2020-02-24 08:47:24
Coralie Duchemin and Nicolai Cramer
Hydroxylamine derivatives are not only important motifs in pharmaceuticals and agrochemicals but as well valuable sub- strates for a variety of complexity-building transformations. For instance, hydroxylamine derivatives have been extensively used as directing groups in catalytic C–H functionalizations and concomitant cleavage of the oxygen–nitrogen bond allowed reoxidation of the catalyst (Scheme 1).3 In this context, phenoxyamides were established as efficient internal oxidants for Rh(III)-catalyzed aryl C(sp2)–H functionalizations.4 Rovis disclosed related Rh(III)-catalyzed vinylic C(sp2)–H functionali- zations using the related enoxyphthalimide motif as the oxi- dative directing group.5 Subsequent trapping reactions with Michael acceptors were used to selectively form trans-5a or cis- cyclopropanes,5c as well as to trigger carboaminations5b Moreover, the ability of oxyamides to engage in radical pro- cesses has been exploited in the decarboxylative functionali- zation of redox active esters triggered either photocatalytically6 or by nickel complexes.7 Along the same lines, Feng reported a photo-catalyzed process for the carboamination of alkenes with N-enoxyphthalimides.8
Despite this steeply increasing synthetic versatility, efficient access to the N-enoxyimide structural motif is still very cum- bersome and represents an unsolved problem. Except for the direct Michael-addition of N-hydroxyimides to acetylene dicar- boxylates,9 the only general reported route involves preparation of the vinylboronic acid in three steps from the corresponding alkene (Scheme 2).5a In turn, the vinylboronic acid is subjected to a copper(I)-mediated coupling with N-hydroxyphthalimide.10 Besides the drawbacks of the low synthetic efficiency of a three-step procedure and the use of copper-mediated reac- tions, this protocol is only suited for N-hydroxy-phthalimides. Related substrates like N-hydroxysuccinimide or N-hydroxy- carbamate do not engage in the transformation.10b
Gold(I)-catalyzed additions of oxygen nucleophiles to term- inal alkynes have become an efficient method for the synthesis of a variety of O-vinyl derivatives.11 For instance, the addition of HOBt to alkynes was reported by Hammond and Xu.12 Zhang disclosed the synthesis of indoles by the addition of aryl hydroxamic acids and N-aryl-N-hydroxycarbamates to alkynes forming labile vinyl ether intermediates which readily rearranged in analogy to the Fischer indole synthesis.13 Moreover, the addition of pyridine N-oxides to alkynes is used for enolate umpolung reactivities.14 Notably, hydroxylamine deriva- tives with two electron-withdrawing substituents on the nitrogen atom such as N-hydroxylimides have not been investigated as suitable nucleophiles. Given the need for an efficient synthetic access and inspired by these reports, we explored the feasibility of a gold(I)-catalyzed efficient single step synthesis of N-enoxy- imides by the addition of N-hydroxyimides to widely accessible terminal alkynes (Scheme 2).
To initially investigate the transformation, we selected 4-methoxyphenyl acetylene (1a) and N-hydroxysuccinimide 2a as the model substrates. The envisioned addition of 1a to the acetylene was accomplished by (PPh3)Au trifluoroacetate in DCE at 90 °C for 6 h. By in situ generation of the gold catalyst, the desired addition product 3aa was obtained in 26% yield (Table 1, entry 1). The yield of 3aa was substantially increased to 65% with a preformed (PPh3)Au trifluoroacetate catalyst (entry 2). The observation points towards decomposition of formed product 3aa under the reaction conditions. Conducting the reaction under strictly anhydrous conditions had no effect on the amount of 4a (entry 3). A five-fold scale- up of the reaction increased the isolated yield of product 3aa to 75% (entry 4). The moderate stability of 3aa which has the tendency to decompose under the reaction conditions to ketone 4a requires striking a fine balance to maximize yield. The reaction outcome proved to be as well highly sensitive to several variables. Notably, replacing the trifluoroacetate anion by weaker coordinating ones such as triflate or triflimide15 was to be detrimental (entries 5 and 6). In these cases, a formal hydration of 1a was dominant yielding exclusively ketone 4a. Subsequent variations of the ligand (IPr or Johnphos) did not substantially improve the reaction outcome (entries 7 and 8).16 Longer reaction times caused a reduction of 3aa and increased the ketone 4a (entry 9), whereas lower reaction temperatures (60 °C) or lower concentrations gave largely reduced conver- sions (entries 10 and 11). Attempts to increase the formation of 3aa by increasing the equivalents of N-hydroxysuccinimide failed (entry 12). Several control experiments were conducted to gauge the relevance of the individual components of the catalyst system. In this respect, the absence of the gold catalyst completely shut down the formation of addition product 3aa (entry 13), which excluded residual Ag(I) salts as the active cata- lyst.17 Omitting the addition of silver salts completely showed that neutral (PPh3)AuCl is not a competent catalyst and caused only unspecific degradation of 1a (entry 14). These experi- ments underlined the relevance of the trifluoroacetate anion in gold(I)-catalyzed transformations to modulate reactivity and selectivity.18,19 Moreover, trifluoroacetic acid itself did not promote the addition of succinimide at all (entry 15).
Next, the scope of the reaction was investigated with opti- mized conditions (Scheme 3). The electronic nature of the aryl substituent of the alkyne was found to have an influence on the reactivity. The reaction works best with alkynes having electron-rich aryl groups and was somewhat less efficient for those with electron-deficient aryl or sterically hindered substi- tuents. Nevertheless, the obtained yield of the single step transformation is still largely competitive with the reported 4-step sequence which is not described for N-hydroxysuccinimdes. Pleasingly, alkenyl- and alkyl-substi- tuted alkynes are particularly well suited for the gold-catalysed addition.
To briefly illustrate the utility of the transformation and the synthetic potential of the enoxyimides, 3ba was used as the substrate in two very recently reported state-of-the-art catalytic transformations. For instance, exposure of N-enoxysuccinimide 3ba to conditions reported by Rovis,5a [Cp*RhCl2]2 in the presence of cesium acetate and ethyl acryl- ate, provided cyclopropane 5 in 72% yield in 61 : 39 cis/trans ratio (Scheme 4). The switch from the original phthalimide group to the succinimide group resulted in a substrate induced cis-dominance. A catalyst-induced cis-selectivity could be obtained for enoxyphthalimide by fine-tuning of the cata- lyst.5c Combined with catalyst engineering, the outlined succi- nimide-type substrate might become useful to boost cis-selecti- vity for refractory substrates or to serve as a suitable platform to develop catalytic enantioselective cyclopropanation reac- tions with chiral cyclopentadienyl complexes.20 Moreover, N-enoxysuccinimide 3ba successfully participated in an iridium-photo-catalyzed carboamination of olefins in analogy to recently disclosed work by Feng.8 For instance, irradiation of a mixture of 3ba ethylvinylether in the presence of an iridium photocatalyst triggered smooth carboamination and formed ketone 6 in 52% yield. To the best of our knowledge, this represents a rare example of an N-oxysuccinimide moiety in a visible light photoinduced radical process.21
Conclusion
In summary, we reported a gold(I)-catalyzed addition of N-hydroxyimides to terminal alkynes. Formal alkyne hydration presumably by decomposition of the desired N-enoxyimide product was mitigated by using a trifluoroacetate counterion on the gold(I) complex. The process accommodates several N-hydroxyimides and a range of aryl, alkenyl and alkyl alkynes decorated with a variety of functional groups including halo- gens, phthalimides, esters and ethers. N-Enoxysuccinimides were shown to be suitable substrates for Rh(III)-catalyzed cyclo- propanations and photo-redox carboaminations. We expect that the reported efficient N-enoxyimide preparation will further enable exploitation of their untapped synthetic potential.
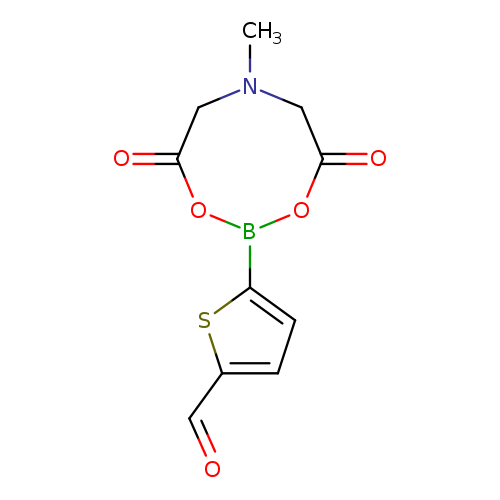
5-(6-Methyl-4,8-dioxo-1,3,6,2-dioxazaborocan-2-yl)thiophene-2-carbaldehydeCatalog No.:AA01FGKG CAS No.:1104637-06-0 MDL No.:MFCD27980601 MF:C10H10BNO5S MW:267.0661 |
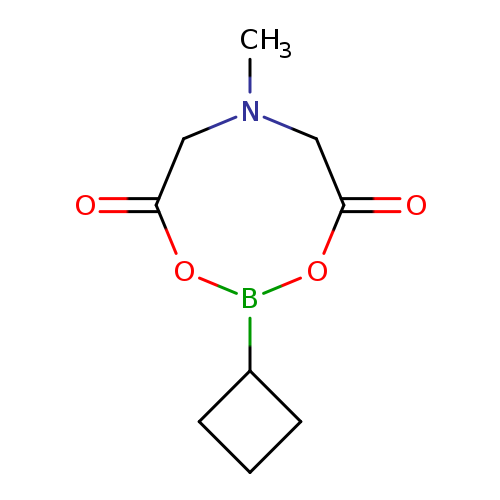
Cyclobutylboronic acid mida esterCatalog No.:AA003P15 CAS No.:1104637-37-7 MDL No.:MFCD15144797 MF:C9H14BNO4 MW:211.0228 |
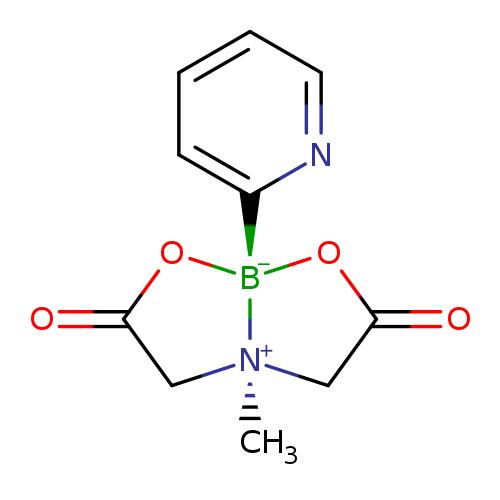
2-Pyridinylboronic acid MIDA esterCatalog No.:AA003HUF CAS No.:1104637-58-2 MDL No.:MFCD16621460 MF:C10H11BN2O4 MW:234.0163 |
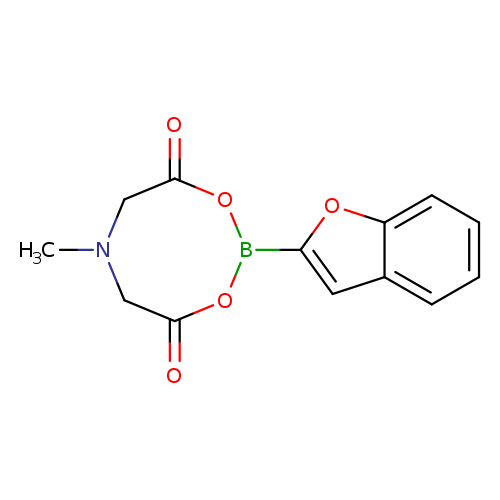
2-Benzofuranylboronic acid mida esterCatalog No.:AA003GHY CAS No.:1104637-65-1 MDL No.:MFCD11215209 MF:C13H12BNO5 MW:273.0491 |
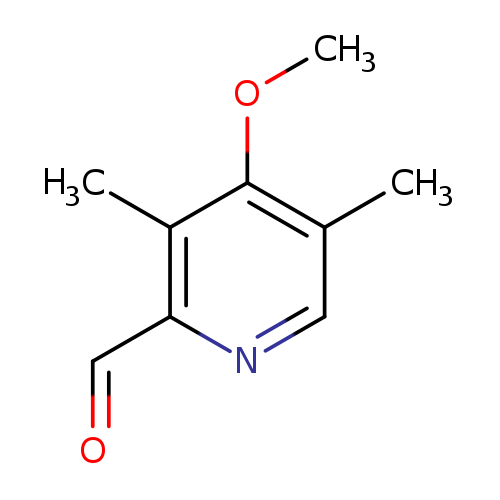
4-Methoxy-3,5-dimethylpyridine-2-carbaldehydeCatalog No.:AA007V16 CAS No.:110464-72-7 MDL No.:MFCD12031924 MF:C9H11NO2 MW:165.1891 |
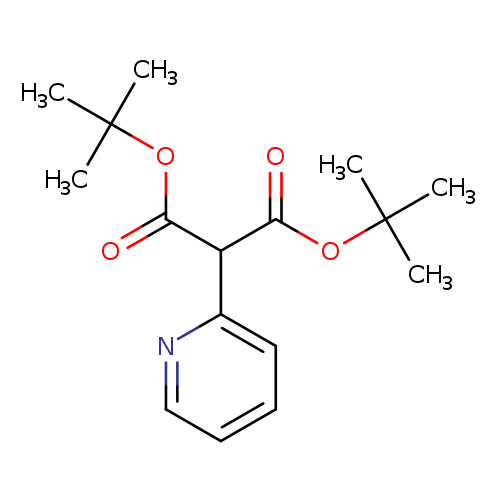
2-Pyridin-2-yl-malonic acid di-tert-butyl esterCatalog No.:AA0093H7 CAS No.:1104643-39-1 MDL No.:MFCD19441885 MF:C16H23NO4 MW:293.3581 |
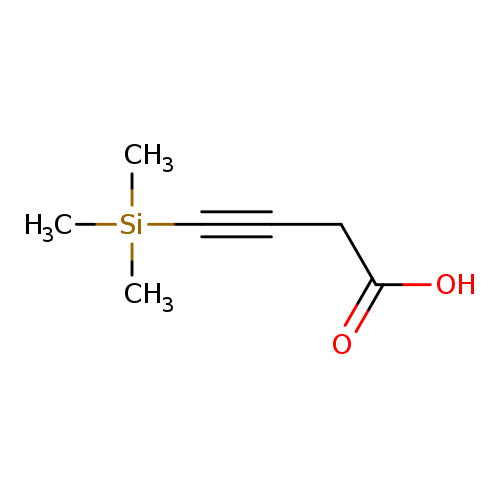
4-(trimethylsilyl)but-3-ynoic acidCatalog No.:AA01BHA2 CAS No.:110469-24-4 MDL No.:MFCD20641728 MF:C7H12O2Si MW:156.2545 |
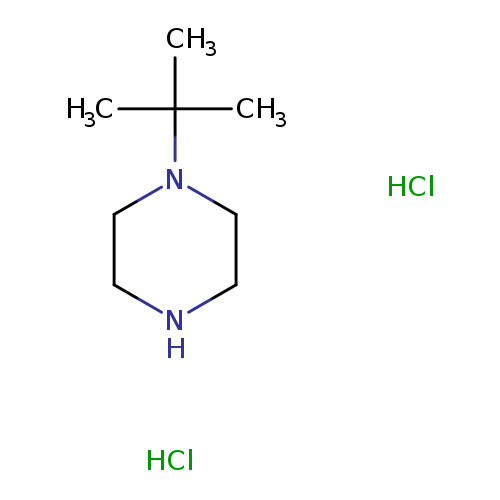
1-tert-Butylpiperazine DiHClCatalog No.:AA00HBNG CAS No.:110469-59-5 MDL No.:MFCD09759216 MF:C8H20Cl2N2 MW:215.1638 |
3-(Piperazin-1-yl)tetrahydrothiophene 1,1-dioxideCatalog No.:AA008V0E CAS No.:110469-63-1 MDL No.:MFCD06655783 MF:C8H16N2O2S MW:204.2898 |
2-(2-Methylbutanoyl)-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acidCatalog No.:AA01A903 CAS No.:1104734-29-3 MDL No.:MFCD09742510 MF:C15H19NO3 MW:261.3163 |
4-amino-1-methyl-1H-imidazole-5-carboxamide hydrochlorideCatalog No.:AA01AC3T CAS No.:110475-17-7 MDL No.:MFCD26407895 MF:C5H9ClN4O MW:176.6042 |
5-ethyl-1H-pyrazol-3-olCatalog No.:AA00J2E8 CAS No.:110475-21-3 MDL No.:MFCD03730222 MF:C5H8N2O MW:112.1298 |
4-Piperidinoaniline, HClCatalog No.:AA009TUB CAS No.:110475-33-7 MDL No.:MFCD02631949 MF:C11H17ClN2 MW:212.7191 |
N-(2-fluorophenyl)-1,2,3,4-tetrahydroquinoline-2-carboxamideCatalog No.:AA01A96T CAS No.:1104793-69-2 MDL No.:MFCD09929174 MF:C16H15FN2O MW:270.3015 |
N-(2,4-dimethylphenyl)-1,3-thiazolidine-4-carboxamideCatalog No.:AA01AA1J CAS No.:1104793-76-1 MDL No.:MFCD09929399 MF:C12H16N2OS MW:236.3332 |
2-amino-2-{4-[(trifluoromethyl)sulfanyl]phenyl}acetic acidCatalog No.:AA01BEFL CAS No.:1104793-77-2 MDL No.:MFCD09929452 MF:C9H8F3NO2S MW:251.2255 |
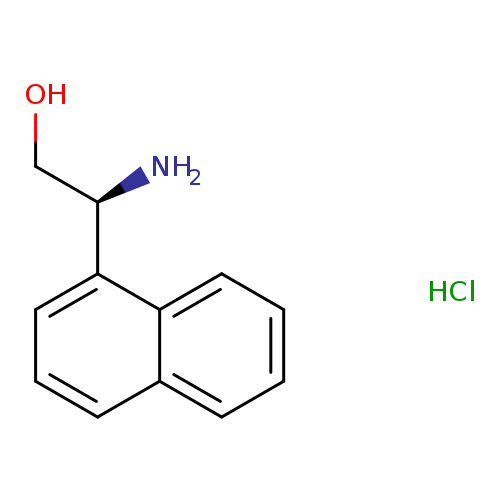
(S)-2-Amino-2-(naphthalen-1-yl)ethanol hydrochlorideCatalog No.:AA009TUD CAS No.:110480-82-5 MDL No.:MFCD09253738 MF:C12H14ClNO MW:223.6987 |
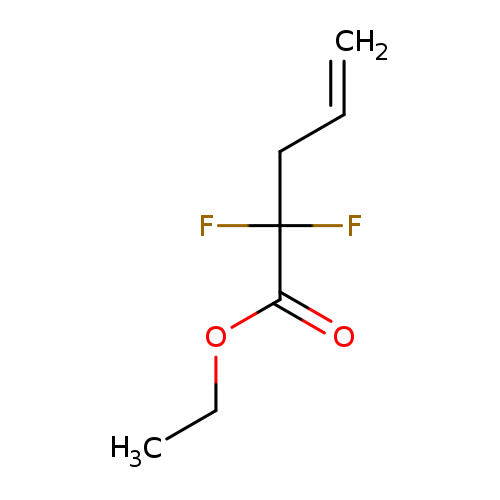
Ethyl 2,2-difluoropent-4-enoateCatalog No.:AA0037X1 CAS No.:110482-96-7 MDL No.:MFCD09763639 MF:C7H10F2O2 MW:164.1499 |
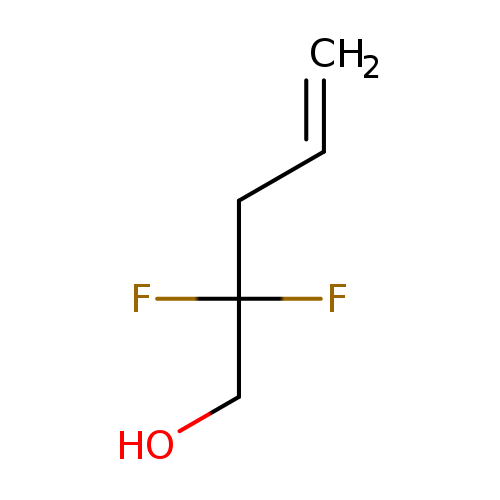
2,2-Difluoropent-4-en-1-olCatalog No.:AA01DXBP CAS No.:110482-97-8 MDL No.:MFCD18433592 MF:C5H8F2O MW:122.1132 |
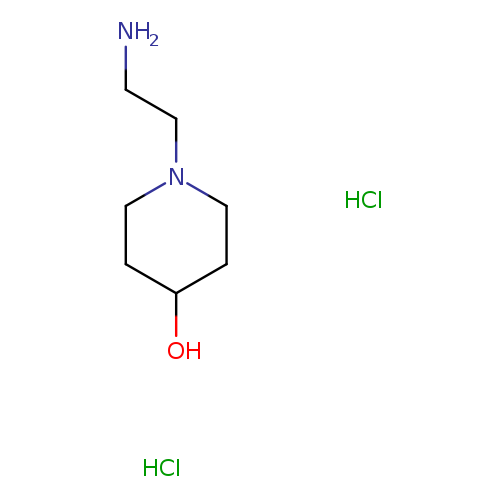
1-(2-Amino-ethyl)-piperidin-4-ol dihydrochlorideCatalog No.:AA007V0W CAS No.:110484-18-9 MDL No.:MFCD10687123 MF:C7H18Cl2N2O MW:217.1366 |
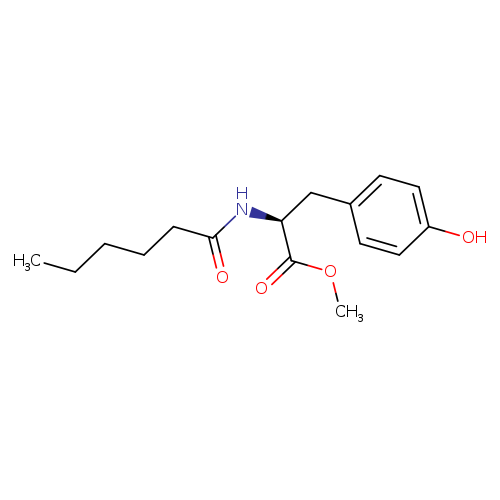
MHPCatalog No.:AA01EOGW CAS No.:1104874-94-3 MDL No.:MFCD31382170 MF:C16H23NO4 MW:293.3581 |
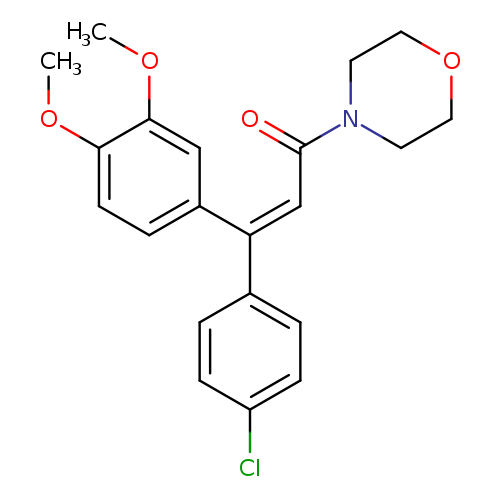
DimethomorphCatalog No.:AA00HBNL CAS No.:110488-70-5 MDL No.:MFCD01632781 MF:C21H22ClNO4 MW:387.8567 |
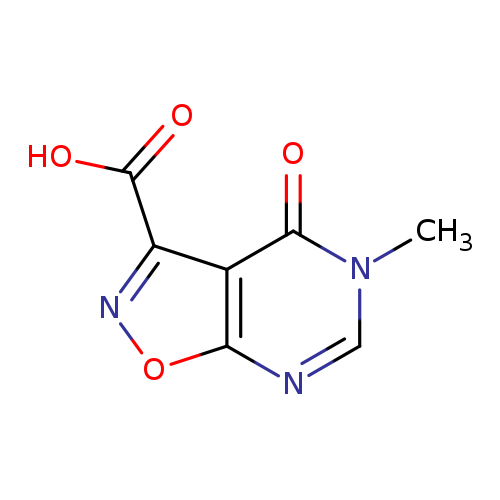
5-Methyl-4-oxo-4,5-dihydroisoxazolo[5,4-d]pyrimidine-3-carboxylic acidCatalog No.:AA008V97 CAS No.:1104927-34-5 MDL No.:MFCD12027392 MF:C7H5N3O4 MW:195.1323 |
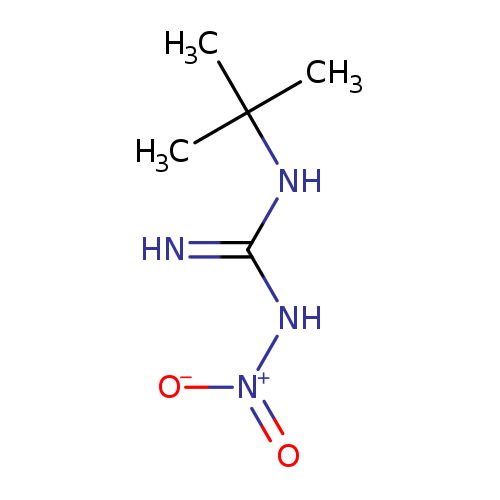
N'-tert-butyl-N-nitroguanidineCatalog No.:AA01DXC1 CAS No.:110499-10-0 MDL No.:MFCD31617468 MF:C5H12N4O2 MW:160.1744 |
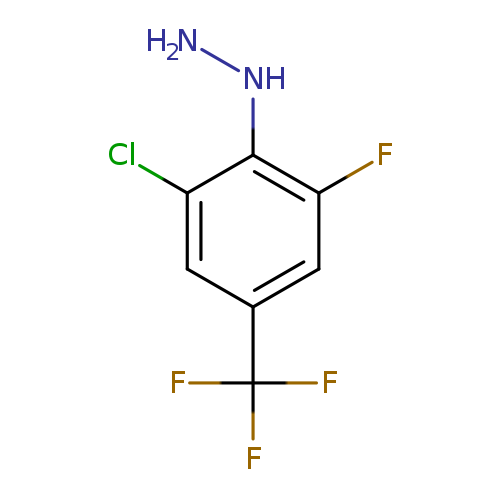
2-Chloro-6-fluoro-4-(trifluoromethyl)phenylhydrazineCatalog No.:AA008UDA CAS No.:110499-66-6 MDL No.:MFCD04972729 MF:C7H5ClF4N2 MW:228.5746 |
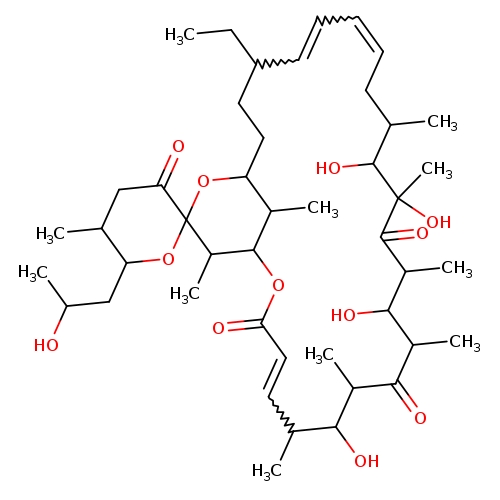
Oligomycin bCatalog No.:AA008RH3 CAS No.:11050-94-5 MDL No.:MFCD00043232 MF:C45H72O12 MW:805.0460 |
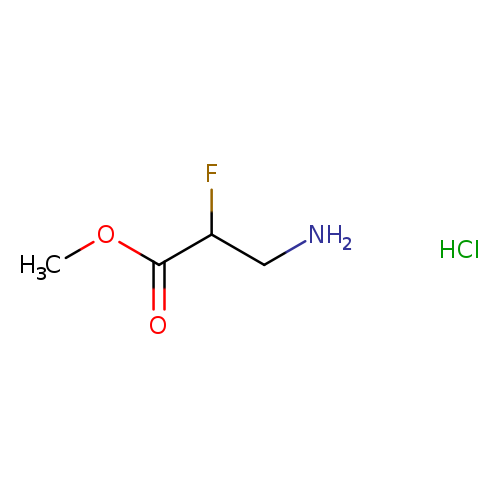
methyl 3-amino-2-fluoropropanoate hydrochlorideCatalog No.:AA01ALDP CAS No.:110501-26-3 MDL No.:MFCD20723408 MF:C4H9ClFNO2 MW:157.5712 |
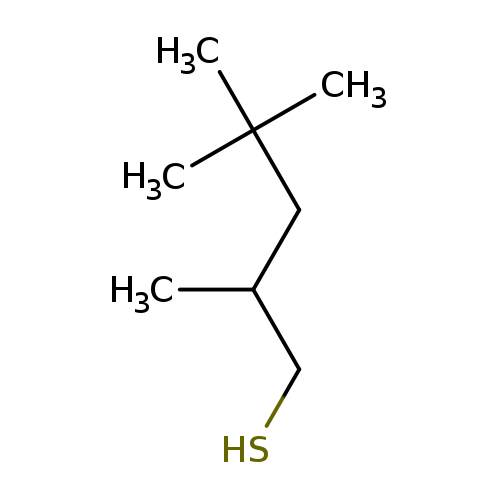
1-Pentanethiol, 2,4,4-trimethyl-, (±)-Catalog No.:AA01FOHY CAS No.:110502-19-7 MDL No.:MFCD00089639 MF:C8H18S MW:146.2935 |
4-hydroxytoremifeneCatalog No.:AA008X4D CAS No.:110503-62-3 MDL No.:MFCD08690443 MF:C26H28ClNO2 MW:421.9590 |
1-(4-Methoxybenzyl)-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1h-pyrazoleCatalog No.:AA00999M CAS No.:1105039-88-0 MDL No.:MFCD16877287 MF:C17H23BN2O3 MW:314.1871 |
1-(4-Methoxybenzyl)-1h-pyrazole-4-carboxylic acidCatalog No.:AA0093NT CAS No.:1105039-93-7 MDL No.:MFCD08456928 MF:C12H12N2O3 MW:232.2353 |
N6-(4-Hydroxybenzyl)-adenosineCatalog No.:AA007CON CAS No.:110505-75-4 MDL No.:MFCD18641957 MF:C17H19N5O5 MW:373.3633 |
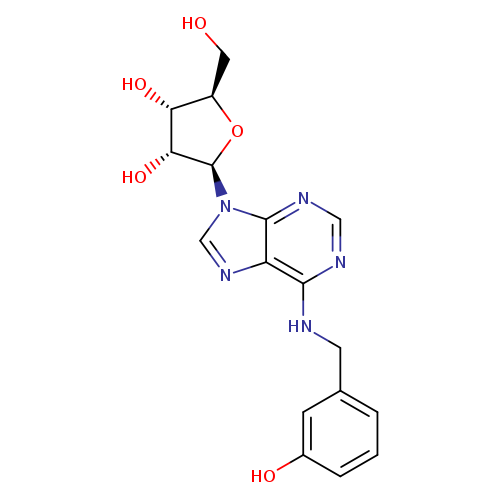
(2R,3R,4S,5R)-2-(6-((3-Hydroxybenzyl)amino)-9h-purin-9-yl)-5-(hydroxymethyl)tetrahydrofuran-3,4-diolCatalog No.:AA007UQV CAS No.:110505-76-5 MDL No.:MFCD28343180 MF:C17H19N5O5 MW:373.3633 |
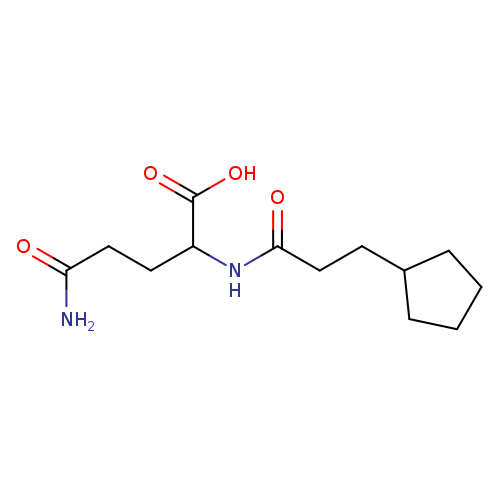
4-carbamoyl-2-(3-cyclopentylpropanamido)butanoic acidCatalog No.:AA01EB3F CAS No.:1105053-08-4 MDL No.:MFCD12444867 MF:C13H22N2O4 MW:270.3248 |
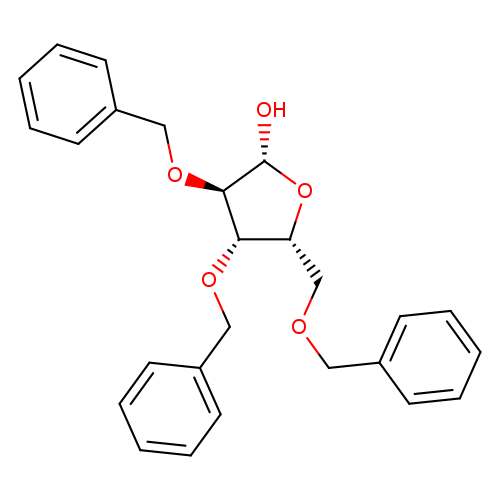
2,3,5-Tri-o-benzyl-beta-d-xylofuranoseCatalog No.:AA00HBNN CAS No.:1105054-66-7 MDL No.:MFCD09864638 MF:C26H28O5 MW:420.4975 |
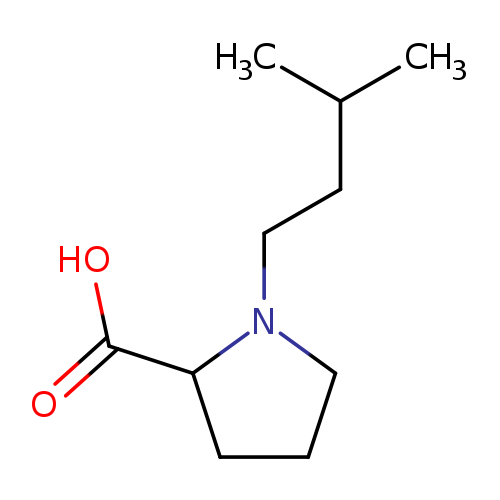
1-(3-methylbutyl)pyrrolidine-2-carboxylic acidCatalog No.:AA01EK5X CAS No.:1105057-50-8 MDL No.:MFCD08442285 MF:C10H19NO2 MW:185.2634 |
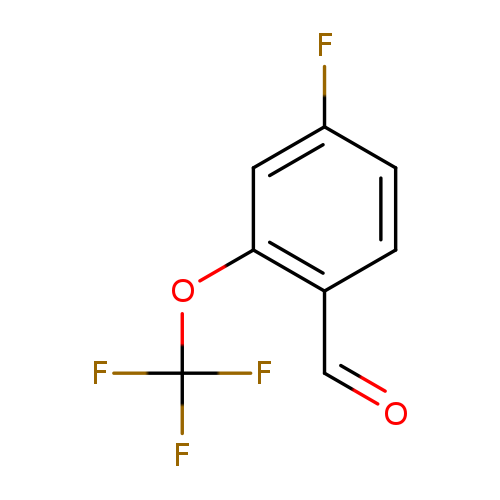
4-Fluoro-2-(trifluoromethoxy)benzaldehydeCatalog No.:AA00HBNR CAS No.:1105060-20-5 MDL No.:MFCD24842738 MF:C8H4F4O2 MW:208.1098 |
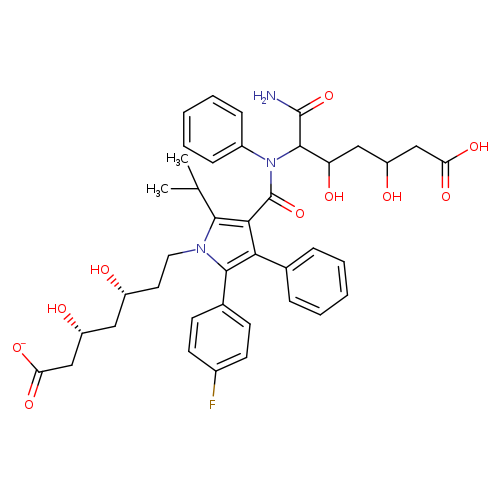
Atorvastatin N-(3,5-Dihydroxy-7-heptanoic Acid)amideCatalog No.:AA008WP4 CAS No.:1105067-87-5 MDL No.: MF:C40H45FN3O10- MW:746.7978 |
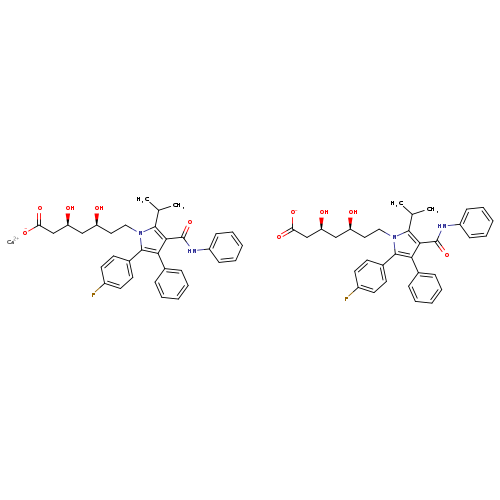
Calcium (3S,5S)-7-(2-(4-fluorophenyl)-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)-1H-pyrrol-1-yl)-3,5-dihydroxyheptanoateCatalog No.:AA008UH7 CAS No.:1105067-88-6 MDL No.:MFCD26142859 MF:C66H68CaF2N4O10 MW:1155.3417 |
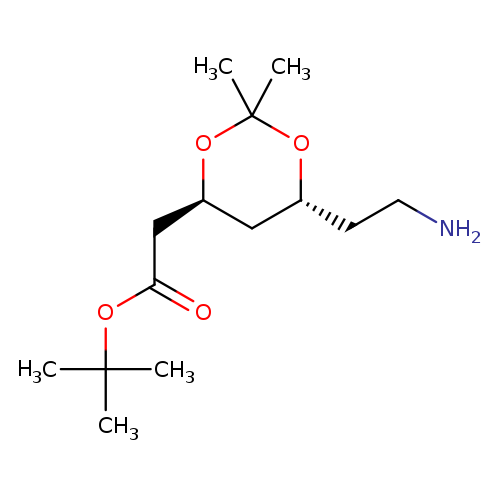
(4S,trans)-1,1-Dimethylethyl-6-aminoethyl-2,2-dimethyl-1,3-dioxane-4-acetateCatalog No.:AA008WHQ CAS No.:1105067-89-7 MDL No.:MFCD22200569 MF:C14H27NO4 MW:273.3685 |
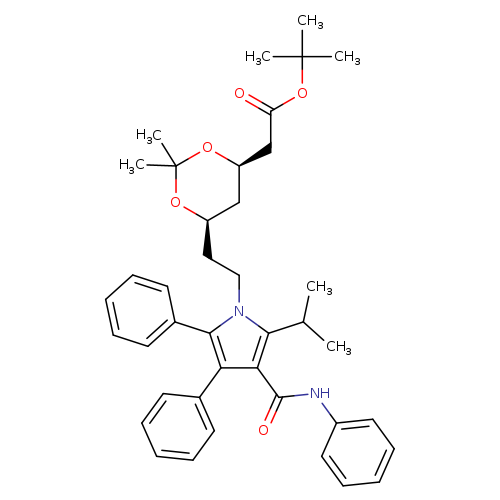
(4R,6R)-2,2-DIMETHYL-6-[2-[2-(1-METHYLETHYL)-4,5-DIPHENYL-3-[(PHENYLAMINO)CARBONYL]-1H-PYRROL-1-YL]ETHYL]-1,3-DIOXANE-4-ACETIC ACID TERT-BUTYL ESTERCatalog No.:AA0083TH CAS No.:1105067-91-1 MDL No.:MFCD23160665 MF:C40H48N2O5 MW:636.8195 |
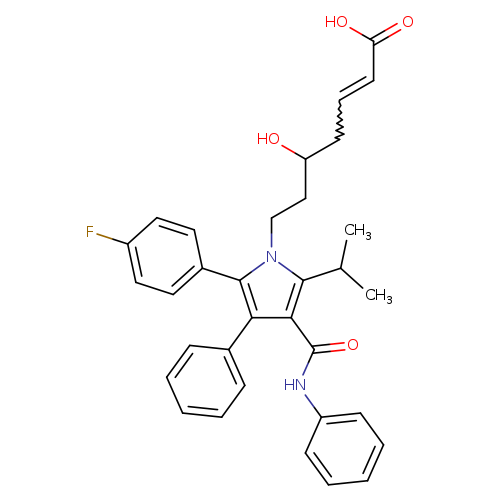
(2E)-2,3-Dehydroxy AtorvastatinCatalog No.:AA01CBAI CAS No.:1105067-93-3 MDL No.: MF:C33H33FN2O4 MW:540.6245 |

AvilamycinCatalog No.:AA0096AX CAS No.:11051-71-1 MDL No.:MFCD21607522 MF:C61H88Cl2O32 MW:1404.2382 |
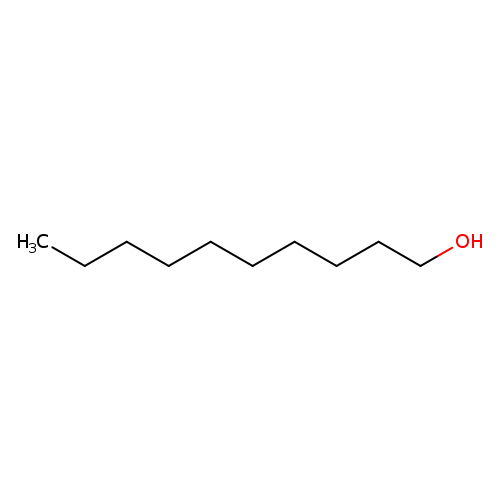
N-DECYL-D21 ALCOHOLCatalog No.:AA008SWR CAS No.:110510-78-6 MDL No.:MFCD00144866 MF:C10H22O MW:158.2811 |
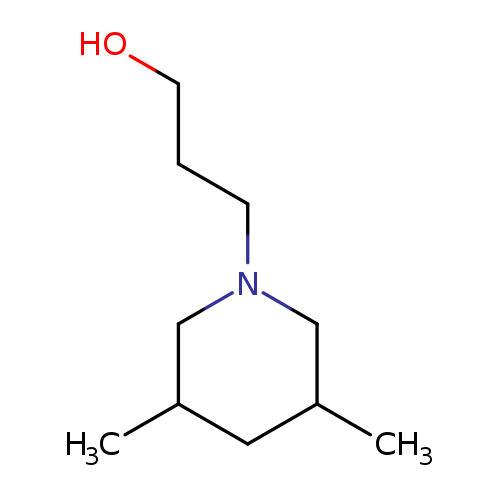
3-(3,5-Dimethylpiperidin-1-yl)propan-1-olCatalog No.:AA007UQO CAS No.:110514-23-3 MDL No.:MFCD08691661 MF:C10H21NO MW:171.2798 |
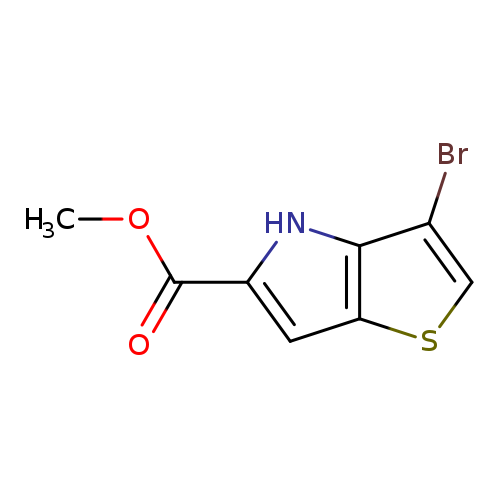
Methyl 3-bromo-4h-thieno[3,2-b]pyrrole-5-carboxylateCatalog No.:AA008U0O CAS No.:1105187-36-7 MDL No.:MFCD11973824 MF:C8H6BrNO2S MW:260.1077 |
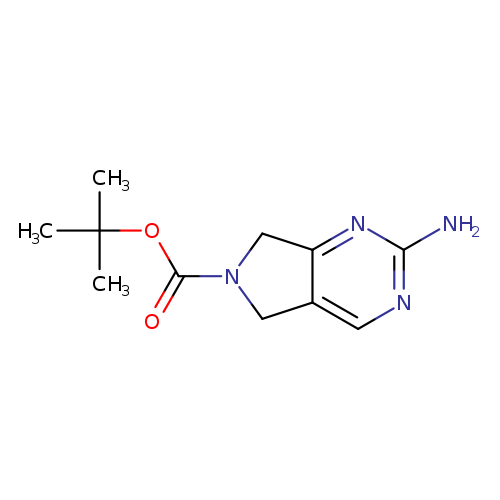
tert-Butyl 2-amino-5h-pyrrolo[3,4-d]pyrimidine-6(7h)-carboxylateCatalog No.:AA007COI CAS No.:1105187-42-5 MDL No.:MFCD08447404 MF:C11H16N4O2 MW:236.2703 |
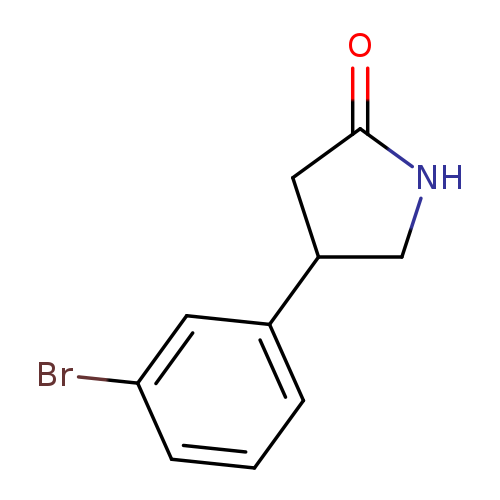
4-(3-Bromophenyl)pyrrolidin-2-oneCatalog No.:AA008U2V CAS No.:1105187-44-7 MDL No.:MFCD11846198 MF:C10H10BrNO MW:240.0965 |
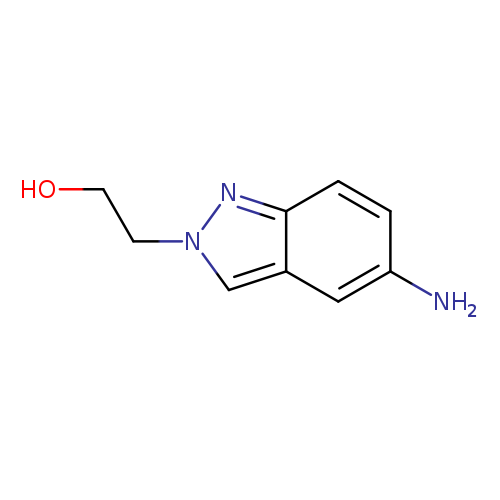
2-(5-Amino-2H-indazol-2-yl)ethanolCatalog No.:AA008TWD CAS No.:1105187-46-9 MDL No.:MFCD11846209 MF:C9H11N3O MW:177.2031 |
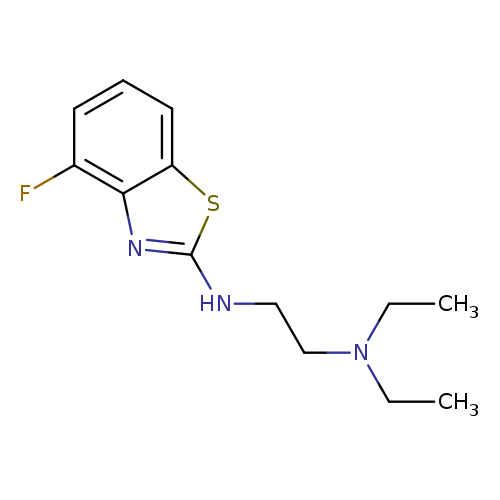
N,N-Diethyl-N'-(4-fluoro-1,3-benzothiazol-2-yl)ethane-1,2-diamineCatalog No.:AA01ARK5 CAS No.:1105188-19-9 MDL No.:MFCD11986989 MF:C13H18FN3S MW:267.3655 |
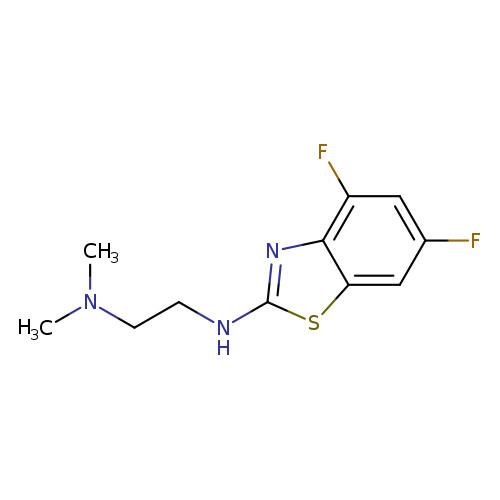
N'-(4,6-Difluoro-1,3-benzothiazol-2-yl)-N,N-dimethylethane-1,2-diamineCatalog No.:AA01ARKQ CAS No.:1105188-23-5 MDL No.:MFCD11986991 MF:C11H13F2N3S MW:257.3028 |
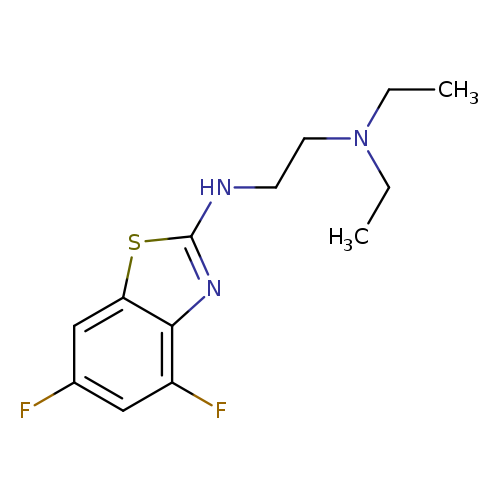
N'-(4,6-Difluoro-1,3-benzothiazol-2-yl)-N,N-diethylethane-1,2-diamineCatalog No.:AA01ARKR CAS No.:1105188-25-7 MDL No.:MFCD11986992 MF:C13H17F2N3S MW:285.3560 |
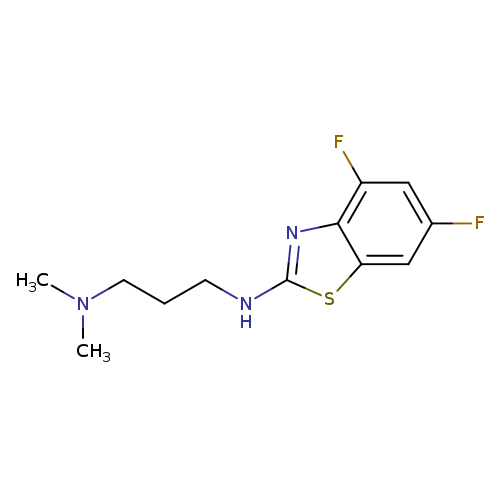
N'-(4,6-Difluoro-1,3-benzothiazol-2-yl)-N,N-dimethylpropane-1,3-diamineCatalog No.:AA01ARKS CAS No.:1105188-28-0 MDL No.:MFCD11986993 MF:C12H15F2N3S MW:271.3294 |
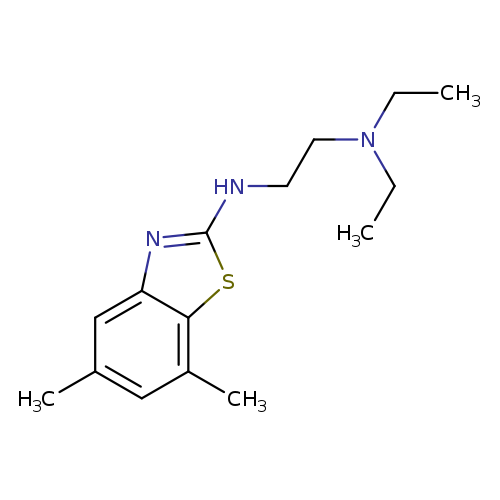
N'-(5,7-Dimethyl-1,3-benzothiazol-2-yl)-N,N-diethylethane-1,2-diamineCatalog No.:AA01ARLG CAS No.:1105188-31-5 MDL No.:MFCD11986994 MF:C15H23N3S MW:277.4282 |
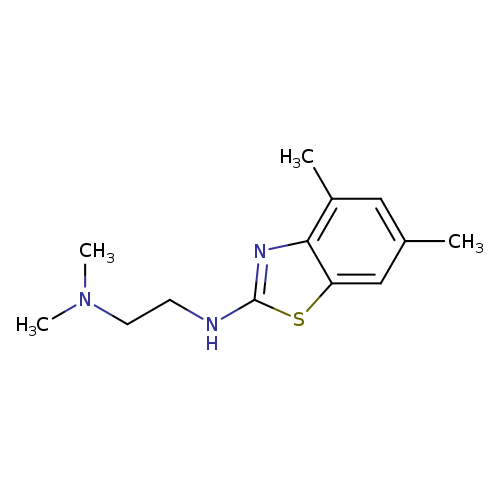
N'-(4,6-Dimethyl-1,3-benzothiazol-2-yl)-N,N-dimethylethane-1,2-diamineCatalog No.:AA01ARLH CAS No.:1105188-33-7 MDL No.:MFCD11986995 MF:C13H19N3S MW:249.3751 |
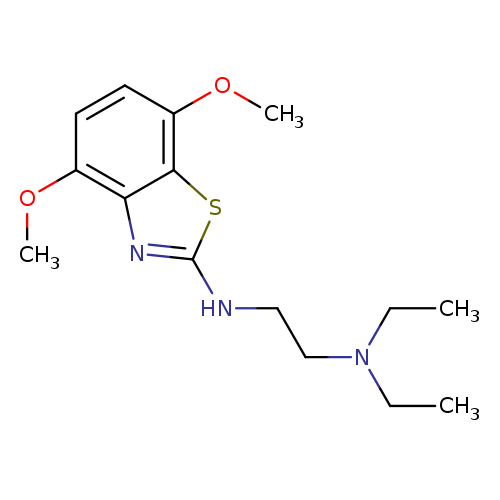
N1-(4,7-Dimethoxybenzo[d]thiazol-2-yl)-N2,N2-diethylethane-1,2-diamineCatalog No.:AA01FMWE CAS No.:1105188-34-8 MDL No.:MFCD11986996 MF:C15H23N3O2S MW:309.4270 |
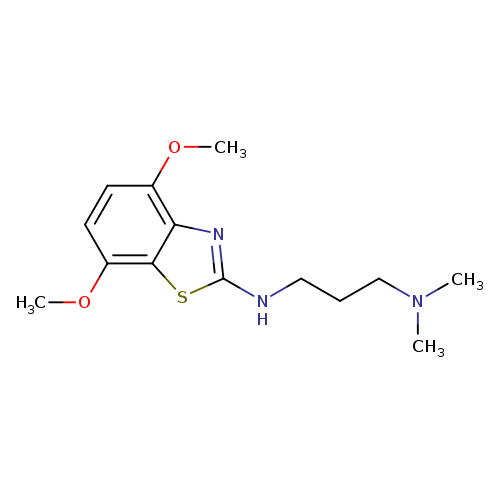
N'-(4,7-Dimethoxy-1,3-benzothiazol-2-yl)-N,N-dimethylpropane-1,3-diamineCatalog No.:AA01ARLI CAS No.:1105188-35-9 MDL No.:MFCD11986997 MF:C14H21N3O2S MW:295.4004 |
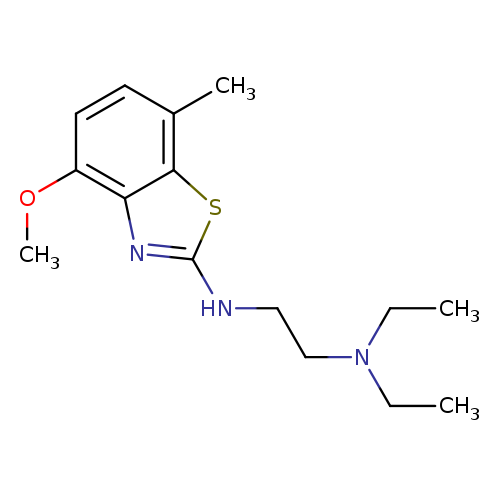
N,N-DIETHYL-N'-(4-METHOXY-7-METHYL-1,3-BENZOTHIAZOL-2-YL)ETHANE-1,2-DIAMI+Catalog No.:AA01ARLJ CAS No.:1105188-36-0 MDL No.:MFCD11986998 MF:C15H23N3OS MW:293.4276 |
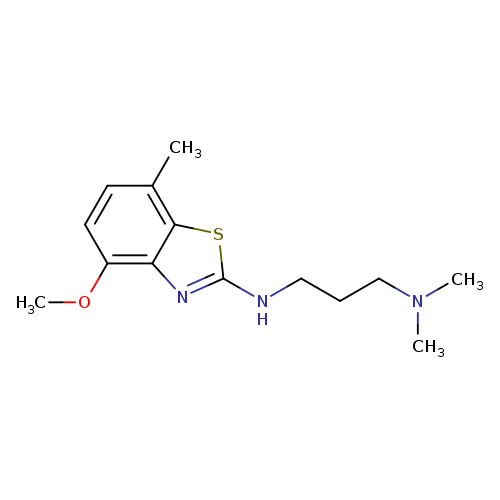
N'-(4-METHOXY-7-METHYL-1,3-BENZOTHIAZOL-2-YL)-N,N-DIMETHYLPROPANE-1,3-DIA+Catalog No.:AA01ARM6 CAS No.:1105188-38-2 MDL No.:MFCD11986999 MF:C14H21N3OS MW:279.4010 |
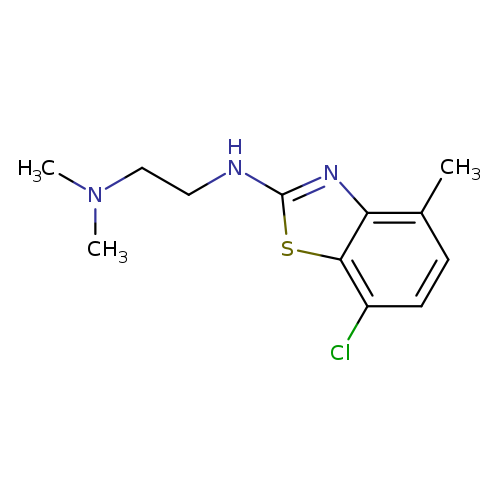
N'-(7-CHLORO-4-METHYL-1,3-BENZOTHIAZOL-2-YL)-N,N-DIMETHYLETHANE-1,2-DIAMI+Catalog No.:AA01ARM7 CAS No.:1105188-40-6 MDL No.:MFCD11987000 MF:C12H16ClN3S MW:269.7935 |
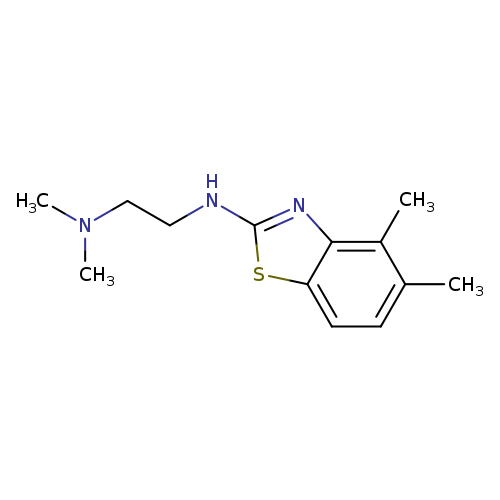
N'-(4,5-Dimethyl-1,3-benzothiazol-2-yl)-N,N-dimethylethane-1,2-diamineCatalog No.:AA01ARM8 CAS No.:1105188-42-8 MDL No.:MFCD11987001 MF:C13H19N3S MW:249.3751 |
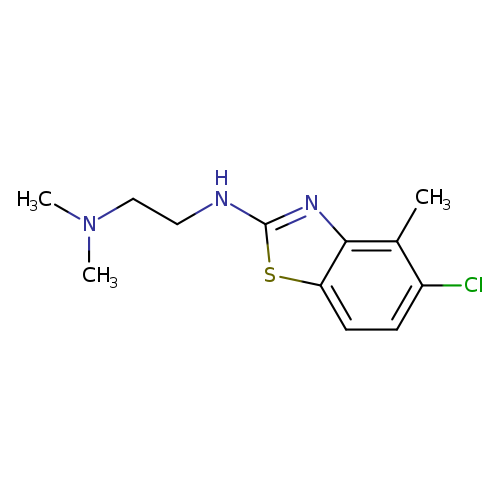
N'-(5-CHLORO-4-METHYL-1,3-BENZOTHIAZOL-2-YL)-N,N-DIMETHYLETHANE-1,2-DIAMI+Catalog No.:AA01ARMA CAS No.:1105188-47-3 MDL No.:MFCD11987003 MF:C12H16ClN3S MW:269.7935 |
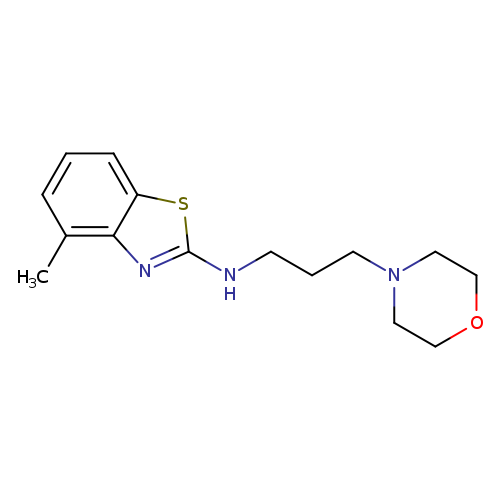
4-Methyl-n-(3-morpholin-4-ylpropyl)-1,3-benzothiazol-2-amineCatalog No.:AA01ARN3 CAS No.:1105188-50-8 MDL No.:MFCD11987004 MF:C15H21N3OS MW:291.4117 |
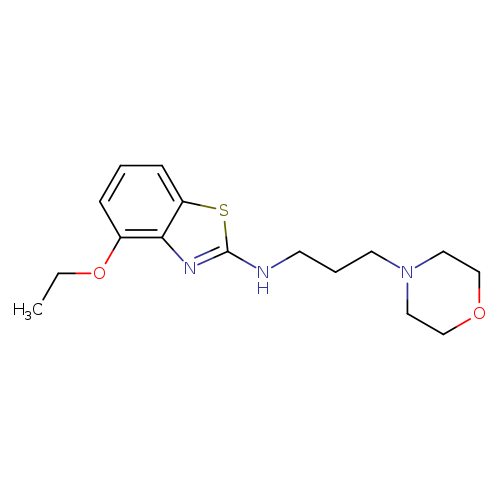
4-ETHOXY-N-(3-MORPHOLIN-4-YLPROPYL)-1,3-BENZOTHIAZOL-2-AMINECatalog No.:AA01ASAN CAS No.:1105188-54-2 MDL No.:MFCD11987005 MF:C16H23N3O2S MW:321.4377 |
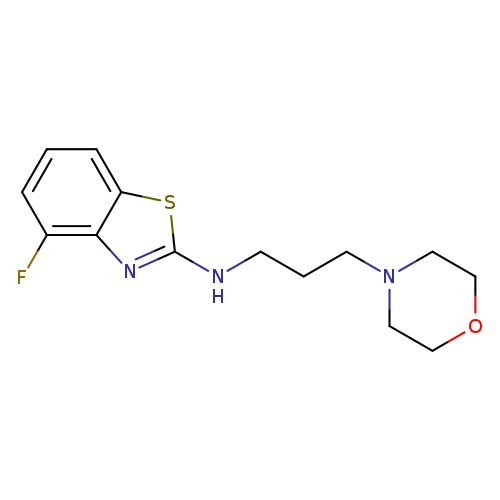
4-Fluoro-N-(3-morpholin-4-ylpropyl)-1,3-benzothiazol-2-amineCatalog No.:AA01ARN4 CAS No.:1105188-56-4 MDL No.:MFCD11987006 MF:C14H18FN3OS MW:295.3756 |
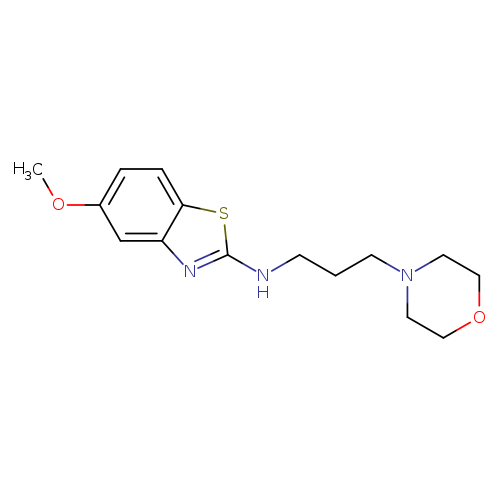
5-Methoxy-N-(3-morpholinopropyl)benzo[d]thiazol-2-amineCatalog No.:AA01FME2 CAS No.:1105188-59-7 MDL No.:MFCD11987007 MF:C15H21N3O2S MW:307.4111 |
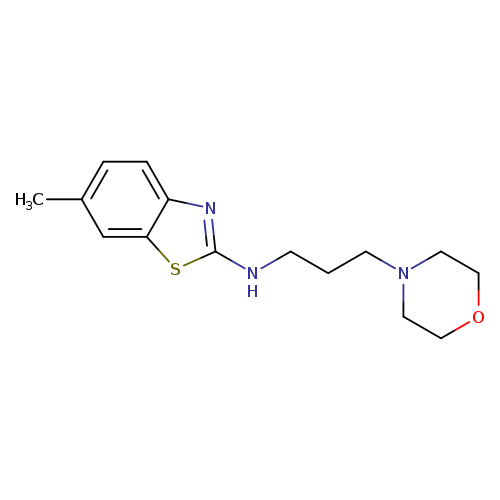
6-Methyl-N-(3-morpholin-4-ylpropyl)-1,3-benzothiazol-2-amineCatalog No.:AA01ARNU CAS No.:1105188-63-3 MDL No.:MFCD11987008 MF:C15H21N3OS MW:291.4117 |
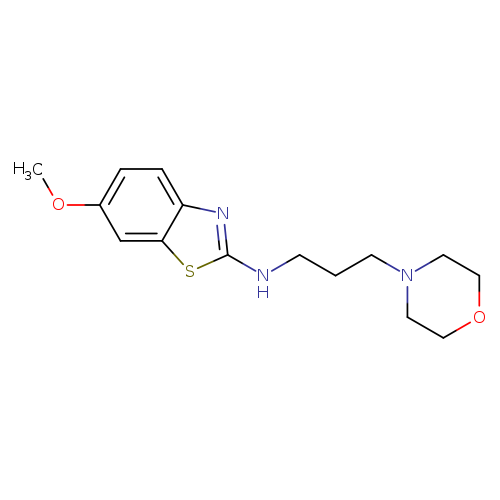
6-Methoxy-N-(3-morpholinopropyl)benzo[d]thiazol-2-amineCatalog No.:AA01FMDV CAS No.:1105188-66-6 MDL No.:MFCD11987009 MF:C15H21N3O2S MW:307.4111 |
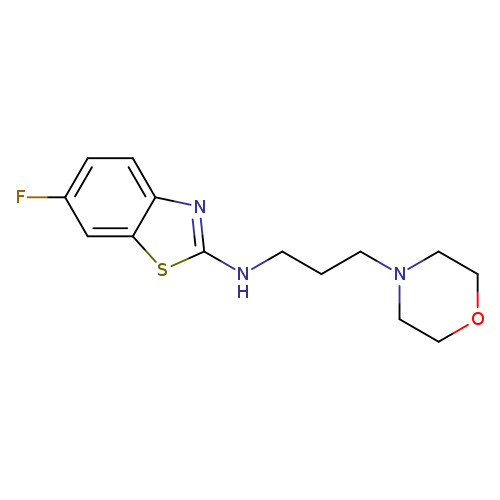
6-Fluoro-N-(3-morpholin-4-ylpropyl)-1,3-benzothiazol-2-amineCatalog No.:AA01ARNV CAS No.:1105188-69-9 MDL No.:MFCD11987010 MF:C14H18FN3OS MW:295.3756 |
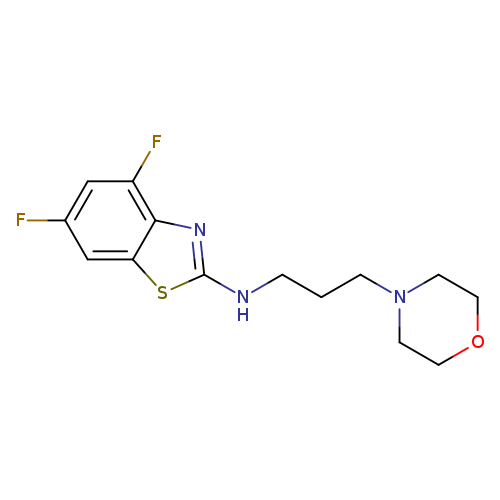
4,6-Difluoro-N-(3-morpholinopropyl)benzo[d]thiazol-2-amineCatalog No.:AA01FMDQ CAS No.:1105188-72-4 MDL No.:MFCD11987011 MF:C14H17F2N3OS MW:313.3661 |
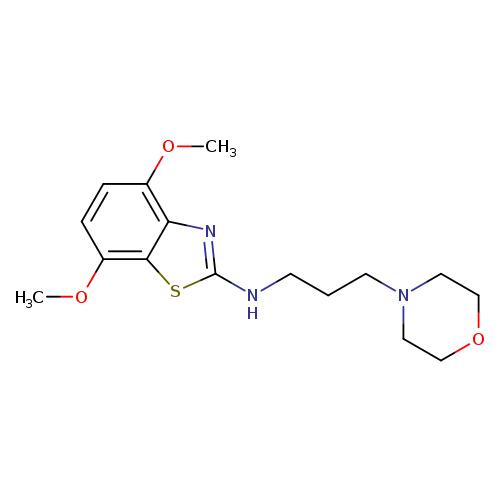
4,7-Dimethoxy-N-(3-morpholinopropyl)benzo[d]thiazol-2-amineCatalog No.:AA01FME1 CAS No.:1105188-79-1 MDL No.:MFCD11987013 MF:C16H23N3O3S MW:337.4371 |
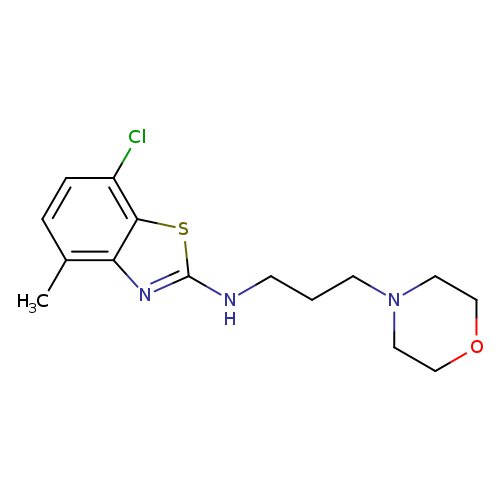
7-Chloro-4-methyl-N-(3-morpholinopropyl)benzo[d]thiazol-2-amineCatalog No.:AA01FMDF CAS No.:1105188-86-0 MDL No.:MFCD11987015 MF:C15H20ClN3OS MW:325.8568 |
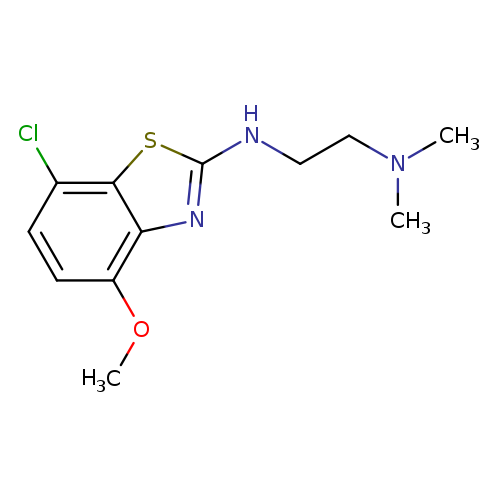
N'-(7-CHLORO-4-METHOXY-1,3-BENZOTHIAZOL-2-YL)-N,N-DIMETHYLETHANE-1,2-DIAM+Catalog No.:AA01AROO CAS No.:1105188-90-6 MDL No.:MFCD11987016 MF:C12H16ClN3OS MW:285.7929 |
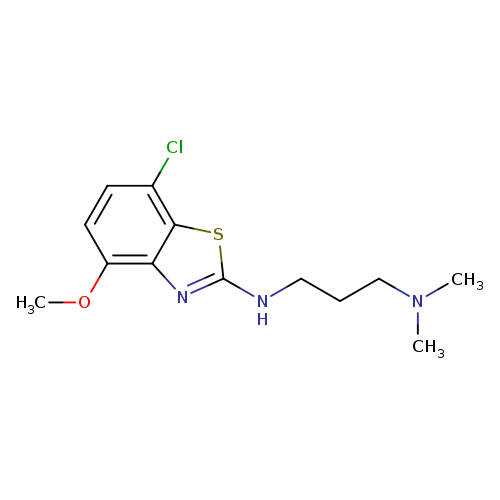
N'-(7-CHLORO-4-METHOXY-1,3-BENZOTHIAZOL-2-YL)-N,N-DIMETHYLPROPANE-1,3-DIA+Catalog No.:AA01AROP CAS No.:1105188-94-0 MDL No.:MFCD11987017 MF:C13H18ClN3OS MW:299.8195 |
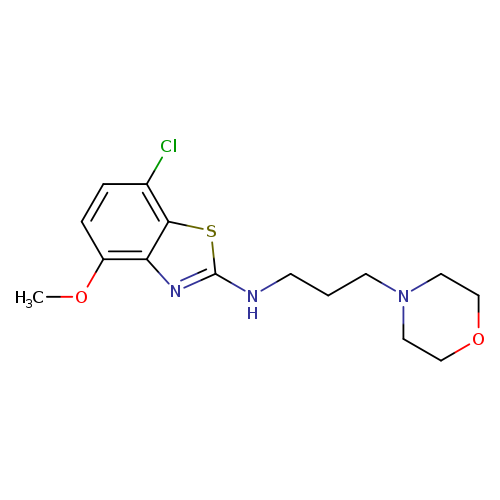
7-Chloro-4-methoxy-N-(3-morpholinopropyl)benzo[d]thiazol-2-amineCatalog No.:AA01FME0 CAS No.:1105188-98-4 MDL No.:MFCD31731182 MF:C15H20ClN3O2S MW:341.8562 |
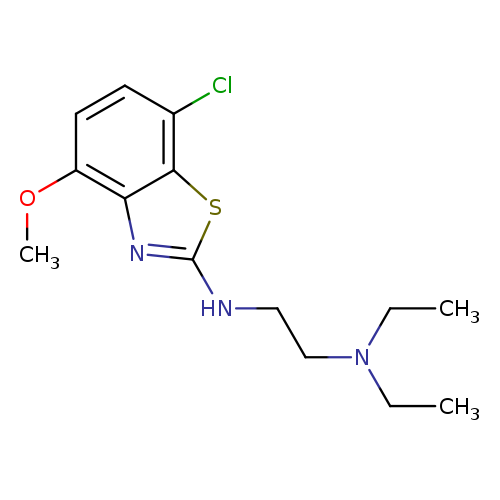
N'-(7-CHLORO-4-METHOXY-1,3-BENZOTHIAZOL-2-YL)-N,N-DIETHYLETHANE-1,2-DIAMI+Catalog No.:AA01AS8X CAS No.:1105189-02-3 MDL No.:MFCD11987019 MF:C14H20ClN3OS MW:313.8461 |
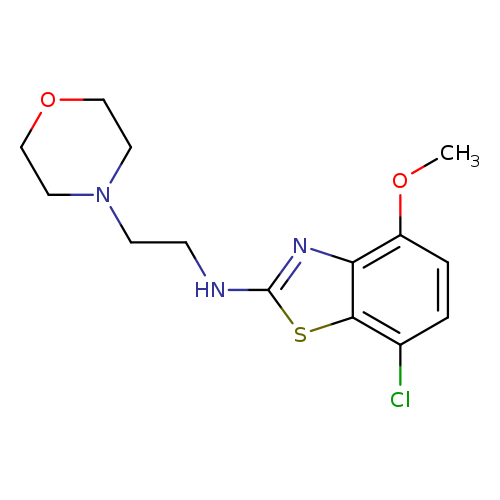
7-Chloro-4-methoxy-N-(2-morpholinoethyl)benzo[d]thiazol-2-amineCatalog No.:AA01FME5 CAS No.:1105189-05-6 MDL No.:MFCD11987020 MF:C14H18ClN3O2S MW:327.8296 |
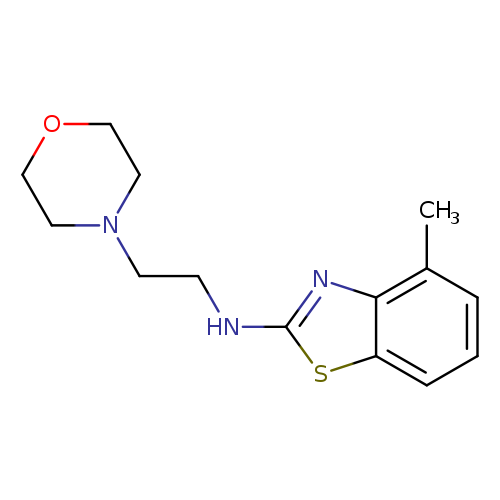
4-Methyl-n-(2-morpholin-4-ylethyl)-1,3-benzothiazol-2-amineCatalog No.:AA01FME6 CAS No.:1105189-08-9 MDL No.:MFCD11987021 MF:C14H19N3OS MW:277.3852 |
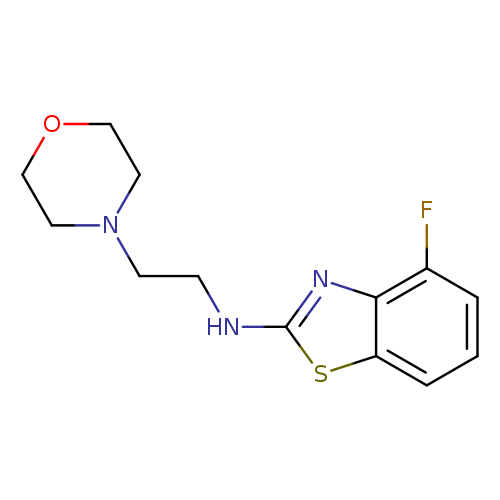
4-Fluoro-N-(2-morpholin-4-ylethyl)-1,3-benzothiazol-2-amineCatalog No.:AA01ARPM CAS No.:1105189-11-4 MDL No.:MFCD11987022 MF:C13H16FN3OS MW:281.3490 |
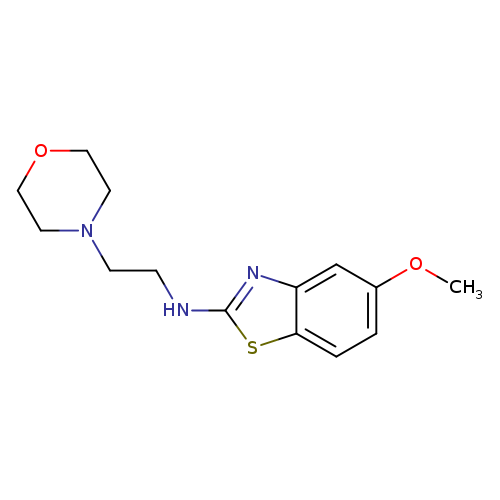
5-Methoxy-N-(2-morpholin-4-ylethyl)-1,3-benzothiazol-2-amineCatalog No.:AA01ARPN CAS No.:1105189-14-7 MDL No.:MFCD11987023 MF:C14H19N3O2S MW:293.3846 |
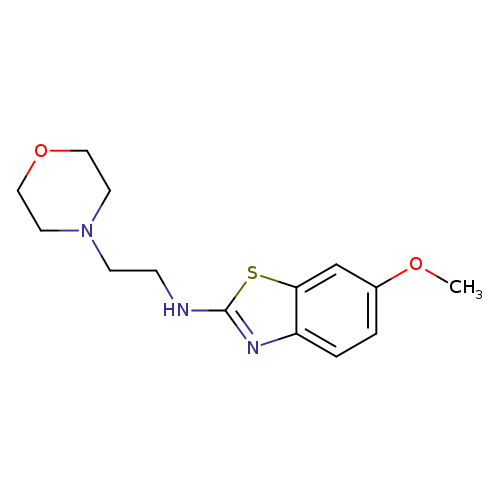
6-Methoxy-N-(2-morpholin-4-ylethyl)-1,3-benzothiazol-2-amineCatalog No.:AA01ARPP CAS No.:1105189-20-5 MDL No.:MFCD11987025 MF:C14H19N3O2S MW:293.3846 |
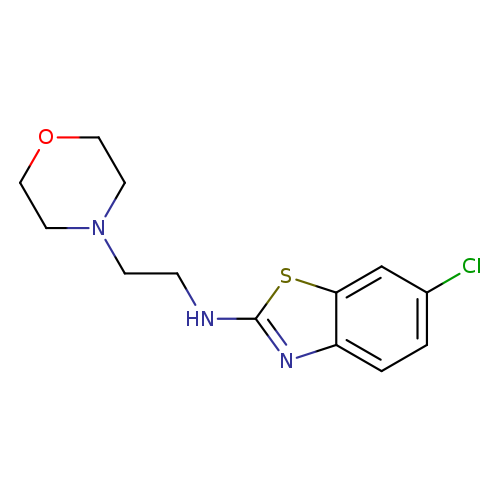
6-Chloro-N-(2-morpholin-4-ylethyl)-1,3-benzothiazol-2-amineCatalog No.:AA01ARPQ CAS No.:1105189-23-8 MDL No.:MFCD11987026 MF:C13H16ClN3OS MW:297.8036 |
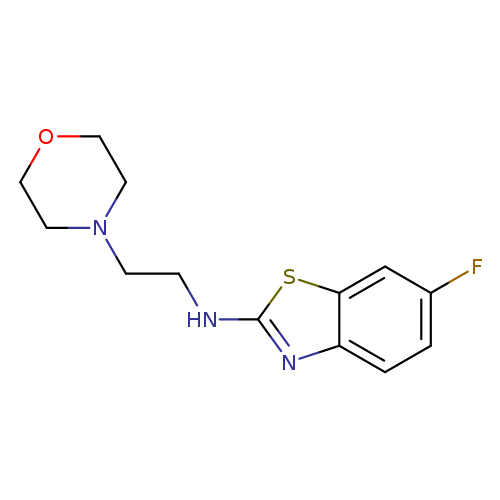
6-Fluoro-N-(2-morpholin-4-ylethyl)-1,3-benzothiazol-2-amineCatalog No.:AA01ARQF CAS No.:1105189-26-1 MDL No.:MFCD11987027 MF:C13H16FN3OS MW:281.3490 |
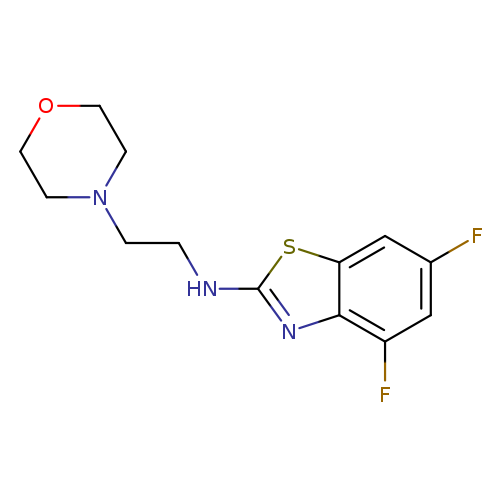
4,6-Difluoro-N-(2-morpholin-4-ylethyl)-1,3-benzothiazol-2-amineCatalog No.:AA01ARQG CAS No.:1105189-32-9 MDL No.:MFCD11987029 MF:C13H15F2N3OS MW:299.3395 |
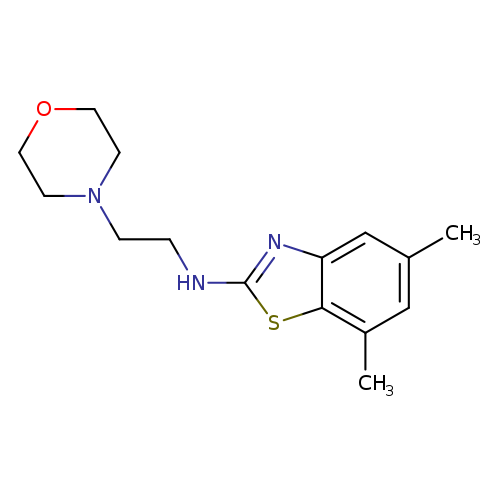
5,7-Dimethyl-N-(2-morpholin-4-ylethyl)-1,3-benzothiazol-2-amineCatalog No.:AA01ARQH CAS No.:1105189-35-2 MDL No.:MFCD11987030 MF:C15H21N3OS MW:291.4117 |
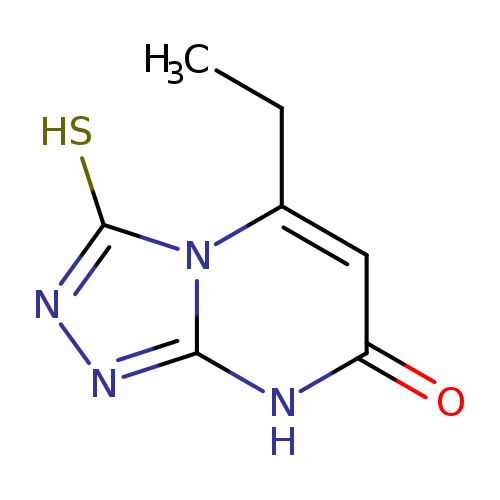
5-Ethyl-3-mercapto[1,2,4]triazolo[4,3-(a)]pyrimidin-7(8(h))-oneCatalog No.:AA01ARP8 CAS No.:1105189-36-3 MDL No.:MFCD11986381 MF:C7H8N4OS MW:196.2296 |
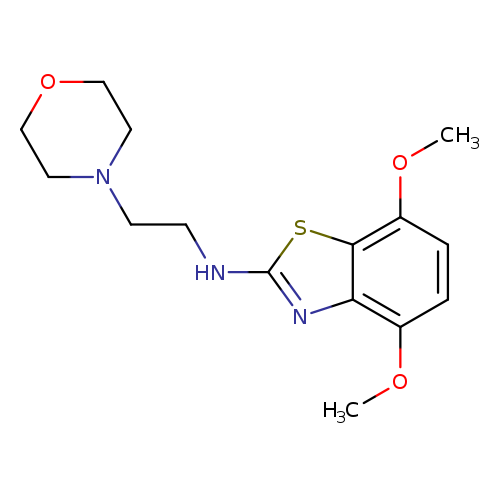
4,7-Dimethoxy-N-(2-morpholinoethyl)benzo[d]thiazol-2-amineCatalog No.:AA01FME4 CAS No.:1105189-39-6 MDL No.:MFCD11987031 MF:C15H21N3O3S MW:323.4105 |
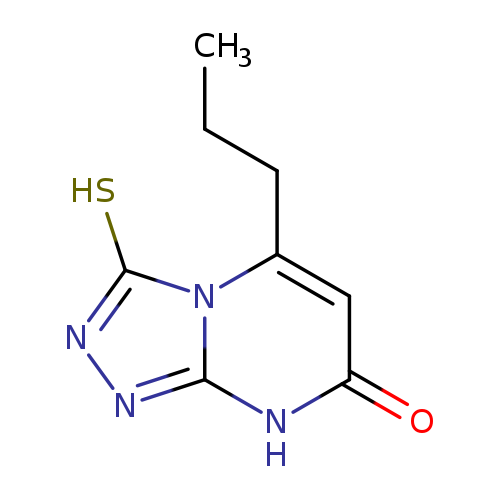
3-MERCAPTO-5-PROPYL[1,2,4]TRIAZOLO[4,3-(A)]PYRIMIDIN-7(8(H))-ONECatalog No.:AA01ARP9 CAS No.:1105189-40-9 MDL No.:MFCD11986382 MF:C8H10N4OS MW:210.2562 |
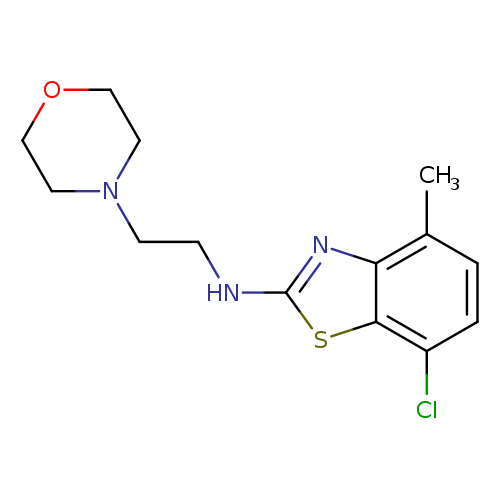
7-Chloro-4-methyl-N-(2-morpholinoethyl)benzo[d]thiazol-2-amineCatalog No.:AA01FMDS CAS No.:1105189-47-6 MDL No.:MFCD11987033 MF:C14H18ClN3OS MW:311.8302 |
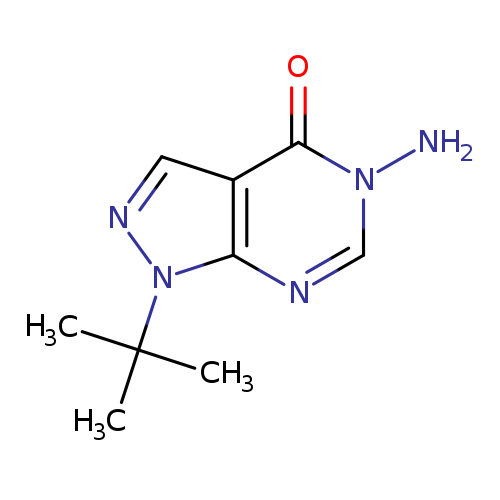
5-Amino-1-tert-butyl-1,5-dihydro-4H-pyrazolo[3,4-d]pyrimidin-4-oneCatalog No.:AA01ARPX CAS No.:1105189-48-7 MDL No.:MFCD11986385 MF:C9H13N5O MW:207.2324 |
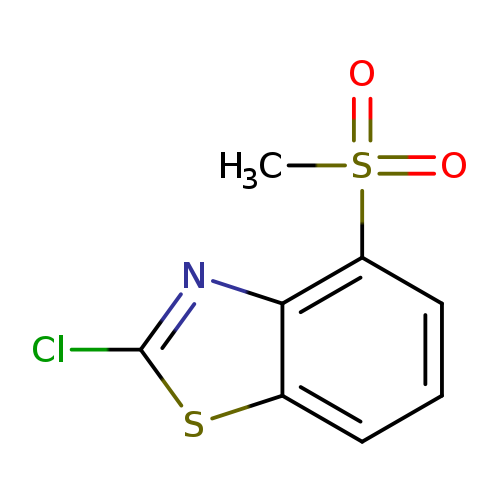
2-chloro-4-methanesulfonyl-1,3-benzothiazoleCatalog No.:AA01ARR5 CAS No.:1105189-51-2 MDL No.:MFCD11987034 MF:C8H6ClNO2S2 MW:247.7217 |
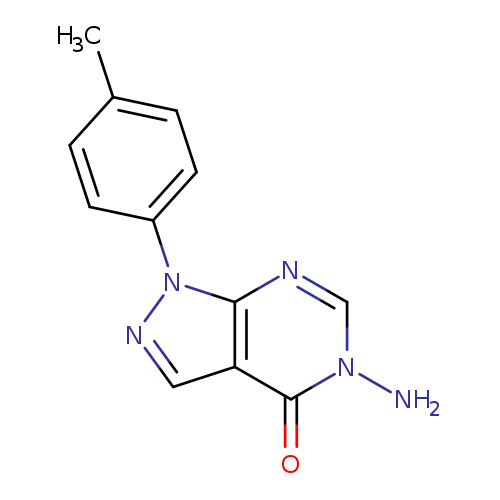
5-Amino-1-(4-methylphenyl)-1,5-dihydro-4h-pyrazolo[3,4-d]pyrimidin-4-oneCatalog No.:AA01ARPY CAS No.:1105189-52-3 MDL No.:MFCD11986386 MF:C12H11N5O MW:241.2486 |
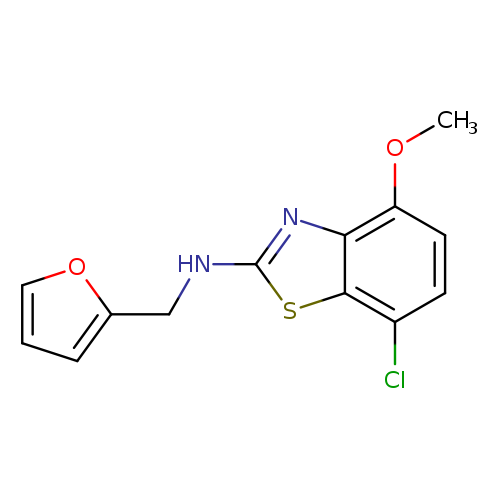
7-Chloro-n-(2-furylmethyl)-4-methoxy-1,3-benzothiazol-2-amineCatalog No.:AA01ARRW CAS No.:1105189-55-6 MDL No.:MFCD11987036 MF:C13H11ClN2O2S MW:294.7566 |
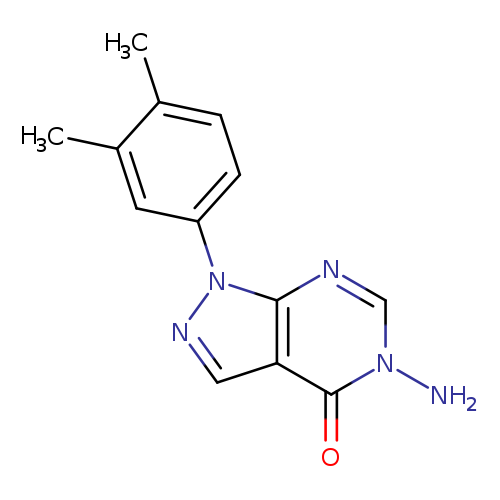
5-AMINO-1-(3,4-DIMETHYLPHENYL)-1,5-DIHYDRO-4H-PYRAZOLO[3,4-D]PYRIMIDIN-4-+Catalog No.:AA01ARPZ CAS No.:1105189-56-7 MDL No.:MFCD11986387 MF:C13H13N5O MW:255.2752 |
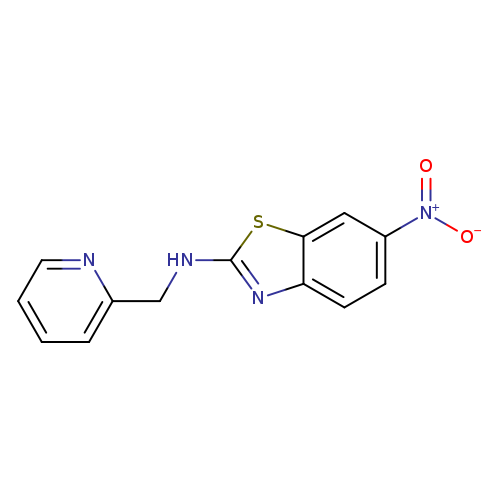
6-Nitro-N-(pyridin-2-ylmethyl)-1,3-benzothiazol-2-amineCatalog No.:AA01ARRX CAS No.:1105189-59-0 MDL No.:MFCD11987037 MF:C13H10N4O2S MW:286.3091 |
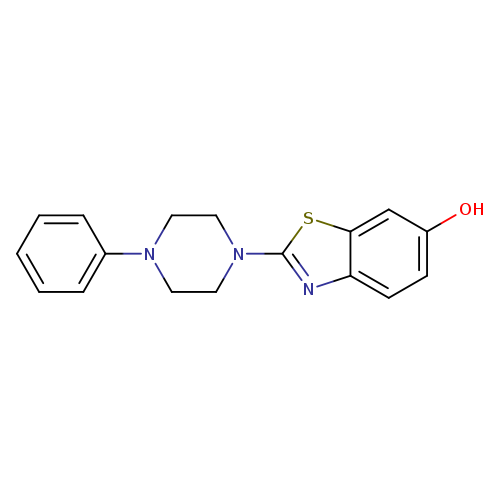
2-(4-Phenylpiperazin-1-yl)benzo[d]thiazol-6-olCatalog No.:AA01FMMG CAS No.:1105189-63-6 MDL No.:MFCD11987040 MF:C17H17N3OS MW:311.4014 |
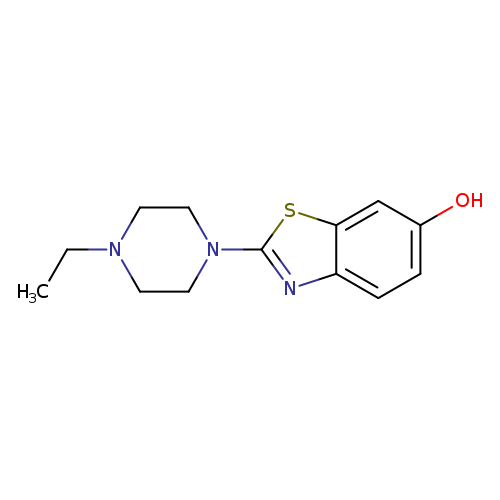
2-(4-Ethylpiperazin-1-yl)-1,3-benzothiazol-6-olCatalog No.:AA01ARSO CAS No.:1105189-67-0 MDL No.:MFCD11987042 MF:C13H17N3OS MW:263.3586 |
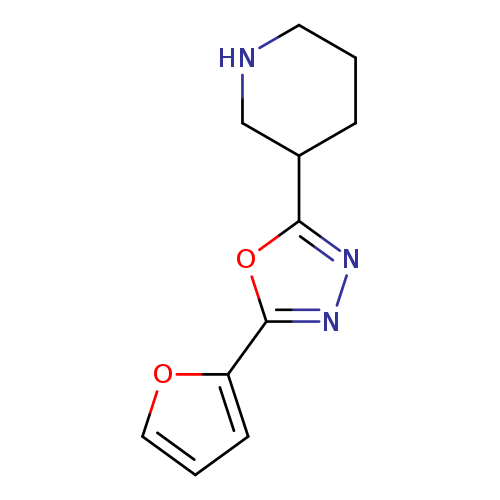
3-[5-(furan-2-yl)-1,3,4-oxadiazol-2-yl]piperidineCatalog No.:AA01ARIJ CAS No.:1105189-75-0 MDL No.:MFCD11937169 MF:C11H13N3O2 MW:219.2398 |
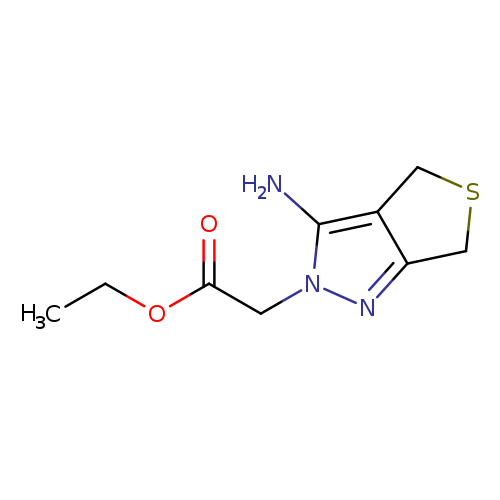
Ethyl (3-amino-4H-thieno[3,4-c]pyrazol-2(6H)-yl)acetateCatalog No.:AA01ARL0 CAS No.:1105190-33-7 MDL No.:MFCD11986669 MF:C9H13N3O2S MW:227.2834 |
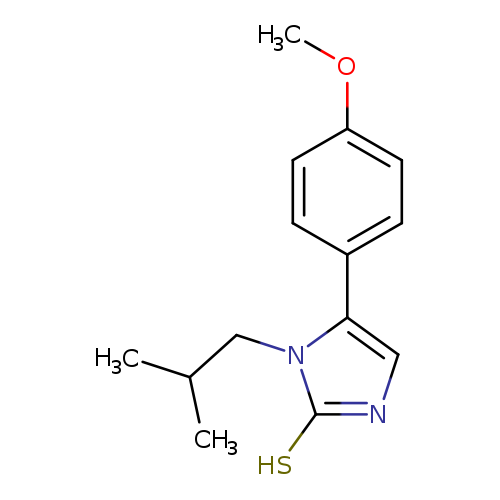
1-Isobutyl-5-(4-methoxyphenyl)-1,3-dihydro-2H-imidazole-2-thioneCatalog No.:AA01AQLT CAS No.:1105190-34-8 MDL No.:MFCD16653449 MF:C14H18N2OS MW:262.3705 |
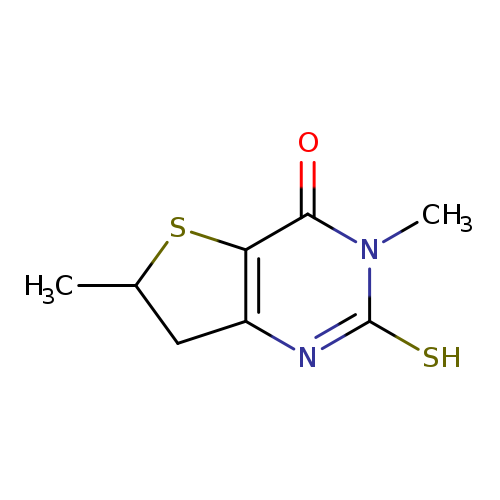
3,6-dimethyl-2-sulfanyl-3H,4H,6H,7H-thieno[3,2-d]pyrimidin-4-oneCatalog No.:AA019TKK CAS No.:1105190-40-6 MDL No.:MFCD11986411 MF:C8H10N2OS2 MW:214.3078 |
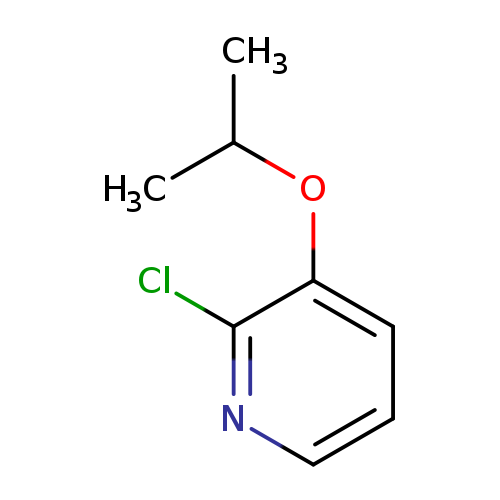
2-Chloro-3-isopropoxypyridineCatalog No.:AA00HBNS CAS No.:1105190-61-1 MDL No.:MFCD16653100 MF:C8H10ClNO MW:171.6241 |
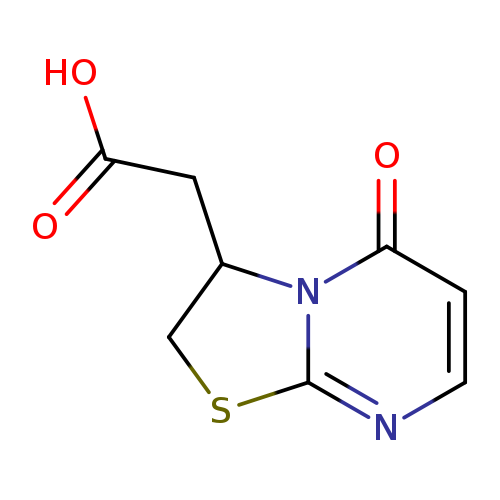
(5-Oxo-2,3-dihydro-5H-[1,3]thiazolo[3,2-a]pyrimidin-3-yl)acetic acidCatalog No.:AA01ARHL CAS No.:1105190-66-6 MDL No.:MFCD11518875 MF:C8H8N2O3S MW:212.2257 |
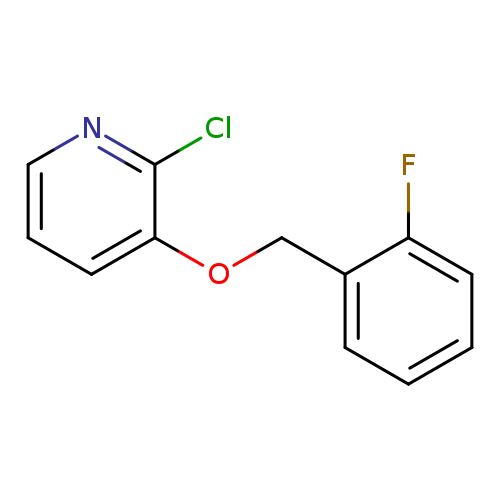
2-Chloro-3-[(2-fluorobenzyl)oxy]pyridineCatalog No.:AA01ARMZ CAS No.:1105190-67-7 MDL No.:MFCD11986848 MF:C12H9ClFNO MW:237.6574 |