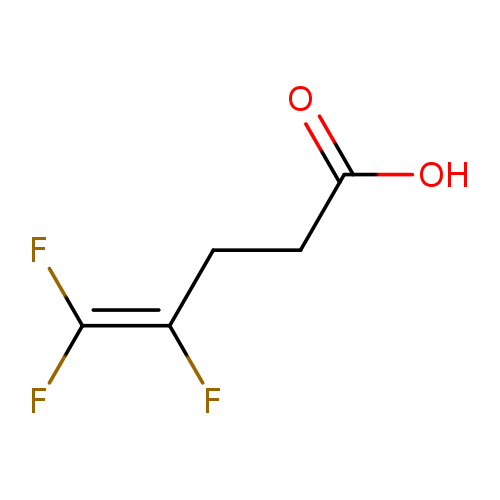
4,5,5-Trifluoropent-4-enoic acidCatalog No.:AA008ULA CAS No.:110003-22-0 MDL No.:MFCD00156072 MF:C5H5F3O2 MW:154.0872 |
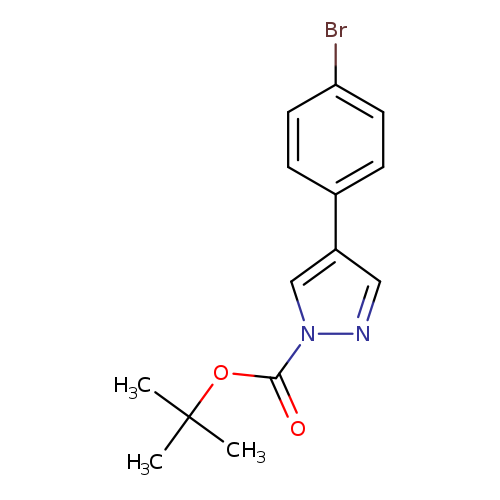
tert-Butyl 4-(4-bromophenyl)pyrazole-1-carboxylateCatalog No.:AA00HBKT CAS No.:1100052-58-1 MDL No.:MFCD28122678 MF:C14H15BrN2O2 MW:323.1851 |
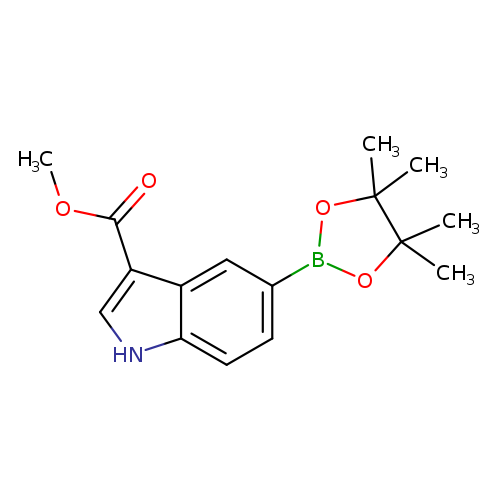
3-(Methoxycarbonyl)indole-5-boronic acid pinacol esterCatalog No.:AA00HBKU CAS No.:1100052-63-8 MDL No.:MFCD20486631 MF:C16H20BNO4 MW:301.1453 |
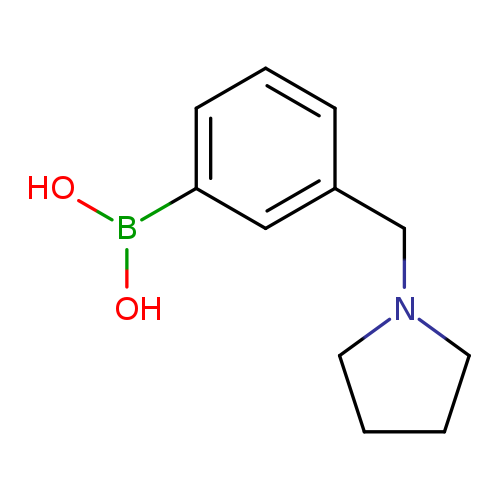
(3-(Pyrrolidin-1-ylmethyl)phenyl)boronic acidCatalog No.:AA0095F1 CAS No.:1100095-09-7 MDL No.:MFCD08060616 MF:C11H16BNO2 MW:205.0612 |
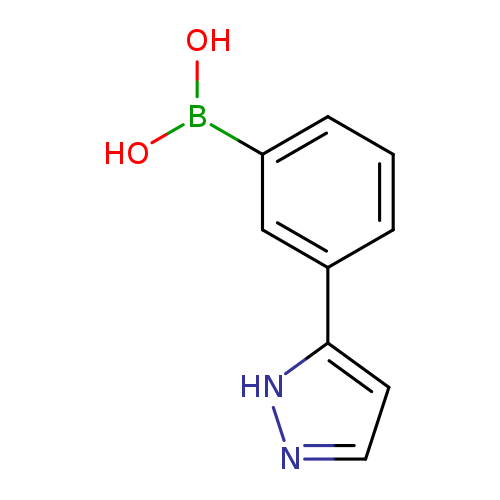
[3-(1H-Pyrazol-5-yl)phenyl]boronic acidCatalog No.:AA00HBKW CAS No.:1100095-25-7 MDL No.:MFCD09701957 MF:C9H9BN2O2 MW:187.9910 |
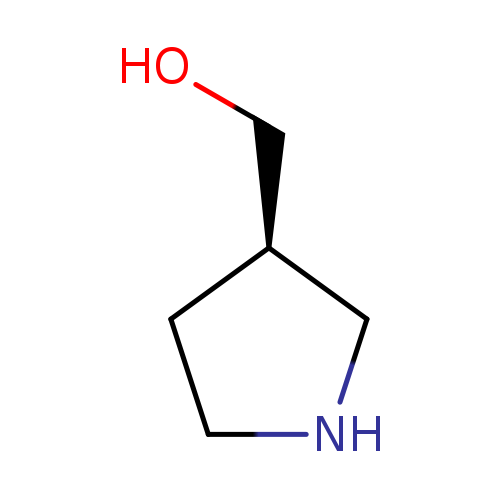
(R)-Pyrrolidin-3-ylmethanolCatalog No.:AA003C6N CAS No.:110013-18-8 MDL No.:MFCD09607969 MF:C5H11NO MW:101.1469 |
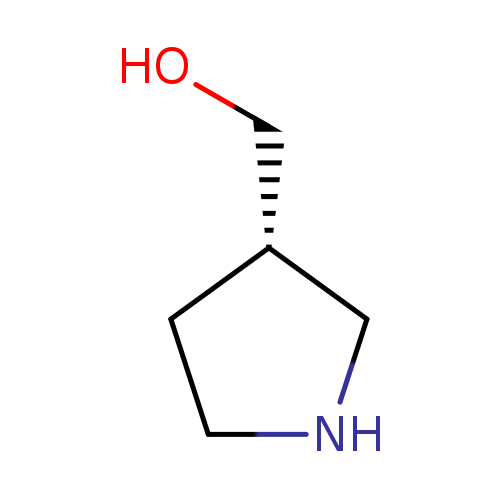
(S)-Pyrrolidin-3-ylmethanolCatalog No.:AA003CIS CAS No.:110013-19-9 MDL No.:MFCD09260722 MF:C5H11NO MW:101.1469 |
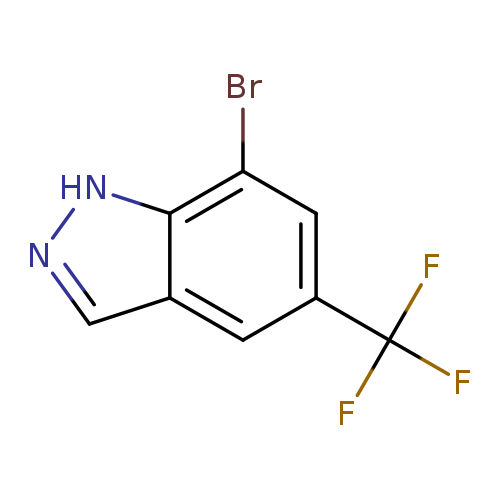
7-Bromo-5-(trifluoromethyl)-1h-indazoleCatalog No.:AA00399M CAS No.:1100212-66-5 MDL No.:MFCD27939136 MF:C8H4BrF3N2 MW:265.0300 |
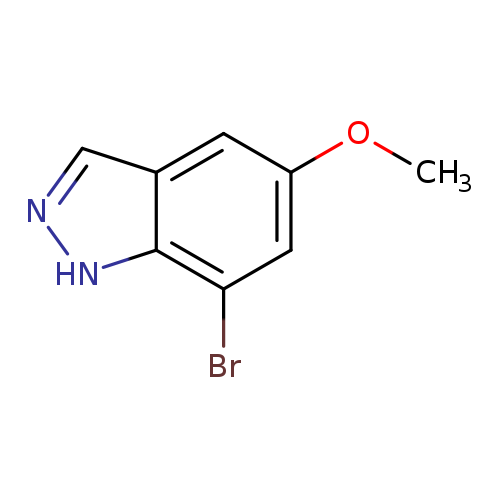
7-Bromo-5-methoxy-1h-indazoleCatalog No.:AA00998M CAS No.:1100214-10-5 MDL No.:MFCD21604728 MF:C8H7BrN2O MW:227.0580 |
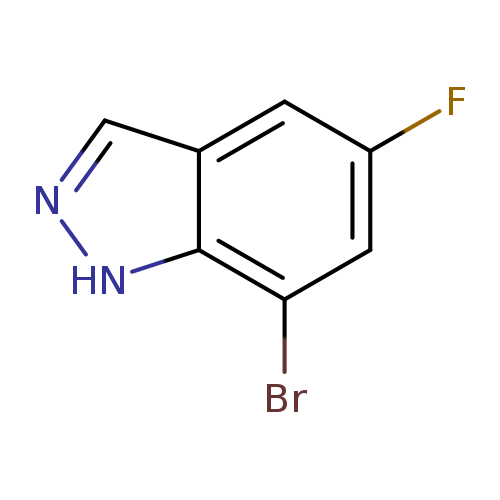
7-Bromo-5-fluoro-1h-indazoleCatalog No.:AA00998N CAS No.:1100214-35-4 MDL No.:MFCD21602945 MF:C7H4BrFN2 MW:215.0225 |
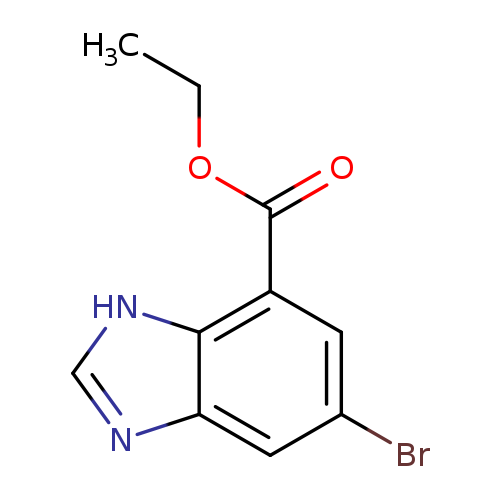
ethyl 5-bromo-1H-1,3-benzodiazole-7-carboxylateCatalog No.:AA01E7PY CAS No.:1100218-00-5 MDL No.:MFCD20501478 MF:C10H9BrN2O2 MW:269.0947 |
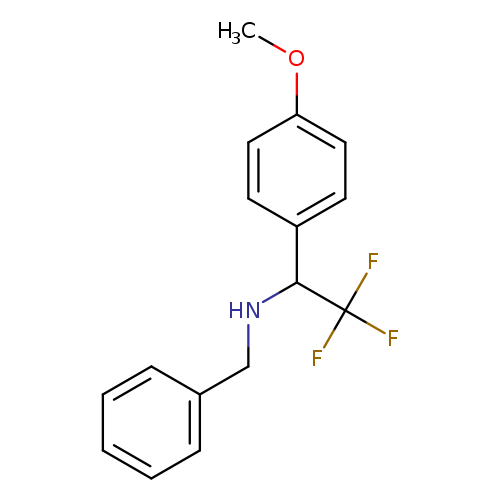
benzyl[2,2,2-trifluoro-1-(4-methoxyphenyl)ethyl]amineCatalog No.:AA01A58W CAS No.:1100245-50-8 MDL No.:MFCD24842862 MF:C16H16F3NO MW:295.2995 |
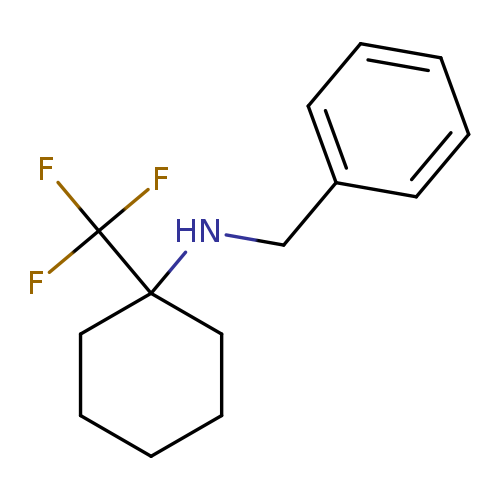
N-benzyl-1-(trifluoromethyl)cyclohexan-1-amineCatalog No.:AA01A5R8 CAS No.:1100246-29-4 MDL No.:MFCD24843035 MF:C14H18F3N MW:257.2946 |
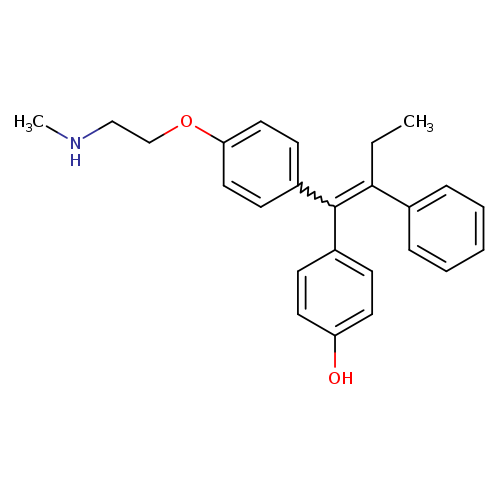
Phenol,4-[1-[4-[2-(methylamino)ethoxy]phenyl]-2-phenyl-1-buten-1-yl]-Catalog No.:AA007E15 CAS No.:110025-28-0 MDL No.:MFCD09840374 MF:C25H27NO2 MW:373.4874 |
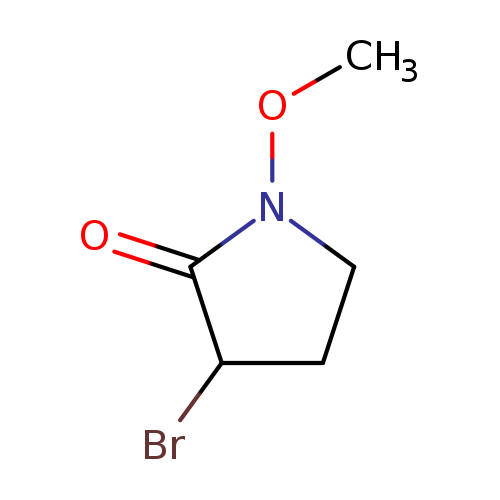
2-Pyrrolidinone, 3-bromo-1-methoxy-Catalog No.:AA00HBL1 CAS No.:110027-11-7 MDL No.:MFCD18762047 MF:C5H8BrNO2 MW:194.0265 |
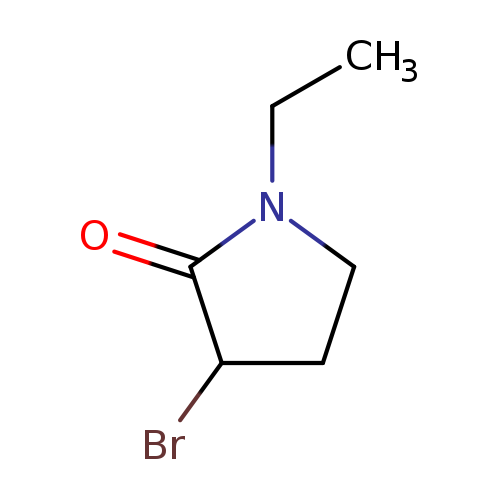
2-Pyrrolidinone, 3-bromo-1-ethyl-Catalog No.:AA00HBL2 CAS No.:110027-12-8 MDL No.:MFCD18886503 MF:C6H10BrNO MW:192.0537 |